Abstract
Twenty-five years ago, immunologists and neuroscientists had little science of mutual interest. This is no longer the case. Neuroscientists now know that the first formally defined cytokine, IL-1, activates a discrete population of hypothalamic neurons. This interaction leads to the release of glucocorticoids from the adrenal gland, a hormone that has a long history in immunoregulation. Immunologists have been surprised to learn that lymphoid cells synthesize acetylcholine, the first formally recognized neurotransmitter. This neurotransmitter suppresses the synthesis of TNF. These discoveries blur the distinction of neuroscience and immunology as distinct disciplines. There are now 37 formally recognized cytokines and their receptors, and at least 60 classical neurotransmitters plus over 50 neuroactive peptides. These findings explain why both immunologists and neuroscientists are getting nervous about immunity and highlight a real need to apply integrative physiological approaches in biomedical research.
Keywords: Immunophysiology, Neuroimmunology, Psychoneuroimmunology, Symptoms, Behavior, Inflammation
1. Introduction
Since the beginning of time, it has been known that animals and humans infected with pathogenic microorganisms become sick. Common symptoms are fever, anorexia, somnolence, psychataxia (inability to concentrate), fatigue, lethargy and pain. All of these are physiological responses that require activation of both the immune and nervous systems. It should therefore come as no surprise that the immune and nervous systems are inextricably linked in ways that dramatically affect our health and well-being.
Regulation of the major organs of the body has long been studied in the discipline of systemic physiology. Indeed, the first issue of the American Journal of Physiology was published in 1898. Seventeen years later, The Journal of Immunology appeared. As specific biomedical disciplines continued to develop, so did more specialized scientific journals. Endocrinology first appeared in 1927, and the Journal of Neuroscience was first published in 1981. During the past 50 years, many major biomedical journals have emphasized the publication of specialty rather that integrative papers. Immunologists, neuroscientists and psychiatrists were focused on their specific disciplines, even though common sense dictated that the brain and immune system must communicate with each other to ultimately change some type of behavior. Integrative physiology research that stressed communication and regulatory systems among major tissues and organs fell out of grace. With the more recent advent of open access journals, this trend toward growth of specialty journals continues.
It was in this historical context that the first issue of Brain, Behavior and Immunity appeared on March 1, 1987. Ader, Cohen and Felten wrote, “As a result, the neurosciences and immunology developed and matured without seriously considering the possibility that there might be channels of communication between these systems that could mutually influence their respective functions.” and “.. ‥the questionable conclusion that the immune system can, and therefore does, operate in an autonomous manner, i.e. independent of other physiological systems.” [1]. Twenty-five years after these words were written, it has become abundantly clear that the immune and central nervous systems are not the sole captains of their own ship. They both regulate actions of the other. The immune system helps to maintain health by interacting in a coordinated fashion with many organ systems in the body, including the nervous system. As such, the immune system should be viewed as just another physiological system, like the cardiovascular, central nervous, renal, musculoskeletal and neuroendocrine systems [2]. All these physiological systems work together to maintain homeostasis and to promote proper body functioning in both health and disease. Integration of the immune system with all other bodily systems defines immunophysiology.
There is ample evidence to conclude that understanding the etiology as well as the treatment of many brain diseases requires a better understanding of immunology. Conversely, pathologies that target peripheral organs are affected by the nervous system. Neuroimmunology research has been classically focused on nervous system diseases such as multiple sclerosis, Parkinson’s disease, Huntington’s disease and Alzheimer’s disease. But, solving and developing effective treatments for these nervous system pathologies requires more knowledge about functional links with the peripheral immune system. As but one example, recent evidence has established that major mental health disorders such as depression, schizophrenia, maniac depression and perhaps autism spectrum disorders are associated with abnormalities in the immune system, even though cause-and-effect relationships are not year clear [3]. Similarly, learning how the nervous and immune systems interact to affect pathologies outside the nervous system is also critical. For example, as discussed below, activation of the vagus nerve reduces tumor necrosis factor (TNFα) synthesis and improves survival during sepsis in animal models [4]. Type II diabetes, obesity, cardiovascular diseases and cancer and its treatments are major health issues of our times. Behavioral changes occur in all of these pathologies, and they can seriously affect quality of life of patients. Most diseases, regardless of whether the primary pathology affects a central or peripheral tissue, are associated with perturbations in communication systems between the immune and nervous systems of animals and humans alike. This link between medicine and physiology is probably one reason why Alfred Nobel did not name one of the five original prizes as the “Nobel Prize in Medicine.” Instead, the name he chose that remains as one of his namesakes is the “Nobel Prize in Medicine or Physiology.”
2. The physiology of immunology
Innate and acquired immunity are very important components of the physiology of the entire body, thus comprising the emerging area of immunophysiology. The flu viruses provide an excellent example of how the body and the brain work together to clear this infection. Influenza viruses generally gain access to the body via mucus membranes, such as those in the eyes and nose. Even though flu viruses normally persist outside the central nervous system, they induce symptoms that involve the brain within a couple days. The classic symptoms are fever, chills, headaches, somnolence and reduced appetite. The resulting malaise and fatigue reduce the motivation of flu patients to perform their normal activities. Although uncomfortable, these symptoms of sickness are generally considered to promote recovery and healing [5,6]. Most of these flu symptoms begin to subside after a couple days and disappear in seven to ten days.
The immune system engages its full armamentarium to remove influenza viruses from the body. Epitope-specific antibodies, CD8+ cytotoxic T cells and natural killer cells are the major effectors. But, various parts of the brain are responsible for regulating body temperature, appetite, sleep, pain and motivational aspects of fatigue. In order to determine if there are detectable changes that occur in the brain following viral infection, Jurgens et al. infected mice with a non-neurotropic strain of influenza [7]. By using very sensitive quantitative real time RT-PCR, no evidence of influenza virus could be detected in the hippocampus. Yet, dramatic changes occurred in the cytokine and neurotrophin mRNA profile of this brain structure: there was an increase in the proinflammatory cytokines interleukin (IL)-1β, tumor necrosis factor (TNF), IL-6 and interferon (IFN) α and a reduction in the expression of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) (Fig. 1). These effects may be related to an increase in the number of activated microglia in the hippocampus, as detected by induction of ionized calcium binding adapter molecule 1 (Iba1) immunohistochemical staining. These data clearly establish that a viral infection in a peripheral organ like the lung causes changes in inflammatory proteins the brain. The resulting changes in behavior, which are cytokine mediated [8], help ensure survival.
Fig. 1.
A peripheral influenza infection regulates RNA expression of numerous cytokines in the brain. Although no virus could be detected in the brain, the influenza virus is able to induce an inflammatory response within the hippocampus. Reprinted with permission from [7].
The physiological circuits described above represent just another example of the benefits of organ systems working together for the benefit of the host. Cells of the immune system are not masters of their own fate. They are regulated by other physiological systems in the body, all of which serve to maintain homeostasis for the benefit of the host. It is not only antigens, but also nerves, hormones and local tissue factors that affect the multitude of activities expressed by lymphoid and myeloid cells, ranging from differentiation to recruitment by tissues to cytokine synthesis. But, an important question that remains is learning how the brain is informed that the body is infected with the flu virus. Several ideas have been proposed over the years, and these communication routes will be discussed below. However, recent advances in understanding immunological privilege of the brain are offering the greatest insights that may ultimately provide an answer to this question. Discoveries such as these are causing both immunologists and neuroscientists to get nervous about immunity.
3. Emerging view of the blood–brain barrier
One of the greatest misconceptions that has impeded progress in understanding reciprocal systems of communication between the immune system and brain is the idea that the blood–brain barrier at the cerebrum is a solid pipe of capillary endothelial cells that absolutely restricts the exchange of large molecular proteins (e.g., cytokines) and cells (e.g., macrophages) between blood and brain. A less recognized restrictive system is the blood–cerebrospinal fluid barrier at the choroid plexus. The blood–brain and blood–cerebrospinal fluid barriers (BBB and BCB, respectively) are generally considered to be the primary structures responsible for immunological privilege of the brain. Recent knowledge is changing this old fashioned view [9,10].
The new concept is that there are both afferent pathways that drain interstitial fluid away from the brain and efferent pathways that permit substances from blood to gain access to the brain parenchyma [11]. Long held dogma was that the major efferent pathway by which large substances gain access to the brain is via specific brain locations that do not form tight junctions that characterize the blood–brain barrier [12]. These brain regions are known as circumventricular organs. Once membrane transport pathways became better understood, a second pathway was identified in the active transport of large, hydrophilic proteins such as cytokines from the luminal (blood) to the abluminal side of the brain (parenchyma) was demonstrated. Twenty years ago, a third efferent pathway identified a fast neural route that was activated following intraperitoneal injections of inflammatory agents such as lipopolysaccharide (LPS) [13]. To date, the major neuronal pathway that has been identified to carry information about the inflammatory status in the periphery to the brain is the vagus nerve [14]. The afferent vagus that transmits information to the brain terminates in the dorsal vagal complex of the brainstem. Fibers then connect to the central nucleus of the amygdala, bed nucleus of the stria terminalis, hypothalamic paraventricular nucleus and ventromedial preoptic area. Blocking transmission of the vagus at the level of the dorsal vagal complex impairs inflammation-induced activation of all these forebrain areas following intraperitoneal injections of LPS [15]. A fourth efferent pathway is just now being defined. Although in its infancy, the central idea is that brain endothelial cells are very active participants in immune–CNS communication. It is now well accepted that a variety of cells within the CNS can synthesize cytokines. These cytokines and chemokines, as well as other inflammatory alarm signals like ATP, can induce the expression of VCAM-1 and ICAM-1 on brain endothelial cells [16]. For example, astrocytic CCL2 (MCP-1) can move from the abluminal to the luminal side of the blood–brain barrier, where it permits entry of leukocytes into the brain parenchyma. Although there is as yet no proof that T cell receptors that are expressed on neurons in the cortex, activation of these receptors has recently be demonstrated to reduce excitability of α7 nicotinic acetylcholine receptors on cortical interneurons [17]. Although the in vivo function of this process is unknown, it is speculated that antigen binding of both the major histocompatibility I proteins and T cell receptors on neurons and microglia protects those neurons during microglial pruning of neuronal spines.
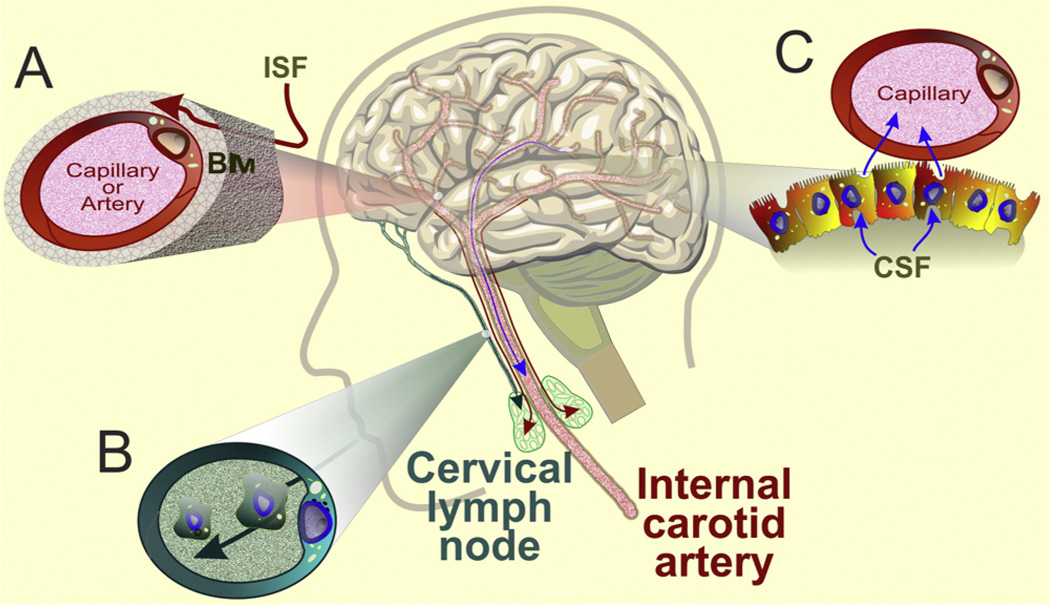
The brain does not contain the rich collection of lymph nodes and lymphatic vessels that characterize the peripheral immune system. This anatomical arrangement once led scientists to dismiss the notion of any type of immune system in the brain. But, as recently described by Carare, Hawkes and Weller [11], interstitial fluid and solutes draining along the walls of cerebral arteries travel along the wall of the internal carotid artery and from there into cervical lymph nodes. Cerebrospinal fluid, as well as lymphoid and myeloid cells in the central nervous system, finds their way to cervical lymph nodes, but through a different route: subarachnoid space→cribriform plate→nasal lymphatics→cervical nodes (Fig. 2). These findings firmly establish an anatomical basis to explain how both soluble antigens and cells from the central nervous system can find their way into lymph nodes in the periphery. These new findings of both afferent pathways that allow antigens and immune cells to drain into peripheral lymph nodes, coupled with newly discovered functions of cerebral endothelial cells that provide efferent information to reach the brain from the periphery, are redefining our understanding of immunological privilege.
Fig. 2.
Drainage from the brain to the periphery. Interstitial fluid (ISF) from the brain, cerebrospinal fluid (CSF), and antigen-presenting cells gain access to the peripheral immune system by three major pathways. (A) Interstitial fluid drains along the basement membrane (BM) of capillaries and cerebral arteries to the internal carotid artery and from there to cervical lymph nodes (red arrows); before eventually entering the circulation. (B) Cerebrospinal fluid and antigen-presenting cells follow a different route by draining through the subarachnoid space through the cribriform plate into nasal lymphatics and from there to cervical lymph nodes (green arrow). (C) CSF also drains into blood vessels through granulations and arachnoid villi by passing through venous endothelial cells (blue arrow).
4. A cytokine stimulates neurons: peripheral IL-1 activates the neuroendocrine stress response
The hypothalamus comprises the floor of the third cerebral ventricle, lying just below the thalamus. It contains specialized neurons that synthesize and release a numerous peptides. The periventricular nucleus contains a variety of neurons. One of these neuronal subpopulations is known as parvocellular neurosecretory cells, and they secrete corticotropin-releasing factor. Classically, an environmental stressor is perceived by the central nervous system, which leads to the release of corticotropin-releasing hormone from the hypothalamus. This corticotropin-releasing hormone travels through short portal vessels to the anterior pituitary gland, where it acts on discrete cells known as corticotrophs to cause the release of adrenocorticotropic hormone. This hormone is transported through the bloodstream to cause the release of glucocorticoid hormones from the cortex of the adrenal gland. The entire concept that only stress activates hypothalamic corticotrophs was turned upside down when a cytokine produced by leukocytes was shown to perform the same action.
In what are now classic papers, two independent research groups showed that a systemic injection of IL-1 caused a dramatic increase in blood adrenocorticotropic hormone [18,19]. When an antibody to corticotropin-releasing hormone was administered prior to injection of IL-1, adrenocorticotropic hormone was barely detectable in the blood of animals injected with either the control buffer or IL-1. These early experiments established that systemic IL-1 acts directly on a part of the brain known as the hypothalamus to induce the release of corticotropin-releasing hormone. This causes an increase in the release of adrenocorticotrophic hormone from the anterior pituitary gland and a subsequent increase in glucocorticoid release from the adrenal gland. Glucocorticoid hormones not only negatively feedback on the hypothalamus to inhibit secretion of corticotropin-releasing hormone from the hypothalamus, but they also have major effects on both myeloid and lymphoid cells. These data clearly established that the very first internationally recognized cytokine, IL-1, induces a classic neuroendocrine stress response. The authors concluded that there must be an immunoregulatory feedback circuit between the immune system and brain.
The finding that IL-1 activates the hypothalamic-pituitary-adrenal axis was quite surprising. At one time, there was no ELISA to measure IL-1. Scientists therefore relied on a thymocyte co-stimulation bioassay to determine the amount of IL-1 activity. In this in vitro assay, dispersed thymocytes were stimulated with a suboptimal amount of mitogen like phytohemagglutinin. When a supernatant was added that contained IL-1, thymocytes incorporated more tritiated thymidine, used as an index of DNA synthesis and cell proliferation, than did controls. Based on this assay, IL-1 was at one time considered as a treatment to improve T cell development. But, when IL-1 was injected systemically in vivo, it caused the thymus to involute [20]. As little as 50 ng of IL-1 injected systemically caused nearly a 50% reduction in the number of thymocytes, and higher doses of IL-1 caused over a 90% reduction. Most of the cells lost were composed of the immature CD4+CD8+ population, just as would be expected following an acute stressor or an injection of glucocorticoid. This finding was a real surprise based upon the distinct in vitro property of IL-1 to cause thymocytes to proliferate. This paradox can be explained by an indirect effect of IL-1 inducing the release of corticosterone in vivo, which could only be detected by using live animals. This scenario reinforces the idea that when immunologists and neuroendocrinologists do not communicate, scientific discovery suffers.
Recent findings have now directly implicated the stress-induced release of glucocorticoids, as well as catecholamines, in the regulation of microglial function [21]. Once considered to be only the major phagocytic cell of the brain, microglia are now well accepted to synthesize and secrete a number of cytokines and to regulate pruning of dendritic spines and synapses, particularly during development. These new results showed that the well-accepted induction of anhedonia that is induced by chronic stress is associated with an increase in the microglial activation in stress-sensitive brain regions such as the medial prefrontal cortex, CA3 region of the hippocampus and amygdala [22].
5. A neurotransmiter suppresses macrophage activation: acetylcholine is synthesized by lymphoid cells
The finding that a protein from the immune system could activate one of the most studied neuroendocrine circuits came as quite a surprise in 1987. Fifteen years later, there was another surprise discovery that further cemented the concept that the brain and immune system communicate with one another. Immunologists designated IL-1 as the first protein formally recognized as a cytokine. Roughly 50 years earlier, the first neurotransmitter to be formally recognized was acetylcholine. It seems rather ironic that the first known neurotransmitter was shown to suppress the activation of macrophages, as assessed by secretion of the cytokine TNF. Tracey and colleagues reported that the first known neurotransmitter, acetylcholine, suppresses the synthesis of macrophage-derived TNF, IL-1, IL-6 and IL-18 [23]. The major neurotransmitter secreted by the vagus nerve, the tenth cranial nerve, is acetylcholine. To test the physiological relevance of their findings, Borovikova et al. electrically stimulated the vagus nerve in vivo prior to the induction of toxic sepsis. This neural stimulation reduced the rise in blood levels of TNF and prevented the drastic reduction in mean arterial blood pressure that characterizes septic shock. This phenomenon of a neurotransmitter suppressing the synthesis of proinflammatory cytokines was coined the “cholinergic anti-inflammatory reflex.”
Since that time, a number of papers have appeared on this topic, as recently reviewed [4]. The broad view is that there is both an afferent arc that carries information about the status of the immune system to the brain, as well as an efferent branch that delivers signals from the brain to cells of the immune system (Fig. 3). In this model, neuronal fibers of the vagus carry both these afferent and efferent signals. The anti-inflammatory properties of vagal nerve stimulation have recently been extended to two murine models of inflammatory bowel disease [24], leading to a reduction in mucosal inflammation. These results are probably not caused by acetylcholine-producing T cells. Instead, a new population of B cells has been discovered that release acetylcholine following stimulation with sulfated cholecystokinin [25]. These cells lead to a reduction in the recruitment of neutrophils to the peritoneum (see [26] for a recent review). It is also noteworthy that in a single case study, computerized electrical stimulation of the vagus nerve relieved joint pain and swelling caused by long-term rheumatoid arthritis and [27]. This was coupled with reduction in blood levels of C reactive protein by greater than 90%. Randomized, blinded, placebo controlled studies will be necessary to determine whether computerized nerve-stimulating devices are efficacious, cost effective and safe.
Fig. 3.
An example of a brain-immune system physiological system: the cholinergic anti-inflammatory reflex. Afferent fibers within the vagus nerve convey information from the intestinal myeloid (such as dendritic cells, DC) and glomus cells to the nucleus of the solitary tract in the brainstem. Following activation of a number of neuronal circuits in medulla, the efferent vagus signals to ganglia in the viscera (e.g., celiac plexus) leading to activation of the splenic nerve. These nerve fibers are noradrenergic, so the release of norepinephrine in the spleen is increased. Norepinephrine stimulates the enzyme choline acetyltransferase (ChAT), an enzyme that is needed for the formation of acetylcholine. Splenic T cells express ChAT, leading to an increase in splenic acetylcholine. This leukocyte-derived neurotransmitter binds to α7-nicotinic acetylcholine receptors (α7nAChR) that are expressed on macrophages, resulting in the inhibition of TNFα synthesis. Note that an intraperitoneal inflammatory stimulus also activates the hypothalamic-pituitary axis, resulting in the release of adrenal glucocorticoids from the adrenal gland. This pathway inhibits dendritic cell and splenic inflammatory responses.
6. Conclusions
The long-standing dogma that immune cells secrete and respond only to cytokines while neurons secrete and respond only to neurotransmitters has undergone a major but poorly recognized revision during the past 25 years. It is now accepted that a complex interaction exists between the brain and the immune system. The brain mirrors peripheral immune responses to infection and stress with changes in cytokine and neurotrophin expression. This results in the brain mediating an orchestrated change in behavior (e.g., sickness behaviors) and physiology (e.g., sleep, anorexia and fever) before it initiates both neural- and endocrine-mediated feedback responses to modify the initial peripheral immune reaction. These responses have evolved to benefit the host and to increase its chances for survival. Although traditionally the immune and central nervous systems have been studied independently, an integrative research approach is needed to fully understand these two physiological systems.
Acknowledgements
This work was supported by grants to KWK (R01 AG 029573, R01 AG 029573- 04S1 and RO1 SUB UT 00000712) and RHM (RO1 MH 083767).
Footnotes
The authors have no conflicting financial interests.
References
- 1.Ader R, Cohen N, Felten DL. Brain, behavior, and immunity. Brain Behav Immun. 1987;1:1–6. doi: 10.1016/0889-1591(87)90001-8. [DOI] [PubMed] [Google Scholar]
- 2.Kelley KW, Johnson RW, Dantzer R. Immunology discovers physiology. Vet Immunol Immunopathol. 1994;43:157–165. doi: 10.1016/0165-2427(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 3.Raison CL, Miller AH. Malaise, melancholia and madness: the evolutionary legacy of an inflammatory bias. Brain Behav Immun. 2013;31:1–8. doi: 10.1016/j.bbi.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012;248:188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, et al. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(Suppl 1):S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 7.Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci. 2012;32:3958–3968. doi: 10.1523/JNEUROSCI.6389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCusker RH, Kelley KW. Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J Expt Biol. 2013;21:684–698. doi: 10.1242/jeb.073411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Mello C, Swain MG. Liver–brain interactions in inflammatory liver diseases: implications for fatigue and mood disorders. Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Lampron A, Elali A, Rivest S. Innate immunity in the CNS: redefining the relationship between the CNS and its environment. Neuron. 2013;78:214–232. doi: 10.1016/j.neuron.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Carare RO, Hawkes CA, Weller RO. Afferent and efferent immunological pathways of the brain. Anatomy, Function and Failure. Brain Behav Immun. 2014;36:9–14. doi: 10.1016/j.bbi.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Quan N, Banks WA. Brain–immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Wan W, Wetmore L, Sorensen CM, Greenberg AH, Nance DM. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res Bull. 1994;34:7–14. doi: 10.1016/0361-9230(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 14.Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 15.Marvel FA, Chen CC, Badr N, Gaykema RP, Goehler LE. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain Behav Immun. 2004;18:123–134. doi: 10.1016/j.bbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Takeshita Y, Ransohoff RM. Inflammatory cell trafficking across the blood–brain barrier: chemokine regulation and in vitro models. Immunol Rev. 2012;248:228–239. doi: 10.1111/j.1600-065X.2012.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komal P, Gudavicius G, Nelson CJ, Nashmi R. T-cell receptor activation decreases excitability of cortical interneurons by inhibiting alpha7 nicotinic receptors. J Neurosci. 2014;34:22–35. doi: 10.1523/JNEUROSCI.2093-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 19.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 20.Morrissey PJ, Charrier K, Alpert A, Bressler L. In vivo administration of IL-1 induces thymic hypoplasia and increased levels of serum corticosterone. J Immunol. 1988;141:1456–1463. [PubMed] [Google Scholar]
- 21.Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14:1262–1276. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010;24:1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 24.Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reardon C, Duncan GS, Brustle A, Brenner D, Tusche MW, Olofsson PS, et al. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc Natl Acad Sci U S A. 2013;110:1410–1415. doi: 10.1073/pnas.1221655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson U, Tracey KJ. A new approach to rheumatoid arthritis: treating inflammation with computerized nerve stimulation. Cerebrum. 2012;2012:3. [PMC free article] [PubMed] [Google Scholar]