Abstract
A multidisciplinary strategy for discovery of new Conus venom peptides combines molecular genetics and phylogenetics with peptide chemistry and neuropharmacology. Here we describe application of this approach to the conantokin family of conopeptides targeting NMDA receptors. A new conantokin from Conus rolani, ConRl-A, was identified using molecular phylogeny and subsequently synthesized and functionally characterized. ConRl-A is a 24-residue peptide containing three gamma-carboxyglutamic acid residues with a number of unique sequence features compared to conantokins previously characterized. The HPLC elution of ConRl-A suggested that this peptide exists as two distinct, slowly exchanging conformers. ConRl-A is predominantly helical (estimated helicity of 50%), both in the presence and absence of Ca++. The order of potency for blocking the four NMDA receptor subtypes by ConRl-A was NR2B>NR2D>NR2A>NR2C. This peptide has a greater discrimination between NR2B and NR2C then any other ligand reported so far. In summary, ConRl-A is a new member of the conantokin family that expands our understanding of structure/function of this group of peptidic ligands targeted to NMDA receptors. Thus, incorporating phylogeny in the discovery of novel ligands for the given family of ion channels or receptors is an efficient means of exploring the megadiverse group of peptides from genus Conus.
Keywords: Conantokin, Molecular phylogeny, Conformational interconversion, Helical peptide, Electrophysiology
INTRODUCTION
The conantokins are a family of peptides found in Conus venoms that are antagonists of N-methyl D-aspartate (NMDA) receptors [1-3]. Nine conantokin peptides from seven Conus species have been documented in the literature: Conantokin-G (ConG) from Conus geographus [4], ConT from Conus tulipa [5], ConR from Conus radiatus [6], ConL from Conus lynceus [7], ConPr-A, Pr-B, Pr-C from Conus parius [8], ConP from Conus purpurascens [9] and ConBr from Conus brettinghami [10]. These peptides comprise the only group of peptidic ligands from animal venoms presently known to target NMDA receptors.
We have recently used a multidisciplinary strategy for discovery from Conus venoms; the general approach combines peptide chemistry (or recombinant expression), molecular genetics and phylogenetics [11-12]. This approach has been used successfully for developing ligands targeted to various nicotinic receptors, resulting in the availability of selective pharmacological agents for diverse molecular isoforms of the nicotinic receptor family [13]. This general approach has not previously been systematically applied to the conantokin family. In this report, we have initiated the concerted discovery strategy for identifying novel conantokin peptides that can more efficiently allow us to assess structure/function relationships in these peptides.
A recently characterized conantokin peptide, ConBr from Conus brettinghami, had a number of divergent characteristics compared to previously described conantokins [10]. We used a phylogenetic approach to find related natural peptides; our goal was a broad assessment of the sequence elements in ConBr that confer its particular selectivity profile. Because Conus rolani, a species not previously investigated, is a member of the same clade as Conus brettinghami, we obtained the sequence of a conantokin peptide from Conus rolani with homology to ConBr, which we designate ConRl-A. The characterization of ConRl-A, and a comparison to ConBr is detailed below. These studies illustrate that molecular phylogeny can be used to quickly identify Conus species that have evolved conantokin peptides useful for targeted structure/function insights. We also describe an unexpected and novel conformational property of ConRl-A that emerged from its characterization.
EXPERIMENTAL PROCEDURES
Phylogenetic analysis
Phylogenetic analysis was performed using combined sequences of 12S, 16S and COI mitochondrial gene regions for each species, as previously described [14]. The lengths of the sequences used for analysis were 585-590, 546-548, and 594-709 base pairs for 12S, 16S, and COI, respectively. The sequences were aligned and neighbor-joining trees generated using ClustalX version 2.0.9 [15] with standard default options.
Preparation of genomic DNA and characterization of clones encoding ConRl-A
Genomic DNA was prepared from 20 mg Conus rolani tissue using the Gentra PUREGENE DNA Isolation Kit (Gentra Systems, Minneapolis, MN) according to the manufacturer's standard protocol. 10 ng of C. rolani genomic DNA was used as a template for polymerase chain reaction (PCR) with oligonucleotides corresponding to conserved regions of the signal sequence (5’ GCG ATG CAA CTG TAC ACG TAT CTG) and 3’ UTR sequence (5’ AAT AAA CAT GAA AGA TTT GGG GAA) of conantokin prepropeptides. The resulting pcr product was purified using the High Pure PCR Product Purification Kit (Roche Diagnostics, Indianapolis, IN) following the manufacturer's suggested protocol. The eluted DNA fragment was annealed to pNEB206A vector using the USER Friendly Cloning kit (New England BioLabs, Inc., Bever1y, MA) following manufacturer's suggested protocol and the resulting product transformed into DH5a competent cells. The nucleic acid sequences of the resulting conantokin toxin-encoding clones were determined according to the standard protocol for Automated sequencing.
Peptide Synthesis
ConRl-A was synthesized using standard Fmoc (N-(9-fluorenyl) methoxycarbonyl) protected amino acids on ABI model 430A peptide synthesizer at the DNA/Peptide Core Synthesis Facility, University of Utah. The side chains of Asp, Glu and Thr residues were protected with tBu group (Novabiochem). The side chains of Lys, Gln and Arg residues were protected with Boc, Trt and Pbf groups (Novabiochem), respectively. The side chains of Gla were protected with tBu groups (Chem-Impex International, Inc.). The peptide was constructed on proline containing preloaded resin, H-Pro-2-ClTrt resin (Novabiochem), using standard DCC/HOBT chemistry. The peptide was cleaved from the resin and simultaneous deprotection of side chains was achieved by agitating 20 mg of the resin in 1 ml of reagent K {TFA/phenol/thioanisole/water/ethanedithiol (82.5:5:5:5:2.5)} at room temperature. The mixture was filtered, precipitated using methyl-tert-butyl ether (MTBE) and spun by centrifugation. The pellet was repeatedly washed with cold MTBE and purified over a Vydac C18 column (10 mm × 250 mm, 5 μm particle size) using ACN/H2O/TFA solvent system. The flow rate was maintained at 3 ml min–1 following a linear gradient of 10–40% ACN over 40 min and the fractions were detected at 220 nm. The purity of conRl-A was confirmed using mass spectrometry. Mass spectra were obtained using a Micromass Quattro II mass spectrometer at the Mass Spectrometry and Proteomics Core Facility of the University of Utah.
Peptide conformation analysis
Conformational analysis of the peptide was achieved using C18 analytical RP-HPLC over the range of temperature from 4-45°C. The desired temperature of the column was achieved by keeping it in a water bath over a period of time. Five nmoles of peptide was dissolved in 50% ACN containing 0.01% TFA and incubated at room temperature for 5 min before injecting to HPLC. The peptide was eluted using a linear gradient of 20-50% Buffer B (90% ACN containing 0.1% TFA) over 35 min. Early-eluting fraction and late-eluting fractions were separated and incubated at 4°C prior to the re-application to column. Distinct fractions were separately ran again under identical chromatographic conditions. Area under early and late eluting conformers was obtained by integrating the chromatograms using data analysis software provided by the manufacturer (Waters Millennium).
Circular Dichrosim Spectroscopy
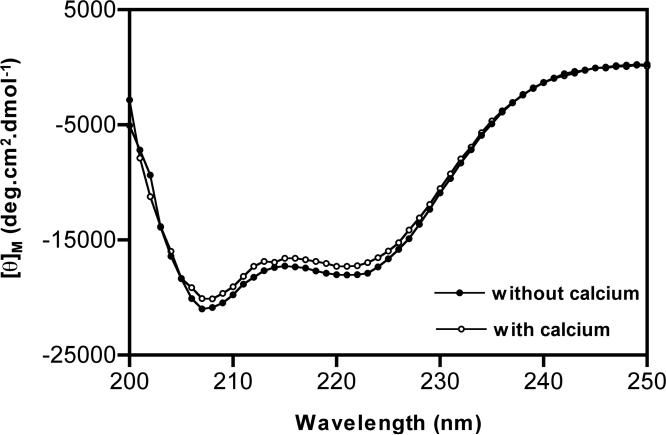
Far-UV CD spectra were recorded with an AVIV Model 62D spectropolarimeter, using a bandwidth of 1 nm, a step size of 1 nM, and an average time of 0.5 sec. Peptide was dissolved in 10 mM HEPES buffer, pH 7.0, with or without 2 mM CaCl2. All measurements were taken at room temperature, over 250-190 nm wavelength range, using cell of 0.1 cm path length. Peptide concentration was 100 μM, as determined by the absorbance at 280 nm. Five independent spectra were collected and averaged for each sample. The contribution of buffer to the CD signal was eliminated by subtracting the peptide CD signal with that of the buffer alone CD signal. All spectral intensities were expressed as mean residue elipticities using the equation reported elsewhere [8]. Molar ellipticity of –35086.66 degrees cm2 dmol–1 was estimated to be a perfect α-helix (100% α-helix). The percent helical conformation was calculated by assuming a linear relationship in comparison with 100% α-helix.
Heterologous expression of NMDA receptors in Xenopus oocytes
The rat NMDA receptor clones used were NR2A, NR2B, NR2C, NR2D, and NR1-2b; GenBank numbers AF001423, U11419, U08259, U08260, and U08264, respectively. We previously used the NR1-3b splice variant to express recombinant NMDA receptors [8-10]; in the present study, we have used NR1-2b slice variant due to its robust expression in the central nervous system [16]. To confirm this splice variant difference does not affect the potency or order of conantokin selectivity toward NMDA receptor complexes, we also assayed conantokins for potency on differing combinations of NR2(A-D) and NR1-3b, and found no difference between splice variants (data not shown). All of the expression clones were driven by a T7 promoter and were used to make capped RNA (cRNA) for injection into the oocytes of Xenopus laevis frogs. cRNA was prepared in-vitro using Ambion RNA transcription kits (Ambion, Inc.) according to manufacturer's protocols. To express NMDA receptors, 2-5 ng of cRNA for each subunit was injected per oocyte. Oocytes were maintained at 18 degrees Celsius in ND96 solution (96.0 mM NaCl, 2.0 mM KCl, 1.8 mM CaCl2, 1mM MgCl2, 5mM HEPES, pH 7.2-7.5) containing antibiotics (septra, amikacin, pen/strep). All voltage-clamp electrophysiology was done using oocytes 1-6 days post-injection.
Two-electrode voltage clamp electrophysiology
All oocytes were voltage clamped at −70mV at room temperature. Oocytes were gravity perfused with Mg2+ -free ND96 buffer (96.0 mM NaCl, 2.0 mM KCl, 1.8 mM CaCl2, 5 mM HEPES, pH 7.2-7.5). Mg2+ was not included in the ND96 buffer because Mg2+ blocks NMDA receptors at the voltage potential used to clamp oocytes (−70mV). To reduce nonspecific absorption of peptide, bovine serum albumin (BSA) was added to ND96 buffer at a final concentration of 0.1mg/ml. To elicit current from oocytes expressing NMDA receptors, one-second pulses of gravity-perfused agonist solution were administered at intervals of 60s, 90s, or 120s, depending on the rate of receptor recovery from desensitization. Agonist solution was comprised of glutamate and co-agonist glycine suspended in Mg2+ -free ND96 buffer at final concentrations of 200 μM and 20 μM, respectively. Buffer was perfused continuously over the oocytes between agonist pulses, except during equilibration periods. During equilibration period, buffer flow was halted for 5 minutes to create a static bath for application of either peptide (suspended in ND96 buffer at various concentrations), or control solution (ND96 buffer alone). The length of equilibration period was equal to or greater than the time necessary to achieve maximal current inhibition at a given concentration. The effect of a peptide on NMDA receptor-mediated current was determined by measuring the amplitude of the first agonist-elicited current pulse immediately following the equilibration period as a percentage of the amplitude of the baseline current (agonist-elicited current immediately preceding equilibration period). Data acquisition was automated by a virtual instrument made by Doju Yoshikami of the University of Utah. Concentration-response curves were generated using Prism software (GraphPad Software, Inc.), using the following equation, where nH is the Hill coefficient and IC50 is the concentration of peptide causing half-maximal block: % Response = 100/{1+([peptide]/IC50)nH}.
RESULTS
Phylogenetic analysis
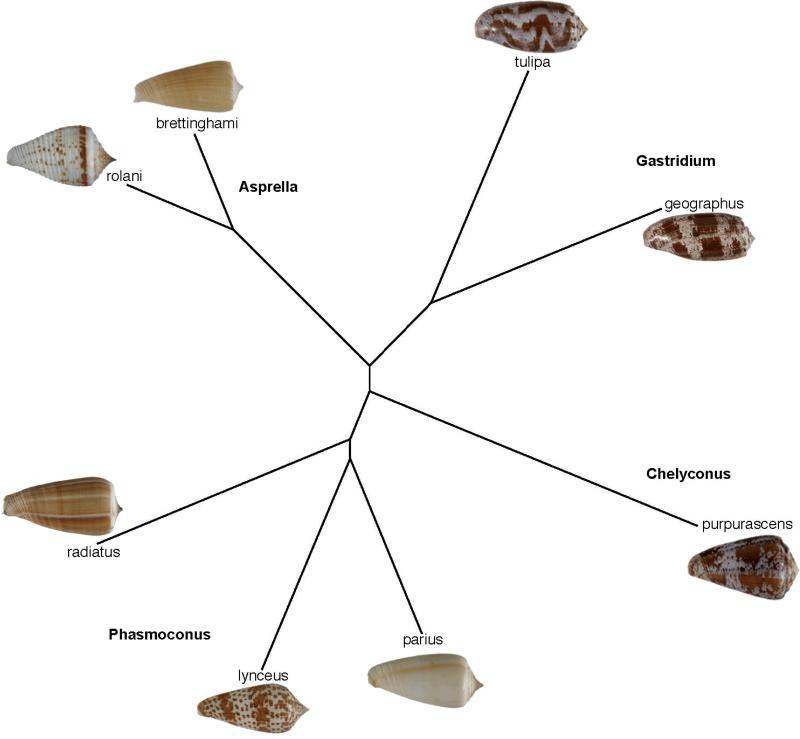
Conus brettinghami is a member of the so-called Conus sulcatus complex, a set of deep-water Conus species all believed to belong to the Asprella clade, with Conus sulcatus as the type species (Asprella is regarded as a subgenus of Conus in most, but not all, taxonomic works). One species in this branch of Conus is Conus rolani; since we had collected this species, genomic DNA clones from Conus rolani were analyzed. C. rolani is also a deep-water piscivorus cone snail collected from the Philippine's coast using tangle nets (ref)”. The phylogenetic relationship of Conus rolani to Conus brettinghami, the source of ConBr, was determined by analysis of the combined sequences of 12s, 16s and COI mitochondrial gene regions and is shown in Figure 1; all Conus species that have yielded other conantokin sequences are shown in the figure.
Figure 1.
Phylogenetic analysis using combined 12S, 16S and COI sequences showing all Conus species from which conantokin peptides have been identified. Conus rolani was collected fom Philippines coast using tangle nets.
Analysis of a clone encoding ConRl-A from Conus rolani
Prior work on α-conopeptides targeted to nicotinic acetylcholine receptors revealed that the analysis of Conus species closely related to each other has potential for identifying homologous peptides with a sufficient level of amino acid sequence divergence to gain structure/function insights. ConBr, a conantokin peptide from Conus brettinghami had some intriguing functional features; to assess structure/function relationships for ConBr, we wanted to identify a homologous peptide with significant amino acid sequence differences.
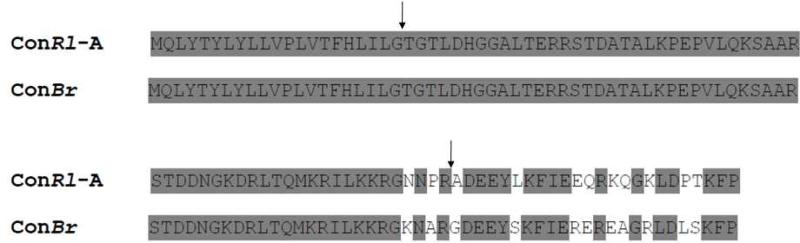
A C. rolani clone encoding a peptide precursor with a high degree of homology to previously identified conantokin precursors was identified and designated ConRl-A. The sequences of the predicted corresponding open reading frames of ConRl-A and ConBr precursors are shown in Figure 2. The striking similarity of the sequences encoding ConRl-A and ConBr establishes that ConRl-A is a member of the conantokin superfamily. A notable feature of ConRl-A is that the predicted amino acid at position 1 of the mature peptide (alanine), differs from that of the canonical N-terminal glycine (or aspartate) at position 1 in all previously identified conantokins.
Figure 2.
Precursor sequences of ConBr and ConRl-A. The arrows indicate the signal sequence/propeptide and propeptide/mature conantokin boundaries.
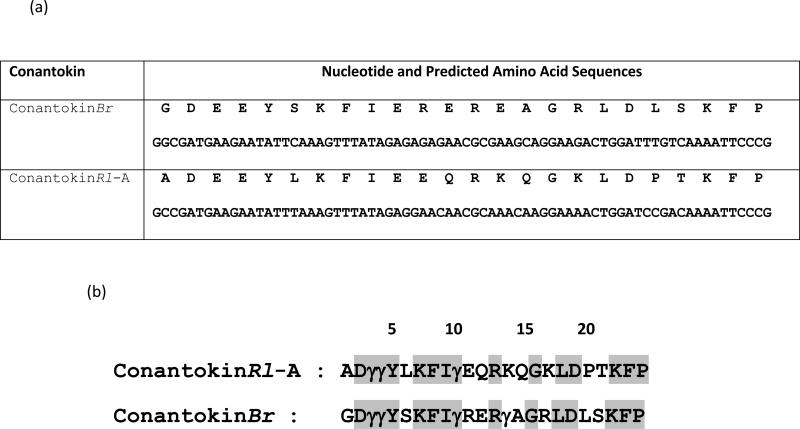
A comparison of the predicted mature sequences of ConRl-A and ConBr after the predicted post-translational modification is shown in Figure 3. In a similar manner to ConBr, the glutamate residues that are known to be γ-carboxylated in members of the conantokin family purified from venom (i.e., glutamates at positions 3-4, and each glu that occurs in a spacing of every 3-4 amino acids thereafter, such that they align on a single side of the helical peptide) are predicted to be post-translationally modified to γ-carboxyglutamate in ConRl-A. However, the glutamate at position 11, which immediately follows the carboxyglutamate predicted at position 10, is not predicted to undergo post-translational modification.
Figure 3.
cDNA sequences encoding conantokins. (a) Nucleotide and predicted amino acid sequences for ConRl-A and ConBr. (b) Predicted mature peptides. Posttranslational modifications (γ: Gla) were assumed based on consistent occurrence of Gla in naturally isolated conantokins and amino acid spacing between Gla residues.
Characterization of ConRl-A
In order to characterize the deduced conantokin from C. rolani, chemical synthesis of the predicted sequence shown in Figure 3 was carried out as described under Methods. The observed mass of the peptide, as revealed from ESI-MS, is 3042.5, 2997.2, 2953.9 and 2909.7. Occurrence of multiple peaks in ESI-MS corresponds to the loss of carboxyl groups, a phenomenon previously observed in Gla containing Conus peptides [9, 17].
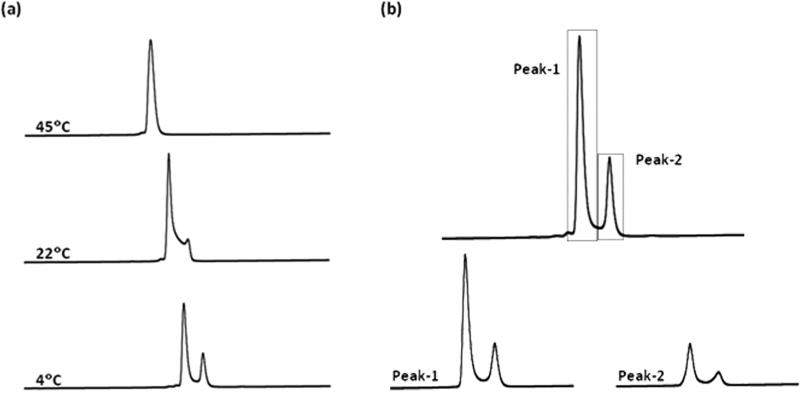
The elution profile of ConRl-A is unusual with two peaks separated by a broad trough. This apparent chromatographic heterogeneity was assessed by eluting the peptide from the HPLC column at different temperature. Figure 4a shows effect of temperature on chromatography of the peptide. At low (4°C) temperature, ConRl-A yields two well-resolved peaks; as the temperature increases (22°C), the trough between two peaks increases and at high temperature (45°C), a single homogeneous peak is found. These observations clearly suggest that ConRl-A exists as two distinct, slow-exchanging conformers in solution. The broad trough in the chromatogram at 22°C can be attributed to interconversion of the two conformers; at higher temperature, conformational interconversion may be sufficiently rapid that the C18 column cannot resolve the two conformers resulting in a single peak in the elution profile. Under these conditions, the conformational interconversion is presumably faster than the chromatographic resolution.
Figure 4.
Conformational analysis of ConRl-A using RP-HPLC. (a) Effect of temperature on chromatography of the peptide at indicated temperature. The temperature of the C18 analytical column was maintained either by incubating in ice (or) water bath of the desired temperature. (b) Elution profiles obtained by reinjection of the fractions corresponding to Peak-1 and Peak-2 of the peptide at 4°C. Both major and minor conformers are represented from each peak.
The conformational equilibrium between two conformers in ConRl-A was probed at 4°C. Figure 4b shows elution profiles of the fractions isolated from the chromatogram of ConRl-A. The early eluting hydrophilic component and late eluting hydrophobic fraction of ConRl-A were separately collected and re-injected onto the column at 4°C. Both components were incubated at 4°C for 20 min prior to re-injection. Re-injection of either of the fraction results in a reappearance of both of the HPLC peaks and a ratio between two peaks of ~ 2.6:1 was obtained in both cases. This observation is consistent with the presence of two interconvertible conformers of ConRl-A in solution, which can be separated upon the appropriate chromatographic conditions at 4°C. Since the ratio of major and minor conformers of ConRl-A is independent of peptide concentration, it is likely that the chromatographic heterogeneity is due to intramolecular changes (and not, for instance, dimerization). For example, proline-containing peptides exhibiting peak-splitting in an RP-HPLC column have been documented in the literature [18-23].
Circular Dichroism Spectroscophy
Specific bands in the far-UV circular dichroism spectra have been widely used for rapid determination of backbone conformation of proteins (or) peptides. Secondary structural information derived from CD spectra can represent qualitatively the overall fold of molecule and such studies have been extensively employed in the characterization of conantokins. Figure 5 shows circular dichroism spectra of ConRl-A. ConRl-A is predominantly alpha helical and estimated helicity is 50%, which is essential same in presence and absence of divalent cation (Ca2+). Molecular modeling of homologues ConBr indicates the presence of helical structure between 2-12 residues of the molecule [10]. ConRl-A contains eight identical residues compared to that of ConBr among structured 2-12 peptide segment. The observed percentage of helicity for ConRl-A and its homology to ConBr strongly suggest that both the peptides may adopt similar helical conformation. Surprisingly ConBr results in a single homogenous peak in RP-HPLC elution profile at room temperature, whereas ConRl-A exhibits pronounced asymmetry.
Figure 5.
Circular dichroism spectra of ConRl-A. Spectra were recorded by dissolving peptide in 10 mM HEPES buffer at pH 7.0, containing either 2 mM CaCl2 (or) absence of CaCl2. Shown is an average spectra obtained from five independent scans. Percentage of helicity of peptide in the presence (or) absence of calcium was found to be 50%.
Functional Characterization of ConRl-A
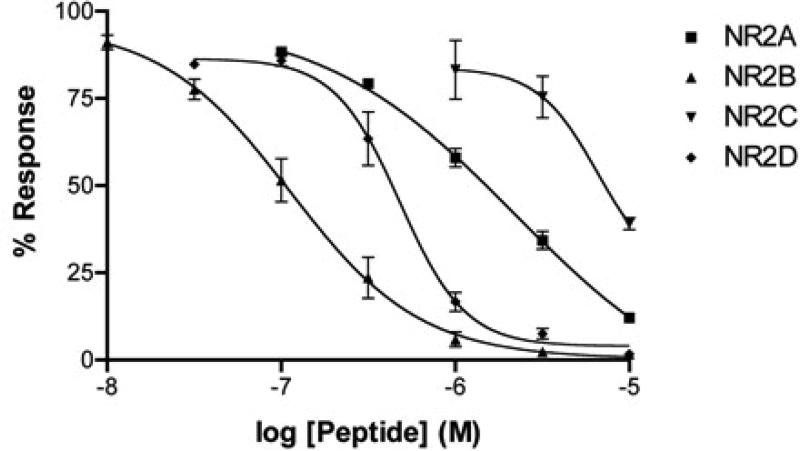
Due to the high degree of homology of ConRl-A with ConBr (a known antagonist of the NR2B and NR2D NMDA receptor subtypes, [10], we assessed the functional antagonism of ConRl-A on the four NMDA receptor subtypes with different NR2 subunits (NR2ANR2D). These were heterologously expressed in Xenopus oocytes in combination with the NR1-2b splice variant. Using two-electrode voltage clamp electrophysiology, we determined the function of ConRl-A by applying the peptide to oocytes and allowing for a 5-minute equilibration period in a static bath. The effect of the peptide was measured by normalizing the agonist-elicited response following equilibration period to baseline response in the absence of peptide. Concentration response curves for ConRl-A on each of the four NMDA receptor subtypes is shown in Figure 6. The order of potency for ConRl-A was NR2B>NR2D>NR2A>NR2C. Interestingly, although 9 amino acids differ between the corresponding positions of ConRl-A and ConBr (Figure 3b), the order of NR2 subunit selectivity was identical for the two peptides, and the degree of potency on each subunit was similar (Table 1).
Figure 6.
Concentration-response curve of ConRl-A on the four different NR2 NMDA receptor subunits separately co-expressed with NR1-2b in Xenopus oocytes. Each data point represents the response obtained from three independent oocytes. Curve fitting was performed using Prism software (GraphPad Software, Inc.).
Table 1.
Comparison of Approximate IC50 (μM) values of ConRl-A and ConBr.
| Peptide | NMDA receptor subtypes NR2(A-D) | |||
|---|---|---|---|---|
| NR2A IC50 | NR2B IC50 | NR2C IC50 | NR2D IC50 | |
| ConBr | 0.68 | 0.14 | 4.9 | 0.31 |
| ConRl-A | 2.1 | 0.11 | 6.1 | 0.48 |
DISCUSSION
We have characterized a novel conantokin, ConRl-A from Conus rolani, a Conus species that belongs to the Asprella clade. We previously characterized ConBr from Conus brettinghami, another species in Asprella; ConBr had a pharmacological profile for the different NMDA receptor subtypes distinct from previously characterized members of the conantokin family. The rationale for focusing on species in the same clade of Conus as Conus brettinghami was to gain structure/function insights using a phylogenetic approach (instead of, for example, using the standard “alanine walk”). Analysis of a Conus rolani library revealed a conantokin peptide that differed in 9 out of 24 amino acids in its primary sequence from ConBr. The conserved sequence features included the Tyr 5 residue previously shown to confer a relatively high affinity for the NR2D subtype [10].
The precursor sequences of ConBr and ConRl-A are typical in the pattern of divergence seen in other conopeptide superfamilies. Thus, the mature toxin sequences are both 24 AA, but the initially translated open reading frame is 103 amino acids. There is a signal sequence of 21 AA that is completely identical in the two predicted precursors. A relatively large propeptide region (58 AA) is found between the signal sequence and the mature conantokin peptide, with a typical proteolytic cleavage site at the C-terminus indicated by the arrow in Figure 2. There are only two divergent AA positions in the 58 amino acid propeptide region (divergence = 3.4%). On the other hand, the very considerable divergence in the mature peptide region (9/24 nonidentical AA; 37.5% divergence) is a characteristic result of the focal hypermutation of conopeptides that has been repeatedly noted [24-26]. These mature peptide regions are among the most rapidly evolving gene sequences known.
The precursors of γ-carboxylated peptides isolated from Conus contain a propeptide sequence that serves as the γ-carboxylase recognition sequence (γ-CRS) for the Conus γ-glutamyl carboxylase. In the case of ConG from Conus geographus, Bandyopadhyay et al [27] demonstrated that the γ-CRS was within the −20 to −1 region of the propeptide sequences within which there are two divergent positions between ConRl-A and conBr. γ-CRS have also been identified in the propeptide regions of other γ-carboxylated peptides e.g. in εTxIX(tx5a) [28- 29] belonging to the T-superfamily and Tx9a [30] in the P-superfamily. The γ-CRS in Gla-TxX and Gla-TxXI is however present as a “post-peptide”.
Since the γ-CRS in peptides belonging to different superfamilies are highly divergent it has not been possible to assign any common sequence features. Czerwiec et al [31] pointed out the presence of conserved basic residues, and α-helicity of the propeptide. A structure-function study of εTxIX [32] suggested the importance of hydrophobic residues. Hydrophobic residues are also important in the mammalian γ-CRS [31]. In the case of the γ-CRS from ConG, substitution of the basic residues by alanine did not have any significant effect [33]. However, the roles of hydrophobic residues in the γ-CRS from conantokins have not been investigated. The putative γ-CRS region of ConBr and ConRl-A are highly similar to that of ConG, and all of the large hydrophobic residues are conserved. Thus, we do not expect the amino acid changes between ConBr and ConRl-A, nor between these peptide precursors and the ConG precursors to affect the function of the γ-CRS.
The characterization of ConRl-A resulted in the unexpected discovery that ConRl-A equilibrates between two conformational states. If the temperature is lowered, the equilibration slows down sufficiently to separate the two conformational forms using standard HPLC conditions. Discrete conformational states have not previously been detected in for any conantokin peptide. Most Conus peptides appear to have a single solution conformation with some notable exceptions [34-39]. The contryphan family of peptides that have a single disulfide bond as well as a D-amino acid, have two interconverting conformational states, dependent on the presence of the D-amino acid [40]. Another peptide with more than one conformational state in solution is α-conotoxin MI; this peptide equilibrated between the two alternative conformational states relatively slowly [41].
In all these prior cases, the conformational equilibrium was in the context of a small peptide with disulfide bonds. What is novel about the discovery of the conformational equilibration between two states in ConRl-A is that this peptide has no disulfide cross-links at all. Given its sequence homology to ConBr, the presence of two conformers was rather unexpected, since the latter peptide elutes from an HPLC column as a single homogenous peak. At present, the molecular basis of unique chromatographic behavior of ConRl-A is unclear.
There are several noteworthy features of ConRl-A. In ConG, the first conantokin characterized, there were 5 γ-carboxyglutamate residues — a number of studies have establishd that these are important for giving the peptide its helical structure in solution [42]. As shown in Table 2, there have been a number of conantokin peptides characterized in which γ-carboxyglutamate residues are substituted with other amino acids. Most notably, the γ7 in ConG is substituted with a lysine residue, in most of the conantokin peptides characterized so far (this is the case for both ConBr and ConRl-A). The literature to date suggests that in conantokins such as ConG with a γ-carboxyglutamate at residue 7, Ca++ is required to maintain the helical conformation [43]; however, the presence of lysine at this position confers stability to the helical conformation, even in the absence of Ca++, as we have demonstrated for ConRl-A using circular dichroism. We note that ConRl-A is the only conantokin peptide characterized so far in which two of the 5 Gla residues in ConG are substituted by Lys; the presence of Lys14 was only found in ConPrB; however, in this peptide, position 7 has Gla rather than Lys.
Table 2.
Comparison of known conantokin sequences
| Conus Clade | Conantokin | Peptide Sequence | Reference |
|---|---|---|---|
| Gastridium | ConT | GEγγ YQK MLγ NLRγ AEVK KNA* | [5] |
| ConG | GEγγ LQγ NQγ LIRγ KSN* | [4] | |
| Phasmoconus | ConL | GEγγ VAK MAAγ LARγ DAVN* | [7] |
| ConR | GEγγ VAK MAAγ LARγ NIAK GCKVNCYP^ | [6] | |
| ConPr-A | GEDγ YAγ GIRγ YQLI HGK I^ | [8] | |
| ConPr-B | DEOγ YAγ AIRγ YQLK YGK I^ | [8] | |
| ConPr-C | GEOγ VAK WAγ GLRγ KAASN* | [8] | |
| Chelyconus | ConP | GEγγ HSK YQγ CLRγ IRVNK VQQγC^ | [9] |
| Asprella | ConBr | GDγγ YSK FIγ RERγ AGR LDLSKFP^ | [10] |
| ConRl-A | ADγγ YLK FIγ EQRK QGK LDPTKFP^ | This work | |
Con, conantokin; γ, γ-carboxyglutamate; O, 4-trans-hydroxyproline
C-terminal amidation
C-terminal free acid.
Another unusual feature of ConRl-A is that it is the first native conantokin sequence characterized with alanine at position 1. Previous mutational studies of ConG, ConT and ConR indicated that an Ala substitution for glycine at position 1 resulted in an ~10-fold reduction in potency [44-46]. However, since the potency of ConRl-A and ConBr do not differ significantly, it would appear that the Ala substitution for Gly in these peptides from Conus species in the Asprella clade is not as functionally significant as was found for the other conantokin peptides, suggesting that there are sequence features of these peptides that allow a substitution at position 1 to be more readily tolerated with respect to its functional activity.
Although the mature ConBr and ConRl-A sequences diverge significantly from each other (37.5% divergence) they are nevertheless much more similar to each other than either peptide is to any other conantokin sequences that have been identified so far (see Table 2). Indeed, the sequence identity of peptides that come from Conus species in the same clade (43%) is generally greater than is found for two conantokin sequences from species that come from different clades (20-25% sequence identity is typical). It is for this reason that a systematic comparison of conantokin sequences from species within a clade is equivalent to a detailed mutational study of a particular peptide, since such a phylogenetically-informed analysis has the potential to reveal which amino acid sequences are stringently conserved, and for which substitutions are acceptable in sets of peptides that may have homologous physiological functions.
The comparison of the sequences of ConBr and ConRl-A, shown in Figure 3 reveals that 15 of the 24 amino acids are identical, but there are 9 substitutions. Three of these (Ala1, Lys17, and Thr21) are conservative substitutions with the other 5 non conservative. Correlating the sequence changes with the functional characterization of ConRl-A suggests that at these 5 positions, particular AA are not stringently required for either potency nor selectivity, since the functional activities of ConBr and ConRl-A are similar (see Table 1). It seems reasonable to regard them as functionally homologous venom components of two different Conus species, given the similarities in potency and selectivity for the 4 different subtypes of NMDA receptors. One of the conserved residues, Tyr 5 was previously shown to be critical for an unusual pharmacological characteristic of these peptides: compared to other conantokins, these peptides exhibit a relatively high affinity for the NR2D receptor subtype[10].
Although the shift in affinity from ConBr is modest, the increase in affinity for NR2B and decrease in NR2C affinity gives the peptide a 55-fold preference for NR2B over NR2C, the greatest discrimination yet found among conantokins for these two NMDA receptor subtypes. This makes ConRl-A a potentially useful pharmacological tool for B/C differentiation.
ACKNOWLEDGEMENT
We thank Drs. Robert Schackmann and Scott Endicott from the DNA/Peptide Synthesis Core Facility at the University of Utah for the synthesis of peptides. Gowd KH acknowledges Pradip K. Bandypadhyay and Russell W. Teichert for useful scientific discussions.
ABBREVIATIONS
- CD spectroscopy
Circular Dichroism spectroscopy
- ConRl-A
ConantokinRl-A
- Gla, γ
γ-carboxyglutamate
- NMDA
N-methyl-D-aspartate
- RP-HPLC
Reverse-Phase High Performance Liquid Chromatography
Footnotes
This work was supported by the grant GM48677 from the National Institute of General Medical Sciences
REFERENCES
- 1.Layer RT, Wagstaff JD, White HS. Conantokins: peptide antagonists of NMDA receptors. Curr Med Chem. 2004;11:3073–3084. doi: 10.2174/0929867043363901. [DOI] [PubMed] [Google Scholar]
- 2.Jimenez EC. Conantokins: from “sleeper” activity to drug development. Philippine Science Letters. 2009 (in press) [Google Scholar]
- 3.Twede VD, Miljanich G, Olivera BM, Bulaj G. Neuroprotective and cardioprotective conopeptides: an emerging class of drug leads. Curr Opin Drug Discov Devel. 2009;12:231–239. [PMC free article] [PubMed] [Google Scholar]
- 4.Mena EE, Gullak MF, Pagnozzi MJ, Richter KE, Rivier J, Cruz LJ, Olivera BM. Conantokin-G: a novel peptide antagonist to the N-methyl-D-aspartic acid (NMDA) receptor. Neurosci. Lett. 1990;118:241–244. doi: 10.1016/0304-3940(90)90637-o. [DOI] [PubMed] [Google Scholar]
- 5.Haack JA, Rivier J, Parks TN, Mena EE, Cruz LJ, Olivera BM. Conantokin-T. A gamma-carboxyglutamate containing peptide with N-methyl-d-aspartate antagonist activity. J. Biol. Chem. 1990;265:6025–6029. [PubMed] [Google Scholar]
- 6.White HS, McCabe RT, Armstrong H, Donevan SD, Cruz LJ, Abogadie FC, Torres J, Rivier JE, Paarmann I, Hollmann M, Olivera BM. In vitro and in vivo characterization of conantokin-R, a selective NMDA receptor antagonist isolated from the venom of the fish-hunting snail Conus radiatus. J. Pharmacol. Exp. Ther. 2000;292:425–432. [PubMed] [Google Scholar]
- 7.Jimenez EC, Donevan S, Walker C, Zhou LM, Nielsen J, Cruz LJ, Armstrong H, White HS, Olivera BM. Conantokin-L, a new NMDA receptor antagonist: determinants for anticonvulsant potency. Epilepsy Res. 2002;51:73–80. doi: 10.1016/s0920-1211(02)00101-8. [DOI] [PubMed] [Google Scholar]
- 8.Teichert RW, Jimenez EC, Twede V, Watkins M, Hollmann M, Bulaj G, Olivera BM. Novel Conantokins from Conus parius Venom are specific antagonists of N-Methyl-D-aspartate receptors. J. Biol. Chem. 2007;282:36905–36113. doi: 10.1074/jbc.M706611200. [DOI] [PubMed] [Google Scholar]
- 9.Gowd KH, Twede V, Watkins M, Krishnan KS, Teichert RW, Bulaj G, Olivera BM. Conantokin-P, an unusual conantokin with a long disulfide loop. Toxicon. 2008;52:203–213. doi: 10.1016/j.toxicon.2008.04.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twede VD, Teichert RW, Walker CS, Gruszczyński P, Kaźmierkiewicz R, Bulaj G, Olivera BM. Conantokin-Br from Conus brettinghami and selectivity determinants for the NR2D subunit of the NMDA receptor. Biochemistry. 2009;48:4063–4073. doi: 10.1021/bi802259a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivera BM. Conus peptides: biodiversity-based discovery and exogenomics. J Biol Chem. 2006;281:31173–31177. doi: 10.1074/jbc.R600020200. [DOI] [PubMed] [Google Scholar]
- 12.Olivera BM, Teichert RW. Diversity of the neurotoxic Conus peptides: a model for concerted pharmacological discovery. Mol Interv. 2007;7:251–260. doi: 10.1124/mi.7.5.7. [DOI] [PubMed] [Google Scholar]
- 13.Olivera BM, Quik M, Vincler M, McIntosh JM. Subtype-selective conopeptides targeted to nicotinic receptors: Concerted discovery and biomedical applications. Channels (Austin) 2008;2:143–152. doi: 10.4161/chan.2.2.6276. [DOI] [PubMed] [Google Scholar]
- 14.Nam HH, Showers-Corneli P, Watkins M, Olivera BM, Bandyopadhyay P. Multiple genes elucidate the evolution of venomous snail-hunting Conus species. Molecular Phylogenetics and Evolution. 2009;53:645–652. doi: 10.1016/j.ympev.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Larkin G, Blackshields NP, Brown 3R, Chenna PA, McGettigan H, McWilliam F, Valentin IMWallace A, Wilm R, Lopez JD, Thompson TJ, Gibson, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics Applications Note. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 16.Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J Neurosci. 1994;14:3180–3194. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakubowski JA, Keays DA, Kelley WP, Sandall DW, Bingham JP, Livett BG, Gayler KR, Sweedler JV. Determining sequences and post-translational modifications of novel conotoxins in Conus victoriae using cDNA sequencing and mass spectrometry. J Mass Spectrom. 2004;39:548–557. doi: 10.1002/jms.624. [DOI] [PubMed] [Google Scholar]
- 18.Gesquiere JC, Diesis E, Cung MT, Tartar A. Slow isomerization of some proline-containing peptides inducing peak splitting during reversed-phase high performance liquid chromatography. J. Chromatogr. 1989;478:121–129. [Google Scholar]
- 19.Trabelsi H, Bouabdallah S, Sabbah S, Raouafi F, Bouzouita K. Study of the cis-trans isomerization of enalapril by reversed-phase liquid chromatography. J Chromatogr A. 2000;871:189–199. doi: 10.1016/s0021-9673(99)01214-5. [DOI] [PubMed] [Google Scholar]
- 20.O'Neal KD, Chari MV, Mcdonald CH, Cook RG, Yu-Lee LY, Morrisett JD, Shearer WT. Multiple cis-trans conformers of the prolactin receptor proline-rich motif (PRM) peptide detected by reverse-phase HPLC, CD and NMR spectroscopy. Biochem J. 1996;315:833–844. doi: 10.1042/bj3150833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francart C, Wieruszeski JM, Tartar A, Lippens G. Structural and dynamic characterization of Pro Cis/Trans Isomerization in a small cyclic peptide. J. Am. Chem. Soc. 1996;118:7019–7027. [Google Scholar]
- 22.Shi T, Spain SM, Rabenstein DL. A striking periodicity of the cis/trans isomerization of proline imide bonds in cyclic disulfide-bridged peptides. Angew. Chem. Int. Ed. 2006;45:1780–1783. doi: 10.1002/anie.200503470. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsen R, Jimenez EC, Grilley M, Watkins M, Hillyard D, Cruz LJ, Olivera BM. The contryphans, a D-tryptophan-containing family of Conus peptides: interconversion between conformers. J. Pept. Res. 1998;51:173–179. doi: 10.1111/j.1399-3011.1998.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 24.Espiritu DJ, Watkins M, Dia-Monje V, Cartier GE, Cruz LJ, Olivera BM. Venomous cone snails: molecular phylogeny and the generation of toxin diversity. Toxicon. 2001;39:1899–1916. doi: 10.1016/s0041-0101(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 25.Olivera BM, Walker C, Cartier GE, Hooper D, Santos AD, Schoenfeld R, Shetty R, Watkins M, Bandyopadhyay P, Hillyard DR. Speciation of cone snails and interspecific hyperdivergence of their venom peptides. Potential evolutionary significance of introns. Ann. N Y. Acad. Sci. 1999;870:223–237. doi: 10.1111/j.1749-6632.1999.tb08883.x. [DOI] [PubMed] [Google Scholar]
- 26.Duda TF, Jr, Palumbi SR. Molecular genetics of ecological diversification: duplication and rapid evolution of toxin genes of the venomous gastropod Conus. Proc Natl Acad Sci U S A. 1999;96:6820–6823. doi: 10.1073/pnas.96.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandyopadhyay PK, Colledge CJ, Walker CS, Zhou LM, Hillyard DR, Olivera BM. Conantokin-G precursor and its role in gamma-carboxylation by a vitamin K-dependent carboxylase from a Conus snail. J. Biol. Chem. 1998;273:5447–5450. doi: 10.1074/jbc.273.10.5447. [DOI] [PubMed] [Google Scholar]
- 28.Rigby AC, Lucas-Meunier E, Kalume DE, Czerwiec E, Hambe B, Dahlqvist I, Fossier P, Baux G, Roepstorff P, Baleja JD, Furie BC, Furie B, Stenflo J. A conotoxin from Conus textile with unusual posttranslational modifications reduces presynaptic Ca2+ influx. Proc Natl Acad Sci U S A. 1999;96:5758–5763. doi: 10.1073/pnas.96.10.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker CS, Steel D, Jacobsen RB, Lirazan MB, Cruz LJ, Hooper D, Shetty R, DelaCruz RC, Nielsen JS, Zhou LM, Bandyopadhyay P, Craig AG, Olivera BM. The T-superfamily of conotoxins. J Biol Chem. 1999;274:30664–30671. doi: 10.1074/jbc.274.43.30664. [DOI] [PubMed] [Google Scholar]
- 30.Lirazan MB, Hooper D, Corpuz GP, Ramilo CA, Bandyopadhyay P, Cruz LJ, Olivera BM. The spasmodic peptide defines a new conotoxin superfamily. Biochemistry. 2000;39:1583–1588. doi: 10.1021/bi9923712. [DOI] [PubMed] [Google Scholar]
- 31.Czerwiec E, Kalume DE, Roepstorff P, Hambe B, Furie B, Furie BC, Stenflo J. Novel gamma-carboxyglutamic acid-containing peptides from the venom of Conus textile. FEBS J. 2006;273:2779–2788. doi: 10.1111/j.1742-4658.2006.05294.x. [DOI] [PubMed] [Google Scholar]
- 32.Bush KA, Stenflo J, Roth DA, Czerwiec E, Harrist A, Begley GS, Furie BC, Furie B. Hydrophobic amino acids define the carboxylation recognition site in the precursor of the gamma-carboxyglutamic-acid-containing conotoxin epsilon-TxIX from the marine cone snail Conus textile. Biochemistry. 1999;38:14660–14666. doi: 10.1021/bi991640l. [DOI] [PubMed] [Google Scholar]
- 33.Huber P, Schmitz T, Griffin J, Jacobs M, Walsh C, Furie B, Furie BC. Identification of amino acids in the gamma-carboxylation recognition site on the propeptide of prothrombin. J Biol Chem. 1990;265:12467–12473. [PubMed] [Google Scholar]
- 34.Jimenez EC, Olivera BM, Gray WR, Cruz LJ. Contryphan is a D - tryptophan containing Conus peptide. J. Biol. Chem. 1996;271:28002–28005. doi: 10.1074/jbc.271.45.28002. [DOI] [PubMed] [Google Scholar]
- 35.Jimenez EC, Craig AG, Watkins M, Hillyard DR, Gray WR, Gulyas J, Rivier JE, Cruz LJ, Olivera BM. Bromocontryphan: Post-translational bromination of tryptophan. Biochemistry. 1997;36:989–994. doi: 10.1021/bi962840p. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez EC, Watkins M, Juszczak LJ, Cruz LJ, Olivera BM. Contryphans from Conus textile venom ducts. Toxicon. 2001;39:803–808. doi: 10.1016/s0041-0101(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 37.Massilia GR, Schinina ME, Ascenzi P, Polticelli F. Contryphan-Vn: A novel peptide from the venom of the Mediterranean snail Conus ventricosus. Biochem. Biophys. Res. Commun. 2001;288:908–913. doi: 10.1006/bbrc.2001.5833. [DOI] [PubMed] [Google Scholar]
- 38.Hanumae Gowd K, Sabareesh V, Sudarslal S, Iengar P, Franklin B, Fernando A, Dewan K, Ramaswami M, Sarma SP, Sikdar SK, Balaram P, Krishnan KS. Novel peptides of therapeutic promise from Indian Conidae. Ann. N.Y. Acad. Sci. 2005;1056:462–473. doi: 10.1196/annals.1352.022. [DOI] [PubMed] [Google Scholar]
- 39.Pallaghy PK, Melnikova AP, Jimenez EC, Olivera BM, Norton RS. Solution structure of contryphan-R, a naturally occurring disulfide-bridged octapeptide containing D-Tryptophan: comparison with protein loops. Biochemistry. 1999;38:11553–11559. doi: 10.1021/bi990685j. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsen RB, Jimenez EC, De la Cruz RG, Gray WR, Cruz LJ, Olivera BM. A novel D-leucine-containing Conus peptide: diverse conformational dynamics in the contryphan family. J Pept Res. 1999;54:93–99. doi: 10.1034/j.1399-3011.1999.00093.x. [DOI] [PubMed] [Google Scholar]
- 41.Gray WR, Rivier JE, Galyean R, Cruz LJ, Olivera BM. Conotoxin MI. Disulfide bonding and conformational states. J Biol Chem. 1983;258:12247–12251. [PubMed] [Google Scholar]
- 42.Prorok M, Castellino FJ. Structure-function relationships of the NMDA receptor antagonist conantokin peptides. Curr Drug Targets. 2001;2:313–322. doi: 10.2174/1389450013348542. [DOI] [PubMed] [Google Scholar]
- 43.Cnudde SE, Prorok M, Dai Q, Castellino FJ, Geiger JH. The crystal structures of the calcium-bound con-G and con-T[K7gamma] dimeric peptides demonstrate a metal-dependent helix-forming motif. J Am Chem Soc. 2007;129:1586–1593. doi: 10.1021/ja065722q. [DOI] [PubMed] [Google Scholar]
- 44.Blandl T, Prorok M, Castellino FJ. NMDA-receptor antagonist requirements in conantokin-G. FEBS Lett. 1998;435:257–262. doi: 10.1016/s0014-5793(98)01077-1. [DOI] [PubMed] [Google Scholar]
- 45.Warder SE, Blandl T, Klein RC, Castellino FJ, Prorok M. Amino acid determinants for NMDA receptor inhibition by conantokin-T. J Neurochem. 2001;77:812–822. doi: 10.1046/j.1471-4159.2001.00281.x. [DOI] [PubMed] [Google Scholar]
- 46.Blandl T, Zajicek J, Prorok M, Castellino FJ. Sequence requirements for the N-methyl-D-aspartate receptor antagonist activity of conantokin-R. J Biol Chem. 2001;276:7391–7396. doi: 10.1074/jbc.M006648200. [DOI] [PubMed] [Google Scholar]