Abstract
Background
The aim of this study was to investigate the effects of Saikosaponin-d (SSd) combined with radiotherapy on SMMC-7721 hepatoma cell lines and its mechanism.
Material/Methods
SMMC-7721 hepatoma cell lines are selected in our research. With MTT (methylthiazolyldiphenyl-tetrazolium-bromide) method, the effects of SSd and radiation on inhibiting SMMC-7721 cell growth were investigated. We also used transmission electron microscopy (TEM) to observe ultrastructural changes of cells. Colorimetry methods were used to measure content changes of glutathione (GSH) and malondialdehyde (MDA) in cells.
Results
Both SSd and radiation inhibited the growth of SMMC-7721 cells. The combination of SSd and radiotherapy had a time-dependent synergistic effect. Radiation caused ultrastructural damage to cells, and the damage was enhanced in combination with SSd. Radiation decreased the GSH content and increased the MDA content in cells, and this effect was suppressed after the intervention of SSd.
Conclusions
SSd can inhibit the growth of SMMC-7721 hepatoma cell lines in vitro. Additionally, it significantly enhances the effects of radiation on inhibiting the growth of SMMC-7721 hepatoma cell lines, and up-regulates the antioxidant level after the radiotherapy. Thus, SSd could be an ideal radiotherapy sensitizer for the treatment of liver cancer.
MeSH Keywords: Saikosaponin-d, Radiation, Hepatocellular Carcinoma, Glutathione
Background
Hepatocellular carcinoma is one of the major diseases that severely threat human health. The morbidity and mortality of liver cancer are increasing [1,2]. According to WHO statistics, there are about 1 million new cases of liver cancer per year world-wide [3,4]. In China, liver cancer accounts for 41% of all new cancer cases annually. With constant technological advancement, radiotherapy has become a key approach of liver cancer treatment. However, liver presents poor tolerance and low radiosensitivity in radiotherapy. The adverse effects of radiation interfere with the smooth implementation of radiotherapy and diminish its effects. Thus, seeking effective radiation sensitizers has become a focus in the research field of liver cancer treatment in recent years.
Saikosaponin-d (SSd) is the dominant active ingredient isolated and extracted from a traditional Chinese medicinal herb Bupleurum. It has various functions like inhibiting tumor cell growth and proliferation [5], inducing tumor cell apoptosis [6], inhibiting tumor metastasis [7], and sensitizing radiotherapy [8,9]. Due to its extremely dynamic antineoplastic activity, SSd is expected to be an effective radiation sensitizer. The present study observed the in vitro effects of SSd and radiation on SMMC-7721 hepatoma cell lines, analyzed its mechanism, and provides certain theoretical contributions for further clinical practice.
Material and Methods
Cells and reagents
SMMC-7721 hepatoma cell lines was purchased from the Medical Experimental Animal Center of the Fourth Military Medical University (Xi’an, China) and SSd was obtained from Sigma Chemical (St. Louis, MO). The Glutathione Malondialdehyde Detection kit was purchased from Nanjing Jiancheng Bioengineering Institute.
Cell culture and experimental groups
The cells were cultured in RPMI-1640 medium (PAA Laboratories GmbH, Austria) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin G, and 100 μg/ml streptomycin sulfate (GIBCO, Invitrogen) in a humidified atmosphere containing 5% CO2 at 37°C. Three groups of SMMC-7721 cells were treated with radiation alone, SSd alone, or a combination of radiation and SSd. Group S is the Saikosaponin-d group; Group R is the radiation group; and Group R+S is the combination of Saikosaponin-d with radiation. Radiation was delivered at a dose of 2 Gy (6 MV, and a dose rate of 400 cGy/min) by using an X-ray linear accelerator at room temperature. SSd was also administered at concentrations (3 μg/ml) as described previously [9]. SSd was added to the cultures at 2 h before irradiation. Control cultures received a carrier solvent consisting of 0.1% DMSO. All study procedures were approved by the Animal Care and Use Committee of Medical College, Xi’an Jiaotong University.
Cell viability determination
Cell viability was detected by MTT assay. SMMC-7721 cells were seeded into a 96-well plate (5×103 cells/well) and incubated at 37°C in 5% CO2 for various periods as desired. MTT solution (5 mg/ml; Sigma, St. Louis, MO) was added (20 μl/well) and the cells were incubated for another 4 h. Supernatants were removed and formazan crystals were dissolved in 200 μl of DMSO. Optical density was determined at 492 nm by using a multi-microplate test system (POLARstar OPTIMA, BMG Labtechnologies, Germany). The assay was conducted in quadruplicate. The inhibition rate (IR) of cell growth was calculated using the following equation: IR=(1-average OD value of experimental group/average OD value of control group) ×100%.
Transmission electron microscope (TEM) observation of ultrastructural changes of hepatoma cells
SMMC-7721 cells in each experimental group were collected, digested by 2.5g/L trypsin, centrifuged at 3000 r/min, washed by PBS and kept in EP tubes. Next, cells were fixed first by 25 g/L glutaraldehyde, then by 10g/L osmic acid, dehydrated by graded ethanol, infiltrated, and embedded in epoxy resin. After slicing by the ultramicrotome, cells were stained by uranyl acetate and lead citrate. Next, a Hitachi H-600 transmission electron microscope was used for observation, filming, and photographing.
Determination of MDA and GSH content in hepatoma cells
Cells at the logarithmic growth phase were taken and digested by 0.25% trypsin. Then, cells were treated by mechanical isolation into single-cell suspension, which was diluted with RPMI 1640 medium to the concentration of 4×104 cell/mL. Next, the suspension was inoculated into a flask of 25 ml, with 3 ml for each tube. All tubes were cultured for 24 h at an incubator with saturation humidity and 5% CO2 at 37°C. After that, the culture solution was replaced by fresh solution. RPMI 1640 was added in Group C and Group R. Additionally, Group R received 2 Gy of radiation at the same time of culture solution replacement. Three mg/L SSd was added in Group S and 3 mg/L SSd combined with 2 Gy of radiation were administered for Group R+S. After 48 h of further culturing, cells were collected and MDA and GSH content were determined according to kit instructions.
Statistical analysis
Quantitative data are presented as the mean ± standard error of the mean (SEM) and analyzed by one-way ANOVA. Statistical analyses were performed using SPSS software (version 13.0). Tukey’s post hoc analyses were conducted to assess the difference between groups. Data were considered significant if P<0.05.
Results
Effects of SSd and radiation on inhibiting hepatoma cell growth
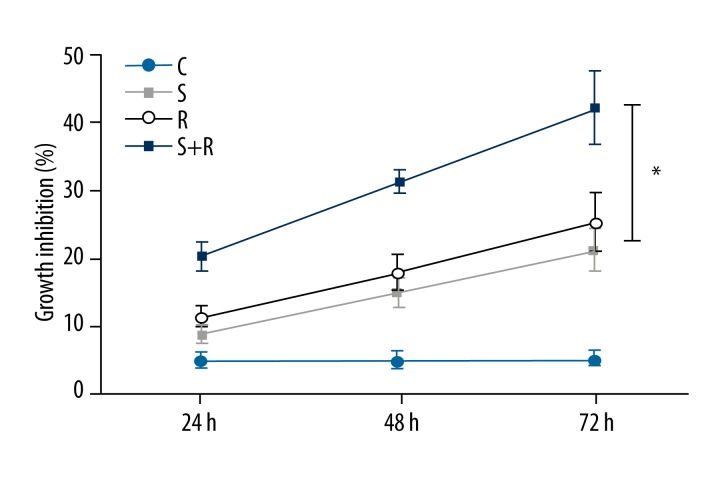
Under a 100× light microscope, the number of cells in each group (Figure 1) was counted. The cell number in the intervention group was less than in the control group. SSd combined with radiation had a significantly greater inhibition of cell growth. MTT demonstrated that SSd and radiation could inhibit the growth of SMMC-7721 cells, and the combined use of both SSd and radiation showed time-dependent synergies, as shown in Figure 2. At different time points, differences in the inhibition rates between Group R+S and Group R were statistically significant (P<0.05).
Figure 1.
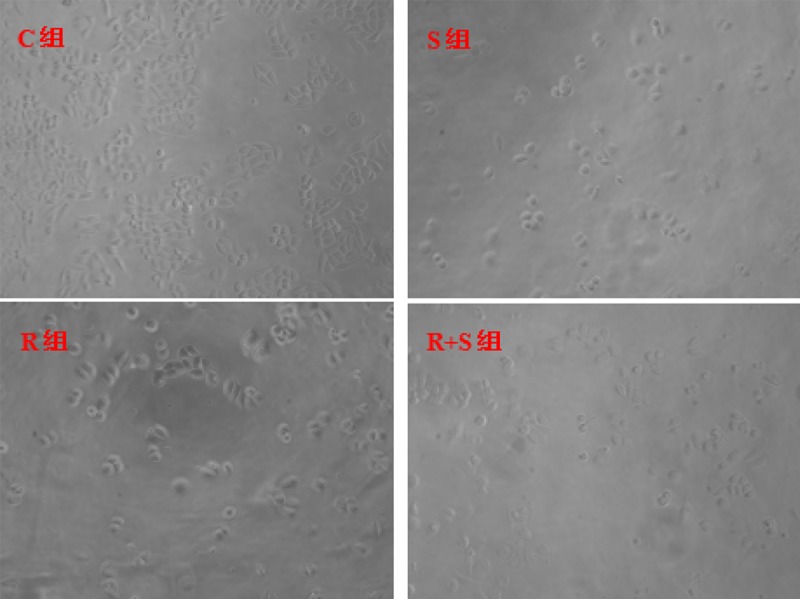
Effects of SSd and radiation on hepatoma cell (×100 in magnification). C – control group; S – Saikosaponin-d group; R – radiation group; R+S – the combined group of Saikosaponin-d with radiation.
Figure 2.
Effects of SSd and radiation on the inhibition ratio of hepatoma cell growth. C – control group; S – Saikosaponin-d group; R – radiationgroup; R+S – the combined group of Saikosaponin-d with radiation. * P<0.05 R+S group vs. R or S group.
Observation of hepatoma cell apoptosis
Under the transmission electron microscope, the shape of control hepatoma cell was regular, with a circular cell nucleus in the center of cells, nucleolus in the center of cell nucleus, and rich organelles. Cell ultrastructure in Group S was similar to that of Group C. The structure of some hepatoma cells in Group R was not clear and some organelles expanded slightly. The cell volume in Group R+S was reduced but the volume of cell nuclei was larger, showing significant deformity. In the nucleus, intranuclear pseudoinclusion was formed. The nucleolus was large and obvious, often locating at the center or moving aside. The number of organelles was reduced and most organelles expanded. A remarkable amount of microvilli-like protrusions in some cytomembranes was also observed. The connecting structures among cells decreased, indicating that radiation induced hepatoma cell damage and the damage was significantly enhanced after the combined application of SSd (Figure 3).
Figure 3.
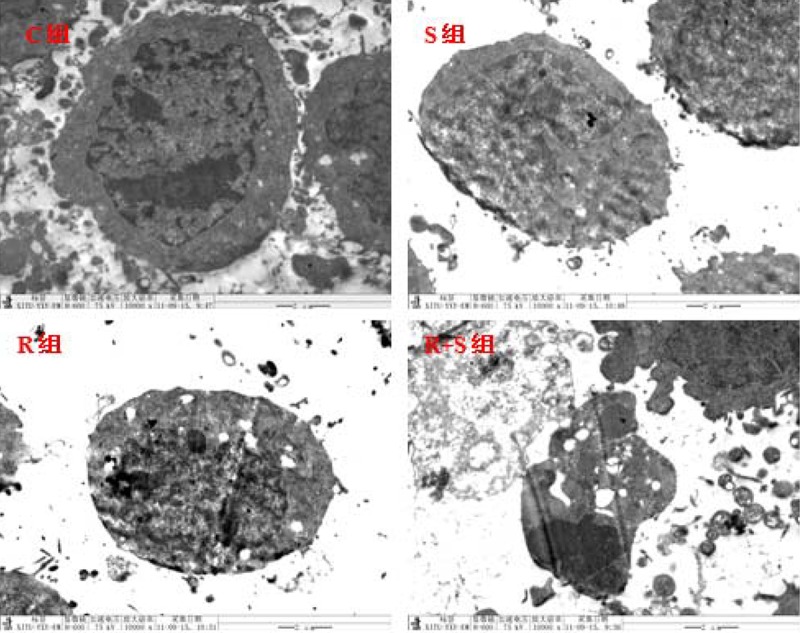
Ultrastructural changes of hepatoma cells after different sets of intervention (×10000 in magnification). C – control group; S – Saikosaponin-d group; R – radiation group; R+S – the combined group of Saikosaponin-d with radiation.
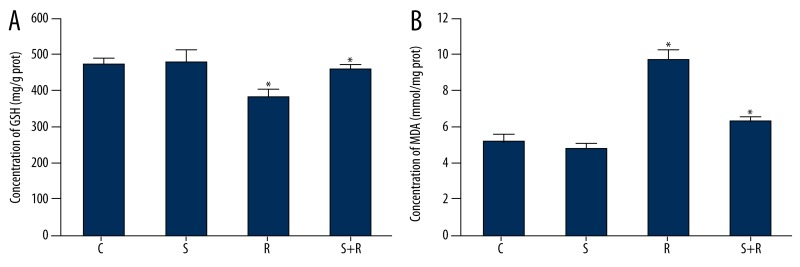
Effects of SSd and radiation on GSH and MDA contents in hepatoma cells
Compared with cells in Group C, GSH contents of cells in Group R decreased from 475.31±67.25 mg/g prot to 396.25±40.32 mg/g prot, and MDA contents rose from 4.95±0.38 mmol/mg prot to 8.96±0.69 mmol/mg prot. With the application of SSd, irradiated cells showed rising GSH and decreasing MDA contents. The difference between the 2 groups was statistically significant (P<0.05). Specific data are shown in Figure 4.
Figure 4.
Effects of SSd and radiation on GSH (A) and MDA (B) contents in cells. C – control group; S – Saikosaponin-d group; R – radiation group; R+S – the combined group of Saikosaponin-d with radiation. * P<0.05 R group vs. control group or S+R group.
Discussion
This research, as well as our previous studies, show that SSd inhibits the growth of SMMC-7721 hepatoma cell lines in vitro, and simultaneously enhanced the effects of radiation on inhibiting the growth of SMMC-7721 hepatoma cell lines. The effects of SSd were time-dependent [6,9]. SSd is believed to be capable of reducing the liver damage caused by oxygen free radicals and other reactive substances, enhancing liver metabolism [10]. Therefore, SSd can protect the healthy liver tissues around the irradiated area. As a result, our research now focuses on determining whether SSd can enhance the killing of tumor cells while protect healthy cells during radiotherapy.
With the further research on effects of oxidative stress in radiotherapy, it has been discovered that under normal physiological conditions, organisms can rapidly eliminate anomaly-free radicals and reactive oxygen by antioxidants and enzymes [11,12], thus maintaining the antioxidant system homeostasis in vivo [13]. After the radiation, a great amount of reactive oxygen can be generated, which is constantly accumulated and induces oxidative stress and upsets the intracellular oxidant/antioxidant balance[14]. Thereby, structural damage of cells and changes in enzyme activity can be generated via chain reaction [15]. MDA is a decomposition product of lipid peroxidation, and MDA content changes reflect the degree to which free radical attack cells. As an indispensible antioxidant enzyme, GSH has become a focus of research in the field of radiosensitization treatment [16]. Experiments in this field have shown that after radiation exposure, oxidative stress of SMMC-7721 hepatoma cells can be produced, thus reducing GSH content and increasing MDA content in cells. However, SSd plays a role in regulating oxidative stress and increasing GSH content. GSH is the most dominant antioxidant system in cells, which can rapidly eliminate excessive ROS. However, the effects of SSd in the sensibilization of radiotherapy remain controversial. Substantial studies have demonstrated that increasing ROS and down-regulating SOD and GSH activities after radiotherapy can accomplish the sensitization of radiotherapy [17]. However, Antonella et al. [18] pointed out that the upregulation of antioxidant expression can enhance the radiosensitivity of tumor cells, while protect healthy tissues from the radiation; our research reached similar findings. Our findings suggest that the antioxidant effects of SSd are capable of regulating intracellular oxidative stress and scavenging free oxygen radicals. However, effects of SSd on enhancing the role of radiotherapy in hepatoma cell inhibition are not correlated with the oxidative stress of organisms. Further experiments are needed to verify the enhancement approach of SSd.
Conclusions
SSd can inhibit the growth of SMMC-7721 hepatoma cell lines in vitro, and simultaneously enhance the effects of radiation on inhibiting the growth of SMMC-7721 hepatoma cell lines. SSd also up-regulates the antioxidant enzyme level after radiotherapy, and may be an ideal liver cancer radiation sensitizer.
Footnotes
Conflict of interest statement
The authors disclose no potential conflicts.
Source of support: National Natural Science Foundation of China (No. 30973810)
References
- 1.Kim DY, Han KH. How to improve treatment outcomes for hepatocellular carcinoma of intermediate and advanced stage. Dig Dis. 2012;30:598–602. doi: 10.1159/000343088. [DOI] [PubMed] [Google Scholar]
- 2.Taura N, Ichikawa T, Miyaaki H, et al. Frequency of elevated biomarkers in patients with cryptogenic hepatocellular carcinoma. Med Sci Monit. 2013;19:742–50. doi: 10.12659/MSM.889361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Ji X-Q, Ruan X-J, Chen H, et al. Somatostatin analogues in advanced hepatocellular carcinoma: An updated systematic review and meta-analysis of randomized controlled trials. Med Sci Monit. 2011;17(8):RA169–76. doi: 10.12659/MSM.881892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu BH, Pu R, Zhang GP, et al. Effect of Saikosaponins-d on reversing malignant phenotype of HepG2 cells in vitro. Zhonghua Gan Zang Bing Za Zhi. 2011;19:764–7. doi: 10.3760/cma.j.issn.1007-3418.2011.10.011. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 6.Wang BF, Dai ZJ, Wang XJ, et al. Saikosaponin-d increases the radiosensitivity of smmc-7721 hepatocellular carcinoma cells by adjusting the g0/g1 and g2/m checkpoints of the cell cycle. BMC Complement Altern Med. 2013;13:263. doi: 10.1186/1472-6882-13-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang BF, Cheng YA, Dang SS. Angiogenesis inhibitory effect of saikosaponin-d on chicken embryo. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:425–29. [in Chinese] [PubMed] [Google Scholar]
- 8.Wang Q, Zheng X-l, Yang L, et al. Reactive oxygen species-mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin-induced cytotoxicity in cancer cells. J Exp Clin Cancer Res. 2010;29:159–65. doi: 10.1186/1756-9966-29-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang BF, Wang XJ, Kang HF, et al. Saikosaponin-D Enhances Radiosensitivity of Hepatoma Cells under Hypoxic Conditions by Inhibiting Hypoxia-Inducible Factor-1α. Cell Physiol Biochem. 2014;33:37–51. doi: 10.1159/000356648. [DOI] [PubMed] [Google Scholar]
- 10.He Y, Hu ZF, Li P, et al. Experimental study of saikosaponin-D (SSd) on lipid peroxidation of hepatic fibrosis on rat. Zhongguo Zhong Yao Za Zhi. 2008;33:915–19. [in Chinese] [PubMed] [Google Scholar]
- 11.Mansour HH. Protective role of carnitine ester against radiation-induced oxidative stress in rats. Pharmacol Res. 2006;54:165–71. doi: 10.1016/j.phrs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Juránek I, Bezek S. Controversy of free radical hypothesis: reactive oxygen species – cause or consequence of tissue injury? Gen Physiol Biophys. 2005;24:263–78. [PubMed] [Google Scholar]
- 13.Maurya DK, Devasagayam TP, Nair CK. Some novel approaches for radioprotection and the beneficial effects of natural products. Indian J Exp Biol. 2006;44(2):93–114. [PubMed] [Google Scholar]
- 14.Srinivasan M, Sudheer AR, Pillai KR, et al. Modulatory effects of curcumin on γ-radiation-induced cellular damage in primary culture of isolated rat hepatocytes. Environ Toxicol Pharmacol. 2007;24:98–105. doi: 10.1016/j.etap.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Jin WS, Kong ZL, Shen ZF, et al. Regulation of hypoxia inducible factor-1α expression by the alteration of redox status in HepG2 cells. J Exp Clin Cancer Res. 2011;30:61. doi: 10.1186/1756-9966-30-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen KC, Willmore WG, Tayabali AF. Cadmium telluride quantum dots cause oxidative stress leading to extrinsic and intrinsic apoptosis in hepatocellular carcinoma HepG2 cells. Toxicology. 2013;306C:114–23. doi: 10.1016/j.tox.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Antonella B, Antonella S, Roberto M. A recombinant MnSOD is radioprotective for normal cells and radiosensitizing for tumor cells. Free Radical Biol Med. 2009;46:110–16. doi: 10.1016/j.freeradbiomed.2008.10.030. [DOI] [PubMed] [Google Scholar]