Abstract

The treatment of Human African trypanosomiasis remains a major unmet health need in sub-Saharan Africa. Approaches involving new molecular targets are important; pteridine reductase 1 (PTR1), an enzyme that reduces dihydrobiopterin in Trypanosoma spp., has been identified as a candidate target, and it has been shown previously that substituted pyrrolo[2,3-d]pyrimidines are inhibitors of PTR1 from Trypanosoma brucei (J. Med. Chem.2010, 53, 221–229). In this study, 61 new pyrrolo[2,3-d]pyrimidines have been prepared, designed with input from new crystal structures of 23 of these compounds complexed with PTR1, and evaluated in screens for enzyme inhibitory activity against PTR1 and in vitro antitrypanosomal activity. Eight compounds were sufficiently active in both screens to take forward to in vivo evaluation. Thus, although evidence for trypanocidal activity in a stage I disease model in mice was obtained, the compounds were too toxic to mice for further development.
Introduction
Parasitic disease remains a major global health problem, especially in tropical and subtropical countries. The availability of curative medicines is extremely limited and, such as are available, are of limited effect. Listed among the most neglected tropical diseases, Human African trypanosomiasis (HAT) continues to threaten tens of millions of people across rural Africa.1 However, concerted efforts in surveillance and intervention have brought the reported incidence to fewer than 7000 cases in 2011, with estimates of the total number of infected people being around 20 000.2 The reduction in cases from an estimated 300 000 at the turn of the century3 has stimulated a campaign to seek elimination of Trypanosoma brucei gambiense-related disease by 2030. The zoonotic nature of Trypanosoma brucei rhodesiense disease makes it more difficult to plan an elimination strategy.
The limitations of drugs currently used for HAT are several: there is a requirement for parenteral administration, severe toxicity is found in some cases, and increasing resistance is emerging in the parasites themselves.3 This means that new drugs remain necessary if elimination is to be achieved and the threat of resurgence is to be removed. It is important to identify and to exploit new molecular approaches for the treatment of HAT and other infectious diseases. Pteridine reductase (PTR1) has been proposed to be a good target in African trypanosomes. The enzyme has been shown to be essential using genetic methods,4 and it has been targeted by inhibitors of several compound classes.5−8 Among these, pyrrolopyrimidines are interesting from the perspective of already possessing biological activity and providing templates for drug discovery; the pyrimidine ring and its substituents readily key into nucleobase and cofactor base binding sites in enzymes, and C5, C6, and N7 are suitable for introducing substituents to control selectivity and physicochemical properties. In the past 2 years alone, papers have appeared where such a scaffold has been exploited for protein kinase inhibition,9−13 topoisomerase inhibition and antibacterial activity,14−16 anti-inflammatory compounds,17 antiparasitic compounds,18 and dipeptidyl peptidase IV inhibitors.19 In addition, pyrrolopyrimidines bring with them the advantage of carrying a pharmacophore with structural similarity to the recognition motif of the parasite’s P2 aminopurine transporter,20 a membrane protein capable of accumulating its substrates to internal levels that exceed external concentrations up to a thousand-fold.21 Previously, we reported that a number of heterocyclic compounds including substituted pyrrolopyrimidines and furopyrimidines are inhibitors of PTR1 from Trypanosoma brucei and Leishmania major.(7,22) Here, we use structure-based design on the pyrrolopyrimidine template, taking into consideration appropriate synthetic strategies, to obtain compounds with anti-trypanosomal activity in vivo.
Compound Design
Crystallographic studies of fused pyrimidines in the active site of the enzyme from T. brucei, TbPTR1, showed binding at the folate site, with the pyrimidine ring sandwiched between the cofactor nicotinamide and a phenylalanine, forming an array of hydrogen bonds with catalytic residues and the cofactor phosphate and ribose.7 Two possible binding poses are observed. One orientation is termed the substrate-like pose and corresponds to how substrates such as oxidized pterins and pteridines bind. The second orientation is that displayed by the archetypal antifolate methotrexate (MTX), in which the pteridine is rotated 180° about the N2–N5 axis in order to maximize hydrogen-bonding capacity.23 In each case, the possibility emerged of introducing substituents at positions 4–7 of the pyrrolopyridine template to direct substituents into hydrophobic pockets near Cys168, Leu209, Pro210, Met213, and Trp221. The requirement for transport into trypanosomes and the possibility that the specific transporters found in trypanosomes that prefer 4-aminopyrimidine substrates might concentrate the inhibitors advantageously were also considered;20,21 2,4-diaminopyrimidines were therefore considered to be important substructures at the outset of this work. Recognizing that physicochemical properties also play an important role in the biological activity of substituted pyrimidines, 4-alkoxy and 4-alkylamino substituents were investigated. To engage the hydrophobic pockets of PTR1 evident from crystallographic studies, 5-alkyl, 5-aryl, 6-alkyl, and 6-aryl pyrrolopyrimidines together with 5,6-disubstituted compounds were all studied (Figure 1); the position and shape of the hydrophobic pockets influenced the selection of substituents in compounds for synthesis. In principle, N7 could also support a substituent, but, as will be shown below, the NH forms an important hydrogen bond with PTR1; consequently, N7 substituents were not investigated.
Figure 1.
Organization of the TbPTR1 active site. (A) A surface representation of the TbPTR1 subunit with cofactor and substrate, folate, bound (PDB 3bmc) showing key residues that create the active site pocket. Potential hydrogen bonds are depicted as magenta dotted lines, and all atoms are colored according to atom type: O, red; N, blue; S, gold; P, orange; C, yellow (NADP+), cyan (TbPTR1), or black (folate). Phe97 is not labeled but is shown as thin cyan lines for clarity. (B) The active site with folate removed and key hydrogen-donor or -acceptor groups circled blue or red, respectively. Phe97 (thin cyan lines) is directly above the nicotinamide in this view. Scaffolds I and II are shown opposite, and possible hydrogen-donor or -acceptor groups are designated D or A, respectively. Arrows on the schematic also indicate the intended direction of R1, R2, and R3 substitutions into hydrophobic parts of the active site.
Synthetic Chemistry
The readily available 4-oxo-5-cyanopyrrolopyrimidine 1 used previously7,23−25 was brominated at C-6 to obtain 2, and a variety of aryl and alkyl substituents were introduced by Suzuki coupling in 10–60% yields (Scheme 1, 3a–c). Similarly, the corresponding 2,4-diamino compounds 6a–d were obtained from 2,4-diaminopyrrolopyrimidines 4 and 5. Hydrogenation of alkene 6b afforded phenylethyl substituted pyrrolopyrimidine 6d.
Scheme 1. 6-Aryl-5-cyano Derivatives.
(i) Glacial acetic acid/bromine, RT; (ii) boronic acid, Pd(PPh3)4, 2-propanol/H2O, t-butylamine, microwave at 16 °C/40 min.
4-Alkylamino- (8a, 8b, 12d) and 4-alkoxypyrrolopyrimidines (12a–c) were prepared from the corresponding 4-chloro derivatives by direct substitution of 4-chloropyrrolopyrimidine 7 with the appropriate amine or alkoxide (Scheme 2). Suzuki coupling of 6-bromopyrrolopyrimidine 14 led to highly substituted inhibitor 15.
Scheme 2. 4-Alkylamino- and 4-Alkoxy Pyrrolopyrimidines.
(i) Amine, triethylamine, 1,4-dioxane, microwave 1 h/200 °C; (ii) acetonitrile, dimethylaniline, TEBACl, POCl3, 90 °C/1 h; (iii) either alcohol, sodium metal, heat 30 °C, or amine, triethylamine, 1,4-dioxane, microwave 20 min, 200 °C; (iv) amine, triethylamine, 1,4-dioxane, microwave 1 h, 20 °C; (v) sodium hydroxide solution, reflux overnight; (vi) glacial acetic acid/bromine, RT; (vii) boronic acid, cesium carbonate, [pd(dppf)Cl2], 2-propanol/H2O, 100 °C overnight. In compound 10, Cl and Br were used in different experiments.
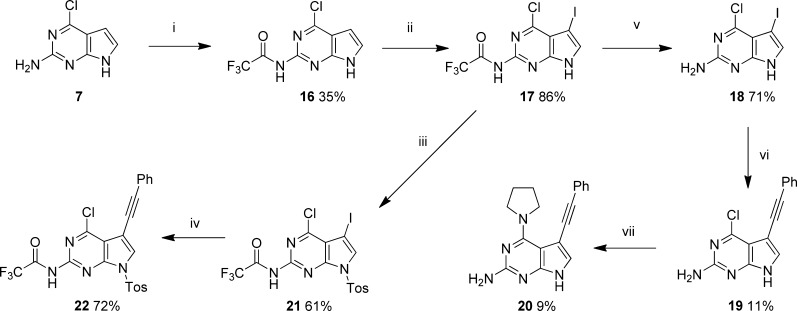
Polysubstituted pyrrolopyrimidines, in general, were found to be most active as inhibitors of PTR1 and of T. brucei in culture. One such compound (20) required a targeted synthesis (Scheme 3). 4-Chloropyrrolopyrimidine 7, protected by trifluoracetylation at N2 (16), was iodinated with N-iodosuccinimide to give 17, which was deprotected to give intermediate 18. Sonogashira coupling followed by nucleophilic aromatic substitution provided 20, which was substantially active both in enzyme assays and against T. brucei in culture. Improved yields in the Songashira coupling with phenylacetylene were obtained when the 7-N-tosyl derivative (22) was used.
Scheme 3.
Recognizing the need for a flexible synthesis leading to pyrrolopyrimidines with 5-hydrophobic substituents, the Michael addition-based synthesis used previously22 was extended to include a wide range of aryl and some aralkyl substitutents in both 4-oxo- and 4-aminopyrimidine series (Scheme 4). Although in principle triaminopyrimidine 24 might be expected to be more reactive than its 4-oxo relative (23), it was found that the opposite was the case; reaction rates were slower and yields were poorer in the preparation of the 2,4-diamino compounds than in the preparation of the 2-amino-4-oxo compounds. It is likely that the mildly basic conditions used for the Michael addition step increased the reactivity of the 2-amino-4-oxo pyrimidine through formation of the anion. In this way, two groups of 5-arylpyrrolopyrimidines, 4-oxo 27a–d and 4-amino 29a–d, were obtained. This reaction was also applicable to the synthesis of 4-alkylamino 31b and 4-alkoxypyrrolopyrimidines 31a and 31c. It is notable in passing that the nitroalkyl intermediates 26, 28, and 30 were found to be trypanocidal, although they are not active as inhibitors of PTR1 (Tables 1 and 2).
Scheme 4.
Table 1. Assay Results for Pyrrolopyrimidines as Inhibitors of PTR1 and Anti-trypanosomal Activity Against T. b. brucei in Culture and Human HEK Cellsa.

| cpd. no. | R1 | R2 | R3 | TbPTR1 (Kiapp μM) | T. b. brucei (IC50 μM) | HEK (IC50 μM) |
|---|---|---|---|---|---|---|
| 3a | OH | CN | 3-MeSO2NH–C6H4 | 0.350 | NT | NT |
| 3b | OH | CN | 3-MeSO2–C6H4 | 0.731 | NT | NT |
| 3c | OH | CN | –CH=CH–Ph | 0.137 | NT | NT |
| 4 | NH2 | CN | H | 4.9 | 2440 | NT |
| 5 | NH2 | CN | Br | 3.317 | 62.36 | NT |
| 6a | NH2 | CN | 3-CHO–C6H4 | 0.230 | 6.03 | NT |
| 6b | NH2 | CN | PhCH=CH– | 0.16 | 72.52 | NT |
| 6c | NH2 | CN | propargyl | 0.24 | 170 | NT |
| 6d | NH2 | CN | Ph(CH2)2 | 0.35 | 17.08 (HMI-9), 8.42 (CMM) | NT |
| 8a | 1-pyrrolidinyl | H | H | 4.2 | NA | NT |
| 8b | 1-thiomorpholinyl | H | H | 8.6 | 80.06 | NT |
| 12a | OMe | CN | H | ∼75 | NA | NT |
| 12b | iPrO | CN | H | >100 | 7.30 | NT |
| 12c | cyclopentyloxy | CN | H | >100 | 1.04 | NT |
| 12d | 1-thiomorpholinyl | CN | H | 8.8 | 14.33 | NT |
| 13 | 1-pyrrolidinyl | CN | H | Insol | 340 | NT |
| 14 | 4-pyrrolidinyl | CN | Br | 0.8 | 139 | NT |
| 15 | 4-pyrrolidinyl | CN | 3-CHO–C6H4– | 0.2 | 7.75 | NT |
| 20 | 1-pyrrolidinyl | propargyl | H | 0.19 | 0.65 | NT |
| 27a | OH | 4-Me–C6H4 | H | 1.2 | 125 | NT |
| 27b | OH | 4-F–C6H4 | H | 1.3 | 25.11 | NT |
| 27c | OH | Ph | H | 1.2 | 53.34 | NT |
| 27d | OH | Ph(CH2)2 | H | 0.27 | 7620 | NT |
| 27e | OH | Me | H | 7.3 | NA | NT |
| 29a | NH2 | 4-Me–C6H4 | H | 0.32 | 170 | NT |
| 29b | NH2 | 4-F–C6H4 | H | 0.48 | 14.47 | NT |
| 29c | NH2 | Ph | H | 0.4 | 41.01 | NT |
| 29d | NH2 | Ph(CH2)2 | H | 0.26 | 93.52 | NT |
| 31a | Me2CHCH2O | 4-F–C6H4 | H | >50 | 1.68 (HMI-9), 2.53 (CMM) | NT |
| 31b | N-cyclohexylamino | 4-F–C6H4 | H | 0.56 | 12.1 (HMI-9), 11.5 (CMM) | 70.77 |
| 31c | i-PrO | 4-F–C6H4 | H | Insol | 1.13 (HMI-9), 1.42 (CMM) | 81.91 |
| 34a | OH | Ph | Ph | 1.17 | 0.64 (HMI-9), 0.41 (CMM) | NT |
| 34b | OH | Me | Ph | 1.06 | 18.2 (HMI-9), 10.4 (CMM) | NT |
| 34c | OH | Ph | 4-F–C6H4 | 0.50 | 0.74 (HMI-9), 0.61 (CMM) | >200 |
| 34d | OH | 4-F–C6H4 | 4-F–C6H4 | 0.76 | 1.39 (HMI-9), 0.74 (CMM) | 160.6 |
| 34e | OH | 4-Cl–C6H4 | 4-Cl–C6H4 | 0.25 | 2.48 (HMI-9), 1.56 (CMM) | 152.4 |
| 34f | OH | 4-OMe–C6H4- | 4-F–C6H4 | Insol | 2.66 (HMI-9), 1.03 (CMM) | 183.6 |
| 34g | OH | 4-Me–C6H4- | 4-Me–C6H4– | >2–10 | 0.94 (HMI-9), 0.43 (CMM) | 124.2 |
| 34h | OH | Ph | 4-Br–C6H4– | 0.230 | 7.38 (HMI-9), 3.20 (CMM) | >100 |
| 34i | OH | Ph(CH2)2 | C6H4- | 0.95 | 0.40 (HMI-9), 0.14 (CMM) | 33.18 |
| 34j | OH | Ph | 4-(Me2CHCH2)–C6H4– | >10 | 3.38 (HMI-9), 2.13 (CMM) | >200 |
| 34k | OH | Ph | 4-MeSO2–C6H4 | Insol | >100 (HMI-9), >100 (CMM) | >200 |
| 34l | OH | 3-Cl–C6H4 | 4-FC6H4 | 0.47 | 1.41 (HMI-9), 0.77 (CMM) | 57.70 |
| 35a | NH2 | Ph | Ph | 0.59 | 2.25 (HMI-9), 0.58 (CMM) | 62.88 |
| 35b | NH2 | Ph | 4-F–C6H4 | 0.24 | 0.32 (HMI-9), 0.08 (CMM) | 49.19 |
| 35c | NH2 | 4-F–C6H4 | 4-F–C6H4 | 0.30 | 0.59 (HMI-9), 0.15 (CMM) | 47.34 |
| 35d | NH2 | 4-Cl–C6H4 | 4-Cl–C6H4 | Insol | 3.55 (HMI-9) 2.02 (CMM) | 53.64 |
| 35e | NH2 | 4-OMe–C6H4- | 4-F–C6H4 | 0.58 | 0.27 (HMI-9), 0.083 (CMM) | 39.14 |
| 35f | NH2 | 4-Me–C6H4- | 4-Me–C6H4– | Insol | 0.65 (HMI-9), 0.19 (CMM) | 47.21 |
| 35g | NH2 | Ph | 4-Br–C6H4– | 0.135 | 0.97 (HMI-9), 0.25 (CMM) | 39.63 |
| 35h | NH2 | Ph | 4-(Me2CHCH2)–C6H4– | 0.58 | 1.97 (HMI-9), 1.39 (CMM) | 32.54 |
| 35i | NH2 | Ph | 4-MeSO2–C6H4 | 1.28 | 7.06 (HMI-9), 6.16 (CMM) | 18.46 |
| 35j | NH2 | 3-Cl–C6H4 | 4-F–C6H4 | 0.29 | 0.39 (HMI-9), 0.19 (CMM) | 34.59 |
| 36a | NMe2 | 4-F–C6H4 | 4–F–C6H4 | Insol | 2.77 (HMI-9), 1.31 (CMM) | 77.10 |
| 36b | NMe2 | 4-Cl–C6H4 | 4-Cl–C6H4 | Insol | 4.96 (HMI-9), 3.08 (CMM) | 356.4 |
| 36c | NMe2 | Ph | 4-F–C6H4 | Insol | 3.03 (HMI-9), 1.34 (CMM) | 53.34 |
| 36d | NMe2 | Ph | Ph | 0.29 | 10.64 (HMI-9), 6.11 (CMM) | 57.77 |
| 36e | NMe2 | 4-OMe–C6H4– | 4-F–C6H4 | 0.30 | 8.55 (HMI-9), 3.43 (CMM) | 45.84 |
| 37 | N-cyclohexylamino | Ph | Ph | 0.20 | 2.32 (HMI-9), 1.78 (CMM) | 25.99 |
| 38 | OH | Ph | 4-(1-morpholinyl propyl)–C6H4– | >10 | 38.1 (HMI-9), 20.1 (CMM) | NT |
| diminazene | 0.027 (HMI-9), 0.007 (CMM) | |||||
| methotrexate | 3.66 (HMI-9), 0.011 (CMM) | |||||
| phenylarsine oxide | 1.49 |
CMM refers to Creek’s minimal medium, and HMI-9 is a commercially available medium (see Experimental Section).
Table 2. Assay Results for Nitroalkylpyrimidines as Inhibitors of PTR1 and Anti-trypanosomal Activity Against T. b. brucei in Culture and Human HEK Cells.

| cpd. no. | R | X | TbPTR1 (Kiapp μM) | T. b. brucei (IC50 μM) | HEK (IC50 μM) |
|---|---|---|---|---|---|
| 26a | OH | 4-Me–C6H4 | >100 | 2.038 | 73.36 |
| 26b | OH | 4-F–C6H4 | >100 | 1.857 | 149.5 |
| 26c | OH | H | >100 | 1.016 | 134.2 |
| 26d | OH | Ph(CH2)2 | >100 | N/A | NT |
| 28a | NH2 | 4-Me–C6H4 | Insol | 0.47 | 32.45 |
| 28b | NH2 | 4-F–C6H4 | ∼50 | 0.16 | 39.11 |
| 28c | NH2 | H | Insol | 0.13 | 34.98 |
| 28d | NH2 | Ph(CH2)2 | >100 | 8.82 | NT |
| 30a | Me2CHCH2O– | 4-F–C6H4– | >100 | 13.9 (HMI-9), 10.6 (CMM) | NT |
| 30b | N-cyclohexyl amino | 4-F–C6H4– | >100 | 0.54 (HMI-9), 0.80 (CMM) | 32.62 |
| 30c | i-PrO | 4-F–C6H4– | Insol | 15.9 (HMI-9), 8.48 (CMM) | NT |
The developing SAR and crystal structure information showed that it was necessary to occupy both hydrophobic pockets at the active site of PTR1 (Figure 1). For this, a new synthesis was necessary. Three types of pyrimidine substrate, 2,6-diamino-4-oxo 23, 2,4,6-triamino 24, and 2,6-amino-4-alkylamino 32, were reacted with diaryl bromoketones 33 prepared by bromination of the corresponding arylmethyl ketones, which were either commercially available or prepared by Friedel–Crafts acylation of a suitably substituted benzene derivative. Condensation of the diarylbromoketones afforded three series of 5,6-diarylpyrrolopyrimidines, 4-oxo 34a–l, 4-amino 35a–j, and 4-dimethylamino 36a–e, compounds in low to moderate yields (Scheme 5.) A single example of a 4-cyclohexylamino analogue (37) and a morpholinoalkyl analogue (38), designed to provide improved solubility, were also prepared using the same methods.
Scheme 5.
Compound Evaluation in Vitro
Target compounds and intermediates were first evaluated by a spectrophotometric enzyme assay that monitored the decrease in absorbance at 340 nm as NADPH is oxidized (Supporting Information). Stock solutions at concentration 100 mM in 100% (v/v) DMSO were prepared and screened in duplicate at 10 and 50 μM against 30 μg mL–1TbPTR1 (0.96 μM). Several compounds that failed to dissolve adequately at the desired concentration were rejected, and this left 102 compounds that were assayed. Inhibition was calculated as a percentage compared to assessment in the absence of inhibitor with background NADPH oxidation subtracted from all measurements. Fifty-four compounds displaying at least 60–70% inhibition of TbPTR1 at 50 μM were further analyzed at concentrations from 0.025 to 100 μM, and Kiapp values were determined, assuming reversible competitive inhibition and 1:1 stoichiometry (Table 1). The Ki of MTX was measured routinely as a standard.26 For consideration for progression to in vivo models of sleeping sickness in mice, targets optimally of less than 100 nM Ki against PTR1 (but acceptably less than 400 nM) and an IC50 of less than 500 nM in an in vitro assay of trypanocidal activity were set. Activity data are reported in Table 1. As noted above, some nitroalkylpyrimidine intermediates (26, 28, 30) also showed activity in the in vitro assay, and the results are reported in Table 2. Selected significant compounds were also assayed for toxicity in human HEK cells.
Structure–Activity Relationships
The first variation explored was the substitution of the 4-amino or 4-oxo group by 4-alkylamino and 4-alkoxy. This was done using 5-cyanopyrrolopyrimidines (3, 12–15), a class of compounds that had been found active in previous work, and two 5-unsubstituted compounds (8a,b), but the general lack of significant activity together with the crystallographic information (see below) implied that a hydrophobic substituent on the pyrrole ring was necessary for activity.
The cyano vector in the crystal structures suggested that an appropriate variation was to examine hydrophobically substituted alkynes with a terminal hydrophobic substituent. The multistep synthesis leading to 20 was not convenient but did lead to a compound with good activity at the PTR1 and acceptable activity against T. brucei in vitro; 20 was taken forward for further evaluation as described below. The importance of a significantly sized hydrophobic substituent was emphasized by the low activity of 5-methylpyrrolopyrimidine 27e in the 4-oxo series and 5-cyanopyrrolopyrimidine 4 in the 4-amino series. In the 4-oxo series, 5-aryl substituents (27) improved the activity in all assays but not sufficiently to give compounds potent enough for progression. There was also the suggestion that a more flexible hydrophobic 5-substituent would not give the required activity, as shown by arylaminomethyl compounds 27d and 29d. The 4-amino series (29a–c), however, had several compounds with significant activity in the PTR1 assay. However, the activity in the cellular assay was disappointing, suggesting that the anticipated enhanced uptake into trypanosomes was not occurring.
When 6-hydrophobic substituents were introduced, compounds with greatly improved PTR1 affinity were obtained. In 4-amino, 4-oxo, and 4-alkylamino series (6, 15), compounds with good inhibitory activity were obtained; however, none of these compounds was sufficiently active in cellular assays to merit progression. Indeed, their activity was exceptionally low. It is possible that the 5-cyano group is sufficiently decreasing the basicity of these compounds so that they are not substrates for the transporters. Several compounds with more extended hydrophobic side chains, notably, phenylethyl, in both the 4-amino and 4-oxo series also had good inhibitory activity against PTR1 (6c, 6d, 27d, 29d). One of these (6c) was active enough in the anti-trypanosomal assay in CMM medium to be considered for in vivo evaluation. More active compounds were found, however. Further investigations of substituent tolerance at C4 showed that alkoxy substitution afforded insoluble or weakly active compounds (31a, 31c) but that significant or good enzyme inhibitory activity was obtained with cyclohexylamino pyrrolopyrimidines (31b, 37). Once again, the cellular activity was lower than required for progression.
5-Methyl-6-phenyl pyrrolopyrimidine 34b was only modestly active, showing that more than a 6-aryl substituent was necessary for useful activity. A significant step forward came when two aryl substituents were introduced at both C5 and C6, as shown first by 5,6-diphenylpyrrolopyrimidines 34a and 35a This led to a clear increase in the anti-trypanosomal assay in CMM medium and, in the 2,4-diamino case (35a), to a compound that was at the margin as a further candidate for progression. A number of such compounds were made, and several of them displayed activity sufficient for further progression to in vivo evaluation. Notable, in terms of efficacy, are 5-phenyl-6-(4-fluorophenyl)pyrrolopyrimidine 35b and 5,6-di(4-fluorophenyl)pyrrolopyrimidine 35c in the diamino series. The 4-dimethylamino compounds (36a–e), on the other hand, were insufficiently active. In the 4-oxo series especially, solubility prevented good enzyme assays from being obtained in some cases, but several compounds appropriate for progression were identified including 34g, 34l, 35f, 35g, and 35j. With the intention of filling the hydrophobic pockets as completely as possible, a branched alkyl substituent was introduced (34j, 35h), but this change did not improve activity. Similarly, the introduction of a strongly electron-withdrawing group (sulfone) gave only weakly active compounds with poor solubility (34k, 35i). An attempt to improve solubility with a flexible, polar ionic substituent (38) gave a compound with very low activity, and this strategy was not investigated further.
Structural Basis of PTR1 Inhibition
In previous work, we developed robust protocols to elucidate high-resolution structures of PTR1.6−8 This allowed us to acquire new structural data that were combined with modeling approaches in an iterative process to guide compound design. Ultimately, this led us to determine isomorphous structures of 23 cocrystal complexes of the PTR1 tetramer (Figures 2 and 3 and Supporting Information). The high-resolution diffraction data and excellent quality of the electron density maps were sufficient to identify examples where multiple conformations and ligand orientations were present, for example, 8b, 29a, and 34i.
Figure 2.
Inhibitors overlaid on a van der Waals surface representation of the TbPTR1 active site. Five amino acids that help to cover the ligand-binding site have been removed for clarity (Phe97, Pro210, Ala212, Met213 and Glu217). NADP+ is shown as yellow sticks. All other atoms are colored according to element (C, gray; N, blue; O, red; S, yellow; F, pale blue; Br, brown). The orientation is similar to that used in Figure 1.
Figure 3.
Difference density omit maps of 23 inhibitors. Difference density (Fo – Fc, where Fo represents the observed and Fc represents the calculated structure factors) maps were calculated (blue chicken wire) with the molecule removed from the final model and are contoured at 3σ. i and ii indicate the primary and secondary molecules of 8b when two were simultaneously observed in the active site, whereas A and B indicate molecules modeled in subunits A or B for 34i. The inhibitors are shown in the same orientation with respect to the active site.
Superposition of the inhibitors as they are positioned in the TbPTR1 active site indicates that the core scaffold position and the hydrogen-bonding interactions of which it is capable are well-conserved. In addition, most of the inhibitors adopt the substrate-like pose described earlier. There are three exceptions: 2,4-diamino-5-phenylethylpyrrolopyrimidine 29d adopts the MTX-type orientation, 2,4-diamino-5-methyl analogue 29a displays both the substrate and MTX-type orientations, and 4-thiomorpholino-substituted compound 12d displays a distinct binding mode shifted away from the cofactor and catalytic residues. In all cases, we attribute the change in orientation to the presence of bulky hydrophobic substituents that would clash with polar components of the active site. 12d is unable to mimic interactions that stabilize substrate binding and therefore is only a modest inhibitor. The other two compounds (29a and 29d) participate in hydrogen bonding and van der Waals interactions similar to substrate and MTX binding poses and retain potency against PTR1.
The compound series that has been developed displayed good levels of PTR1 inhibition that are based on exploiting previously recognized molecular features, for example, by fulfilling the hydrogen-bonding capacity at the catalytic center. The widely dispersed appearance of the mainly hydrophobic substituents on the core scaffold (Figure 2) demonstrates the conformational diversity available to interrogate the different regions of the active site, and this enhanced affinity for the target in some cases. In general, the key to improving potency, as exemplified by 3c, 20, and 35g, has been to incorporate a sufficiently bulky group capable of additional van der Waals interactions in the hydrophobic cavities adjacent to the substrate-binding site.
The 3-formylphenyl substituted compounds (6a and 15) are worth specific mention. They were designed to adopt the substrate-like pose but with the appropriate substituents that might form a covalent attachment to Cys168. This is indeed observed with stable thioester linkages being formed, presumably via oxidation of a first formed thioacetal adduct. The success here indicates that covalent modification might be exploited in future.
Compound Evaluation in Vitro
Compounds were tested for activity, in vitro, against T. b. brucei using the Alamar blue assay, which demonstrates cell viability. Cells were tested in both rich HMI-9 medium and also a minimal medium, CMM, that does not contain added folate or biopterin. Results are shown in Tables 1 and 2. When inhibitors of dihydrofolate reductase (DHFR) are assessed in the latter medium, they are several hundred-fold more active than when tested in the rich medium, presumably due to the ability of the abundant folate to compete for binding sites on the target enzyme. The fact that for our compounds there was little difference in potency when using CMM or HMI-9 media would indicate that they are not exerting their activity through inhibition of DHFR, a fact consistent with structural predictions based on modeling within the DHFR-binding site that suggest regions of steric clash (data not shown).
Compounds were also tested against additional T. b. brucei strains that are defective in transporter proteins that might be expected to contribute to their uptake.20,21 The TbAT1 knockout (KO) line has lost the P2 aminopurine transporter, which is also known to carry melaminophenylarsenicals and diamidines,27 whereas the B48 line has additionally lost another diamidine/melaminophenylarsenical transporter, the HAPT1 carrier (now known to be an aquaglyceroporin designated AQP2).28,29 Trypanocidal activity on these two transporter mutants showed hardly any changes as compared with that of wild-type trypanosomes (data not shown), indicating that our compounds enter via routes other than, or in addition to, the P2 transporter and HAPT1. PTR1 inhibitors were also tested against related leishmania parasites (Leishmania mexicana M379, data not shown), where activity was moderate and weaker, indicating differences in its PTR1 from that of trypanosomes and in its biological context.
Pharmacokinetic and in Vivo Evaluation
Progression of compounds into in vivo evaluation was based on a target property profile that included the following data: (1) affinity of compound for TbPTR1 preferably <100 nM but acceptably lower than 400 nM. Several compounds were found with Ki in the range 120–140 nM, but selection of compounds for in vivo evaluation also took strongly into account the activity against T. brucei in culture. The use of a high substrate concentration to ensure a robust assay implies equally a high value for Ki in the enzyme assays. (2) Selectivity of compound with respect to human dihydrofolate reductase, which was inferred from the relative activity in HDMI and CMM media. (3) Time required to kill T. brucei in culture desirably within 6 h but acceptably within 48 h; many compounds were found that killed within 48 h, but none was as rapid as 6 h (kill curves and the methods used are described in the Supporting Information). (4) Physicochemical parameters (Table 3): logP range desirably of 2–3.5 and pKa between 4.5 and 9.2. For the most active compounds, clogP was in the range 3–3.6 and calculated pKa was typically 4–5 for 4-amino compounds and 9–9.6 for 4-oxo compounds (Pipeline Pilot, Accelrys Inc., was used for both logP and pKa estimation, see the Supporting Information). (5) Metabolic stability in microsomal assay: desirably t1/2 > 6 h but acceptable >2 h; t1/2 for the most active compound (35b) was >8 h. (6) In vitro trypanocidal activity desirably IC50 < 100 nM but acceptably <800 nM; several compounds were obtained with IC50 < 500 nM (Table 1).
Table 3. Pharmacokinetic Properties of Selected PTR1 Inhibitorsa.
| TbPTR1 | T. b. brucei HMI-9 | T. b. brucei CMM | HEK | Cli | plasma bind | t1/2 | clogP | LE PTR1 | LE CMM | |
|---|---|---|---|---|---|---|---|---|---|---|
| Kiapp μM | IC50 μM | IC50 μM | IC50 μM | mL/min/g | fu | min | ||||
| 20 | 0.19 | 0.65 | 5.6 | 3.35 | 0.4 | |||||
| 29b | 0.48 | 14.5 | 1.7 | 1.84 | 0.48 | |||||
| 34a | 1.17 | 0.640 | 0.407 | 1.17 | 0.029 | ∼130 | 2.75 | 0.35 | 0.38 | |
| 34c | 0.50 | 0.738 | 0.614 | >200 | 0.17 | 0.017 | 2.90 | 0.36 | 0.35 | |
| 34i | 0.95 | 0.396 | 0.135 | 33.2 | 2.5 | 3.58 | 0.33 | 0.37 | ||
| 34l | 0.47 | 1.405 | 0.767 | 57.7 | <0.5 | 3.26 | 0.35 | 0.33 | ||
| 35a | 0.59 | 2.247 | 0.583 | 62.9 | 5.26 | 0.035 | ∼100 | 3.41 | 0.37 | 0.37 |
| 35b | 0.24 | 0.321 | 0.082 | 49.2 | 1.90 | 0.011 | >500 | 3.57 | 0.38 | 0.4 |
Blank cells imply not tested in relevant assay. Kiapp is the apparent dissociation constant for the enzyme inhibitor complex, before correction for the inhibition modality-specific influence of substrate concentration relative to Km. As the inhibitors compete for binding with the pterin substrate, Ki can be calculated according to the equation Kiapp=Ki /(1 + S × Km–1), where S and Km refer to the pterin substrate. Kiapp and IC50 values refer to the data presented in Tables 1 and 2. Cli is the clearance in vivo. fu is the fraction unbound by plasma. t1/2 is the half-life of the compound. clogP and ligand efficiency (LE) were obtained using Pipeline Pilot (Accelrys Inc).
Compounds for which PK data were obtained are shown in Table 3. These showed selectivity for killing parasites over a human cell line (HEK) in culture, again indicative of on-target activity and at an appropriate level of discrimination to justify further progression. Ligand efficiencies with respect to both PTR1 inhibition and in vitro anti-trypanosomal activity were all good, and the PK properties were acceptable for in vivo evaluation. LC–MS measurements showed significant accumulation of 34a and 35a into trypanosomes. The PK data showed good stability and relatively slow clearance, particularly for 35b, one of the most potent compounds. Four compounds in the 4-amino series (35b, 35e, 35g, 35j) have been evaluated for efficacy in vivo. On observation of toxicity in the first round of experiments, MTD studies with 35b, 35e, and 35g showed severe toxicity and fatality at 100 mg/kg, some transient clinical signs at 30 mg/kg, but no clinical signs at 10 mg/kg; therefore, 30 mg/kg was selected as the dose for in vivo efficacy studies. Infected mice were found to be more sensitive to the toxic effects of the drugs, but once daily dosing only up to 4 days was found to be tolerated. In the experiments with 35b, 35e, and 35g, one mouse in each group survived long enough to demonstrate reduction of parasitaemia from ∼108 to below detectable limits. The surviving mice receiving 35e and 35g showed a relapse of parasitaemia on day 10. The 4-oxo series was also evaluated with the hope that toxicity would be reduced. 34h and 34i did not show acute toxicity when administered at a single dose of 30 mg/kg. However, 34i was not curative, and toxicity was still observed with 34h, although parasitaemia was greatly reduced with this compound.
It is evident that there is significant host toxicity in both the 4-oxo and 2,4-diamino series of 5,6-diarylpyrrolopyrimidines that could not be removed by aryl substituent modification. This series of compounds is nevertheless trypanotoxic and inhibits PTR1 in in vitro assays. The detailed information obtained from the crystallographic studies provides a strong basis for the design of further series of compounds based on interactions other than those in the hydrophobic pocket, for example, the covalent binding described above. Future work will address whether they actually act through inhibiting this target in vivo. The compounds demonstrate mammalian toxicity, possibly through interaction with a CNS receptor, that must also be addressed in future chemical modifications.
Experimental Section
Cell culture
Trypanosomes
Bloodstream form T. b. brucei (strain Lister 427) was cultured at 37 °C in a humidified 5% CO2 environment in either HMI-9 (Gibco) or Creek’s minimal medium (CMM)30 supplemented with 10% FBS. Cells were routinely diluted when reaching the mid-log-phase concentration of 2 × 106 cells/mL and maintained in culture for no more than 30 passages.
Human Cells
The human embryonic kidney cell line HEK 293T was cultured in DMEM (Sigma-Aldrich) supplemented with penicillin (100 units/ml), streptomycin (0.1 mg/mL), l-glutamine (2 mM), and 10% FBS. Cells were grown in a humidified atmosphere at 37 °C and 5% CO2 and split when 80–85% confluent.
Alamar Blue Assay
Against Trypanosomes
Compounds’ potency against trypanosomes was assessed by Alamar blue assay.31 Cells were seeded at a final concentration of 2 × 104 cells/mL into serial dilutions of the test compound. After 48 h incubation at 37 °C and 5% CO2, 20 μL of resazurin dye (Sigma-Aldrich) solution at 0.49 mM was added, and cells were incubated for a further 24 h. The reduction of resazurin was measured with a fluorimeter (FLUOstar Optima, BMG Labtech) using 544 nm excitation and 590 nm emission wavelengths. Output was plotted using the IC50 determination algorithm of the GraphPad Prism 5.0 software. All experiments were carried out in duplicate and repeated on at least three independent occasions.
Against HEK Cells
To test compounds’ activity against HEK cells, 3 × 105 cells/mL were seeded in a 96-well plate and allowed to adhere for 3 h at 37 °C and 5% CO2. An equal volume of doubling serial dilutions of test compound was then added to the wells, and cells were incubated for a further 16 h before addition of resazurin (20 μL of a 0.49 mM solution). After 24 h, fluorescence was measured and data analyzed as described above.
In Vivo Experimental Protocol
Experimental groups of three ICR mice were infected with 1 × 105 bloodstream T. b. brucei strain 427 obtained from a donor mouse. Twenty-four hours postinfection mice were injected intraperitoneally with 30 mg/kg daily of test compound (repeated for four consecutive days), prepared in a 10% v/v DMSO, 40% v/v PEG, and 50% v/v water solution. Parasitaemia was monitored daily by tail venipuncture. Infected, untreated mice were used as control. All procedures were carried out by licensed animal workers and complied with the UK Animals (Scientific Procedures) Act, 1986, and with the national and University of Glasgow maintenance and care guidelines.
Compound Synthesis
General
1H and 13C NMR spectra were measured on a Bruker DPX 400 MHz spectrometer with chemical shifts given in ppm (δ values) relative to proton and carbon traces in solvent. Coupling constants are reported in Hz. IR spectra were recorded on Shimadzu IRAffinity-1 spectrometer. Elemental analysis was carried out on a PerkinElmer 2400 analyzer series 2. Accurate mass was measured using Thermo Exactive MS and a Thermo U3000 HPLC system. Ionization was carried out by an ESI source (not heated). Anhydrous solvents were obtained from a Puresolv purification system, from Innovative Technologies, or purchased as such from Aldrich. Melting points were recorded on a Reichert hot-stage microscope and are uncorrected. Chromatography was carried out using 200–400 mesh silica gels, or using reverse-phase HPLC on a Waters system using a C18 Luna column.
Declaration of Purity
All final compounds were equal to or more than 95% pure by HPLC and 1H NMR (400 MHz).
2-Amino-4-oxo-6-[(E)-2-phenylethenyl]-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile (3c)
(E)-2-Phenylethenylboronic acid (93 mg, 0.630 mmol), 2-amino-6-bromo-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile (80 mg, 0.315 mmol), and cesium carbonate (513 mg, 1.575 mmol) were suspended in isopropyl alcohol/water (2:1) (6 mL), to which was added (1,1′-bis(diphenylphosphino)ferrocene)-dichloropalladium(II) [Pd(dppf)Cl2] (29 mg, 0.0395 mmol). The reaction mixture was purged with nitrogen for 20 min and then heated overnight at 100 °C [oil bath temperature] in a sealed tube. Silica gel was added to the reaction mixture, and the solvents were removed under reduced pressure. The residue was partitioned between ethyl acetate and water, the organic layer was collected, and the solvent was removed. The crude material was purified by HPLC. Fractions containing the required material were collected and freeze-dried to give the desired product as a yellow solid (20 mg, 23%), mp > 230 °C. 1H NMR (DMSO-d6): 12.28 (1H, s), 10.73 (1H, s), 7.55 (2H, d, J = 7.4 Hz), 7.42–7.30 (4H, m), 6.99 (1H, d, J = 16.4 Hz), 6.50 (2H, br). IR (KBr): 2209, 1701, 1609, 1580, 1488, 1419, 1273, 1037, 954, 791, 753, 695 cm.–1 HRESIMS: calculated for C15H12N5O, 278.1036; found, 278.1039.
3a,b were similarly prepared.
2,4-Diamino-6-bromo-7H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile (5)
To a suspension of 2,4-diamino-7H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile (1.087 g, 6.24 mmol) in glacial acetic acid (70 mL) was added bromine (480 μL, 9.40 mmol, 1.5 equiv). The reaction mixture was stirred at room temperature for 20 h and then heated at 60 °C for 14 h. The solvent was removed under reduced pressure, and the solid material obtained was dried in vacuo for 12 h to give the required product as a dark brown solid (1.240 g, 79%) with no distinct melting point. 1H NMR (DMSO-d6): 7.49 (2H, br, NH2), 7.00 (2H, br, NH2). IR (KBr): 3332, 3168, 2225, 1662, 1583, 1514, 1430, 1385, 1229, 875, 767 cm.–1
2,4-Diamino-6-[(E)-2-phenylethenyl]-7H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile (6b)
(E)-2-Phenylethenylboronic acid (117 mg, 0.790 mmol), 2,4-diamino-6-bromo-7H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile (100 mg, 0.395 mmol), and cesium carbonate (643 mg, 1.975 mmol) were suspended in isopropyl alcohol/water (2:1) (6 mL), to which was added (1,1′-bis(diphenylphosphino)ferrocene)-dichloropalladium(II) [Pd(dppf)Cl2] (29 mg, 0.0395 mmol). The reaction mixture was purged with nitrogen for 20 min and then heated overnight at 100 °C [oil bath temperature] in a sealed tube. Silica gel was added to the reaction mixture, and the solvents were removed under reduced pressure. The residue was applied to a silica gel column chromatography and eluted with [1] n-hexane and [2] ethyl acetate [Rf = 0.1]. Fractions containing the required material were collected, and the solvent was removed under reduced pressure to give the desired product as a yellow solid (65 mg, 60%), mp > 230 °C. 1H NMR (DMSO-d6): 12.11 (1H, s), 7.57 (2H, d, J = 7.Hz), 7.42–7.37 (3H, m), 7.34(1H, t, J = 7.4 Hz), 7.05 (1H, d, J = 16.4 Hz), 6.21 (2H, br), 6.01 (2H, br). IR (KBr): 2209, 1701, 1609, 1580, 1488, 1419, 1273, 1037, 954, 791, 753, 695 cm.–1 HRESIMS: calculated for C15H13N6, 277.1196; found, 277.1199.
6a and 6c–e were similarly prepared.
4-(1-Pyrrolidinyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (8a)
A mixture of 4-chloro-7H-pyrrolo[2,3-d]pyrimidin-2-amine (100 mg, 0.593 mmol), pyrolidine (44 mg, 0.622 mmol, 102 μL, d 0.860), and triethylamine (63 mg, d 0.726, 87 μL, 0.720 mmol) were dissolved in 1,4-dioxane (2 mL, dry). The reaction mixture was heated in a microwave reactor for 1 h at 200 °C. The reaction mixture was diluted with methanol, and then silica gel was added. Solvents and excess reagents were removed under reduced pressure. The residue was applied to a silica gel column and eluted with methanol/ethyl acetate (1:9), Rf = 0.2. The required product was obtained as light brown solid (80 mg, 66%), mp > 230 °C. 1H NMR (DMSO-d6): 10.72 (1H, s), 6.67 (1H, q, J = 1.7 Hz), 5.40 (2H, br), 6.34 (1H, q, J = 1.1 Hz), 3.64 (4H, br), 1.93 (4H, br). IR (KBr): 1605, 1564, 1506, 1457, 1407, 1349, 1195, 1098, 1032, 905, 823, 696 cm.–1 HRESIMS: calculated for C10H14N5, 204.1244; found, 204.1242.
8b was similarly prepared.
N-[5-Cyano-4-(1-pyrrolidinyl)-7H-pyrrolo[2,3-d]pyrimidin-2-yl]-2,2-dimethylpropanamide (11)
N-(4-Chloro-5-cyano-7H-pyrrolo[2,3-d]pyrimidin-2-yl)-2,2-dimethylpropanamide (350 mg, 1.260 mmol) was suspended in butanol (20 mL), to which was added pyrrolidine (269 mg, 313 μL, 3 mol equiv, 3.78 mmol). The reaction mixture was heated at 90 °C in a sealed tube for 48 h. The reaction mixture was left to cool to room temperature, and the precipitate was filtered, washed with water, and dried. The required product (0.290 g) was obtained as brown solid with no distinct melting point. The filtrate was collected, the organic layer was separated, and butanol was removed in vacuo and then triturated with small amount of ethyl acetate and filtered to give an additional amount of the required material. The total amount obtained was 0.330 g, 84%. 1H NMR (DMSO-d6): 12.52 (1H, br), 9.17 (1H, s), 8.11 (1H, s), 3.78 (4H, t, J = 6.5 Hz), 1.98 (4H, t, J = 6.5 Hz), 1.20 (9H, s). IR: 2220, 1706, 1579, 1546, 1425, 1380, 1172, 1105, 807, 788 cm–1 HRESIMS: calculated for C16H19ON6, 311.1626; found, 311.1628.
2-Amino-4-(1-pyrrolidinyl)-7H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile (13)
N-[5-Cyano-4-(1-pyrrolidinyl)-7H-pyrrolo[2,3-d]pyrimidin-2-yl]-2,2-dimethylpropanamide (330 mg, 1.057 mmol) was suspended in a solution of ethanol (2 mL), sodium hydroxide (85 mg, 2.113 mmol), and water (2 mL). The reaction mixture was heated under reflux overnight. Solvents were removed under reduced pressure, and the crude material was suspended in water and ethyl acetate. The solid material was filtered of to give the required product (230 mg, 95%) as white solid with no distinct melting point. TLC [ethyl acetate: Rf = 0.5]. 1H NMR (DMSO-d6): 12.49 (1H, br), 7.99 (1H, s), 6.85 (2H, br), 3.77 (4H, s), 1.99 (4H, s). IR: 2226, 1685, 1633, 1591, 1427, 1207, 1137, 841, 803, 762, 724 cm–1 HRESIMS: calculated for C11H13N6, 229.1196; found, 229.12.
2-Amino-6-bromo-4-(1-pyrrolidinyl)-7H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile (14)
2-Amino-4-(1-pyrrolidinyl)-7H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile (230 mg, 1.008 mmol) was dissolved in glacial acetic acid (20 mL), to which was added bromine (78 μL, 242 mg, 1.512 mmol). The reaction mixture was stirred at room temperature overnight. The solvent was evaporated, and the crude material was purified by HPLC. The required product was obtained as yellow solid (90 mg, 29%), mp > 230 °C. 1H NMR (DMSO-d6): 6.77 (1H, br), 4.74 (2H, br), 3.75 (4H, br). IR (KBr): 2226, 1679, 1633, 1584, 1452, 1391, 1200, 1137 cm.–1 HRESIMS: calculated for C11H12N6Br, 307.0301; found, 307.0297.
2-Amino-6-(3-formylphenyl)-4-(1-pyrrolidinyl)-7H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile (15)
3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzaldehyde (76 mg, 0.326 mmol), 2-amino-6-bromo-4-(1-pyrrolidinyl)-7H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile (50 mg, 0.163 mmol), and cesium carbonate (266 mg, 0.815 mmol) were suspended in isopropyl alcohol/water (2:1) (6 mL), to which was added (1,1′-bis(diphenylphosphino)ferrocene)-dichloropalladium(II) [Pd(dppf)Cl2] (12 mg, 0.0163 mmol). The reaction mixture was purged with nitrogen for 20 min then heated overnight at 100 °C [oil bath temperature] in a sealed tube. Solvents were removed under reduced pressure, and the residue was purified by HPLC. The freeze-dried material was obtained as a white solid (10 mg, 19%) with no distinct melting point. 1H NMR (DMSO-d6): 13.06 (1H, br), 10.11 (1H, s), 8.34 (1H, t, J = 1.5 Hz), 8.15 (1H, t, J = 1.2 Hz), 8.07 (1H, t, J = 1.2 Hz), 7.83 (1H, d, J = 7.7 Hz), 6.84 (2H, br), 3.84 (4H, s), 2.02 (4H, s). IR (KBr): 2221, 1659, 1555, 1463, 1388, 1110, 1139, 892, 835, 802, 763, 721 cm.–1 HRESIMS: calculated for C18H17N6O, 333.1458; found, 333.1460.
N-(4-Chloro-7H-pyrrolo[2,3-d]pyrimidin-2-yl)-2,2,2-trifluoroacetamide (16)
4-Chloro-7H-pyrrolo[2,3-d]pyrimidin-2-amine (1.20 g, 7.118 mmol) was dissolved in pyridine (6 mL, dry), to which was added trifluoroacetic anhydride (TFAA) (1.3 mL, 9.35 mmol) dropwise within 5 min, and the mixture was stirred for 3 h at room temperature. The solvent was removed under reduced pressure to yield an amber solid, which was coevaporated twice with water (5 mL). The resulting material was filtered, washed with cold water, and then dried to give the required product as light brown solid (0.660 g, 35%), mp 227–229 °C (dec). 1H NMR (DMSO-d6): 12.65 (1H, s), 12.20 (1H, s), 7.66 (1H, dd, J = 3.5 and 1.5 Hz), 6.61 (1H, q, J = 1.7 Hz). IR (KBr): 1732, 1582, 1425, 1338, 1287, 1203, 1159, 925, 887, 823, 772, 737 cm.–1 HRESIMS: calculated for C8H5ON435ClF3, 265.0098; found, 265.0098.
N-(4-Chloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidin-2-yl)-2,2,2-trifluoroacetamide (17)
To a suspension of 4-chloro-7H-pyrrolo[2,3-d]pyrimidin-2-amine (0.660 g, 2.494 mmol) in DCM (20 mL, dry) was added N-iodosuccinimide (0.971 g, 4.315 mmol), and the reaction mixture was heated under reflux for 12 h. The suspension was filtered, and the solid was washed with hot water. The product was obtained as gray solid (0.840 g, 86%), mp > 230 °C. 1H NMR (DMSO-d6): 13.00 (1H, s), 12.27 (1H, s), 7.89 (1H, d, J = 2.4 Hz). IR (KBr): 1733, 1576, 1527, 1452, 1422, 1334, 1264, 1186, 1166, 965, 924, 767 cm.–1 HRESIMS: calculated for C8H4ON435ClF3I, 390.9065; found, 390.9064.
4-Chloro-5-(phenylethynyl)-7H-pyrrolo[2,3-d]pyrimidin-2-ylamine (19)
4-Chloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidin-2-amine (300 mg, 1.02 mmol), phenyl acetylene (125 mg, 1.22 mmol, 112 μL, 1.2 mol equiv), Cu(I)I (10 mg), tetrakis (32 mg), and TEA (206 mg, 2.04 mmol, 287 μL, 2 mol equiv) were dissolved in DMF (10 mL, dry). The reaction mixture was stirred at room temperature overnight. Brine was added to the reaction mixture (the reaction was slightly exothermic). The brown solid precipitated was filtered, washed with water, and dried. This was then triturated with boiling methanol and filtered hot to give (0.2350 g, 86%) as brown solid. The filtrate was concentrated and applied to a silica gel column and eluted with ethyl acetate. The pure material was obtained as pale yellow solid (31 mg, 11%), mp > 230 °C. 1H NMR (DMSO-d6): 11.87, 7.54 (1H, d, J = 2.3 Hz), 7.49–7.47 (2H, m), 7.41–7.36 (3H, m), 6.68 (2H, s). IR (KBr): 2215, 1633, 1560, 1488, 1448, 1404, 1260, 1020, 926, 819, 787, 752, 689 cm.–1 HRESIMS: calculated for C14H10ClN4, 269.0589; found, 269.0592.
5-(Phenylethynyl)-4-(1-pyrrolidinyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine (20)
4-Chloro-5-(phenylethynyl)-7H-pyrrolo[2,3-d]pyrimidin-2-ylamine (100 mg, 0.372 mmol), pyrolidine (100 μL, 86 mg, 1.21 mmol), and TEA (100 μL, 72.6 mg, 0.719 mmol) were added to 1,4-dioxane (4 mL, dry). The reaction mixture was heated in a microwave reactor for 1 h at 200 °C. The reaction mixture was diluted with methanol, and then silica gel was added. Solvents and excess reagents were removed under reduced pressure. The residue was applied to a silica gel column and eluted with methanol/ethyl acetate (1:1) containing 1% TEA. The crude material obtained was further purified by HPLC to give the required product as light brown solid after freeze-drying (10 mg, 9%), mp > 230 °C. 1H NMR (DMSO-d6): 12.20 (1H, br), 7.52–7.38 (6H, m), 7.17 (2H, br), 3.93 (4H, br), 2.02 (4H, br). IR (KBr): 2211, 1678, 1635, 1201, 1181, 1136, 884, 836, 800, 758, 722, 688 cm.–1 HRESIMS: calculated for C18H18N5, 304.1557; found, 304.1561.
N-{4-Chloro-5-iodo-7-[(4-methylphenyl)sulfonyl]-7H-pyrrolo[2,3-d]pyrimidin-2-yl}-2,2,2-trifluoroacetamide (21)
Sodium hydride (48 mg, 1.20 mmol, 60% in oil, 1.2 mol equiv) was added to THF (20 mL, dry) under nitrogen. To this was added the starting material (0.3905 g, 1.00 mmol) while the reaction mixture was cooled to 0 °C with stirring. The reaction mixture was left stirring for a further 15 min after completion of the addition. p-Toluenesulfonyl chloride (229 mg, 1.2 mmol, 1.2 mol equivalents) was added to the reaction mixture portionwise while keeping the temperature between 5 and 10 °C. After the addition had ended, the mixture was allowed to come to 20 °C, and the stirring was continued at this temperature for a further 1 h. The reaction mixture was extracted with aq. NaHCO3 (saturated) solution and ethyl acetate and extracted. The organic layers were combined, dried (Na2SO4), and filtered, and the solvent was removed under reduced pressure. The required material was obtained after recrystallization from ethyl acetate/n-hexane (0.330 g, 61%), mp 215–218 °C. 1H NMR (DMSO-d6): 12.64 (1H, s), 8.32 (2H, d, J = 8.3 Hz), 8.19 (1H, s), 7.47 (2H, d, J = 8.3 Hz), 2.38(3H, s). IR (KBr): 1753, 1595, 1569, 1451, 1375, 1218, 1174, 1141, 1087, 916, 813 cm.–1 HRESIMS: calculated for C15H10ClF3IN4O3S, 544.9153; found, 544.9155.
N-[4-Chloro-7-[(4-methylphenyl)sulfonyl]-5-(phenylethynyl)-7H-pyrrolo[2,3-d]pyrimidin-2-yl]-2,2,2-trifluoroacetamide (22)
N-{4-Chloro-5-iodo-7-[(4-methylphenyl)sulfonyl]-7H-pyrrolo[2,3-d]pyrimidin-2-yl}-2,2,2-trifluoroacetamide (300 mg, 0.551 mmol) was dissolved in DMF (10 mL, dry), to which were added Cu(I)I (10.6 mg, 0.055 mmol, 10% molar equivalents), phenylacetylene (68 mg, 0.661 mmol, 73 μL, 1.2 mol equiv), tetrakis(triphenylphosphine)palladium(0) (64 mg, 0.055 mmol, 10% molar equivalents), and triethylamine (112 mg, 1.102 mmol, 2 mol equiv) at room temperature with stirring under nitrogen. The reaction mixture was left stirring at room temperature overnight. Brine was added to the reaction mixture, and the precipitated brown solid was filtered, washed with water, and dried to give the desired product as brown solid (0.2070 g, 72%), mp > 230 °C. 1H NMR (DMSO-d6): 8.10 (2H, d, J = 8.4 Hz), 7.87 (1H, s), 7.55–7.43 (6H, m), 7.34 (2H, s), 2.40 (3H, s). IR (KBr): 1631, 1602, 1541, 1487, 1378, 1178, 1112, 1013, 786, 755 cm.–1 HRESIMS: calculated for C7H7O2S, 363.0273; found, 363.0275.
2-Amino-5-phenyl-3,7-dihydro-4H-pyrrolo[2,3-d]pyrimidin-4-one (27c)32
To an aqueous solution of NaOH (0.330 g, 8.25 mmol) in 5 mL of water was added 2,6-diamino-5-(2-nitro-1-phenylethyl)-4(3H)-pyrimidinone (0.398 g, 1.53 mmol) at room temperature. The mixture was stirred for 2 h and then was slowly added to an aqueous solution of 1.37 g (14 mmol) of sulfuric acid (98%) in 5 mL of water at 0 °C [the sequence of the addition is important]. The resulting mixture was stirred at 0 °C for 1 h and at room temperature overnight. The solid material was filtered, washed with water, and dried. The crude material was purified by HPLC, and fractions containing the required product were collected and freeze-dried to give lilac colored solid (50 mg, 15%), mp > 230 °C. 1H NMR (DMSO-d6): 11.26 (1H, s), 10.43 (1H, s), 7.95 (2H, dd, J = 1.24 and 8.4 Hz), 7.31 (2H, t, J = 7.52 Hz), 7.16 (1H, t, 7.4 Hz), 7.03 (1H, d, J = 2.4 Hz), 6.25 (2H, br). IR (KBr): 1719, 1684, 1657, 1210, 1181, 1146, 763, 723, 705 cm.–1 HRESIMS: calculated for C12H11ON4, 227.0927; found, 227.0925.
27a,b,d were similarly prepared.
5-(2-Nitro-1-phenylethyl)-2,4,6-pyrimidinetriamine (28c)
2,4,6-Pyrimidinetriamine (0.575 g, 4.60 mmol) and [(E)-2-nitroethenyl]benzene (0.783 g, 5.25 mmol) were suspended in a mixture of water (20 mL) and ethyl acetate (20 mL) at room temperature. The resulting mixture was left stirring at room temperature for 18 h. The reaction mixture was extracted with ethyl acetate and dried (Na2SO4). The crude material obtained was purified by column chromatography using silica gel, methanol/ethyl acetate [1:9, Rf = 0.5], to give the required product as yellow solid (0.500 g, 40%), mp 110–113 °C (transparent). 1H NMR (DMSO-d6): 7.36–7.22 (5H, m), 5.48–5.41 (7H, m, containing 3 × NH2, exchangeable), 5.18–5.04 (2H, m). IR (KBr): 1619, 1567, 1442, 1377, 1254, 1032, 802, 743, 701 cm.–1 HRESIMS: calculated for C12H15O2N6, 275.1251; found, 275.1244.
28a,b,d were similarly prepared.
5-Phenyl-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamine (29c)32
To a solution of NaOH (0.330 g, 8.25 mmol) in water (5 mL) was added 5-(2-nitro-1-phenylethyl)-2,4,6-pyrimidinetriamine (0.441 g, 1.53 mmol) at room temperature. The mixture was stirred for 2 h and then was slowly added to a solution of sulfuric acid (98%, 1.37 g, 14 mmol) in water (5 mL) at 0 °C [the sequence of the addition is important]. The resulting mixture was stirred at 0 °C for 1 h and at room temperature overnight. The solid material was filtered, washed with water, and dried to give the crude product as brown solid (285 mg). The crude material was purified by HPLC, and fractions containing the required product were collected and freeze-dried to give pale yellow solid (50 mg, 29%), mp 200–203 °C. 1H NMR (DMSO-d6): 11.91 (1H, s), 7.48–7.34 (5H, m), 7.20 (4H, br), 7.08 (1H, d, J = 1.8 Hz). IR (KBr): 1694, 1651, 1543, 1453, 1390, 1207, 1131, 826, 800, 759, 725 cm.–1 HRESIMS: calculated for C12H12N5, 226.1087; found, 226.1082.
29a,b,d were similarly prepared.
5-[1-(4-Fluorophenyl)-2-nitroethyl]-6-isopropyloxy-2,4-pyrimidinediamine (30c)
6-Isopropyloxy-2,4-pyrimidinediamine (250 mg, 1.486 mmol) and 1-fluoro-4-[(E)-2-nitroethenyl]benzene (248 mg, 1.486 mmol) were dissolved in ethyl acetate (20 mL). The reaction mixture was placed in a sealed tube and heated at 50 °C for 4 days. Solvent was removed under reduced pressure, and the crude material was applied to a silica gel column chromatography. Elution with ethyl acetate/n-hexane 1:1 (Rf = 0.1) gave the required product (100 mg, 20%) as white solid, mp 148–150 °C. 1H NMR (DMSO-d6): 7.37 (2H, dd, J = 5.5 Hz, J = 8.7 Hz), 7.12 (2H, t, J = 8.9 Hz), 6.14 (2H, s), 5.78 (2H, s), 5.25–5.13 (3H, m), 4.82 (1H, t, J = 8.0 Hz), 1.25 (3H, d, J = 6.2 Hz), 1.08 (3H, d, J = 6.2 Hz). IR (KBr): 798, 818, 1005, 1109, 1140, 1198, 1231, 1375, 1422, 1445, 1508, 1539, 1566, 1603, 1653 cm–1. HRESIMS: calculated for C15H19FN5O3, 336.1466; found, 336.1469.
5-(4-Fluorophenyl)-4-isopropyloxy-7H-pyrrolo[2,3-d]pyrimidin-2-amine (31c)
5-[1-(4-Fluorophenyl)-2-nitroethyl]-6-isopropyloxy-2,4-pyrimidinediamine (65 mg, 0.194 mmol) was dissolved in a solution of NaOH (215 mg, 5.375 mmol) in water (5 mL). The reaction mixture was left stirring at room temperature for 2 h (with occasional gentle heating to help the starting material to go into solution). This solution was added dropwise to a cooled solution of sulfuric acid (0.70 g) in water (5 mL) at 0 °C with stirring, after which time the reaction mixture was left stirring at room temperature overnight. The white solid material formed was filtered, washed with water, and dried. HPLC purification of this material afforded the required product (10 mg, 18%) as white solid after freeze-drying with no distinct melting point. The starting material was also recovered (10 mg, 15%) as pale yellow solid. 1H NMR (DMSO-d6): 11.50 (1H, s), 7.68 (2H, dd, J = 5.6 Hz, J = 8.9 Hz), 7.20 (2H, t, J = 8.9 Hz), 7.11 (1H, d, J = 2.3 Hz), 6.58 (2H, br), 5.47 (1H, septet, J = 6.2 Hz), 1.33 (6H, d, J = 6.2 Hz). IR (KBr): 1720, 1684, 1658, 1213, 1177, 1141, 833, 723, 707, 686 cm.–1 HRESIMS: calculated for C15H16FN4O, 287.1303; found, 287.1307.
31a,b were similarly prepared.
Leading to 34c, 35b, and 36c
1-(4-Fluorophenyl)-2-phenylethanone33
Aluminum chloride (16.0 g, 0.119 mmol, 1.2 mol equiv) was added to fluorobenzene (50 mL, 51.2 g, 0.533 mmol, 5.0 mol equiv) with stirring and cooling with ice water under nitrogen. Phenacyl chloride (13.8 mL, 16.13 g, 0.104 mmol, 1.05 mol equiv) was added dropwise while keeping the temperature below 20 °C. The reaction mixture was stirred for a further 15 min, and then it was heated at 50 °C for 5 h, after which time the reaction mixture was left stirring at room temperature for 9 h. Hydrolysis was carried out by diluting with dichloromethane, pouring the reaction mixture onto crushed ice (50 g), and extracting the resulting suspension with HCl (2M, 30 mL). The organic phase was then cautiously washed with a saturated aqueous solution of sodium hydrogen carbonate and brine. The organic layer was dried (Na2SO4) and filtered, and the solvent was removed under reduced pressure to give solid material, which was washed with n-hexane. The desired material (21.00 g, 94%) was obtained as a pale yellow solid, mp 83–85 °C (Lit. mp 82 °C),33Rf = 0.5 (1:6 ethyl acetate/n-hexane). 1H NMR (DMSO-d6): 8.16 (2H, dd, J = 6.0 Hz, J = 8.3 Hz), 7.39–7.24 (7H, m), 4.39 (2H, s). IR: 710, 722, 744, 792, 830, 860, 990, 1093, 1147, 1191, 1233, 1333, 1413, 1502, 1593, 1677 cm.–1 HRESIMS: calculated for C14H12FO, 215.0867; found, 215.0870.
1,2-Bis(4-fluorophenyl)ethanone, 1,2-bis(4-chlorophenyl)ethanone, 1-(4-fluorophenyl)-2-(4-methoxyphenyl)ethanone, 1,2-bis(4-methylphenyl)ethanone, 1-(4-bromophenyl)-2-phenylethanone, 1-(4-isobutylphenyl)-2-phenylethanone, 1-[4-(methylsulfonyl)phenyl]-2-phenylethanone, and 2-(3-chlorophenyl)-1-(4-fluorophenyl)ethanone, and 1-[4-(3-chloropropyl)phenyl]-2-phenylethanone were similarly prepared.
2-Bromo-1-(4-fluorophenyl)-2-phenylethanone (33c)
1-(4-Fluorophenyl)-2-phenylethanone (5.46 g, 25.49 mmol) was dissolved in chloroform (58 mL), to which was added a hydrobromic acid solution 30% in acetic acid (0.140 mL, 1 mol equiv) at room temperature with stirring. Bromine (1.32 mL) was dissolved in chloroform (5 mL) and added to the reaction mixture dropwise with stirring. At the end of the reaction, a slight bromine coloration should remain. Aqueous sodium sulfite (10%) solution was added, and the reaction mixture was then extracted. The organic layer was collected and washed with a saturated aqueous solution of sodium hydrogen carbonate followed by brine, the organic layer was dried (Na2SO4) and filtered, and the solvent was removed under reduced pressure to give the required product (7.20 g, 96%) as a reddish-brown oil that solidified on standing, mp 45–46 °C (Lit. mp 46 °C).331H NMR (CDCl3): 8.07 (2H, dd, J = 5.4 Hz, 9.0 Hz), 7.55–7.53 (2H, m), 7.43–7.34 (3H, m), 7.17 (2H, dd, J = 8. 5 Hz, 8.8 Hz), 6.34 (1H, s). IR: 729, 771, 797, 827, 852, 991, 1005, 1103, 1155, 1186, 1213, 1234, 1271, 1302, 1408, 1454, 1495, 1504, 1593, 1688 cm.–1 HRESIMS: calculated for C14H1179BrFO, 292.9972; found, 292.9974.
2-Bromo-1,4-diphenyl-1-butanone (33a), 2-bromo-1,2-bis(4-fluorophenyl)ethanone (33d), 2-bromo-1,2-bis(4-chlorophenyl)ethanone (33e), 2-bromo-1-(4-fluorophenyl)-2-(4-methoxyphenyl)ethanone (33f), 2-bromo-1,2-bis(4-methylphenyl)ethanone (33g), 2-bromo-1-(4-bromophenyl)-2-phenylethanone (33h), 2-bromo-1-(4-isobutylphenyl)-2-phenylethanone (33j), 2-bromo-1-[4-(methylsulfonyl)phenyl]-2-phenylethanone (33k), 2-bromo-2-(3-chlorophenyl)-1-(4-fluorophenyl)ethanone (33l), and 2-bromo-1-{4-[3-(4-morpholinyl)propyl]phenyl}-2-phenylethanone (33m) were similarly prepared.
2-Amino-5,6-diphenyl-3,7-dihydro-4H-pyrrolo[2,3-d]pyrimidin-4-one (34a)
2,6-Diamino-4(3H)-pyrimidinone (0.290 g, 2.00 mmol) and desyl bromide (0.550 g, 2.00 mmol) were dissolved in DMF (2 mL, dry). The reaction mixture was heated at 60 °C for 4 days. The solvent was removed in vacuo, and the residue was applied to a silica gel column and eluted with 1:9 methanol/ethyl acetate. The product was obtained as yellow solid after trituration with hot methanol (230 mg, 38%). 1H NMR (DMSO-d6): 11.44 (1H, s), 10.27 (1H, s), 7.31–7.18 (10H, m), 6.13 (2H, s). IR: 698, 724, 758, 783, 835, 880, 1157, 1225, 1377, 1441, 1506, 1516, 1543, 1599, 1634 cm.–1 HRESIMS: calculated for C18H15N4O, 303.1240; found, 303.1242.
2-Amino-6-(4-fluorophenyl)-5-phenyl-3,7-dihydro-4H-pyrrolo[2,3-d]pyrimidin-4-one (34c)
2-Bromo-1-(4-fluorophenyl)-2-phenylethanone (2.324 g, 7.929 mmol) and 2,6-diamino-4(3H)-pyrimidinone (1.00 g, 7.929 mmol) were dissolved in DMF (4 mL, dry). The reaction mixture was heated at 60 °C for 4 days with stirring under nitrogen. DMF was removed in vacuo, and the residue was applied to a silica gel column chromatography and eluted with methanol/ethyl acetate (1:9; Rf = 0.5). The product was obtained as an orange solid (0.880 g, 35%), mp > 230 °C. Some of this material was further purified by HPLC. 1H NMR (DMSO-d6): 11.48 (1H, s), 10.30 (1H, s), 7.29–7.19 (7H, m), 7.10 (2H, t, J = 8.9 Hz), 6.16 (2H, br). IR: 722, 758, 784, 811, 835, 879, 1155, 1225, 1377, 1441, 1506, 1516, 1543, 1600, 1633 cm.–1 HRESIMS: calculated for C18H14ON4, 321.1146; found, 321.1145.
34b–l were similarly prepared.
5,6-Diphenyl-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamine (35a)
2,4,6-Pyrimidinetriamine (0.250 g, 2.00 mmol) and desyl bromide (0.550 g, 2.00 mmol) were dissolved in DMF (2 mL, dry). The reaction mixture was heated at 60 °C for 4 days. The solvent was removed in vacuo, and the residue was applied to a silica gel column chromatography and eluted with 1:9 methanol/ethyl acetate, Rf = 0.4. The product was obtained as yellow solid (68 mg, 11%). Some of this material was further purified by HPLC, mp 130–133 °C. 1H NMR (DMSO-d6): 12.26 (1H, s), 7.48–7.25 (12H, m), 6.67 (2H, br). IR: 1647, 1443, 1389, 1194, 1136, 1076, 1017, 972, 918, 835, 810, 766, 694 cm–1 HRESIMS: calculated for C18H16N5, 302.1400; found, 302.1396.
6-(4-Fluorophenyl)-5-phenyl-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamine (35b)
2-Bromo-1-(4-fluorophenyl)-2-phenylethanone (2.324 g, 7.929 mmol) and 2,4,6-pyrimidinetriamine (1.000 g, 7.991 mmol) were dissolved in DMF (4 mL, dry). The reaction mixture was heated at 60 °C for 4 days with stirring under nitrogen. DMF was removed in vacuo, and the residue was applied to a silica gel column chromatography and eluted with methanol/ethyl acetate (1:9; Rf = 0.4). The product was obtained as yellow solid (0.270 g, 11%), mp 125–130 °C. Some of this material was further purified by HPLC. IR: 721, 758, 783, 810, 835, 879, 1157, 1225, 1377, 1441, 1506, 1516, 1543, 1599 cm.–1 1H NMR (DMSO-d6): 12.32 (1H, s), 7.48–7.26 (9H, m), 7.18 (2H, t, J = 8.9 Hz), 6.79 (2H, br). HRESIMS: calculated for C18H15N5F, 320.1306; found, 320.1307.
35c–j were similarly prepared.
N4,N4-Dimethyl-5,6-diphenyl-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamine (36d)
N4,N4-Dimethyl-2,4,6-pyrimidinetriamine (0.500 g, 3.26 mmol) and 2-bromo-1,2-diphenylethanone [desyl bromide] (0.898 g, 3.26 mmol) were dissolved in DMF (4 mL, dry), to which were added potassium iodide (0.541 g, 3.26 mmol) and cesium carbonate (1.062 g, 3.26 mmol). The reaction mixture was heated at 60 °C for 24 h. DMF was removed in vacuo, and the residue was dissolved in ethyl acetate and methanol, to which was added silica gel, and the solvents were removed under reduced pressure. The residue was applied to a silica gel column chromatography and eluted with ethyl acetate (Rf = 0.3). The required product was obtained as a yellow solid (0.310 g, 29%), mp > 230 °C. 1H NMR (DMSO-d6): 12.26 (1H, s), 7.48–7.25 (12H, m), 6.67(2H, br). IR: 1591, 1541, 1479, 1433, 1394, 1323, 1276, 1058, 1028, 869, 767, 690 cm–11H NMR (DMSO-d6): 11.35 (1H, s), 7.36–7.17 (10H, m), 5.74 (2H, s), 2.50 (6H, s). HRESIMS: calculated for C20H20N5, 330.1713; found, 330.1710.
36a–c,e were similarly prepared.
2-Amino-6-{4-[3-(4-morpholinyl)propyl]phenyl}-5-phenyl-3,7-dihydro-4H-pyrrolo[2,3-d]pyrimidin-4-one trifluoroacetate (38)
1-{4-[3-(4-Morpholinyl)propyl]phenyl}-2-phenylethanone
1-[4-(3-Chloropropyl)phenyl]-2-phenylethanone (5.50 g, 0.02 mol) was dissolved in toluene (15 mL, dry), to which was added morpholine (5.20 g, 0.06 mol) with stirring at room temperature. The reaction mixture was heated under reflux overnight, and then the solvent and excess morpholine were removed under reduced pressure. The crude material obtained was dissolved in ether and extracted with 30% NaOH (aqueous). The organic layer was extracted with water, dried (Na2SO4), and filtered, and the solvent was removed under reduced pressure to give the required product as a thick brown oil. The crude product was applied to a silica gel column chromatography and eluted with ethyl acetate to give pale yellow thick oil (6.325 g, 98%). 1H NMR as HCl salt (DMSO-d6): 8.00 (2H, d, J = 8.2 Hz), 7.41 (2H, d, J = 8.2 Hz), 7.31–7.20 (5H, m), 4.35 (2H, s), 3.92–3.82 (4H, m), 3.39 (2H, d, J = 12.2 Hz), 3.07–3.01 (4H, m), 2.73 (2H, t, J = 7.7 Hz), 2.08 (2H, qt, J = 4.2 Hz). IR: 717, 740, 788, 806, 842, 991, 1180, 1215, 1317, 1356, 1405, 1433, 1567, 1601, 1682 cm–1
2-Bromo-1-{4-[3-(4-morpholinyl)propyl]phenyl}-2-phenylethanone
1-{4-[3-(4-Morpholinyl)propyl]phenyl}-2-phenylethanone (0.900 g, 2.783 mmol) was dissolved in chloroform (25 mL). Hydrobromic acid in acetic acid (33%, 1 mL) was added at room temperature with stirring. Bromine (0.5 mL) in chloroform (10 mL) was added to the reaction mixture at room temperature with stirring. The dropwise addition continued until slight bromine coloration remained. The stirring was continued for further 30 min. Aqueous sodium sulfite (10%) solution was added, and the reaction mixture was then extracted. The organic layer was collected and washed with a saturated solution of sodium hydrogen carbonate followed by brine, the organic layer was dried (Na2SO4) and filtered, and the solvent was removed under reduced pressure to give the required product (1.020 g, 91%) as pale yellow oil. This material was used in the next experiment without further purification.
2-Amino-6-{4-[3-(4-morpholinyl)propyl]phenyl}-5-phenyl-3,7-dihydro-4H-pyrrolo[2,3-d]pyrimidin-4-one trifluoroacetate (38)
2,6-Diamino-4(3H)-pyrimidinone (0.160 g, 1.268 mmol) and 2-bromo-1-{4-[3-(4-morpholinyl)propyl]phenyl}-2-phenylethanone (0.501 g, 1.832 mmol) were dissolved in DMF (5 mL, dry) with stirring. The reaction mixture was heated at 60 °C for 24 h. HPLC purification gave the required product as light brown solid (50 mg, 7%) with no distinct melting point. 1H NMR (DMSO-d6): 11.41 (1H, s), 10.26 (1H, s), 9.50 (1H, br), 7.29–7.08 (9H, m), 6.11 (2H, br), 3.10 (4H, m), 2.59 (2H, m), 1.94 (2H, m). IR: 696, 719, 765, 796, 835, 1132, 1194, 1433, 1495, 1657 cm–1. HRESIMS: calculated for C25H28N5O2, 430.2238; found, 430.2242.
Acknowledgments
Financial support was provided by the Medical Research Council (UK) through grant no. G0901426/1 and the Wellcome Trust, grant nos. 094090 and 100476. We thank Craig Irving, Patricia Keating, Gavin Blackburn, and Gavin Bain for their help and support, which they provided during the course of this research work, and Blair Johnston for advice in the preparation of the manuscript.
Glossary
Abbreviations Used
- AQP2
aquaglyceroporin 2
- CMM
Creek’s minimal medium
- DHFR
dihydrofolate reductase
- HAT
Human African Trypanosomiasis
- HPLC
high-performance liquid chromatography
- HRCIMS
high-resolution chemical ionization mass spectroscopy
- HREIMS
high-resolution electron ionization mass spectroscopy
- HRESIMS
high-resolution electrospray ionization mass spectroscopy
- HRFABMS
high-resolution fast atom bombardment mass spectroscopy
- KO
knockout
- MTX
methotrexate
- PTR1
pteridine reductase 1
- TbPTR1
Trypanosoma brucei pteridine reductase 1
Supporting Information Available
Synthetic methods and characterization of analogues of compounds described herein; protein preparation and purification; spectrophotometric assay methods; and ligand cocrystallization and structure determination. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
The version of this paper that was published ASAP on July 29, 2014, contained incorrect versions of both Scheme 4 and Scheme 5. The corrected version was reposted July 30, 2014.
Supplementary Material
References
- Brun R.; Blum J.; Chappuis F.; Burri C. Human African trypanosomiasis. Lancet 2010, 375, 148–159. [DOI] [PubMed] [Google Scholar]
- Simarro P. P.; Cecchi G.; Franco J. R.; Paone M.; Diarra A.; Ruiz-Postigo J. A.; Fèvre E. M.; Mattioli R. C.; Jannin J. G. Estimating and mapping the population at risk of sleeping sickness. PLoS Neglected Trop. Dis. 2012, 6, e1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett M. P.; Boykin D. W.; Brun R.; Tidwell R. R. Human African trypanosomiasis: pharmacological re-engagement with a neglected disease. Br. J. Pharmacol. 2007, 152, 1155–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienkiewicz N.; Ong H. B.; Fairlamb A. H. Trypanosoma brucei pteridine reductase 1 is essential for survival in vitro and for virulence in mice. Mol. Microbiol. 2010, 77, 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinks D.; Ong H. B.; Mpamhanga C. P.; Shanks E. J.; Robinson D. A.; Collie I. T.; Read K. D.; Frearson J. A.; Wyatt P. G.; Brenk R.; Fairlamb A. H.; Gilbert I. H. Design, synthesis and biological evaluation of novel inhibitors of Trypanosoma brucei pteridine reductase 1. ChemMedChem. 2011, 6, 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzuti A.; Paglietti G.; Hunter W. N.; Gamarro F.; Piras S.; Loriga M.; Allecca S.; Corona P.; McLuskey K.; Tulloch L.; Gibellini F.; Ferrari S.; Costi M. P. Discovery of potent pteridine reductase inhibitors to guide antiparasite drug development. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 1448–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch L. B.; Martini V. B.; Iulek J.; Huggan J. K.; Lee J.-H.; Gibson C. L.; Smith T. K.; Suckling C. J.; Hunter W. N. Design of pteridine reductase inhibitors; early stage drug development targeting African sleeping sickness and Leishmaniasis. J. Med. Chem. 2010, 53, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpamhanga C. P.; Spinks D.; Tulloch L. B.; Shanks E. J.; Robinson D. A.; Collie I. T.; Fairlamb A. H.; Wyatt P. G.; Frearson J. A.; Hunter W. N.; Gilbert I. H.; Brenk R. One scaffold, three binding modes: novel and selective pteridine reductase 1 inhibitors derived from fragment hits discovered by virtual screening. J. Med. Chem. 2009, 52, 4454–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursavich M. G.; Dastrup D.; Shenderovich M.; Yager K. M.; Cimbora D. M.; Williams B.; Kumar D. V. Novel Mps1 kinase inhibitors: from purine to pyrrolopyrimidine and quinazoline leads. Bioorg. Med. Chem. Lett. 2013, 23, 6829–6933. [DOI] [PubMed] [Google Scholar]
- Dincer S.; Cetin K.; Taylan O-B.A.; Olgen S. Synthesis, biological evaluation and docking studies of new pyrrolo[2,3-d]pyrimidine derivatives as Src family-selective tyrosine kinase inhibitors. J. Enzyme Inhib. Med. Chem. 2013, 28, 1080–1087. [DOI] [PubMed] [Google Scholar]
- Arcari J. T.; Beebe J. S.; Berliner M. A.; Bernardo V.; Boehm M.; Borzillo G. V.; Clark T.; Cohen B. D.; Connell R. D.; Frost H. N.; Gordon D. A.; Hungerford W. M.; Kakar S. M.; Kanter A.; Keene N. F.; Knauth E. A.; LaGreca S. D.; Lu Y.; Martinez-Alsina L.; Marx M. A.; Morris J.; Patel N. C.; Savage D.; Soderstrom C. I.; Thompson C.; Tkalcevic G.; Tom N. J.; Vajdos F. F.; Valentine J. J.; Vincent P. W.; Wessel M. D.; Chen J. M. Discovery and synthesis of novel 4-aminopyrrolopyrimidine Tie-2 kinase inhibitors for the treatment of solid tumors. Bioorg. Med. Chem. Lett. 2013, 23, 3059–3063. [DOI] [PubMed] [Google Scholar]
- Addie M.; Ballard P.; Buttar D.; Crafter C.; Currie G.; Davies B. R.; Debreczeni J.; Dry H.; Dudley P.; Greenwood R.; Johnson P. D.; Kettle J. G.; Lane C.; Lamont G.; Leach A.; Luke R. W. A.; Morris J.; Ogilvie D.; Page K.; Pass M.; Pearson S.; Ruston L. Discovery of 4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide (AZD5363), an orally bioavailable, potent inhibitor of Akt kinases. J. Med. Chem. 2013, 56, 2059–2073. [DOI] [PubMed] [Google Scholar]
- Le Brazidec J.-Y.; Pasis A.; Tam B.; Boykin C.; Wang D.; Marcotte D. J.; Claassen G.; Chong J.-H.; Chao J.; Fan J.; Khanh N.; Silvian L.; Ling L.; Zhang L.; Choi M.; Teng M.; Pathan N.; Zhao S.; Li T.; Taveras A. Structure-based design of 2,6,7-trisubstituted-7H-pyrrolo[2,3-d]pyrimidines as Aurora kinases inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 4033–4037. [DOI] [PubMed] [Google Scholar]
- Tessier P. R.; Nicolau D. P. In vitro activity of novel gyrase inhibitors against a highly resistant population of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 2887–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tari L. W.; Trzoss M.; Bensen D. C.; Li X.; Chen Z.; Thanh L.; Zhang J.; Creighton C. J.; Cunningham M. L.; Kwan B.; Stidham M.; Shaw K. J.; Lightstone F. C.; Wong S. E.; Nguyen T. B.; Nix J.; Finn J. Pyrrolopyrimidine inhibitors of DNA gyrase B (GyrB) and topoisomerase IV (ParE). Part I: structure guided discovery and optimization of dual targeting agents with potent, broad-spectrum enzymatic activity. Bioorg. Med. Chem. Lett. 2013, 23, 1529–1536. [DOI] [PubMed] [Google Scholar]
- Trzoss M.; Bensen D. C.; Li X.; Chen Z.; Thanh L.; Zhang J.; Creighton C. J.; Cunningham M. L.; Kwan B.; Stidham M.; Nelson K.; Brown-Driver V.; Castellano A.; Shaw K. J.; Lightstone F. C.; Wong S. E.; Nguyen T. B.; Finn J.; Tari L. W. Pyrrolopyrimidine inhibitors of DNA gyrase B (GyrB) and topoisomerase IV (ParE), Part II: development of inhibitors with broad spectrum, Gram-negative antibacterial activity. Bioorg. Med. Chem. Lett. 2013, 23, 1537–1543. [DOI] [PubMed] [Google Scholar]
- Mohamed M. S.; Kamel R.; Abd El-hameed R. H. Evaluation of the anti-inflammatory activity of some pyrrolo[2,3-d]pyrimidine derivatives. Med. Chem. Res. 2013, 22, 2244–2252. [Google Scholar]
- Kaspersen S. J.; Sundby E.; Charnock C.; Hoff B. H. Activity of 6-aryl-pyrrolo[2,3-d]pyrimidine-4-amines to Tetrahymena. Bioorg. Chem. 2012, 44, 35–41. [DOI] [PubMed] [Google Scholar]
- Xie H.; Zeng L.; Zeng S.; Lu X.; Zhang G.; Zhao X.; Cheng N.; Tu Z.; Li Z.; Xu H.; Yang L.; Zhang X.; Huang M.; Zhao J.; Hu W. Novel pyrrolopyrimidine analogues as potent dipeptidyl peptidase IV inhibitors based on pharmacokinetic property-driven optimization. Eur. J. Med. Chem. 2012, 52, 205–212. [DOI] [PubMed] [Google Scholar]
- Barrett M. P.; Fairlamb A. H. The biochemical basis of arsenical-diamidine cross resistance in African trypanosomes. Parasitol. Today 1999, 15, 136–140. [DOI] [PubMed] [Google Scholar]
- Carter N. S.; Berger B. J.; Fairlamb A. H. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 1995, 270, 28153–28157. [DOI] [PubMed] [Google Scholar]
- Gourley D. G.; Schüttelkopf A. W.; Leonard G. A.; Luba J.; Hardy L. W.; Beverley S. M.; Hunter W. N. Pteridine reductase mechanism correlates pterin metabolism with drug resistance in trypanosomatid parasites. Nat. Struct. Biol. 2001, 8, 521–525. [DOI] [PubMed] [Google Scholar]
- Gibson C. L.; Huggan J. K.; Kennedy A.; Kiefer L.; Lee J.-H.; Suckling C. J.; Clements C.; Harvey A. L.; Hunter W. N.; Tulloch L. B. Diversity oriented syntheses of fused pyrimidines designed as potential antifolates. Org. Biomol. Chem. 2009, 7, 1829–1842. [DOI] [PubMed] [Google Scholar]
- Gangjee A.; Vidwans A.; Elfatih E.; McGuire J. J.; Queener S. F.; Kisliuk R. L. Synthesis, antifolate, and antitumor activities of classical and nonclassical 2-amino-4-oxo-5-substituted-pyrrolo[2,3-d]pyrimidines. J. Med. Chem. 2001, 44, 1993–2003. [DOI] [PubMed] [Google Scholar]
- Zhu G.; Liu Z.; Xu Y.; Mao Z. Synthesis of pyrrolo[2,3-d]pyrimidine analogues of the potent antitumor agent N-{4-[3-(2,4-diamino-7H-pyrrolo-[2,3-d]pyrimidin-5-yl)propyl]benzoyl}-l-glutamic acid (TNP-351). Heterocycles 2008, 75, 1631–1638. [Google Scholar]
- Dawson A.; Gibellini F.; Sienkiewicz N.; Tulloch L. B.; Fyfe P. K.; McLuskey K.; Fairlamb A. H.; Hunter W. N. Structure and reactivity of Trypanosoma brucei pteridinereductase: inhibition by the archetypal antifolate methotrexate. Mol. Microbiol. 2006, 61, 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matovu E.; Stewart M. L.; Geiser F.; Brun R.; Maser P.; Wallace L. J.; Burchmore R. J.; Enyaru J. C.; Barrett M. P.; Kaminsky R.; Seebeck T.; de Koning H. P. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryotic Cell 2003, 2, 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D. J.; Gould M. K.; Nerima B.; Maser P.; Burchmore R. J.; de Koning H. P. Loss of the high-affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol. Pharmacol. 2007, 71, 1098–1108. [DOI] [PubMed] [Google Scholar]
- Baker N.; Glover L.; Munday J. C.; Aguinaga A. D.; Barrett M. P.; de Koning H. P.; Horn D. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 10996–11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creek D. J.; Nijagal B.; Kim D. H.; Rojas F.; Matthews K. R.; Barrett M. P. Metabolomics guides rational development of a simplified cell culture medium for drug screening against Trypanosoma brucei. Antimicrob. Agents Chemother. 2013, 57, 2768–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz B.; Iten M.; Grether-Buhler Y.; Kaminsky R.; Brun R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [DOI] [PubMed] [Google Scholar]
- Taylor E. C.; Liu B. A New and efficient synthesis of pyrrolo[2,3-d]pyrimidine anticancer agents: Alimta (LY231514, MTA), Homo-Alimta, TNP-351, and some aryl 5-substituted pyrrolo[2,3-d]pyrimidines. J. Org. Chem. 2003, 68, 9938–9947. [DOI] [PubMed] [Google Scholar]
- Schoening K.-U.; Hartwig J.. Preparation process for atorvastatin, an inhibitor of HMG-CoA reductase, via an improved process for the preparation of the key intermediate 2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide. PCT Int. Appl. WO 2003004457 A2 20030116, 2003.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.