Abstract
Hypersensitivity to mechanical stimuli following surgery has been reported in patients who subsequently develop chronic pain after surgery. In animals, peripheral injury increases prostaglandin production in the spinal cord, and spinal cyclooxygenase inhibitors reduce hypersensitivity after injury. We therefore tested the hypothesis that spinal ketorolac reduces hypersensitivity and acute and chronic pain after hip arthroplasty (www.clinicaltrials.gov NCT 00621530). Sixty-two patients having total hip arthroplasty with spinal anesthesia were randomized to receive 13.5 mg hyperbaric bupivacaine with spinal saline or 13.5 mg hyperbaric bupivacaine with 2 mg preservative-free ketorolac. The primary outcome was area of hypersensitivity surrounding the wound 48 hr after surgery, but this only occurred in 4 patients, precluding assessment of this outcome. The groups did not differ in acute pain, acute opioid use, or pain incidence or severity 2 and 6 months after surgery. There were no serious adverse events. Our results suggest that a single spinal dose of ketorolac does not substantially reduce acute surgical pain, and is thus unlikely to reduce the risk of persistent incisional pain.
Keywords: Ketorolac, Postoperative analgesia, Spinal cord, Pain Mechanisms, intrathecal injection, human
A role of prostaglandin production in pain is well established. However, the importance of prostaglandins in many chronic pain conditions remains unknown, as do the sites where prostaglandins act. Cyclooxygenase inhibitors reduce peripheral inflammation and pain, potentially in part by an action in the spinal cord [1,2]. In humans, however, intrathecal injection of the cyclooxygenase inhibitor, ketorolac, did not reduce pain to various acute and subacute experimental treatments and to acute pain following vaginal hysterectomy.[3,4]
Postoperative pain is frequently accompanied by hypersensitivity [5] and the primary goal of the current study was to determine whether intrathecal ketorolac reduces postoperative hypersensitivity. The degree of acute and sub-acute hypersensitivity following surgery correlates with the risk of chronic pain after surgery.[6,7] Therefore, a secondary goal was to determine whether ketorolac reduces the incidence of pain 6 months after surgery. Finally, cerebrospinal fluid concentrations of inflammatory mediators are increased in patients with arthritis[8] and increased PGE2 concentrations in cerebrospinal fluid patients correlate with degree of postoperative pain.[9] A final aim of the current study was to test whether some patients coming for hip arthroplasty may have increased PGE2 concentrations and might uniquely benefit from intrathecal ketorolac.
We enrolled patients having American Society of Anesthesiologists (ASA) physical status 1, 2, or 3 patients, age 18 years or above, who were scheduled for primary unilateral total hip arthroplasty with spinal anesthesia at Wake Forest University or the Cleveland Clinic. The protocol was approved by the National Institutes of Health Clinical Trials Unit, the Institutional Review Boards at Wake Forest School of Medicine and the Cleveland Clinic, and the Food and Drug Administration, and was registered prior to patient recruitment at www.clinicaltrials.gov (NCT00621530). Written consent was obtained from participating patients. Patients routinely taking opioids for pain other than their primary hip pain were excluded, as were those taking more than an equivalent of 10 mg per day of oxycodone. Patients taking pregabalin or gabapentin stopped these medications three days before surgery, and those taking tramadol stopped this medication 24 hours before surgery. We did not record use of analgesics after hospital discharge.
A day before surgery, patients completed the Neuropathic Pain Symptom Inventory[10] the McGill Short Form Pain Questionnaire, and rated their level of anxiety and pain at rest and with ipsilateral hip movement with a 10-cm-long visual analog scales anchored at “not anxious at all” to “as anxious as possible” and “no pain” to “worst possible pain.” On the day of surgery, patients took 1 gm acetaminophen orally and were randomized to receive 13.5 mg hyperbaric bupivacaine spinally plus 0.4 ml saline or preservative free ketorolac (Acular PF, Allergan, Irvine, CA; 5 mg/ml = 2.0 mg) under IND 62,179 from the Food and Drug Administration. The trial was thus fully double-blinded. Following intravenous sedation with midazolam and fentanyl, a 25-guage Whitacre spinal needle was inserted in a lumbar interspace and one milliliter of cerebrospinal fluid (CSF) sampled. The study medication was then. Intraoperative anesthetic management was routine. Analgesia in the first 24 postoperative hours was provided by intravenous Patient Controlled Analgesia (PCA) with morphine 1 mg/ml or hydromorphone 0.2 mg/ml (1.5 ml dose, 10 minute lockout interval, 6 ml hourly limit, no basal infusion), with subsequent oral oxycodone 5–15 mg. Presence and area of hyperalgesia and allodynia surrounding the wound was obtained at 48 hours postoperatively, once with a 225-mN von Frey filament and once with a cotton tipped swab and patients reported average pain for each 24 hr period. Patients were contacted by telephone at 8 weeks and 6 months after surgery and queried regarding presence of new pain since surgery. Based on presence of hyperalgesia at 48 hours of 11 [9, 17] cm (median [25th, 75th percentile) beyond the wound in this surgical population,[11] we estimated that 50 patients per group would allow us to detect a reduction in the area of hyperalgesia by as little as 25% at alpha of 0.5. PGE2 in CSF was measured using the Parameter ELISA kit from R&D Systems (Minneapolis, MN).
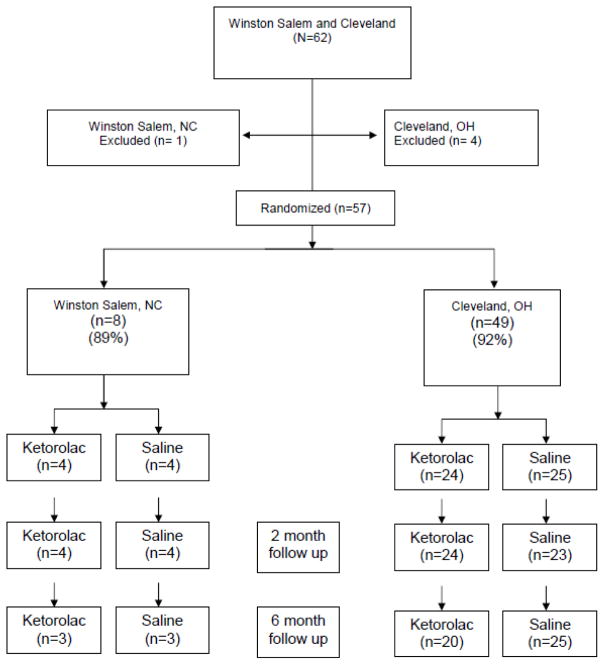
The study was stopped early, after recruitment of only 62 patients, when the only manufacturer of preservative-free ketorolac stopped making the product (Figure 1). Ketorolac and saline groups were well matched regarding preoperative characteristics, with the exception of anxiety, which was higher in the ketorolac group (5.0 [2.8, 7.3]) than in the saline group (2.0 [0,4]), (P< 0.05 by Mann Whitney U test). Ketorolac and saline groups did not differ regarding surgical procedure and time or intraoperative dose of sedation or analgesics. Although ephedrine administration was similar in patients given intrathecal ketorolac or saline (10 [0,25] and 0 [0,15] mg respectively), more phenylephrine was given to patients assigned to ketorolac [150 [0,365] and 0 [0,63] μg, (P=0.009)]. Blood pressure and heart rate decreased similarly after spinal injection in both groups. Cephalad level of sensory anesthesia did not differ between groups.
Figure 1.
Patient recruitment and disposition
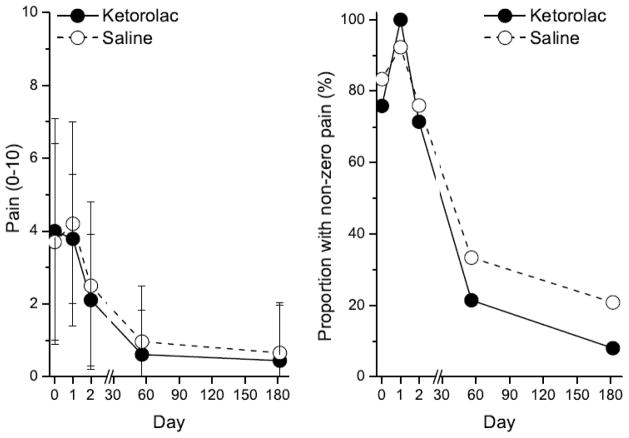
Only 4 of 57 subjects (2 each in the saline and ketorolac groups) exhibited hyperalgesia or allodynia 48 hours after surgery. Pain during the first two days after surgery did not differ between the ketorolac and saline groups at this time or up to 6 months later (Figure 1). Similarly, opioid use did not differ between the ketorolac and saline groups, either by PCA (43 [23 to 76] vs 49 [32 to 73] mg morphine equivalents) or total use, including rescue and oral medications (76 [48 to 105] vs 83 [63 to 107] mg morphine equivalents). Only 15% of the study population had residual pain 6 months after surgery, with no difference between groups (Figure 1). Finally, the groups did not differ 2 or 6 months after surgery in neuropathic pain scores. Cerebrospinal fluid concentrations of PGE2 were within the normal range, defined as the 95th percentile in healthy volunteers[3,12] in all but 4 individuals (3 randomized to placebo, 1 to ketorolac). Compared to the other patients in the study, patients with high spinal PGE2 concentrations did not differ in preoperative characteristics, including pain assessments or neuropathic pain symptom inventory, or postoperative pain or opioid use; and none of them reported pain 6 months following surgery.
Our study was critically based on a previous report which showed a high incidence of large areas of hypersensitivity 48 hr after hip arthroplasty.[11] Because the incidence we observed was much lower in our patients — and because the drug became unavailable—we were unable to test our primary hypothesis that intrathecal ketorolac reduces postoperative hypersensitivity.
Why hyperalgesia in our study was lower than previously reported remains unclear. This might reflect the use of spinal anesthesia in our patients and general anesthesia in theirs, since neuraxial anesthesia improves many aspects of acute recovery following hip surgery.[13,14]. Intrathecal ketorolac also failed to reduce pain or opioid requirements in the first 2 days after surgery, which is similar to results reported in women having vaginal hysterectomies.[4] Systemic cyclooxygenase inhibitors are effective in this setting, suggesting that they are acting at non-spinal sites. Based on observations of increased IL-1β in patients with chronic arthritis,[8] we expected to observe high concentrations of PGE2 in CSF in our patients who had chronic arthritis. Yet only 4 of 62 subjects had higher concentrations than normal volunteers, perhaps reflecting a difference between production of these inflammatory mediators in the central nervous system or a difference in patient populations (rheumatoid arthritis[8] compared to primarily osteoarthritis in our study).
We observed an overall incidence of pain of approximately 15% six months after total hip arthroplasty. This result in our prospective study is thus substantially less than the 27% at three years previously reported in a postal questionnaire,[15] likely reflecting response bias using the latter methodology. Although fewer patients given ketorolac reporting pain 6 months after surgery, the difference was not statistically significant and the study was inadequately powered to assess this outcome. Given the higher baseline anxiety scores in the ketorolac group, we would have expected a higher incidence of pain 6 months later rather than lower. Further testing of intrathecal ketorolac must await either re-introduction of a preservative free product or neurotoxicity testing of the new formulation.
In summary, intrathecal ketorolac did not alter the characteristics of bupivacaine spinal anesthesia in patients undergoing total hip arthroplasty. We observed only a tiny amount of hyperalgesia to mechanical testing in this patient population 48 hr after surgery, and were thus unable to adequately test the effect of intrathecal ketorolac on hyperalgesia. However, the failure of ketorolac to alter acute pain or opioid requirements or the incidence of pain 6 months after surgery, combined with the largely negative previous studies of intrathecal ketorolac in volunteers with experimental pain and hypersensitivity, and in patients with postoperative or chronic neuropathic pain questions the role of spinal cyclooxygenase in human pain as has been suggested in preclinical studies. Given the failure of a recent clinical trial in patients with post-herpetic neuralgia to target these inflammatory processes[16] it is quite conceivable that preclinical studies in male rodents with focus on withdrawal threshold have defined mechanisms which are irrelevant to human pain.
Figure 2.
Mean ± SD pain intensity score (left) and proportion of patients with pain (right) following surgery on day 0 in patients receiving spinal injection of saline placebo (open circles) or 2 mg preservative-free ketorolac (filled circles). See Figure 1 for number of subjects over time. No differences between groups.
Acknowledgments
The authors wish to thank Professor Torsten Gordh, Jr, MD and Anne-Li Lind at Uppsala University, Uppsala, Sweden for helpful comments on the study and manuscript and for performing the PGE2 assays.
Supported in part by grant GM48085 from the National Institute of Health, Bethesda, MD and by the Uppsala Berzelii Center For Neurodiagnostics -Pain Theme, Uppsala, Sweden.
References
- 1.Malmberg AB, Yaksh TL. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- 2.Malmberg AB, Yaksh TL. Pharmacology of the spinal action of ketorolac, morphine, ST-91, U50488H, and L-PIA on the formalin test and an isobolographic analysis of the NSAID interaction. Anesthesiology. 1993;79:270–281. doi: 10.1097/00000542-199308000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Eisenach JC, Curry R, Tong C, Houle TT, Yaksh TL. Effects of intrathecal ketorolac on human experimental pain. Anesthesiology. 2010;112:1216–1224. doi: 10.1097/ALN.0b013e3181d94d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenach JC, Curry R, Rauck R, Pan P, Yaksh TL. Role of spinal cyclooxygenase in human postoperative and chronic pain. Anesthesiology. 2010;112:1225–1233. doi: 10.1097/ALN.0b013e3181d94dc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilder-Smith OH. Chronic pain and surgery: a review of new insights from sensory testing. J Pain Palliat Care Pharmacother. 2011;25:146–159. doi: 10.3109/15360288.2010.505256. [DOI] [PubMed] [Google Scholar]
- 6.Eisenach JC. Preventing chronic pain after surgery: who, how, and when? Reg Anesth Pain Med. 2006;31:1–3. doi: 10.1016/j.rapm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Kaasa T, Romundstad L, Roald H, Skolleborg K, Stubhaug A. Hyperesthesia one year after breast augmentation surgery increases the odds for persistent pain at four years. A prospective four-year follow-up study. Scandinavian Journal of Pain. 2010;1:75–81. doi: 10.1016/j.sjpain.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Lampa J, Westman M, Kadetoff D, Agreus AN, Le ME, Gillis-Haegerstrand C, Andersson M, Khademi M, Corr M, Christianson CA, et al. Peripheral inflammatory disease associated with centrally activated IL-1 system in humans and mice. Proc Natl Acad Sci U S A. 2012;109:12728–12733. doi: 10.1073/pnas.1118748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buvanendran A, Kroin JS, Berger RA, Hallab NJ, Saha C, Negrescu C, Moric M, Caicedo MS, Tuman KJ. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104:403–410. doi: 10.1097/00000542-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, et al. Development and validation of the neuropathic pain symptom inventory. Pain. 2004;108:248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Martin F, Cherif K, Gentili ME, Enel D, Abe E, Alvarez JC, Mazoit JX, Chauvin M, Bouhassira D, Fletcher D. Lack of impact of intravenous lidocaine on analgesia, functional recovery, and nociceptive pain threshold after total hip arthroplasty. Anesthesiology. 2008;109:118–123. doi: 10.1097/ALN.0b013e31817b5a9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenach JC, Curry R, Hood DD, Yaksh TL. Phase I safety assessment of intrathecal ketorolac. Pain. 2002;99:599–604. doi: 10.1016/S0304-3959(02)00208-7. [DOI] [PubMed] [Google Scholar]
- 13.Memtsoudis SG, Sun X, Chiu YL, Nurok M, Stundner O, Pastores SM, Mazumdar M. Utilization of critical care services among patients undergoing total hip and knee arthroplasty: epidemiology and risk factors. Anesthesiology. 2012;117:107–116. doi: 10.1097/ALN.0b013e31825afd36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuman MD, Silber JH, Elkassabany NM, Ludwig JM, Fleisher LA. Comparative effectiveness of regional versus general anesthesia for hip fracture surgery in adults. Anesthesiology. 2012;117:72–92. doi: 10.1097/ALN.0b013e3182545e7c. [DOI] [PubMed] [Google Scholar]
- 15.Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152:566–572. doi: 10.1016/j.pain.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Landry RP, Jacobs VL, Romero-Sandoval EA, DeLeo JA. Propentofylline, a CNS glial modulator does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages. Exp Neurol. 2012;234:340–350. doi: 10.1016/j.expneurol.2011.11.006. [DOI] [PubMed] [Google Scholar]