Abstract
Objective
To investigate a new wide bone-anchored hearing implant considering initial stability, stability over time, implant loss, and skin reaction.
Study Design
Consecutive, prospective case series.
Setting
Tertiary referral center.
Patients
Twenty adult patients were enrolled. All operations were 1-stage, single-incision technique with subcutaneous reduction.
Intervention(s)
Measurement of implant stability.
Main Outcome Measure(s)
Implant stability quotient (ISQ) values were recorded using resonance frequency analysis at the time of implantation and at 10 days, 6 weeks, 6 months, and 1 year after surgery. Skin and soft tissue reactions according to Holgers grading system.
Results
Implant stability quotient measurements revealed a significant increase in ISQ during the first 10 days after operation, and the ISQ values continued to rise throughout the 1-year observation period. No implants were lost. Skin and soft tissue reactions were rare and minor, as no reaction was seen in 93% of the follow-up examinations and no grade 4 reactions occurred.
Conclusion
The new wide implant showed good stability at surgery. Osseointegration was fast, and implant stability increased throughout the 1-year observation period. No implants were lost. Skin and soft tissue reactions were rare and minor.
Key Words: Bone-anchored hearing system, Resonance frequency analysis, Implant, Implant stability quotient, Osseointegration, Skin reaction
Bone-anchored hearing systems (BAHS) were introduced in 1977 by Tjellström and colleagues (1) and have now been used clinically for more than 30 years. More than 100,000 patients have been fitted with BAHS worldwide. Generally, the outcomes are good, and several studies have shown improved audiologic and quality-of-life outcomes (for a summary, see, e.g., 2).
The principle of the BAHS is that sound vibrations are led directly to the inner ear via the mastoid bone, bypassing the middle ear. This is achieved via an osseointegrated implant and a skin penetrating abutment. Results from large populations have been published recently, including a systematic review of 20 articles on 2,310 implants (3) and a retrospective study of more than 1,000 implants (4). Both studies report high success rates and a majority of complications as typically minor in nature. The most commonly reported complications are related to the skin and soft tissue surrounding the abutment, such as inflammation and abutment site infection and soft tissue overgrowth. The reported overall implant survival rate range is 82.6% to 98.4% (3), with higher failure rates in children (3,4) and irradiated patients (5).
Wider diameter implants have recently been introduced by both manufacturers of bone-anchored hearing systems (Oticon Medical AB, Askim, Sweden and Cochlear BAS, Mölnlycke, Sweden). Whereas the previous generation implants were 3.75 mm in diameter, the current implants are 4.5 mm. A wider diameter implant provides larger initial surface to bone contact, leading to higher initial stability (6). This opens the possibility to load the implant with the hearing aid at an early time point in patients with normal bone quality without impairing osseointegration and implant survival. The broader abutment also allows a longer abutment to be used (12 mm), which is important when new surgical techniques without subcutaneous reduction are performed (7).
This case series prospectively investigated the clinical outcomes of the 4.5-mm wide Ponto implant from Oticon Medical. The focus has been on implant stability and survival, as well as skin and soft tissue reactions.
MATERIALS AND METHODS
The study is a consecutive, prospective case series of patients operated at our tertiary referral center (University Hospital). The patients were operated from November 15, 2011, to September 28, 2012, and the last data were collected on September 30, 2013.
To be included in the study, the patients had to be at least 18 years and eligible for bone conduction implant surgery. No exclusion criteria were used. Twenty patients were enrolled. The study was reviewed by the regional ethical committee.
Procedures
All operations were performed under local anesthesia, and all patients were operated by the same surgeon (first author). The single-incision technique with subcutaneous skin reduction was applied in all cases (8,9).
Skin incision was approximately 4 cm long and to the periosteum. After the periosteum was removed, a hole was drilled and widened with a 3.8-mm diameter countersink. The implant was placed single stage with application of 50 Ncm torque. After the implant was placed, subcutaneous tissue was removed, and the incision was sutured. A separate hole was made with a biopsy punch, allowing the abutment to penetrate the skin (Fig. 1). Finally, the implant stability quotient (ISQ) was measured (see below) (Fig. 2).
FIG. 1.
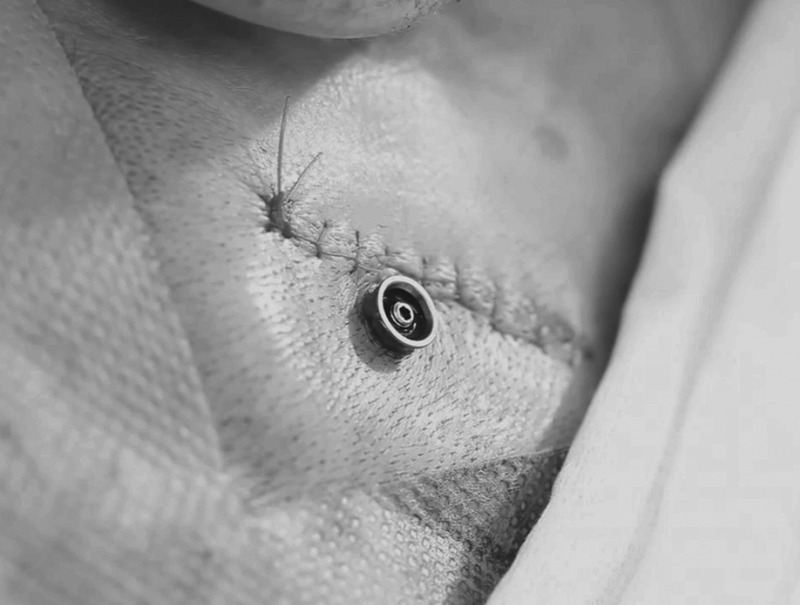
Operation field at end of the single-incision technique with subcutaneous skin reduction.
FIG. 2.
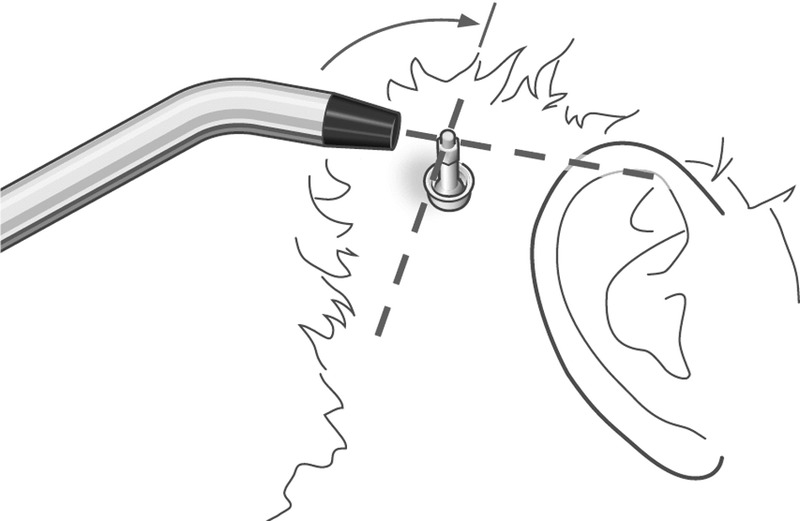
Implant stability quotient (ISQ) measurement. The SmartPeg is excited by a magnetic pulse from the measurement probe. The resonance frequency, which is the measure of implant stability, is calculated from the response signal.
Implant
The implant used was a Ponto Wide implant (diameter, 4.5 mm; length, 4 mm). The implant has a greater diameter (4.5 mm versus 3.75 mm) and a different cutting geometry and threading when compared with the previous generation model. Ponto abutments of lengths 6, 9, and 12 mm were used, depending on skin thickness. The implant is equipped with a traditional Brånemark type machined titanium implant surface.
Postoperative Care and Fitting
At the end of surgery, a healing cap was fixed to the abutment and gauze imbibed with antibiotic-steroid ointment circulated around the abutment, under the cap. A pressure head dressing was applied overnight to prevent hematoma. The healing cap and gauze were removed after approximately 10 days. The sound processor was fitted from 6 weeks after surgery.
Prospective Follow-up Examinations
Patients were reevaluated in the clinic after 10 days, 6 weeks, 6 months, and 12 months.
Soft tissue reactions around the abutment were classified according to Holgers grading system (10).
Osstell ISQ (Osstell, Göteborg, Sweden) is a portable, handheld instrument that involves the use of the noninvasive technique, resonance frequency analysis, for measuring stability of the bone-implant complex. The system includes the use of a SmartPeg attached to the abutment by means of an integrated screw. The SmartPeg is excited by a magnetic pulse from the measurement probe on the handheld instrument (Fig. 2). The resonance frequency, which is the measure of implant stability, is calculated from the response signal. Results are displayed as the implant stability quotient (ISQ), which is scaled from 1 to 100. The greater the number, the greater the stability. The Osstell ISQ instrument has an accuracy of ±2 ISQ.
Two ISQ measurements were performed and recorded at every visit. A Smartpeg type 55 (Osstell, Göteborg, Sweden) was applied on the internal screw of the abutment, and ISQ measures were obtained along the anteroposterior and superoinferior axes (Fig. 2). The mean of the 2 measurements of the ISQ value was used for the analysis. The same Smartpeg was used on the individual patient throughout the study to minimize the measurement variability.
Statistics
For comparison over time, the Wilcoxon signed rank test was used for continuous variables. A significance level of 0.05 was adopted.
RESULTS
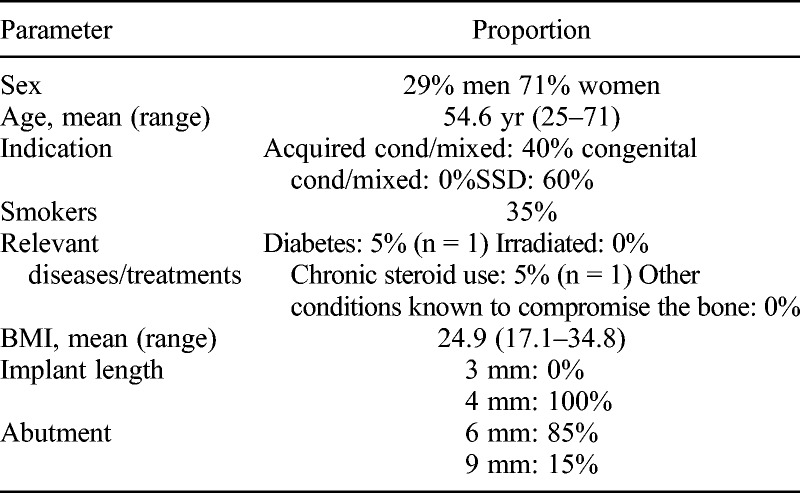
All operations were without complications. Patient characteristics are reported in Table 1. The most common reason for implantation was single-sided deafness (SSD, 12 patients), the remaining 8 had conductive or mixed hearing loss. One patient had Crohn’s disease and received per oral steroid treatment. All patients received a 4-mm-long implant. Three patients received a 9-mm abutment at surgery because of the thick subcutaneous tissue. Body mass index of these 3 patients was 31.0, 32.1, and 27.7, respectively. The remaining 17 patients received a 6-mm abutment. One patient (BMI, 28.7) was shifted to a 9-mm abutment at the 6-month follow-up and again from 9 to 12 mm at the 12-month follow-up visit because of contact between the processor and the skin caused by angling of the implant.
TABLE 1.
Baseline patient characteristics and implants used (n = 20)
The number of patients attending each visit, together with the average time after surgery is summarized in Table 2. The sound processor fitting was done in average, 7.2 weeks (range, 5.7–9.1 weeks) after operation (n = 19). One patient attended only 1 follow-up visit. This patient was excluded from stability analysis but otherwise included in the data analysis. (This patient was loaded 30 weeks after operation for reasons not related to the bone anchored implant. Including this patient, the average loading time for the full group (n = 20) was 8.5 wk).Three patients missed 1 follow-up visit. For stability data (ISQ), a last-observation-carried-forward was used for missing data.
TABLE 2.
Overview of follow-up visits

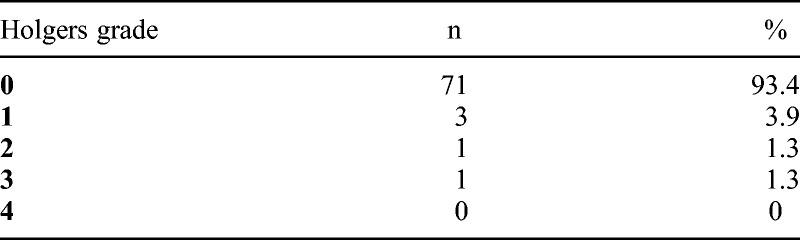
The skin reactions according to Holgers grading system are summarized in Table 3. Two adverse skin reactions (Holgers ≥2) were observed at a total of 76 follow up visits, corresponding to 2.6%. In 93.4% of the visits, there was no reaction (Holgers grade 0). The most severe skin reaction was the patient with Crohn’s disease, who had infection and formation of granulation tissue (Holgers grade 3) around the abutment at 1-year follow-up. The complication was treated successfully with lapis 10% and antibiotic ointment. No case of skin overgrowth of the abutment occurred and no additional surgery was performed.
TABLE 3.
Distribution of skin reactions (Holgers grading system) at follow-up examinations
Mean intraindividual (2 measurements; anteroposterior and superoinferior axes) and mean interindividual implant stability quotient (ISQ) per visit are shown in Figure 3, revealing a significant increase in ISQ during the first 10 days after operation. After loading of the implants (Visit 2), there was an increase in ISQ at both 6 and 12 months (p = 0.0012 and p = 0.0024, respectively). Two patients were excluded from Figure 3 (the patient with only 1 follow-up visit and the patient with change of abutment).
FIG. 3.
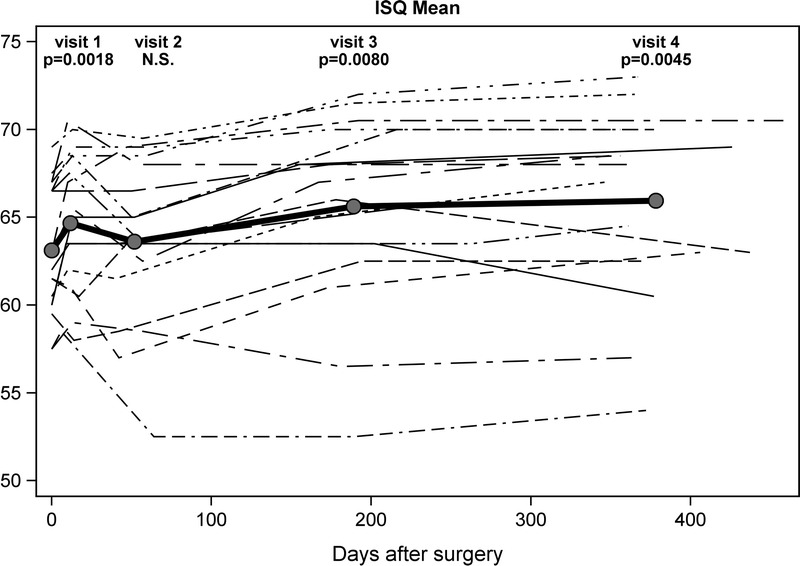
Mean intraindividual and mean interindividual (bold) implant stability quotient (ISQ) after surgery (n = 18).
No implant was lost.
DISCUSSION
The present study was undertaken to monitor complications and make the first tests of osseointegration on a new wide implant. These first data on the implant showed that the stability at surgery was good, that osseointegration was fast, and that implant stability increased throughout the first year after surgery. Soft tissue reactions were rare and minor, and no implants were lost.
The stability of an implant immediately after surgical placement is defined as primary implant stability. Primary implant stability is affected by several factors, including bone quality, bone quantity, implant geometry, and the relationship between the pilot hole/tapped channel and the implant diameter (11). The Ponto Wide implant has a new cutting geometry (OptiGrip design), which allows a reduction of the diameter of the drilled hole (3.8 mm versus 4.0 mm for the other available wide implant). The ISQ measurements are also heavily affected by the length of the abutment. For instance, the 2 patients with the clearly lowest ISQ values in Figure 3 both use 9 mm long abutments.
A wide implant (diameter, 4.5 mm) gives better initial stability in rabbits compared with the traditional implant (diameter, 3.75 mm) (12). The larger radius of the implant provides an increase of the surface area in contact with bone, and this improves stability (13). The Ponto Wide implant with a diameter of 4.5 mm has thus been designed to enhance stability at implantation and over time. It is difficult to compare the absolute ISQ-measurements between studies because of individual differences in bone quality and thickness and also the use of different lengths of implants and abutments. In the present study, we registered high ISQ values at the time of implantation, comparable with previous observations on wide implants (6,14).
A postimplant decrease in ISQ after 10 days or later has been demonstrated in other studies on temporal bone implantation (6), as well as maxilla and mandible implantation (e.g., 15,16). As noted, this does not occur with the wide implant used in the present patient series. This is corroborated by 3 other studies using wide implants for bone-anchored hearing systems ( 14,17,18). The higher insertion torque (50 Ncm) allowed by the wide implant and the altered diameter ratio between the drilled hole and the implant are the likely explanations, as these features affect the initial bone-remodeling process induced by the insertion trauma.
After loading the processor on the wide implant on average 7 weeks after operation, we did not observe a fall in ISQ. Conversely, we registered a continuous increase of the ISQ, as shown in other studies of wide implants (6,14,17,18). Loading at around 6 weeks after surgery has previously been reported as safe in adult patients (e.g., 2,4,19), and this is reinforced by this study on the Ponto Wide implant. Given the high initial stability, and the fact that ISQ values are increased already at 10 days after implantation, earlier loading seems feasible, at least for patients with normal bone quality. Indeed, successful loading of wide diameter implants 3 to 4 weeks after surgery has recently been reported (18,20). There is some stability variance between the patients; this variance is mainly caused because of abutment length 9 mm on 3 patients. For example, the patient who shifted the abutment from 6 to 9 mm at 6-month follow-up, the ISQ was reduced from 69 to 61 after placement of the 9 mm long abutment. Differences in quality and quantity of bone between the patients can also explain this stability variance.
At the follow-up visits, it was an interesting observation that he patients appreciated being subjected to the reassuring ISQ measurements, and this was found to be an additional incentive to appear.
In conclusion, these first published ISQ measurements on the Ponto Wide implant showed high initial stability and good osseointegration the first year after implantation. No decrease in ISQ was observed after implant loading. As expected, soft tissue reactions rarely occurred and were of only minor severity.
Footnotes
Conflicts of Interest and Source of Funding: None declared
REFERENCES
- 1. Tjellström A, Lindström J, Hallén O, Albrektsson T, Brånemark PI. Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol 1981; 2: 304– 10 [PubMed] [Google Scholar]
- 2. Snik AF, Mylanus EA, Proops DW, et al. Consensus statements on the BAHA system: where do we stand at present? Ann Otol Rhinol Laryngol Suppl 2005; 195: 2– 12 [DOI] [PubMed] [Google Scholar]
- 3. Kiringoda R, Lustig LR. A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol 2013; 34: 790– 4 [DOI] [PubMed] [Google Scholar]
- 4. Dun CA, Faber HT, de Wolf MJ, Mylanus EA, Cremers CW, Hol MK. Assessment of more than 1,000 implanted percutaneous bone conduction devices: skin reactions and implant survival. Otol Neurotol 2012; 33: 192– 8 [DOI] [PubMed] [Google Scholar]
- 5. Granström G. Osseointegration in irradiated cancer patients: an analysis with respect to implant failures. J Oral Maxillofac Surg 2005; 63: 579– 85 [DOI] [PubMed] [Google Scholar]
- 6. Dun CA, de Wolf MJ, Hol MK, et al. Stability, survival, and tolerability of a novel baha implant system: six-month data from a multicenter clinical investigation. Otol Neurotol 2011; 32: 1001– 1007 [DOI] [PubMed] [Google Scholar]
- 7. Hultcrantz M. Outcome of the bone-anchored hearing aid procedure without skin thinning: a prospective clinical trial. Otol Neurotol 2011; 32: 1134– 9 [DOI] [PubMed] [Google Scholar]
- 8. Mylanus EA, Cremers CW. A one-stage surgical procedure for placement of percutaneous implants for the bone-anchored hearing aid. J Laryngol Otol 1994; 108: 1031– 5 [DOI] [PubMed] [Google Scholar]
- 9. van der Pouw CT, Mylanus EA, Cremers CW. Percutaneous implants in the temporal bone for securing a bone conductor: surgical methods and results. Ann Otol Rhinol Laryngol 1999; 108: 532– 6 [DOI] [PubMed] [Google Scholar]
- 10. Holgers KM, Tjellström A, Bjursten LM, Erlandsson BE. Soft tissue reactions around percutaneous implants: a clinical study of soft tissue conditions around skin-penetrating titanium implants for bone-anchored hearing aids. Am J Otol 1988; 9: 56– 9 [PubMed] [Google Scholar]
- 11. O’Sullivan D, Sennerby L, Jagger D, Meredith N. A comparison of two methods of enhancing implant primary stability. Clin Implant Dent Relat Res 2004; 6: 48– 57 [DOI] [PubMed] [Google Scholar]
- 12. Sennerby L, Gottlow J, Rosengren A, Flynn M. An experimental evaluation of a new craniofacial implant using the rabbit tibia model: Part II. Biomechanical findings. Otol Neurotol 2010; 31: 840– 5 [DOI] [PubMed] [Google Scholar]
- 13. Ivanoff CJ, Sennerby L, Johansson C, Rangert B, Lekholm U. Influence of implant diameters on the integration of screw implants. An experimental study in rabbits. Int J Oral Maxillofac Surg 1997; 26: 141– 8 [DOI] [PubMed] [Google Scholar]
- 14. D’Eredità R, Caroncini M, Saetti R. The new Baha implant: a prospective osseointegration study. Otolaryngol Head Neck Surg 2012; 146: 979– 83 [DOI] [PubMed] [Google Scholar]
- 15. Barewal RM, Oates TW, Meredith N, Cochran DL. Resonance frequency measurement of implant stability in vivo on implants with a sandblasted and acid-etched surface. Int J Oral Maxillofac Implants 2003; 18: 641– 51 [PubMed] [Google Scholar]
- 16. Han J, Lulic M, Lang NP. Factors influencing resonance frequency analysis assessed by Osstell mentor during implant tissue integration: II. Implant surface modifications and implant diameter. Clin Oral Implants Res 2010; 21: 605– 11 [DOI] [PubMed] [Google Scholar]
- 17. Marsella P, Scorpecci A, D’Eredità R, Della Volpe A, Malerba P. Stability of osseointegrated bone conduction systems in children: a pilot study. Otol Neurotol 2012; 33: 797– 803 [DOI] [PubMed] [Google Scholar]
- 18. McLarnon CM, Johnson I, Davison T, et al. Evidence for early loading of osseointegrated implants for bone conduction at 4 weeks. Otol Neurotol 2012; 33: 1578– 82 [DOI] [PubMed] [Google Scholar]
- 19. Wazen JJ, Gupta R, Ghossaini S, Spitzer J, Farrugia M, Tjellstrom A. Osseointegration timing for Baha system loading. Laryngoscope 2007; 117: 794– 6 [DOI] [PubMed] [Google Scholar]
- 20. Faber HT, Dun CA, Nelissen RC, Mylanus EA, Cremers CW, Hol MK. Bone-anchored hearing implant loading at 3 weeks: stability and tolerability after 6 months. Otol Neurotol 2013; 34: 104– 10 [DOI] [PubMed] [Google Scholar]


