Abstract
Objectives
To fabricate biodegradable polydioxanone (PDS II®) electrospun periodontal drug delivery systems (hereafter referred to as matrices) containing either metronidazole (MET) or ciprofloxacin (CIP) and to investigate the effects of antibiotic incorporation on both non-periodontitis and periodontitis related bacteria.
Materials and methods
Fibrous matrices were processed from PDS polymer solution by electrospinning. Antibiotic-containing PDS solutions were prepared to obtain four distinct groups: 5 wt.% MET, 25 wt.% MET, 5 wt.% CIP, 25 wt.% CIP. Pure PDS was used as a control. High performance liquid chromatography (HPLC) was done to evaluate MET and CIP release. Dual-species biofilms formed by Lactobacillus casei (Lc) and Streptococcus salivarius (Ss) were grown on the surface of all electrospun matrices. After 4 days of biofilm growth, the viability of bacteria on biofilms was assessed. Additionally, antimicrobial properties were evaluated against periodontopathogens Fusobacterium nucleatum (Fn) and Aggregatibacter actinomycetemcomitans (Aa) using agar diffusion assay.
Results
A three-dimensional interconnected porous network was observed in the different fabricated matrices. Pure PDS showed the highest fiber diameter mean (1158±402 nm) followed in a descending order by groups 5 wt.% MET (1108±383 nm), 25 wt.% MET (944±392 nm), 5 wt.% CIP (871±309 nm), and 25 wt.% CIP (765±288 nm). HPLC demonstrated that groups containing higher amounts (25 wt.%) of incorporated drugs released more over time while those with lower levels (5 wt.%) the least. No inhibitory effect of the tested antibiotics was detected on biofilm formation by the tested non-periodontitis related bacteria. Meanwhile, CIP-containing matrices inhibited growth of Fn and Aa.
Conclusion
CIP-containing matrices led to a significant inhibition of periodontal pathogens without negatively impairing the growth of periodontal beneficial bacteria.
Clinical relevance
Based on the proven in vitro inhibition of periodontitis-related bacteria, future in vivo research using relevant animal models is needed to confirm the effectiveness of these drug delivery systems.
Keywords: Drug delivery, Periodontitis, Antibiotics, Electrospinning, Biofilm, Bacteria
Introduction
Antimicrobials have been used as an adjunct to periodontal procedures, such as surgery and mechanical debridement of root surfaces in an effort to reduce bacterial recolonization of root and tissue surfaces [1]. Metronidazole is one of the most frequently indicated antibiotics for the treatment of periodontal disease [2]. However, given the increased microbial resistance related to its use [3–4], fluoroquinolones, such as ciprofloxacin, have been suggested as a potential substitute.
It is well established that the long-term use of antimicrobials might eradicate normal flora, which induces an imbalance in microbial composition by killing predominantly susceptible strains and selecting resistant ones [5]. The presence of normal microbiota is essential for health maintenance, since it acts as a barrier protecting the host from exogenous microbial colonization as well as from already present potential pathogens [6]. Nonetheless, it is expected that the eradication of normal microbiota might create conditions for emergence and colonization of opportunistic and resistant pathogens posing an increased risk to disease development. In terms of oral health, it has been suggested that some oral commensal microorganisms, such as Lc and Ss, might have an antagonistic relationship with some periodontal pathogens [7–8].
Guided tissue regeneration (GTR) membranes have also been adopted in periodontal treatment to promote tissue regeneration by avoiding migration of epithelial cells into the periodontal pocket [9]. Interestingly, the incorporation of metronidazole to these membranes [10] has given promising results in terms of inhibition of Porphyromonas gingivalis (Pg) growth [9]. In this context, a study has shown that the use of localized drug delivery systems for the treatment of periodontal disease resulted in lower selection of resistant species when compared to systemically delivery drug systems [11]. Accordingly, the use of a periodontal biodegradable matrix able to release antimicrobials directly to the gum surface without systemic drug exposure seems to be a wise approach preventing both bacteria resistance [12–13] and drug related systemic side-effects [14]. Moreover, it is highly desirable that this system does not interfere with the growth of oral commensal microorganisms. Therefore, the purpose of this work was to fabricate biodegradable polydioxanone (PDS II®) based electrospun periodontal drug delivery systems (hereafter referred to as matrices) containing either metronidazole (MET) or ciprofloxacin (CIP) and to investigate the effects of antibiotic incorporation on both periodontopathogens and commensal oral bacteria.
Materials & Methods
Materials
Violet colored polydioxanone (PDS II®, Ethicon Inc., Somerville, NJ) monofilament surgical sutures were used. 1,1,1,3,3,3-hexafluoro-2-propanol (HFP, Sigma-Aldrich, St. Louis, MO) was purchased and employed as a solvent for PDS II®. Therapeutic agents (i.e., metronidazole and ciprofloxacin) were obtained from Sigma-Aldrich. The distinct bacteria, namely Lc (ATCC 4646), Ss (ATCC 25975), Fn (ATCC 10953) and Aa (ATCC 33384) were obtained from American Type Culture Collection (ATCC). Tryptic Soy Broth (TSB) culture media, Rogosa agar and Columbia Blood Agar base were obtained from Difco (Sparks, MD, USA). Sucrose, phosphate buffer saline (PBS), sodium chloride and paraformaldehyde were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Defibrinated sheep blood was obtained from Carolina Biological Supply (Burlington, NC, USA). Hexamethyldisilazane (HMDS) was obtained from Sigma-Aldrich. All materials and chemicals were used as-received without any additional purification.
Electrospinning of nanofibrous antibiotic-containing matrices
An in-house conventional electrospinning system consisting of a (1) a high-voltage source (ES50P-10W/DAM, Gamma High-Voltage Research Inc., FL), (2) a syringe pump (Legato 200, KD Scientific Apparatus, Holliston, MA), and a (3) grounded stainless steel collecting drum (ϕ = 4 cm) connected to a high-speed mechanical stirrer (BDC6015, Caframo, Wiarton, ON) was employed to obtain distinct nanofibrous matrices [9, 10]. Briefly, after needles removal, PDS pieces (~ 3 cm in length) were placed in a glass vial containing methylene chloride (Sigma-Aldrich) to eliminate the violet dye [15]. Subsequently, clear PDS was dissolved at 100 mg mL−1 overnight in HFP under stirring conditions [15]. Antibiotic-containing (5 and 25 wt.% based on the weight of PDS) solutions made by adding either one of the drugs directly to the PDS solution were prepared under vigorous stirring overnight to obtain four distinct experimental groups of antibiotic-containing fibers as follows: 5 wt.% MET, 25 wt.% MET, 5 wt.% CIP, 25 wt.% CIP. Pure PDS was also electrospun as a control. Pure PDS or the antibiotic-containing formulations were individually loaded in a plastic syringe fitted with a 27-gauge stainless steel needle and electrospun directly over glass slides (ϕ = 12 mm, # 12-545-80, Fisherbrand, Fisher Scientific, Fair Lawn, NJ, USA). The fibers were collected at room temperature (RT) on the aluminum foil covered rotating mandrel containing the glass slides using optimized parameters (i.e., 15 kV power voltage to the needle tip, 20 cm collection distance and a flow rate of 1 ml h−1). Samples were kept at RT for 2 days in a vacuum desiccator to remove any residual solvent. A similar procedure was carried out to obtain nanofibrous matrices over aluminum foil only to be used in both the antibiotic release and agar diffusion assays. The average fiber diameter was calculated based on three distinct SEM micrographs and a total of 90 measurements using an image analysis software (ImageJ, NIH, Bethesda) [10]. Electrospun fiber morphology was imaged at 15 keV under a scanning electron microscope (SEM, JSM-5310LV, JEOL, Tokyo, Japan) after mounting and sputter coating with Au-Pd.
In-vitro antibiotic release profile
The antibiotic-containing electrospun matrices were cut into 1 × 1 cm2 squares. The drug content was calculated as a function of matrices weight [16]. Each sample (n=4) was incubated in 20.0 mL of PBS at 37 °C. 1 mL aliquots of each sample were collected at days 1, 3, and 7. Equal amounts of fresh PBS were added back to the incubation media following collection of each sample to maintain the same volume [16]. High performance liquid chromatography (HPLC) equipped with a UV-Vis detector (Perkin-Elmer, Shelton, CT, USA) was used to determine the drug content using maximal absorption peaks for CIP and MET at 275 and 280 nm, respectively. CIP and MET standards were run on the HPLC at known concentrations. Linear calibration curves were used to calculate antibiotic concentrations in the aliquots. Antibiotic release (%) was then calculated based on the initial weight of the drug incorporated in the matrices [16]. Data are given as the mean value plus or minus the standard deviation (±SD) of the mean.
In-vitro biofilm model – Non-periodontitis related bacteria
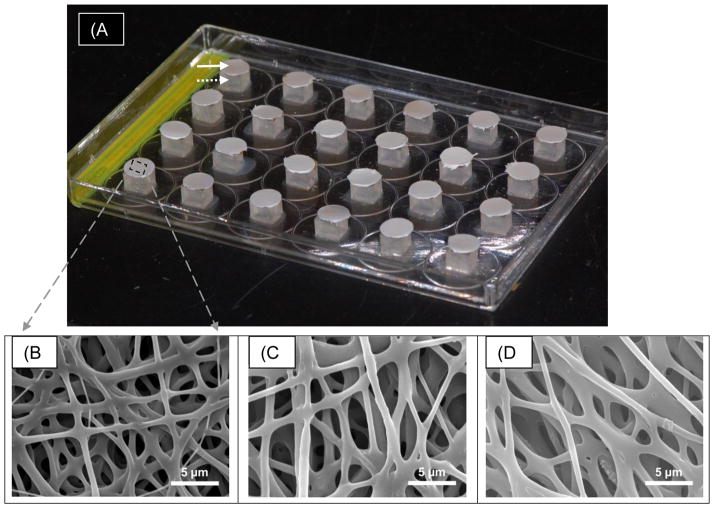
Dual-species biofilms were grown on the surface of all electrospun samples. Glass slides containing PDS matrices were mounted using wax on acrylic cubes attached to the lid of 24-well cell culture plates (Fig. 1a). Prior to biofilm formation, mounted samples were disinfected by immersion in 70% ethanol for 30 min and by exposure to ultra-violet light for 2 h. For biofilm formation, Lc and Ss were cultivated on Columbia Blood Agar (CBA) supplemented with 5% of sheep blood. Colony-forming units (CFU) were transferred from CBA plates to tubes containing TSB supplemented with sucrose and incubated at 37°C with 5% TSB supplemented with 5% sucrose was then added to each well of the culture plate. An aliquot of 500 μL of a multispecies suspension formed by aliquots of same optical density of the above mentioned strains [17] was added to each well and the plate was kept under constant agitation during 2 h at 37°C. Matrices were then transferred to another plate containing fresh artificial saliva [18] and kept at 37°C and 5% pCO2.
Fig. 1.
(a) Macrophotograph of the dual-species biofilm experimental set up on modified 24-wells tissue culture plates. Note the distinct PDS-based nanofibrous samples that were electrospun directly on cover borosilicate glass (solid arrow) slips attached to acrylic blocks (dotted arrow) using dental wax previously glued to the lid of the tissue culture plate. Representative SEM micrographs (b–d) of electrospun PDS matrices. (b) PDS control, (c) PDS + 25 wt.% MET, and (d) PDS + 25 wt.% CIP. Note the three-dimensional nanofibrous structure with an evident interconnected porous network.
After approximately 16 h, matrices were transferred to a new plate containing TSB supplemented with 5% sucrose and incubated at 37°C and 5% pCO2 for 45 min. They were then dip-washed 3 times in sterile saline (0.9% NaCl), immersed in fresh artificial saliva and incubated at 37°C, 5% pCO2. The same procedure was repeated 4 and 8 h later to mimic the feast and famine periods present in oral cavity. The matrices were then transferred back to the same saliva provided early in the morning. This protocol was repeated for the following 3 days.
Subsequent to biofilm formation, electrospun matrices (n=3/group) were aseptically removed from the lids and individually transferred to tubes containing 1 mL of sterile 0.9% NaCl. The biofilm suspension was vortexed for 30 seconds and sonicated for 30 seconds at 7W. Aliquots were inoculated on CBA plates for counts of total bacteria and Ss and Rogosa SL agar for counts of Lc using a spiral plater. Plates were incubated at 37°C for 48 h under 5% pCO2. Colony-forming units (CFU) were examined under stereomicroscope and the results expressed as CFU/mL.
After biofilm formation, electrospun matrices (n=2/group) were fixed with 4% paraformaldehyde for 30 min and rinsed once in sterile 1× PBS for 5 min. Samples were initially dehydrated in a graded ethanol series from 50% to 100%, dried using ethanol:HDMS solutions of 3:1, 1:1 and 1:3 and finally in 100% HMDS [19]. The samples were dried at RT overnight and stored in a vacuum desiccator. Samples were imaged at 15 keV under a SEM after mounting and sputter coating with Au-Pd [19].
Agar diffusion
The agar diffusion test was used to demonstrate the antimicrobial effect of antibiotic-containing electrospun matrices on periodontopathogens, namely Fn and Aa. Sterile 6 mm disk-shaped (n=4) samples of all experimental groups and the control were placed on blood agar plates containing bacterial lawns of Fn and Aa [19, 20]. 5 days after incubation the inhibition zones (in mm) were measured [19, 20].
Statistical analysis
Analysis of variance (ANOVA) was used to determine the effect of each tested condition on counts of viable cells on biofilms and inhibition zones. One-way ANOVA followed by Tukey’s multiple comparisons procedure were used to compare the groups for differences in bacterial counts and inhibition zones. Analyses were performed on the natural logarithm of the data to satisfy the ANOVA assumptions. Statistical significance was accepted at the p<0.05 level.
Results
Representative SEM micrographs of PDS and antibiotic-containing electrospun fibers are presented in Figure 1(B–D). A three-dimensional interconnected porous network was observed in all fabricated electrospun matrices. Electrospun fiber diameter for the PDS samples with or without the antimicrobial presented a submicron array with values ranging from 477 nm to 1560 nm. Pure PDS showed the highest fiber diameter mean (1158±402 nm) followed in a descending order by groups 5 wt.% MET (1108±383 nm), 25 wt.% MET (944±392 nm), 5 wt.% CIP (871±309 nm), and 25 wt.% CIP (765±288 nm).
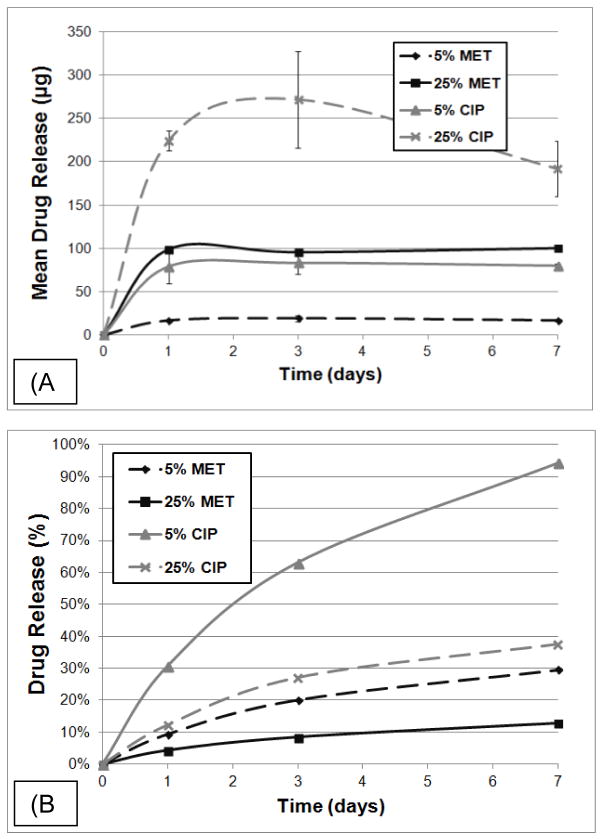
Antibiotic release profiles from each group of electrospun matrices containing either MET or CIP were examined using HPLC. Figure 2a presents the mean amount of drug released (in μg) at 1, 3, and 7 days. Groups containing higher amounts (i.e., 25 wt.%) of incorporated drugs released more over time while those with lower levels (i.e., 5 wt.%), the least. Moreover, Figure 2a shows that 5 wt.% CIP released (~ 75 μg) approximately three times more drug over the 7 days period when compared to its MET counterpart (~ 25 μg). It is interesting to note a similar pattern of drug release, in other words, when comparing 5 wt.% and 25 wt.% concentrations, both MET and CIP yielded approximately four (5 and 25 wt. MET) and five (5 and 25 wt.% CIP) times more drug. Figure 2b shows the cumulative drug release as a percentage of the total weight recorded prior to the study. The overall percentage of drug released by each group began to diverge after 72 h. After 7 day 5 wt.% CIP had a cumulative release of about 92%, as opposed to ~ 40%, ~ 30%, and 12% respectively for 25 wt.% CIP, 5 wt.% MET, and 25 wt.% MET.
Fig. 2.
(a) Mean drug release ± SD (in μg) and the (b) percentage of drug release from the antibiotic-containing PDS-based electrospun matrices based on initial sample weight.
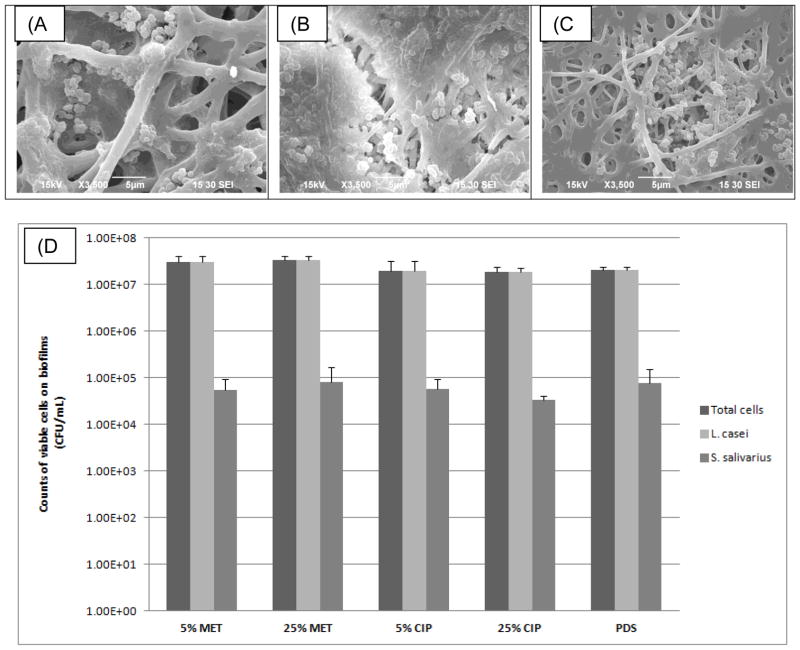
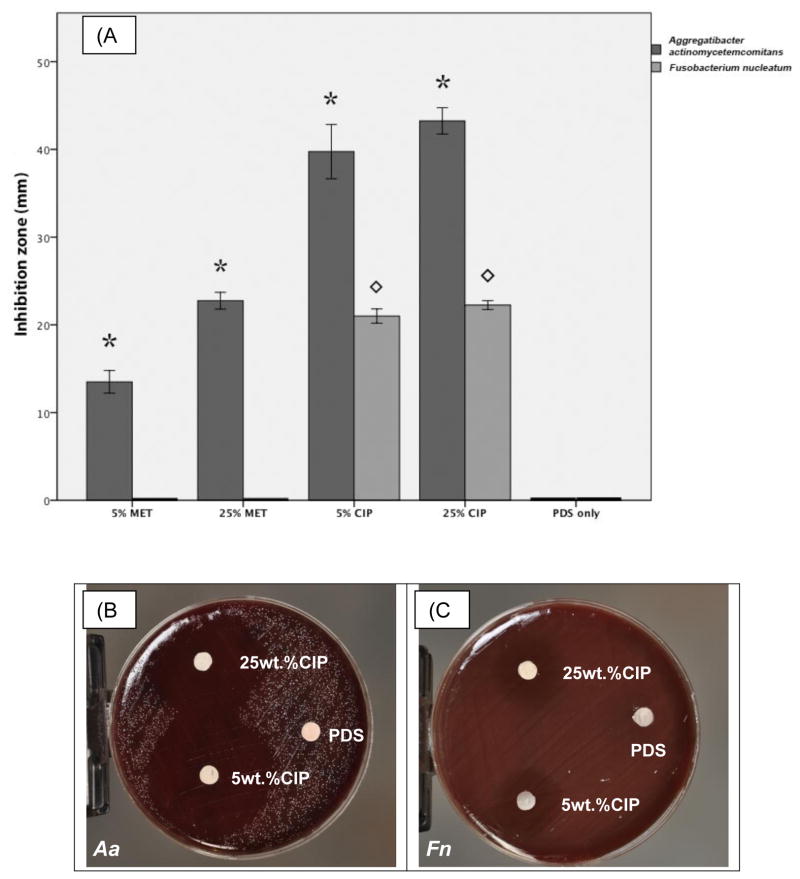
In respect to bacterial cell viability, no inhibitory effect of the tested drugs was detected on biofilm formation by the tested non-periodontitis related bacteria. SEM images showed the colonization of PDS matrices by the dual-species biofilm (Figure 3a–c). No significant differences in counts of Lc and Ss were found among the tested conditions (Figure 3d). Agar diffusion data revealed that CIP-containing matrices inhibited growth of both Fn and Aa. Figure 4 shows clearly a dose dependent inhibition for Fn (p= 0.04). MET-containing matrices had no effect on Fn growth, whereas dose dependent inhibition was detected for Aa (p< 0.0003). Overall, MET was less effective than CIP for growth inhibition of Aa.
Fig. 3.
Representative SEM micrographs of the electrospun PDS matrices following exposure to the dual-species biofilm. (a) PDS control, (b) PDS + 25% MET, and (c) PDS + 25% CIP. Note the presence of biofilm over the nanofibrous structure regardless of the material composition. (d) Mean counts of viable cells with error bars indicating SD (CFU/mL) on biofilms. No significant differences in counts of L. casei and S. salivarius were found among the tested conditions (p>0.05).
Fig. 4.
(a) Growth inhibition of Aa and Fn following 5 days exposure to electrospun matrices incorporated with 5 or 25 wt.% of MET or CIP. *represents a statistical difference of groups tested against Aa in comparison to pure PDS used as a negative control. designates a statistical difference of groups tested against Fn and the control. Data was generated from a one-way ANOVA followed by Tukey’s (p<0.05). (b–c) Representative macrophotographs showing growth inhibition of Aa and Fn, respectively.
Discussion
Normal flora of human oral cavity is comprised by approximately seven hundred species [21]. These different strains interact with the environment and each other by synergistic or antagonistic interactions [22], which modulate the metabolic function of the microbial community and are vital for the maintenance of the ecological microbial balance in that specific ecosystem. However, the prolonged and indiscriminate use of systemically used antimicrobials may disrupt this microbial balance by the selecting resistant strains [5]. Therefore, site-specific drug-delivery system for treatment of periodontal disease should not impair the growth of unrelated periodontal pathogenic bacteria. As expected, our data clearly suggested that under the concentrations used in the present study there was no detectable effect of the tested antimicrobials on counts of Lc and Ss (Figure 3).
In terms of interspecies interactions, there is evidence showing the importance between the natural interplay between oral bacteria and healthy environments. Koll-Klais and colleagues [7] showed that Lactobacilli spp. inhibited the growth of periodontal disease associated-microorganisms, such as Aa, Pg and Prevotella intermedia (Pi) [23]. Additionally, it has been shown that S. salivarius interferes with the colonization of hard tissues and epithelial cells by Aa [24–25] and with the colonization of surfaces by Pg [8]. The importance of commensal microbiota on oral health status has also been suggested by Suci & Young [26] that verified unaffected levels of S. sanguinis after continued use of ciprofloxacin prevents further establishment of a mature Aa biofilm. Maintaining the viability of these “antagonistic” bacteria using the tested drug-delivery system provides an additional effect to the mechanical treatment, since it could impair further hard- and soft- tissue recolonization by periodontal pathogens.
In a previous study carried out by Bottino and colleagues [19] antibacterial properties of the MET- and CIP-containing electrospun matrices against Pg and Ef were evaluated using both biofilm and agar diffusion assays. Here, CIP and MET were tested on the growth inhibition of two other bacteria involved in periodontal infections, Fn and Aa. The agar diffusion test has proved the efficacy of CIP-containing matrices towards bacteria used, placing it as a universal antimicrobial which could be used in eradication of both endodontic and periodontal infections.
Successful drug release from the electrospun matrices containing MET and CIP were observed through HPLC experiments, agreeing with previous results [19]. Considerable similarities in fiber diameter and morphology amongst the groups suggest these differences are not likely due to the structure of the matrices. More importantly, other factors including but not limited to degree of drug hydrophilicity, drug molecular weight, optimal pH for release, drug-matrix physicochemical interactions are known [16, 19] to play a role on kinetics of drug release and therefore deserve further investigation. A sustained drug release was seen for 5 wt.% MET, 5 wt.% CIP and 25 wt.% MET (Figure 2a) over 7 days. Although one might consider a week a relatively short period of time, current commercially available products (e.g., ELYZOL®, a metronidazole-containing gel) are normally applied over 7 day periods, normally two applications within 6 months of initial therapy [27]. Taken together, among the drug-containing matrices evaluated, it is safe to say, based on the HPLC data, that the amount of drugs released are well above the minimum inhibitory concentration (MIC) of the periodontopathogens (Fn – MET sensitive at MIC 0.2–0.4 μg, and Aa – sensitive to CIP at MIC below 1 μg) [28, 29].
Current adjunct treatments of periodontal disease commonly paired with scaling and root planning (SRP) lack discriminatory advantage against the culprit pathogens. A recent meta-analysis demonstrates the importance of adjunct treatment in dealing with periodontal disease [30]. An increasing reliance on adjunct treatments to prevent further infection necessitates comparisons to what is currently available. One group investigating periodontal disease and local tetracycline therapy, utilizing Actisite®, described its broad number of bacterial targets and the negative impact on some potentially beneficial bacteria. This is in addition to an increased selection for tetracycline resistant pathogenic bacteria detectable in as little as 2 weeks, such as Pg and Aa, which persisted after six months of treatment [31]. Another potential supplemental treatment utilizing 25% MET incorporated into a gel, Elyzol®, has been shown to eliminate some periodontal pathogens but was unable to remove Aa in a clinical setting [32, 33]. This could be attributable to its quick diffusion and inability to apply consistent local antibiotic treatment.
The structure and composition of our electrospun matrices provide a valuable addition to current adjunct treatments for periodontitis. Most importantly, the combination of antibiotics and the release by a stable polymer-based electrospun matrix allows for extended local treatment. Our system is derived from a biocompatible, FDA-approved biodegradable polymer (i.e., polydioxanone) commonly used in medical and dental applications as suture material. According to the literature [34], upon degradation, 50% of the suture strength is reduced after three weeks, and by six months the polymer is fully resorbed with low inflammatory response when compared to other polymers such as (poly(glycolic-co-lactic acid) and poly(glycolic acid)). Hence, this drug delivery system allows for a more targeted elimination of harmful bacteria over some broad spectrum antibiotic treatments available. Furthermore, this lowers the potential for antibiotic selection of dangerous bacteria based on resistance in the periodontal environment. We hypothesize that under this condition, periodontal pockets could be colonized by these antagonistic microorganisms and reduce the competitive advantage of periodontal pathogens. This outcome is desirable given that it could help promote tissue healing. It would also avoid commensal microorganisms of normal flora from indiscriminate elimination.
Conclusions
The presence of the tested antimicrobials did not impair the growth of periodontal beneficial bacteria; however it demonstrated an inhibitory effect on periodontopathogens. Future in vivo research using relevant animal models is needed to confirm the effectiveness of these drug delivery systems before its clinical use as a potential adjunct to SRP in the treatment of periodontal disease.
Acknowledgments
This work was supported in part by the Research Support Funds Grant (RSFG) at Indiana University Purdue University Indianapolis (IUPUI), by start-up funds from the IU School of Dentistry and the NIH-NIDCR (Grant # DE023552) (all to M.C.B.).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Walker CB, Karpinia K. Rationale for use of antibiotics in periodontics. J Periodontol. 2002;73:1188–1196. doi: 10.1902/jop.2002.73.10.1188. [DOI] [PubMed] [Google Scholar]
- 2.Xajigeorgiou C, Sakellari D, Slini T, Baka A, Konstantinidis A. Clinical and microbiological effects of different antimicrobials on generalized aggressive periodontitis. J Clin Periodontol. 2006;33:254–264. doi: 10.1111/j.1600-051X.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 3.Serrano C, Torres N, Valdivieso C, Castano C, Barrera M, Cabrales A. Antibiotic resistance of periodontal pathogens obtained from frequent antibiotics users. Acta Odontol Latinoam. 2009;22:99–104. [PubMed] [Google Scholar]
- 4.Ardila CM, Granada MI, Guzman IC. Antibiotic resistance of subgingival species in chronic periodontitis patients. J Periodontol Res. 2010;45:557–563. doi: 10.1111/j.1600-0765.2010.01274.x. [DOI] [PubMed] [Google Scholar]
- 5.Aminov RI. The role of antibiotics and antibiotic resistance in nature. Environm Microbiol. 2009;11:2970–2988. doi: 10.1111/j.1462-2920.2009.01972.x. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis. 2001;1:101–114. doi: 10.1016/S1473-3099(01)00066-4. [DOI] [PubMed] [Google Scholar]
- 7.Koll-Klais P, Mandar R, Leibur E, Marcotte H, Hammarstrom L, Mikelsaar M. Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol Immunol. 2005;20:354–361. doi: 10.1111/j.1399-302X.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 8.van Hoogmoed CG, Geertsema-doornbusch GI, Teughels W, Quirynen M, Busscher HJ, van der Mei HC. Reduction in periodontal pathogens adhesion by antagonistic strains. Oral Microbiol Immunol. 2008;23:43–48. doi: 10.1111/j.1399-302X.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 9.Bottino MC, Thomas V, Schmidt G, Vohra YK, Chu TM, Kowolik MJ, Janowski GM. Recent advances in the development of GTR/GBR membranes for periodontal regeneration--a materials perspective. Dent Mater. 2012;28:703–21. doi: 10.1016/j.dental.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Bottino MC, Thomas V, Janowski GM. A novel spatially designed and functionally graded electrospun membrane for periodontal regeneration. Acta Biomater. 2011;7:216–24. doi: 10.1016/j.actbio.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues RMJ, Goncalves C, Souto R, Feres-Filho EJ, Uzeda M, Colombo APV. Antibiotics resistance profile of the subgingival microbiota following systemic or local tetracycline therapy. J Clin Periodontol. 2004;31:420–427. doi: 10.1111/j.1600-051X.2004.00493.x. [DOI] [PubMed] [Google Scholar]
- 12.Killoy WJ. Local delivery of antimicrobials: a new era in the treatment of adult periodontitis. Compend Contin Educ Dent. 1999;20:13–18. [PubMed] [Google Scholar]
- 13.Killoy WJ. The clinical significance of local chemotherapies. J Clin Periodontol. 2002;29:22–29. [PubMed] [Google Scholar]
- 14.Rams TE, Slots J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol 2000. 1996;10:139–159. doi: 10.1111/j.1600-0757.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 15.Thomas V, Zhang X, Vohra YK. A biomimetic tubular scaffold with spatially designed nanofibers of protein/PDS bio-blends. Biotechnol Bioeng. 2009;104:1025–1033. doi: 10.1002/bit.22467. [DOI] [PubMed] [Google Scholar]
- 16.Cui W, Li X, Zhu X, Yu G, Zhou S, Weng J. Investigation of drug release and matrix degradation of electrospun poly(DL-lactide) fibers with paracetanol inoculation. Biomacromolecules. 2006;7;5:1623–1629. doi: 10.1021/bm060057z. [DOI] [PubMed] [Google Scholar]
- 17.Guggenheim B, Giertsen E, Schupbach P, Shapiro S. Validation of an in vitro biofilm model of supragingival plaque. J Dent Res. 2001;80:363–70. doi: 10.1177/00220345010800011201. [DOI] [PubMed] [Google Scholar]
- 18.Stookey GK, Stahlman DB. Enhanced fluoride uptake in enamel with a fluoride-impregnated prophylactic cup. J Dent Res. 1976;55:333–341. doi: 10.1177/00220345760550030801. [DOI] [PubMed] [Google Scholar]
- 19.Bottino MC, Kamocki K, Yassen GH, Platt JA, Vail MM, Ehrlich Y, Spolnik KJ, Gregory RL. Bioactive nanofibrous scaffolds for regenerative endodontics. J Dent Res. 2013;92;11:963–969. doi: 10.1177/0022034513505770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reise M, Wyrwa R, Müller U, Zylinski M, Völpel A, Schnabelrauch M, Berg A, Jandt KD, Watts DC, Sigusch BW. Release of metronidazole from electrospun poly(L-lactide-coD/L-lactide) fibers for local periodontitis treatment. Dent Mater. 2012;28;2:179–88. doi: 10.1016/j.dental.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2009;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan K, Sibley CD, Davidson CJ, Surette MG. Chemical interactions between organisms in microbial communities. Contrib Microbiol. 2009;16:1–17. doi: 10.1159/000219369. [DOI] [PubMed] [Google Scholar]
- 23.Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 24.Teughels W, Kinder Haake S, Sliepen I, Pauwels M, van Eldere J, Cassiman J, Quirynen M. Bacteria interfere with A. actinomycetemcomitans colonization. J Dent Res. 2007;86:611–617. doi: 10.1177/154405910708600706. [DOI] [PubMed] [Google Scholar]
- 25.Sliepen I, Hofkens J, van Essche M, Quirynen M, Teughels W. Aggregatibacter actinomycetemcomitans adhesion inhibited in a flow cell. Oral Microbiol Immunol. 2008;23:520–524. doi: 10.1111/j.1399-302X.2008.00456.x. [DOI] [PubMed] [Google Scholar]
- 26.Suci P, Young M. Selective killing of Aggregatibacter actinomycetemcomitans by ciprofloxacin during development of a dual species biofilm with Streptococcus sanguinis. Arch Oral Biol. 2011;56:1055–1063. doi: 10.1016/j.archoralbio.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Elyzol® Colgate. [Accessed 21 January 2014]; http://colgate-sensitive-pro-relief.colgateprofessional.ro/products/Colgate-Elyzol-25-Dental-Gel/faqs.
- 28.Chow AW, Patten V, Guze LB. Susceptibility of anaerobic bacteria to metronidazole: relative resistance of non-spore-forming gram-positive baccilli. J Infect Dis. 1975;131;2:182–5. doi: 10.1093/infdis/131.2.182. [DOI] [PubMed] [Google Scholar]
- 29.Shaddox LM, Walker C. Microbial testing in periodontics: value, limitations and future directions. Periodontol 2000. 2009;50:25–38. doi: 10.1111/j.1600-0757.2008.00285.x. [DOI] [PubMed] [Google Scholar]
- 30.Kalsi R, Vandana K, Prakash S. Effect of local drug delivery in chronic periodontitis patients: A meta-analysis. J Indian Soc Periodontol. 2011;15;4:304–309. doi: 10.4103/0972-124X.92559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodrigues R, Gonçalves C, Souto R, Feres-Filho E, Uzeda M, Colombo A. Antibiotic resistance profile of the subgingival microbiota following systemic or local tetracycline therapy. J Clin Periodontol. 2004;31;6:420–427. doi: 10.1111/j.1600-051X.2004.00493.x. [DOI] [PubMed] [Google Scholar]
- 32.Riep B, Purucker P, Bernimoulin J. Repeated local metronidazole-therapy as adjunct to scaling and root planing in maintenance patients. J Clin Periodontol. 1999;26;11:710–715. doi: 10.1034/j.1600-051x.1999.t01-2-261101.x. [DOI] [PubMed] [Google Scholar]
- 33.Salvi G, Mombelli A, Mayfield L, Rutar A, Suvan J, Garrett S, Lang N. Local antimicrobial therapy after initial periodontal treatment. Journal Of Clinical Periodontol. 2002;29;6:540–550. doi: 10.1034/j.1600-051x.2002.290611.x. [DOI] [PubMed] [Google Scholar]
- 34.Boland ED, Coleman BD, Barnes CP, Simpson DG, Wnek GE, Bowlin GL. Electrospinning polydioxanone for biomedical applications. Acta Biomater. 2005;1;1:115–23. doi: 10.1016/j.actbio.2004.09.003. [DOI] [PubMed] [Google Scholar]