Abstract
Background
The aim of this initial study was to evaluate the clinical and radiological effectiveness of radioembolization (RE) using 188Re-Human Serum Albumin (HSA) microspheres in patients with advanced, progressive, unresectable primary or secondary liver cancers, not suitable to any other form of therapy.
Material/Methods
Overall, we included 13 patients with 20 therapy sessions. Clinical and radiological responses were assessed at 6 weeks after therapy, and then every 3 months. The objective radiological response was classified according to Response Evaluation Criteria in Solid Tumors (RECIST) v.1.0 by sequential MRI. Adverse events were evaluated using NCI CTCAE v.4.03.
Results
There were 4 patients with hepatocellular carcinoma (HCC), 6 with metastatic colorectal cancer (mCRC), 2 with neuroendocrine carcinoma (NEC), and 1 patient with ovarian carcinoma. Mean administered activity of 188Re HSA was 7.24 GBq (range 3.8–12.4) A high microspheres labeling efficacy of over 97±2.1% and low urinary excretion of 188Re (6.5±2.3%) during first 48-h follow-up. Median overall survival (OS) for all patients was 7.1 months (CI 6.2–13.3) and progression-free survival (PFS) was 5.1 months (CI 2.4–9.9). In those patients who had a clinical partial response (PR), stable disease (SD), and disease progression (DP) as assessed 6 weeks after therapy, the median OS was 9/5/4 months, respectively, and PFS was 5/2/0 months, respectively. The treatment adverse events (toxicity) were at an acceptable level. Initially and after 6 weeks, the CTC AE was grade 2, while after 3 months it increased to grade 3 in 4 subjects. This effect was mostly related to rapid cancer progression in this patient subgroup.
Conclusions
The results of this preliminary study indicate that RE using 188Re HSA is feasible and a viable option for palliative therapy in patients with extensive progressive liver cancer. It was well tolerated by most patients, with a low level of toxicity during the 3 months of follow-up.
MeSH Keywords: Injections, Intra-Arterial, Liver Neoplasms - radiotherapy, Radioisotopes
Background
Radioembolization (RE) using radioactive microspheres in patients with advanced unresectable primary or secondary liver cancers is currently used as a palliative therapy, especially in those patients who have no other option of further effective therapy [1]. Most studies concerning Selective Internal Radiation Therapy (SIRT) concentrate on the use of yttrium (90Y)-labeled resin or glass spheres [2–4]. This type of treatment can be effective even for tumor burden within the liver because direct administration of the radiolabeled particles via the hepatic artery results in deposition of the radioisotope, primarily into the tumor vascular bed. Consequently, the release of high-energy β-particles (electrons) can result in an effective tumor treatment and, since there is less particle deposition within the normal liver, toxicity is reduced [1–4].
However, the use of 90Y spheres has some drawbacks, the most common in many countries being the high cost of the commercially available products, and unreliable overseas shipments. Additionally, since 90Y is a pure beta emitter, only bremsstrahlung post-therapy imaging is possible, which is not optimal for precise evaluation of radioactive spheres deposition within tumor lesions [1]. This is also why it is very difficult to perform robust personal internal dosimetry, as the images of radiotracer biodistribution are blurry. The situation would be much improved if the radionuclide embolization agent emitted gamma radiation, which would allow for good imaging and subsequent dosimetry. Additionally, local production of this agent would improve its availability and reduce the cost of therapy [5–10].
In this respect, 188Re seems to be a very attractive radioisotope for this type of therapy. It is generator-produced, so it can be available in a standard nuclear medicine department. Additionally, its labeling is relatively easy using kits such as human serum albumin (HSA) [6,7]. Another advantage of 188Re HSA is its high in vitro and in vivo stability [6–8,10]. Usually radiochemical purity greater than 95% is achieved, which is better than in other radiopharmaceuticals such as 188Re-HDD/lipiodol [9,10].
Currently there are only a few publications on the use of 188Re HSA spheres in patients with advanced primary and secondary liver cancer [8,10]. In this study, we present our experience using 188Re HSA microspheres in the therapy of patients with advanced, nonresectable primary and secondary liver cancer, and the effect of this treatment on survival.
Material and Methods
General
This was a prospective single institution open-label phase I–II study, which was approved by the Clinical Ethics Committee of the Central Clinical Hospital Ministry of Internal Affairs. Prior to recruitment, all patients understood the experimental nature of the treatment and provided their written consent.
Patients
The studied group was 13 patients (7 females) with a mean age of 55.7 years (range 26–78 years), with a histological diagnosis of primary or metastatic cancer within the liver. All patients enrolled in this study had failed standard therapeutic approaches. Each case was evaluated by a clinical oncologist, surgeon, and interventional radiologist before being accepted for radioembolization.
Study
None of the patients had received prior radioembolization treatment. All had evidence of clinical, biochemical, or radiological progression evaluated by CT or MRI based on Response Evaluation Criteria in Solid Tumors (RECIST) v.1.0. Exclusion criteria were: Hemoglobin (Hb) <8 g/dL, White Blood Count (WBC) <2×103/mL, Neutrophils Counts (ANC) <1.5×103/mL, Platelets (Plt) <80×103/mL, creatinine level >1.2 mg/dL or GFR <30 mL/min, and poor performance status (WHO status 3 and 4). In hepatocellular cancer (HCC) the Child-Pugh status was B >8 PN and total bilirubin >3.0 mg/dL. Before being included in the study, each patient’s liver-lung shunt was assessed using whole body imaging after intra-arterial infusion of 99mTc HSA microspheres (B20, 2.5 mg), injected within 2 h after preparation, 120 MBq into the right hepatic artery and 80 MBq into the left hepatic artery (ROTOP Pharmaka AG, Germany). A shunt of less than 20% was the necessary criterion for treatment. Other contraindications for treatment included pregnancy, established myelosuppression, renal failure, heart insufficiency [New York Heart Association class III or IV], unstable Coronary Artery Disease (CAD), uncontrolled hypertension, ascites, evident lung metastases, or other extra-liver significant metastatic disease except single bone metastases, single abdominal lymph nodes involvement, pulmonary disease (e.g., severe asthma/chronic obstructive pulmonary), survival expectancy of less than 3 months, the presence of other cancers (excluding in situ skin cancer and ca coli uteri), HIV infection, and known immunosuppression.
Radiopharmaceutical
The physical characteristics of 188Re are as follows: the half-life is equal to 16.9 h and the maximum energy of beta emission is 2.1 MeV. Additionally, a gamma emission of 155 keV (15% abundance), which accompanies 188Re beta decay, provides opportunity for post-therapeutic imaging, allowing determination of biodistribution of the radiotracer and personalized dosimetry.
The method for preparation of 188Re microspheres was based on the procedure described earlier by Wunderlich et al. [6–8] with some modifications. Briefly, to make the labeling procedure easier to perform in the hospital, the components of labeling mixture – 2,5 dihydroxybenzoic acid (Fluka AG) SnCl2 anhydrous (Sigma-Aldrich) and potassium sodium tartrate (POCH Gliwice, Poland) – were prepared under aseptic conditions in the lyophilized form, each in a separate vial. Prior to use for injection, the content of these vials was dissolved in water. Carrier-free [188Re] NaReO4 was obtained in 1–4 mL of normal saline from an alumina based 3.7 GBq 188W/188Re generator (NCNR POLATOM, Poland). The labeling was carried out in the presence of stannous chloride using HSA microspheres B20; 2.5 mg, 300 000–500 000 particles (ROTOP Pharmaka AG, Germany).
For radiolabeling, 2 mL of the solution containing 4.7 mg/mL of 2,5 dihydroxybenzoic acid and 5 mg/mL of SnCl2 were added to each of 1–3 vials of B20 HSA microspheres and their content was resuspended by gently shaking. The desired quantity of [188Re] perrhenate in saline in the range from 3 to 4.5 GBq in 1–4 mL was added to the microspheres vials and the content incubated at 95°C for 1 h. When incubation was completed, 0.6 mL of 42 mg/mL potassium sodium tartrate solution was added and the contents shaken again for 5 min without heating. The radiolabeling yield was tested by filtration using Sterifix Paed 0.2 μm (B. Braun, Germany). The preparation was ready for injection when more than 95% of [188Re] radioactivity was bound to microspheres.
Radiation treatment planning
All patients underwent CT angiography and an additional MR before and after intravenous (i.v.) contrast enhancement to calculate the volume of the right and left lobes of the liver, as well tumor volume for each lobe. The ratio of tumor volume to the normal liver was measured to determine the planned injection activity. In case of bilobar tumor involvement, the second RE was performed 2–4 weeks after the first RE procedure. The data from the pre-treatment “scout” planar scan, following administration of 99mTc-microspheres (MAA) through a selective liver catheter, was done separately for the left hepatic artery (mean 99mTc activity 80 MBq) and right hepatic artery (mean 99mTc activity 120 MBq). These data were used to determine the liver-lung shunt and to visually assess accumulation of MAA spheres within the liver. In the case of liver-lung shunt exceeding 20%, the patient would not be treated, but in this group the liver-lung shunt was always below 5%. The activity of 188Re HSA to be injected was calculated based on an empirical method [11]. In patients who had a ratio of tumor to liver volume above 50%, the total injection activity of 188Re HSA was higher than 9.0 GBq, in those between 25% to 50% it was 6.0–9.0 GBq, and those with a ratio below 25% received 3.0–6.0 GBq. These highest activities correspond to the sum of the 2 injections used for the 7 patients with bilobar disease.
Radioembolization – procedure
A standard radiological method using femoral artery puncture with a 4F angiographic sheath (Balton, Poland) was used. Angiography of abdominal aorta, celiac trunk, common hepatic artery, and superior mesenteric artery was performed using power injection and 4F SIM catheter (Cordis, USA) [1–4]. Before the RE, the liver and abdominal vasculature was identified using hepatic mapping angiograms. In each case, collateral vessels supplying extra-hepatic structures such as the stomach and pancreas were identified and embolized, especially the gastroduodenal (GDA) and right gastric (RGA) arteries. Using a coaxial method, the microcatheter (Microferret, Cook Medical Inc., USA) was inserted into the right gastric artery and gastroduodenal artery and the embolization was done using coils (Hilal, Tornado, Cook Medical Inc. USA). In 4 cases, the embolization of GDA was performed using the Amplatzer Vascular Plug II (AGA Medical, now part of St. Jude; USA).
The same method without embolization of splanchnic vessels was used during RE with selective catheterization of the left or right hepatic artery. Patient preparation performed 30 min before RE included i.v. administration of Ondansetron [8 mg], Dexaven [8 mg], and Ranitidine [50 mg]. The activity of 188Re HSA (calculated according to the empirical method discussed above) placed in approximately 10 ml of buffer solution was administered using a modified delivery set, via a microcatheter placed into the left or right lobe of the liver. In each case, 188Re HSA high-active solution was diluted by saline and slowly infused for a mean duration of 45 min. During RE, potential arterial spasm and reverse back-flow was carefully monitored using sequential low-contrast volume fluoroscopy. In case vascular spasm and significant back flow occurred, the procedure was discontinued. In patients with involvement of both lobes, sequential second lobe therapy was performed over the next 2–4 weeks.
Image processing
The imaging protocol for each patient included a standard CT scan performed before RE, a single whole-body (WB) scan done 6 h after treatment, and 2 or 3 SPECT scans of the abdomen performed at 6–10 h, 18–24 h, and 48 h after treatment. These multiple scans were performed to investigate potential release of 188Re from the site of injection. A standard double-head camera (e-cam; Siemens; USA) equipped with a medium-energy low-penetration (MELP) collimator was used for SPECT and WB acquisitions. The data were collected in 2 energy windows: window 1 (W1) (155 keV ±10%) measured the 188Re photopeak (abundance of 15%) and window 2 (W2) (190 keV ±7.5%) was used to measure the bremsstrahlung contribution. For SPECT imaging, the camera made 128 stops (64 stops per head) of 15 s each.
Images were reconstructed from the W1 data using the Ordered Subsets Expectation Maximization (OSEM) algorithm, in which the W2 data was included to account for bremsstrahlung [5]. The patient-specific corrections for attenuation, scatter, and resolution loss were applied. For attenuation and scatter corrections, attenuation maps were created from the pre-therapy CT images. The conversion to absolute activity was done using experimentally determined camera efficiency and dead-time.
Evaluation of response
The radiological response to therapy (RECIST v.1.0, considering the change in the sum of diameters of the dominant liver lesions) was assessed using MRI. These MRI studies were performed 2–6 weeks after the treatment and then repeated every 3 months during the follow-up period up to 1 year, then in 6-month intervals. Additional maximum percent change from baseline in the sum of the biggest volume diameters of target lesions (the same as RECIST) were evaluated at 3 months after RE using commercial software Syngo-Via v.1.2 (Siemens, Germany). Clinical response and performance status (WHO – Zubrod) after RE were evaluated based on physician interviews. These were conducted before the RE and then at 1, 3, and 6 weeks after completion of therapy, and later at 3-month intervals. Assessment included appetite; malaise; weight loss; and the presence, intensity, and frequency of abdominal pain, diarrhea, nausea, vomiting, and fever. The use of any analgesic and other drugs before and after treatment was also recorded.
Adverse events
Adverse events (AE; toxicity) were graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events v.4.03 (NCI CTCAE). For gastrointestinal and general adverse events, a standardized set of questions (including appetite; malaise; weight loss; and the presence, intensity, and frequency of abdominal pain, diarrhea, nausea, vomiting, and fever). Acute adverse events within 1 week, 6 weeks, and 3 months after RE were acquired.
Statistical analysis
Efficacy was assessed in the intention-to-treat population on the basis of investigator-assessed tumor response. Estimated median overall survival (OS) and progression-free survival (PFS) were evaluated using the Kaplan-Meier method, with corresponding 2-sided 95% confidence intervals. Potential differences in OS and PFS between groups of patients based on histology of metastatic tumors vs. primary (HCC) were evaluated using the Cox-Mantel test. The potential differences between groups of parameters, such as performance status (WHO) before therapy and 6 weeks after therapy, or potential changes of biochemical profile during therapy and at 6 weeks and during follow-up, were evaluated using a Wilcoxon matched-pair test. The value of P<0.05 was considered as statistically significant.
Results
The group of 13 patients that were treated included: 4 patients with hepatocellular carcinoma (HCC), 6 subjects with metastatic into liver colorectal carcinoma (mCRC), 2 patients with neuroendocrine carcinoma (NEC G3), and 1 patient with metastatic ovarian carcinoma. Clinical characteristics of the patients, including type of cancer and initial performance status (PS) based on WHO, are presented in Table 1. Table 2 presents tumor and liver characteristics for all patients enrolled in this study, including subgroups of those with primary and metastatic disease. Table 3 summarizes previous patient therapies, including surgery, systemic chemotherapy, and (in a case of HCC) transarterial embolization (TACE).
Table 1.
Evaluation before therapy of clinical characteristics of patients with primary or metastatic liver neoplasm (N=13) that were enrolled in this study.
| Patients clinical characteristics | All (N=13) | mCRC (N=6) | HCC (N=4) | Others (N=3) |
|---|---|---|---|---|
| Mean age | 55.7 | 53.5 | 59.0 | 51 |
| Range of age | 26–78 | 26–78 | 44–71 | 38–75 |
| Atandard deviation (SD) | 16.8 | 18.3 | 10.3 | 18.8 |
| Female/male | 7/6 (46/54%) | 2/4 (33/67) | 1/3 (25/75%) | 2/1 (33/77) |
| Initial performance status | ||||
| WHO status=0 | 1 (8%) | 0 | 0 | 1 (33%) |
| WHO status=1 | 8 (62%) | 5 (83%) | 2 (50%) | 1 (33%) |
| WHO status=2 | 4 (30%) | 1 (17%) | 2 (50%) | 1 (33%) |
| HCC – Child-Pugh A | 2 (50%) | |||
| HCC – Child-Pugh B | 2 (50%) | |||
HCC – hepatocellular carcinoma; mCRC – metastatic colorectal carcinoma.
Table 2.
Tumor and liver volume characteristic in all patients enrolled in this study.
| Tumor characteristics | Grade of tumor cells G1/2/3 | Tumor volume (mean mL) | Tumor volume (range mL) | Liver volume (mean mL) | Liver volume (range mL) | Mean tumor/ liver ratio |
|---|---|---|---|---|---|---|
| HCC (N=4) | 2/2/0 | 1135.0 | 303–2100.0 | 2458.5 | 1432–3147 | 0.41 |
| mCRC (N=6) | 0/4/2 | 548.5 | 145–1837.0 | 2359.3 | 1432–2880 | 0.23 |
| Others (N=3)* | 0/1/2 | 412.7 | 357–770.0 | 1841.2 | 1310–2347 | 0.22 |
| All (N=13) | 2/7/4 | 697.0 | 145–2100.0 | 2270.2 | 1310–3147 | 0.28 |
Others included 2 subjects with neuroendocrine carcinoma NEC (WHO 2010), G3 with Ki-67 >45% (gastric) and an unknown origin and also single ovarian carcinoma G2 (moderately differentiated).
Table 3.
Summary of patients’ previous therapies, including surgery, systemic chemotherapy, and, in the case of HCC, transarterial embolization – TACE.
| Previous therapy | Surgery | Systemic chemotherapy | Embolization | |||
|---|---|---|---|---|---|---|
| No | Type | No | type | No | Type | |
| HCC (N=4) | 2 | Hemihepatectomy | 3 | Sorafenib | 3 | TACE |
| mCRC (N=6) | 6 | Hemicolectomy left (N=4) and right (N=2) | 2 | FOLFOX-4 (1st line) FOLFIRI (2nd) | ||
| 2 | FOLFOX-4 (1st line), FOLFIRI (2nd) & Cetuximab (3rd) | |||||
| 4 | 2 | FOLFOX-4 (1st line), FOLFIRI (2nd) & Retuximab (3rd) | ||||
| NEC G3) (N=2) | 1 | Gastrectomy | 2 | PE (1st & 2nd line) | ||
| Ovarian ca (N=1), | 1 | Uterine, ovary and omentectomy | 1 | DDP + Taxol (1st line) & DDP + Gemcitabine (2nd) | ||
HCC – hepatocellular carcinoma; mCRC – metastatic Colorectal Cancer; NEC – neuroendocrine carcinoma (G3 – poorly differentiated); FOLFOX-4 - standard first- or second-line chemotherapy in patients with mCRC, this regimen consists of Folinic acid (leucovorin) + Fluorouracil (5FU) and Oxaliplatin; FOLFIRI – standard first or second line chemotherapy in patients with mCRC, this regiment consists of: Folinic acid (leucovorin) + Fluorouracil (5FU) and Irinotecan; PE – standard chemotherapy in advanced neuroendocrine carcinomas (poorly differentiated G3) – this regimen consists of Cisplatin and Etoposide; DDP+Taxol – standard chemotherapy in advanced ovarian cancer, this regiment consists of: Cisplatin and Taxol; DDP + Gemcitabine – second-line chemotherapy in patients with advanced ovarian cancer, this regiment consists of: Cisplatin and Gemcitabine.
A total of 20 therapies were given with a mean administered activity of 7.24 GBq (range 3.8–12.4 GBq) per patient (mean 4.71 GBq per therapy session). A summary of therapy data is presented in Table 4. Seven patients (4 with mCRC, 2 with HCC, and 1 with NEC) had 2 therapy sessions due to both left and right lobe liver involvement. A second embolization was performed within 2–6 weeks after initial RE.
Table 4.
Summary of patient distribution in 188Re HSA microspheres therapy.
| All (N=13) | HCC (N=4) | mCRC (N=6) | Others (N=3) | |
|---|---|---|---|---|
| Mean (range) | Mean (CI ±95%) | Mean (CI ±95%) | Mean (CI ±95%) | |
| Therapy sessions (N=20) | 1.38 (1–2) | 1.75 (1–2) | 1.5 (1–2) | 1.33 (1–2) |
| Injected activity per therapy (GBq) | 4.71 (3.0–7.4) | 5.71 (3.4–7.2) | 4.61 9 (3.2–5.3) | 4.23 (3–5) |
| Cumulative dose to the patient (GBq) | 7.24 (3.8–12.4) | 8.95 (5.8–12.4) | 6.91 (3.8–11.4) | 5.63 (4.3–8.0) |
| Mean liver-lung shunt in% | 6.1 | 4.2 | 6.8 | 5.4 |
A preliminary analysis of the sequential SPECT studies indicated no reduction or only a slight reduction of 188Re accumulation in the liver over time (data were corrected for physical decay). The results of the quantitative analysis of these data together with patients’ personalized dosimetry calculations, are reported in our previous publication [12].
Bio-distribution of microspheres and internal dosimetry
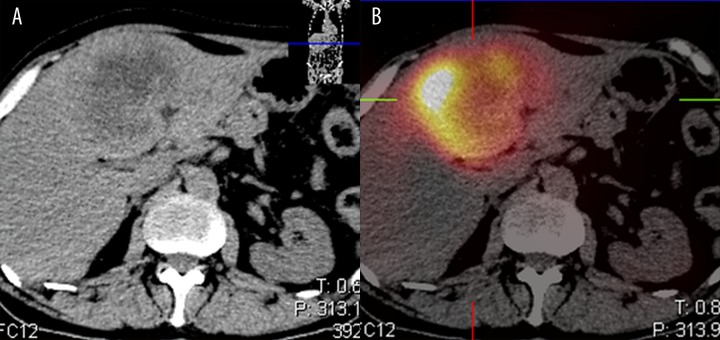
The distribution of 188Re-HSA microspheres in the post-therapy images (WB and SPECT) showed good localization of radiotracer within the liver tumors. Figure 1 presents an example of a contrast-enhanced CT image and SPECT bremsstrahlung image fused with CT of a 52-year-old male with neuroendocrine carcinoma. Analysis of different image processing techniques (Figure 2) demonstrated the importance of applying the aforementioned corrections for attenuation, scatter, resolution loss, and bremsstrahlung. The zoomed transaxial slices of SPECT images pertaining to the same patient are presented. The reconstructions with limited corrections (columns 1 and 2) and all corrections (column 3) are compared. The most advanced reconstruction technique resulted in high-quality representation of the biodistribution of microspheres, in spite of the low abundance (15%) of the 188Re photopeak and high bremsstrahlung background (Figure 2, last column).
Figure 1.
An example of a contrast-enhanced CT (A) and fused CT and bremsstrahlung image (B) of a 52-year-old male with neuroendocrine carcinoma. NEC G3 (WHO 2010). The patient was after primary tumor surgery, currently with liver metastasis and chemotherapy refractory disease. RECIST DP (liver metastasis), not suitable for 90Y Sir-spheres, due to high bilirubin level (>1.5 xULN). RE using 188Re HSA microspheres (segment 4 hepatic artery administration of 4.8 GBq of 188Re HSA microspheres.
Figure 2.
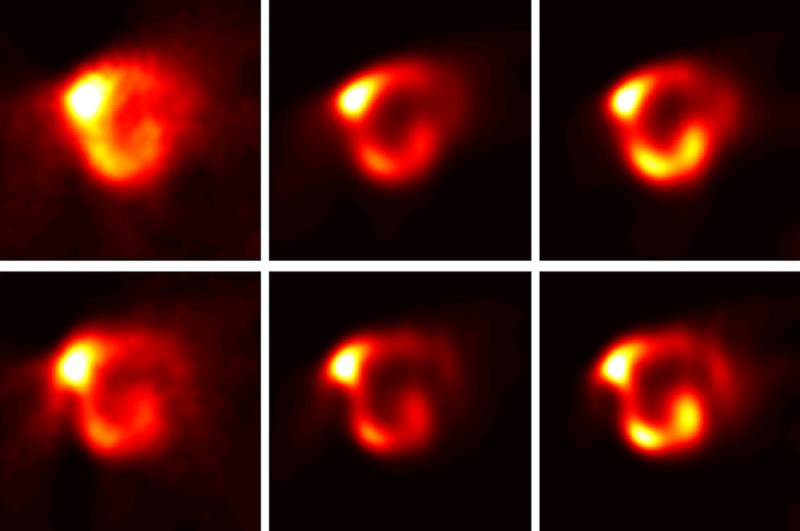
The zoomed transaxial slices of SPECT images pertaining to the same patient are presented. The reconstructions with different time points upper row 9 h after therapy and lower row 20 h after therapy. First column: no correction, second column correction for bremsstrahlung and resolution loss, and third column correction for bremsstrahlung, resolution loss, attenuation and scatter correction as well. There is limited corrections (columns 1 and 2) compare to all corrections (column 3). The most advanced reconstruction technique resulted in high-quality representation of the biodistribution of microspheres in spite of the low abundance (15%) of the 188Re photopeak and high bremsstrahlung background.
Adverse avents (toxicity)
No major acute adverse events were recorded during the therapy sessions or within 48 h of administration of the 188Re HSA microspheres. In a single case, a mild allergic reaction with increase of breathing and heart rate, and vascular spasm occurred during fluoroscopic control angiography with low-volume intra-arterial radiological contrast. The RE was terminated and standard steroid and antihistamines were given, resulting in relief of the allergy symptoms.
In terms of general toxicity, a mild post-embolization syndrome occurred in 8 patients (76%). Fatigue and grade 1 fever was observed in 5 patients and grade 2 in 1 patient. Weight loss was observed in 3 patients. Reported gastrointestinal toxicity mostly consisted of nausea (6 patients) and abdominal pain (5 patients) (Table 5). The single patient with the allergic reaction required 48-h hospitalization. All other patients remained in the hospital for 24 h, this being governed by radiation protection requirements and not by the clinical state of the patient. Within 1 week after therapy, the liver function test showed grade 2 toxicity, with a rise in bilirubin level and alkaline phosphatase in 2 subjects (15%), and in 1 patient with grade 2 reduction in fibrinogen (Table 6). Most reported cases of grade 2 toxicity were seen 6 weeks after treatment, although a few additional cases of grade 3 toxicity (increased bilirubin in 2 patients and alkaline phosphates in 2 patients) were seen after 3 months. However, all these cases were related to further rapid disease progression.
Table 5.
A summary of the general adverse events (toxicity and specific gastrointestinal toxicity) NCI CTCAE v.3.1; acute within 1 week of therapy as assessed during the clinical follow-up interview.
| Type of adverse event (toxicity) NCI CTCAE v.3.1 |
Number of patients (%) |
|---|---|
| Gastrointestinal | |
| Nausea (grade 1) | 6 (46) |
| Abdominal pain (grade 1) | 5 (38) |
| General toxicity | |
| Fever (grade 1/grade 2) | 5 (38)/1 (8) |
| Fatigue (grade 1) | 8 (76) |
| Weight loss (grade 1/grade 2) | 3 (23) |
Table 6.
Significant biochemical and hematological adverse events: toxicity (NCI-CTCAE v.3.1) grade 2 and 3 observed during therapy (initial), early (6 weeks after therapy), and late (after 3 months).
| Characteristics of toxicity (NCI CTC v.3.1) | Initial (N=13) No. of patients (%) |
Early 6 weeks (N=13) No. of patients (%)) |
Late 3 months (N=13) No. of patients (%) |
|---|---|---|---|
| Bilirubin | |||
| Grade 2 | 2 (15%) | 2 (15%) | 0 |
| Grade 3 | 2 (15%) | ||
| AP | |||
| Grade 2 | 2 (15%) | 3 (23%) | 3 (23%) |
| Grade 3 | 0 | 2 (15%) | |
| Albumin | |||
| Grade 2 | 1 (7.5%) | 2 (15%) | |
| Grade 3 | |||
| Fibrinogen | |||
| Grade 2 | 1 (7.5%) | 1 (7.5%) | 1 (7.5%) |
Clinical benefit
In terms of clinical response, including general condition before therapy, patient performance status (PS WHO) was assessed as follows: WHO grade 0 in 1 patient (8%), WHO grade 1 in 8 patients (62%), and WHO 2 in 4 patients (31%). At the assessment performed 6 weeks after the therapy, the performance status was noted as PS WHO 0 in 5 patients (38%), WHO grade 1 in 6 patients (46%), and WHO grade 2 in 2 patients (15%). This improvement was statistically significant (Wilcoxon matched pair test P=0.01). Clinical improvement was also noted in most patients during follow-up visits. After 6 weeks, 12 patients demonstrated some benefits in terms of clinical response or disease stability and only 1 patient had progressive disease. At 3 months after treatment, clinical response or stability was seen in 11 patients, with just 2 having progressive symptoms. At 6 months, there were 8 survivors, 6 of whom were clinically stable, but 2 patients had clinical progression.
Radiological response
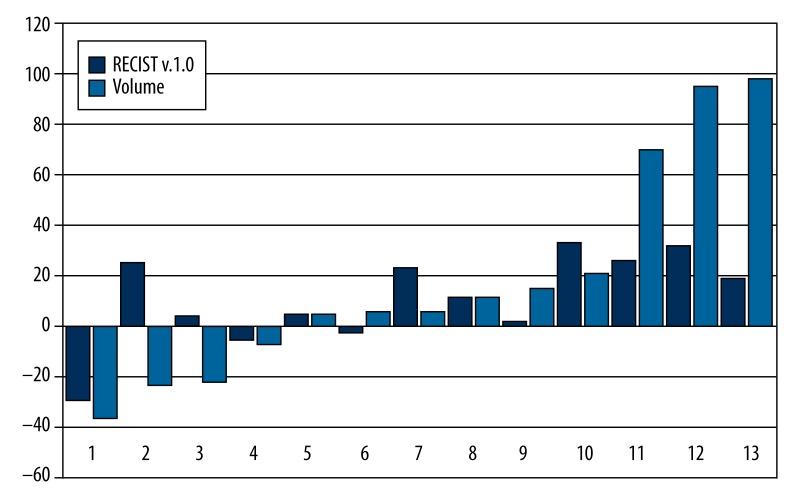
Radiologic response assessment using RECIST v.1.0 on MRI, and in a single case CT at 6 weeks after therapy, showed stable disease in 12 patients and disease progression in just 1 patient. At 3 months, 1 patient had a partial response, 7 patients were stable, and 5 had progressive disease (Table 7). Figure 3 shows the maximum percent change in the sum of the longest diameters of target lesions, evaluated using RECIST v.1.0 and volumetric analysis, in the sum of the biggest volume of the same target lesions, evaluated from baseline and after 3 months after therapy using sequential MRI in all 13 patients.
Table 7.
Early clinical and radiological response (RECIST v.1.0) to RE using 188Re HSA microspheres in patients with advanced progressive primary and secondary liver cancers evaluated after 6 weeks, 3 months, and 6 months.
| Clinical No. of patients (%) |
RECIST v.1.0 No. of patients (%) |
|
|---|---|---|
| Early 6 weeks (N=13) | ||
| PR | 7 (54) | 0 |
| SD | 5 (38) | 12 (92) |
| DP | 1 (8) | 1 (8) |
| At 3 months (N=13) | ||
| PR | 5 (38) | 1 (8) |
| SD | 6 (46) | 7 (54) |
| DP | 2 (15) | 5 (38) |
| At 6 months (N=8) | ||
| PR | 2 (25) | 0 |
| SD | 4 (50) | 6 (75) |
| DP | 2 (25) | 2 (25) |
RECIST v.1.0. DP – disease progression; PR – partial response; N/A – not applicable; SD – stable disease.
Figure 3.
The maximum percent change in the sum of the longest diameters of target lesions evaluated using RECIST v.1.0 and volumetric analysis in the sum of the biggest volume of the same target lesions, evaluated from baseline and after 3 months post-therapy using sequential MRI in all 13 patients.
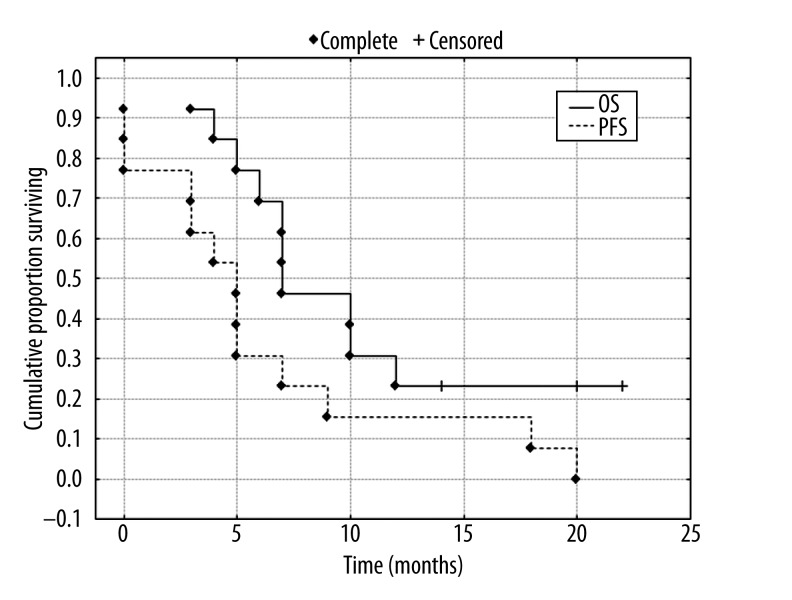
PFS and OS
Median PFS calculated for all 13 subjects was 5.1 months (±95% CI 2.4–9.9), median OS was 7.1 months (±95%CI 6.2–13.3) (Figure 4). In patients who had a clinical partial response (PR), stable disease (SD), and disease progression (DP) as assessed 6 weeks after therapy, the median OS was 9/5/4 months, respectively, and PFS was 5/2/0 months, respectively.
Figure 4.
Cumulative proportion surviving (Kaplan-Mayer) for patients (N=13) after RE of liver tumor. Median overall survival – OS 7.1 months (CI±95% 6.2–13.3 months) and median progression-free survival – PFS 5.1 months (CI±95% 2.4–9.9M).
Discussion
The results of this study indicate that 188Re-HSA can be considered to be a safe and effective treatment in patients with advanced primary or metastatic liver tumors that are resistant to standard treatment. Post-treatment symptoms were mild and manageable. Although some late toxicity was seen in the majority of patients, this was due primarily to disease progression and did not appear to be the result of the treatment. Previously published reports using intra-arterial 188Re products have concentrated on the use of HDD Lipiodol to treat primary HCC [9,13–16]. The results of several of these studies were slightly better than observed in our patient group, but their patients often had just HCC, which has a relatively better prognosis than many metastatic cancers with liver involvement [10,13–16]. However, a common trait observed in these trials is patients’ high level of tolerance for the treatments, with only a mild post-embolization syndrome. The results of our trial are also similar to those reported in other small-scale trials using 188Re-labelled microspheres administered into the liver via the hepatic artery [8,10].
At present, the most commonly used form of radioembolization uses 90Y-labeled microspheres, which has been shown to be effective against both metastatic cancer of the colon and hepatocellular cancer. In a randomized control trial, it was shown that the sequential use of 90Y resin RE and 5FU treatment increased medical overall survival from 3 months to 18 months in patients with liver metastases from colon cancer [17]. Further phase II trials have shown that similar prolonged survivals can be obtained when using RE in combination with more modern chemotherapy regiments. However, chemotherapy often results in adverse events (toxicity), which limit treatment outcomes [17,18]. A recent randomized controlled trial comparing the use of 90Y resin microspheres with and without chemotherapy in mCRC is being undertaken in Europe and may provide more complete data on what response can be expected from this treatment method.
Although there have not been any phase III trials in HCC with 90Y resin microspheres, there are 2 ongoing phase III trials (1 in Europe and 1 in East Asia), and the results of multicenter trials presented in a recent European paper with pooled results from centers in Spain, Italy, and Germany suggest that a median overall survival of 12 months can be achieved with minimal toxicity [19].
However, the overriding issue with therapies that use 90Y particulates is their cost (normally of the order of €10,000 per treatment), which limits access to this therapy in many countries, especially now that healthcare budgets are restricted (20). The result of our study suggests that 188Re-HSA could produce similar effects to 90Y RE, at about one-forth the cost. It is important to note that from the physics point of view, the energy and the range of 188Re beta emissions are very similar to those of 90Y; therefore, their dose depositions must also be similar. Also, the advantage of a generator-produced radioisotope is that greater usage leads to lower per-unit cost [21]. In this study, the 188W generator was held in a central facility and its long half-life (69.8 d) of 188Re allowed this 1 facility to provide treatment doses to many regional hospitals, as has been done in other trials using 188Re-HDD Lipiodol [14,15]. This again could improve cost efficiency.
As this was a phase I-II trial, only patients with advanced disease who had failed standard treatment were recruited. The poor health status of this patient population to some extent explains why the survival data was worse than in other trials treating less advanced patients. The preliminary dosimetry calculations performed as part of this study (to be reported in the separate publication) suggest, however, that tumor doses well over 100Gy can be achieved, which is higher than in other similar studies [22]. This finding offers promise that if the therapy was extended to less advanced patients, response rates would improve.
The lack of radiological response in our study is of less concern than would be expected, because it has become established that a formal disease response as measured by RECIST is not required for improved survival in patients treated with radionuclides [23,24]. Some groups have suggested that 18F FDG PET is a better method by which to monitor these patients [25], but this approach was not available to us. Furthermore, as some patients’ clinical condition improved after treatment, this is evidence that this improvement is the best predictor of survival in patients treated with radionuclide therapy [17–19,23,24].
Conclusions
The results of our study demonstrated that 188Re HSA microspheres RE is a practical method by which primary and secondary tumors in the liver can be treated. It was well tolerated and showed evidence of symptomatic response in many patients. There were minimal adverse events (toxicity), and early dosimetric analysis suggests that treatment doses of over 100 Gy can be achieved. It is expected that if this treatment is applied to patients with less advanced disease, better survival can be obtained.
Footnotes
Source of support: Departmental sources
References
- 1.Kennedy AS, Coldwell D, Nutting C, et al. Resin Y-90-microsphere brachytherapy for unresectable colorectal liver metastases: Modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65:412–25. doi: 10.1016/j.ijrobp.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 2.Lau WY, Leung WT, Ho S, et al. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer. 1994;70:994–99. doi: 10.1038/bjc.1994.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stubbs RS, Cannan RJ, Mitchell AW. Selective internal radiation therapy (SIRT) with 90Yttrium microspheres for extensive colorectal liver metastases. Hepatogastroenterology. 2001;48:333–37. [PubMed] [Google Scholar]
- 4.Salem R, Thurston KG, Carr BI, et al. Yttrium-90 microspheres: Radiation therapy for unresectable liver cancer. J Vasc Interv Radio. 2002;13:S223–29. doi: 10.1016/s1051-0443(07)61790-4. [DOI] [PubMed] [Google Scholar]
- 5.Shcherbinin S, Celler A, Belhocine T, et al. Accuracy of Quantitative Reconstructions in SPECT/CT Imaging. Phys Med Biol. 2008;53:4595–604. doi: 10.1088/0031-9155/53/17/009. [DOI] [PubMed] [Google Scholar]
- 6.Wunderlich G, Drews A, Kotzerke J. A kit for labeling of [188Re] human serum albumin microspheres for therapeutic use in nuclear medicine. Appl Radiat Isot. 2005;62:915–18. doi: 10.1016/j.apradiso.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Wunderlich G, Pinkert J, Andreeff M, et al. Preparation and biodistribution of rhenium-188 labeled albumin microspheres B 20: a promising new agent for radiotherapy. Appl Radiat Isot. 2000;52:63–68. doi: 10.1016/s0969-8043(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 8.Liepe K, Brogsitter C, Leonhard J, et al. Feasibility of high activity rhenium-188-microsphere in hepatic radioembolization. Jpn J Clin Oncol. 2007;37:942–50. doi: 10.1093/jjco/hym137. [DOI] [PubMed] [Google Scholar]
- 9.Lambert B, Bacher K, Defreyne L, et al. 188Re-HDD/lipiodol therapy for hepatocellular carcinoma: a phase I clinical trial. J Nucl Med. 2005;46:60–66. [PubMed] [Google Scholar]
- 10.Liepe K, Kotzerke J, Lambert B. Advantage of 188Re-radiopharmaceuticals in hepatocellular cancer and liver metastases. J Nucl Med. 2005;46:1407–8. [PubMed] [Google Scholar]
- 11.Billbao JI, Kennedy AS. In: Liver Radioembolization with 90Y Microspheres. Bilbao JI, Reiser MF, editors. Springer-Verlag; 2008. [Google Scholar]; Kennedy AS, Dezarn WA, McNeillie P. Empiric Method Calculation. Dosimetry and Dose Calculation; pp. 51–59. [Google Scholar]
- 12.Shcherbinin S, Grimes J, Bator A, et al. Three-dimensional personalized dosimetry for 188Re liver selective internal radiation therapy based on quantitative post-treatment SPECT studies. Phys Med Biol. 2014;59:119–34. doi: 10.1088/0031-9155/59/1/119. [DOI] [PubMed] [Google Scholar]
- 13.Keng GH, Sundram FX, Yu SW, et al. Preliminary experience in radionuclide therapy of hepatocellular carcinoma using hepatic intra-arterial radio-conjugates. Ann Acad Med Singapore. 2002;31:382–86. [PubMed] [Google Scholar]
- 14.Bernal P, Raoul JL, Vidmar G, et al. Intra-arterial rhenium-188 lipiodol in the treatment of inoperable hepatocellular carcinoma: results of an IAEA-sponsored multination study. Int J Radiat Oncol Biol Phys. 2007;69:1448–55. doi: 10.1016/j.ijrobp.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Bernal P, Raoul JL, Stare J, et al. International Atomic Energy Agency-sponsored multination study of intra-arterial rhenium-188-labeled lipiodol in the treatment of inoperable hepatocellular carcinoma: results with special emphasis on prognostic value of dosimetric study. Semin Nucl Med. 2008;38:S40–45. doi: 10.1053/j.semnuclmed.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Srivastava DN, Chau TT, et al. Inoperable hepatocellular carcinoma: transarterial 188Re HDD-labeled iodized oil for treatment – prospective multicenter clinical trial. Radiology. 2007;243:509–14. doi: 10.1148/radiol.2432051246. [DOI] [PubMed] [Google Scholar]
- 17.Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88:78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 18.Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25:1099–106. doi: 10.1200/JCO.2006.08.7916. [DOI] [PubMed] [Google Scholar]
- 19.Sangro B, Carpanese L, Cianni R, et al. European Network on Radioembolization with Yttrium-90 Resin Microspheres (ENRY) Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–78. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 20.Ray CE, Jr, Battaglia C, Libby AM, et al. Interventional radiologic treatment of hepatocellular carcinoma-a cost analysis from the payer perspective. J Vasc Interv Radiol. 2012;23:306–14. doi: 10.1016/j.jvir.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Knapp FF, Jr, Beets AL, Guhlke S, et al. Availability of rhenium-188 from the alumina-based tungsten-188/rhenium-188 generator for preparation of rhenium-188-labeled radiopharmaceuticals for cancer treatment. Anticancer Res. 1997;17(3B):1783–95. [PubMed] [Google Scholar]
- 22.Lambert B, Bacher K, Defreyne L, et al. (188)Re-HDD/lipiodol therapy for hepatocellular carcinoma: an activity escalation study. Eur J Nucl Med Mol Imaging. 2006;33:344–52. doi: 10.1007/s00259-005-1954-1. [DOI] [PubMed] [Google Scholar]
- 23.Navalkissoor S, Alhashimi DM, Quigley AM, et al. Efficacy of using a standard activity of (131)I-MIBG therapy in patients with disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2010;37:904–12. doi: 10.1007/s00259-009-1326-3. [DOI] [PubMed] [Google Scholar]
- 24.Cwikla JB, Sankowski A, Seklecka N, et al. Efficacy of radionuclide treatment DOTATATE Y-90 in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs): a phase II study. Ann Oncol. 2010;21:787–94. doi: 10.1093/annonc/mdp372. [DOI] [PubMed] [Google Scholar]
- 25.Szyszko T, Al-Nahhas A, Canelo R, et al. Assessment of response to treatment of unresectable liver tumours with 90Y microspheres: value of FDG PET versus computed tomography. Nucl Med Commun. 2007;28:15–20. doi: 10.1097/MNM.0b013e328011453b. [DOI] [PubMed] [Google Scholar]