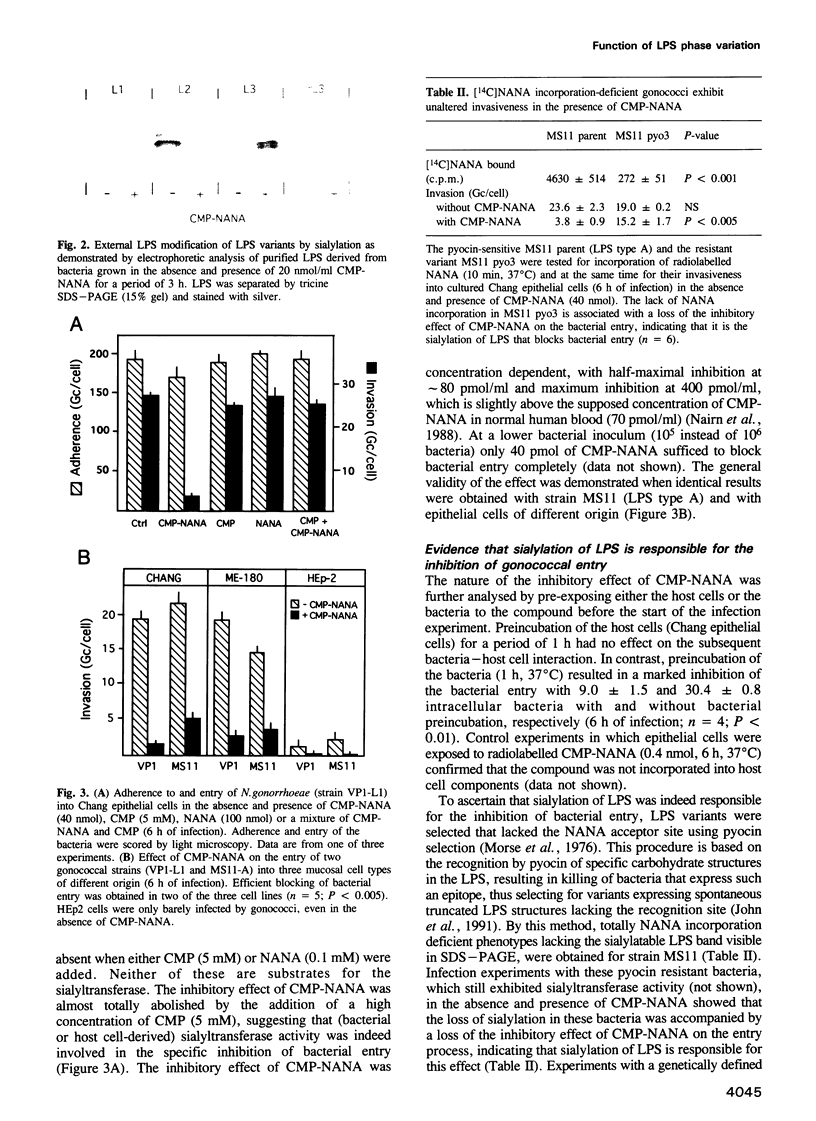
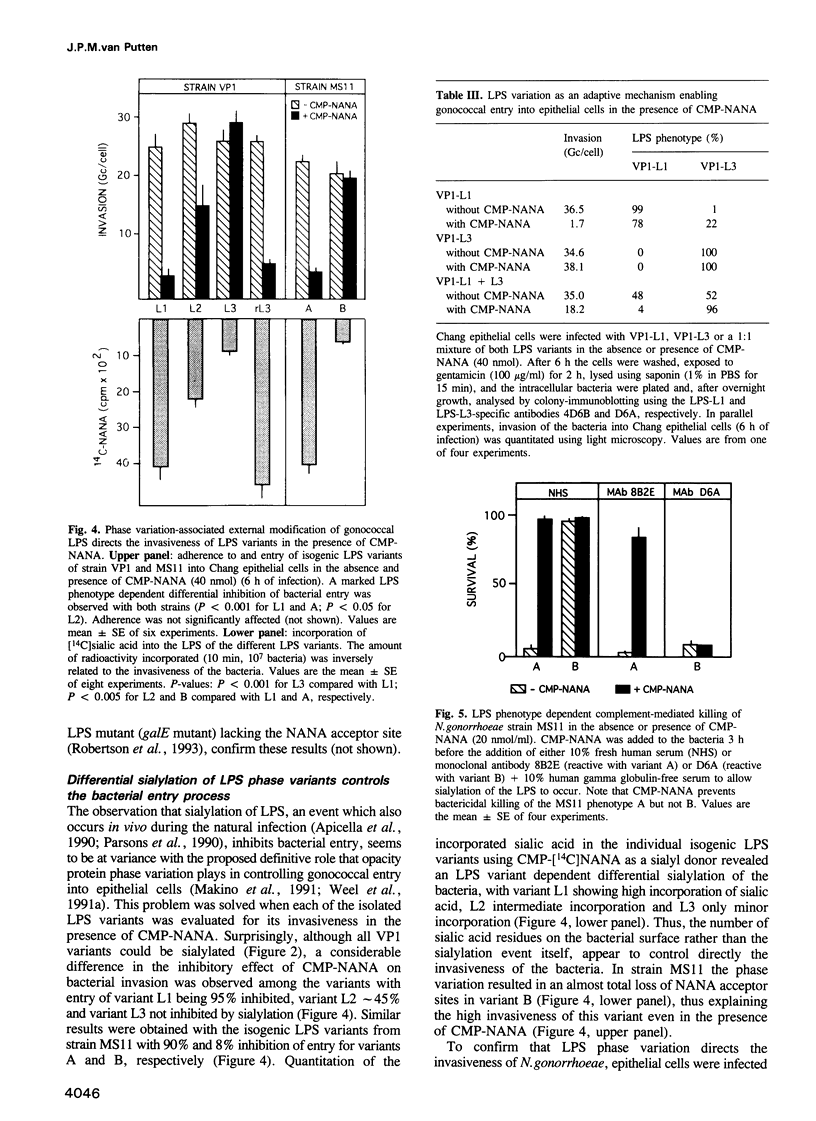
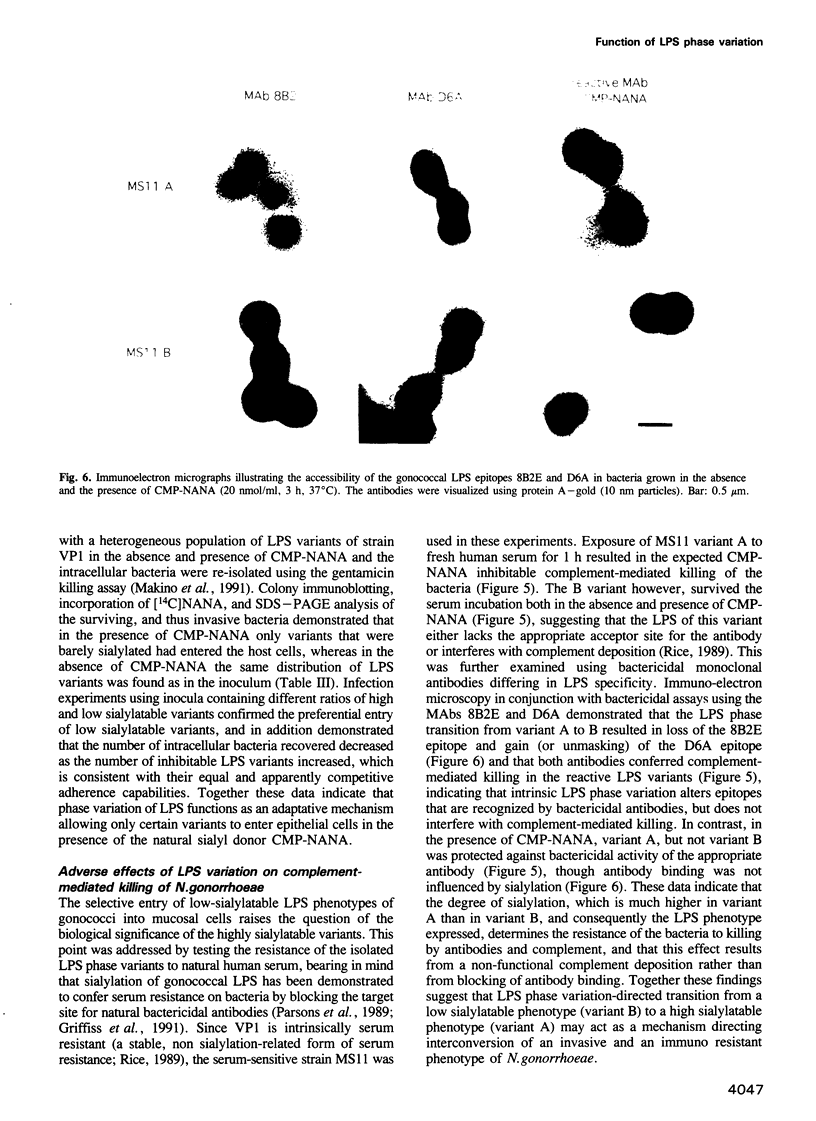
Abstract
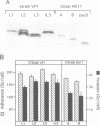
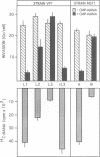
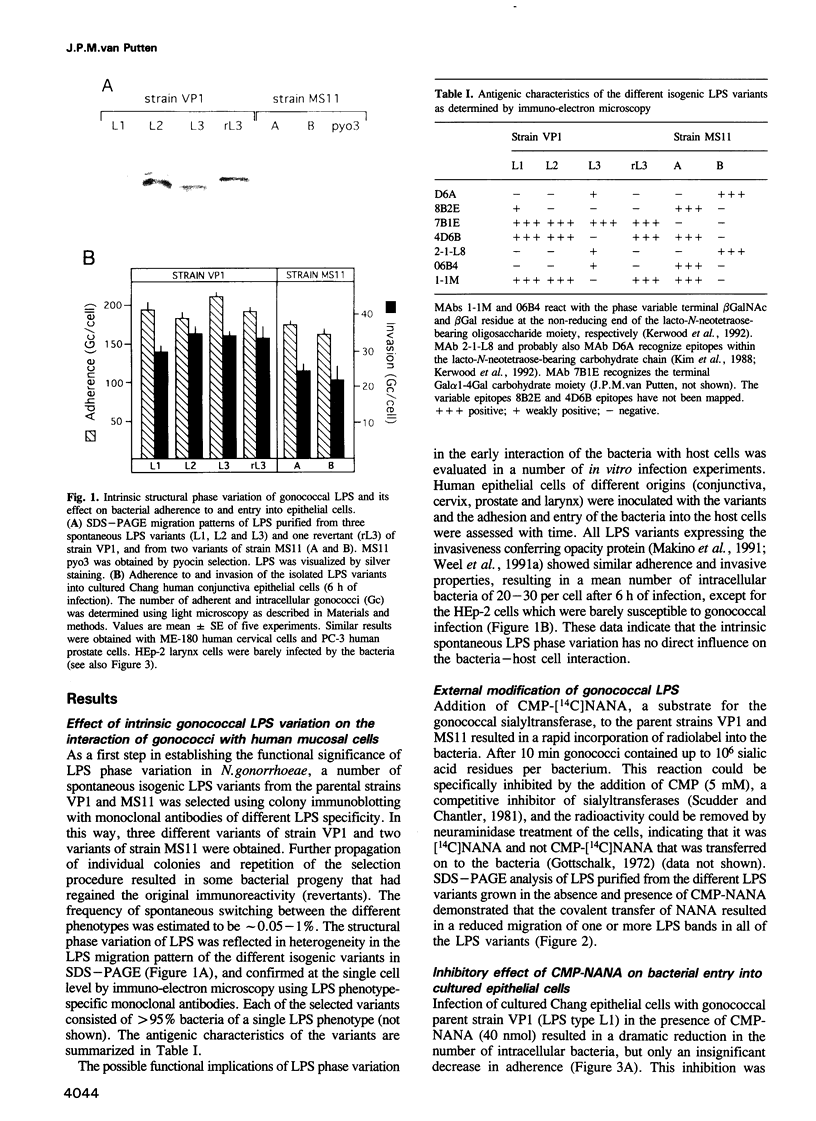
Phase variation of Neisseria gonorrhoeae lipopolysaccharide (LPS) controls both bacterial entry into human mucosal cells, and bacterial susceptibility to killing by antibodies and complement. The basis for this function is a differential sialylation of the variable oligosaccharide moiety of the LPS. LPS variants that incorporate low amounts of sialic acid enter human mucosal epithelial cells very efficiently, but are susceptible to complement-mediated killing. Phase transition to a highly sialylated LPS phenotype results in equally adhesive but entry deficient bacteria which, however, resist killing by antibodies and complement because of dysfunctional complement activation. Phase variation of N. gonorrhoeae LPS thus functions as an adaptive mechanism enabling bacterial translocation across the mucosal barrier, and, at a later stage of infection, escape from the host immune defence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Mandrell R. E., Shero M., Wilson M. E., Griffiss J. M., Brooks G. F., Lammel C., Breen J. F., Rice P. A. Modification by sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J Infect Dis. 1990 Aug;162(2):506–512. doi: 10.1093/infdis/162.2.506. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Shero M., Jarvis G. A., Griffiss J. M., Mandrell R. E., Schneider H. Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide. Infect Immun. 1987 Aug;55(8):1755–1761. doi: 10.1128/iai.55.8.1755-1761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Sparling P. F. Mucosal infection with Neisseria gonorrhoeae. Bacterial adaptation and mucosal defenses. J Clin Invest. 1992 Jun;89(6):1699–1705. doi: 10.1172/JCI115770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarco de Hormaeche R., Jessop H., Senior K. Gonococcal variants selected by growth in vivo or in vitro have antigenically different LPS. Microb Pathog. 1988 Apr;4(4):289–297. doi: 10.1016/0882-4010(88)90089-7. [DOI] [PubMed] [Google Scholar]
- Dudas K. C., Apicella M. A. Selection and immunochemical analysis of lipooligosaccharide mutants of Neisseria gonorrhoeae. Infect Immun. 1988 Feb;56(2):499–504. doi: 10.1128/iai.56.2.499-504.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins C., Carbonetti N. H., Varela V. A., Stirewalt D., Klapper D. G., Sparling P. F. Antibodies to N-terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated. Mol Microbiol. 1992 Sep;6(18):2617–2628. doi: 10.1111/j.1365-2958.1992.tb01439.x. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Jarvis G. A., O'Brien J. P., Eads M. M., Schneider H. Lysis of Neisseria gonorrhoeae initiated by binding of normal human IgM to a hexosamine-containing lipooligosaccharide epitope(s) is augmented by strain-specific, properdin-binding-dependent alternative complement pathway activation. J Immunol. 1991 Jul 1;147(1):298–305. [PubMed] [Google Scholar]
- Griffiss J. M., O'Brien J. P., Yamasaki R., Williams G. D., Rice P. A., Schneider H. Physical heterogeneity of neisserial lipooligosaccharides reflects oligosaccharides that differ in apparent molecular weight, chemical composition, and antigenic expression. Infect Immun. 1987 Aug;55(8):1792–1800. doi: 10.1128/iai.55.8.1792-1800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines K. A., Reibman J., Tang X. Y., Blake M., Weissmann G. Effects of protein I of Neisseria gonorrhoeae on neutrophil activation: generation of diacylglycerol from phosphatidylcholine via a specific phospholipase C is associated with exocytosis. J Cell Biol. 1991 Aug;114(3):433–442. doi: 10.1083/jcb.114.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- John C. M., Griffiss J. M., Apicella M. A., Mandrell R. E., Gibson B. W. The structural basis for pyocin resistance in Neisseria gonorrhoeae lipooligosaccharides. J Biol Chem. 1991 Oct 15;266(29):19303–19311. [PubMed] [Google Scholar]
- Jones D. M., Borrow R., Fox A. J., Gray S., Cartwright K. A., Poolman J. T. The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb Pathog. 1992 Sep;13(3):219–224. doi: 10.1016/0882-4010(92)90022-g. [DOI] [PubMed] [Google Scholar]
- Judd R. C., Shafer W. M. Topographical alterations in proteins I of Neisseria gonorrhoeae correlated with lipooligosaccharide variation. Mol Microbiol. 1989 May;3(5):637–643. doi: 10.1111/j.1365-2958.1989.tb00211.x. [DOI] [PubMed] [Google Scholar]
- Kerwood D. E., Schneider H., Yamasaki R. Structural analysis of lipooligosaccharide produced by Neisseria gonorrhoeae, strain MS11mk (variant A): a precursor for a gonococcal lipooligosaccharide associated with virulence. Biochemistry. 1992 Dec 29;31(51):12760–12768. doi: 10.1021/bi00166a008. [DOI] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Hu Z., Westerink M. A., Poolman J. T., Griffiss J. M. Electromorphic characterization and description of conserved epitopes of the lipooligosaccharides of group A Neisseria meningitidis. Infect Immun. 1988 Oct;56(10):2631–2638. doi: 10.1128/iai.56.10.2631-2638.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Zhou D., Mandrell R. E., Griffiss J. M. Effect of exogenous sialylation of the lipooligosaccharide of Neisseria gonorrhoeae on opsonophagocytosis. Infect Immun. 1992 Oct;60(10):4439–4442. doi: 10.1128/iai.60.10.4439-4442.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupsch E. M., Knepper B., Kuroki T., Heuer I., Meyer T. F. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 1993 Feb;12(2):641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford P. R., Moxon E. R. Growth of Haemophilus influenzae type b in continuous culture: effect of dilution rate on outer-membrane protein and lipopolysaccharide expression. FEMS Microbiol Lett. 1992 May 15;72(1):43–47. doi: 10.1016/0378-1097(92)90487-9. [DOI] [PubMed] [Google Scholar]
- Lesse A. J., Campagnari A. A., Bittner W. E., Apicella M. A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990 Jan 24;126(1):109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Lynch E. C., Blake M. S., Gotschlich E. C., Mauro A. Studies of Porins: Spontaneously Transferred from Whole Cells and Reconstituted from Purified Proteins of Neisseria gonorrhoeae and Neisseria meningitidis. Biophys J. 1984 Jan;45(1):104–107. doi: 10.1016/S0006-3495(84)84127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., van Putten J. P., Meyer T. F. Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. EMBO J. 1991 Jun;10(6):1307–1315. doi: 10.1002/j.1460-2075.1991.tb07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E. Further antigenic similarities of Neisseria gonorrhoeae lipooligosaccharides and human glycosphingolipids. Infect Immun. 1992 Jul;60(7):3017–3020. doi: 10.1128/iai.60.7.3017-3020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Kim J. J., John C. M., Gibson B. W., Sugai J. V., Apicella M. A., Griffiss J. M., Yamasaki R. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol. 1991 May;173(9):2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Lesse A. J., Sugai J. V., Shero M., Griffiss J. M., Cole J. A., Parsons N. J., Smith H., Morse S. A., Apicella M. A. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J Exp Med. 1990 May 1;171(5):1649–1664. doi: 10.1084/jem.171.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., McLaughlin R., Aba Kwaik Y., Lesse A., Yamasaki R., Gibson B., Spinola S. M., Apicella M. A. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialylated. Infect Immun. 1992 Apr;60(4):1322–1328. doi: 10.1128/iai.60.4.1322-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Mertsola J., Cope L. D., Sáez-Llorens X., Ramilo O., Kennedy W., McCracken G. H., Jr, Hansen E. J. In vivo and in vitro expression of Haemophilus influenzae type b lipooligosaccharide epitopes. J Infect Dis. 1991 Sep;164(3):555–563. doi: 10.1093/infdis/164.3.555. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Gibbs C. P., Haas R. Variation and control of protein expression in Neisseria. Annu Rev Microbiol. 1990;44:451–477. doi: 10.1146/annurev.mi.44.100190.002315. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Mintz C. S., Sarafian S. K., Bartenstein L., Bertram M., Apicella M. A. Effect of dilution rate on lipopolysaccharide and serum resistance of Neisseria gonorrhoeae grown in continuous culture. Infect Immun. 1983 Jul;41(1):74–82. doi: 10.1128/iai.41.1.74-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Vaughan P., Johnson D., Iglewski B. H. Inhibition of Neisseria gonorrhoeae by a bacteriocin from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Aug;10(2):354–362. doi: 10.1128/aac.10.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn C. A., Cole J. A., Patel P. V., Parsons N. J., Fox J. E., Smith H. Cytidine 5'-monophospho-N-acetylneuraminic acid or a related compound is the low Mr factor from human red blood cells which induces gonococcal resistance to killing by human serum. J Gen Microbiol. 1988 Dec;134(12):3295–3306. doi: 10.1099/00221287-134-12-3295. [DOI] [PubMed] [Google Scholar]
- Norrod E. P., Burnham J. S., Williams R. P., Ding M. J. Induced changes in the surface of Neisseria gonorrhoeae. Can J Microbiol. 1983 May;29(5):584–592. doi: 10.1139/m83-091. [DOI] [PubMed] [Google Scholar]
- Parsons N. J., Andrade J. R., Patel P. V., Cole J. A., Smith H. Sialylation of lipopolysaccharide and loss of absorption of bactericidal antibody during conversion of gonococci to serum resistance by cytidine 5'-monophospho-N-acetyl neuraminic acid. Microb Pathog. 1989 Jul;7(1):63–72. doi: 10.1016/0882-4010(89)90112-5. [DOI] [PubMed] [Google Scholar]
- Ray A., Redhead K., Selkirk S., Poole S. Variability in LPS composition, antigenicity and reactogenicity of phase variants of Bordetella pertussis. FEMS Microbiol Lett. 1991 Apr 15;63(2-3):211–217. doi: 10.1016/0378-1097(91)90088-r. [DOI] [PubMed] [Google Scholar]
- Rest R. F., Frangipane J. V. Growth of Neisseria gonorrhoeae in CMP-N-acetylneuraminic acid inhibits nonopsonic (opacity-associated outer membrane protein-mediated) interactions with human neutrophils. Infect Immun. 1992 Mar;60(3):989–997. doi: 10.1128/iai.60.3.989-997.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A. Molecular basis for serum resistance in Neisseria gonorrhoeae. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S112–S117. doi: 10.1128/cmr.2.suppl.s112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. D., Frosch M., van Putten J. P. The role of galE in the biosynthesis and function of gonococcal lipopolysaccharide. Mol Microbiol. 1993 May;8(5):891–901. doi: 10.1111/j.1365-2958.1993.tb01635.x. [DOI] [PubMed] [Google Scholar]
- Robertson B. D., Meyer T. F. Genetic variation in pathogenic bacteria. Trends Genet. 1992 Dec;8(12):422–427. doi: 10.1016/0168-9525(92)90325-x. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Boslego J. W., Hitchcock P. J., Zahos K. M., Apicella M. A. Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men. J Exp Med. 1991 Dec 1;174(6):1601–1605. doi: 10.1084/jem.174.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Hammack C. A., Apicella M. A., Griffiss J. M. Instability of expression of lipooligosaccharides and their epitopes in Neisseria gonorrhoeae. Infect Immun. 1988 Apr;56(4):942–946. doi: 10.1128/iai.56.4.942-946.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder P. R., Chantler E. N. Glycosyltransferases of the human cervical epithelium. II. Characterization of a CMP-N-acetylneuraminate: galactosyl-glycoprotein sialyltransferase. Biochim Biophys Acta. 1981 Jul 24;660(1):136–141. doi: 10.1016/0005-2744(81)90118-2. [DOI] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Barrera O., Sola J., Boslego J. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J Exp Med. 1988 Dec 1;168(6):2121–2129. doi: 10.1084/jem.168.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Boykins R., Frasch C. E. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983 Aug;155(2):498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Virji M., Heckels J. E. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leucocytes. J Gen Microbiol. 1986 Feb;132(2):503–512. doi: 10.1099/00221287-132-2-503. [DOI] [PubMed] [Google Scholar]
- Weel J. F., Hopman C. T., van Putten J. P. Bacterial entry and intracellular processing of Neisseria gonorrhoeae in epithelial cells: immunomorphological evidence for alterations in the major outer membrane protein P.IB. J Exp Med. 1991 Sep 1;174(3):705–715. doi: 10.1084/jem.174.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weel J. F., Hopman C. T., van Putten J. P. In situ expression and localization of Neisseria gonorrhoeae opacity proteins in infected epithelial cells: apparent role of Opa proteins in cellular invasion. J Exp Med. 1991 Jun 1;173(6):1395–1405. doi: 10.1084/jem.173.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weel J. F., Hopman C. T., van Putten J. P. Stable expression of lipooligosaccharide antigens during attachment, internalization, and intracellular processing of Neisseria gonorrhoeae in infected epithelial cells. Infect Immun. 1989 Nov;57(11):3395–3402. doi: 10.1128/iai.57.11.3395-3402.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weel J. F., van Putten J. P. Fate of the major outer membrane protein P.IA in early and late events of gonococcal infection of epithelial cells. Res Microbiol. 1991 Nov-Dec;142(9):985–993. doi: 10.1016/0923-2508(91)90009-y. [DOI] [PubMed] [Google Scholar]
- Weiser J. N., Love J. M., Moxon E. R. The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell. 1989 Nov 17;59(4):657–665. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- Weiser J. N., Williams A., Moxon E. R. Phase-variable lipopolysaccharide structures enhance the invasive capacity of Haemophilus influenzae. Infect Immun. 1990 Oct;58(10):3455–3457. doi: 10.1128/iai.58.10.3455-3457.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzler L. M., Barry K., Blake M. S., Gotschlich E. C. Gonococcal lipooligosaccharide sialylation prevents complement-dependent killing by immune sera. Infect Immun. 1992 Jan;60(1):39–43. doi: 10.1128/iai.60.1.39-43.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Putten J. P., Hopman C. T., Weel J. F. The use of immunogold-silver staining to study antigen variation and bacterial entry into eukaryotic cells by conventional light microscopy. J Med Microbiol. 1990 Sep;33(1):35–41. doi: 10.1099/00222615-33-1-35. [DOI] [PubMed] [Google Scholar]