Abstract
The Culicine mosquito, Aedes aegypti, is both a major vector of arthropod-borne viruses (arboviruses) and a genetic model organism for arbovirus transmission. TALE nucleases (TALENs), a group of artificial enzymes capable of generating site-specific DNA lesions, consist of a non-specific FokI endonuclease cleavage domain fused to an engineered DNA binding domain specific to a target site. While TALENs have become an important tool for targeted gene disruption in a variety of organisms, application to the mosquito genome is a new approach. We recently described the use of TALENs to perform heritable genetic disruptions in Ae. aegypti. Here, we provide detailed methods that will allow other research laboratories to capitalize on the potential of this technology for understanding mosquito gene function. We describe target site selection, transient embryo-based assays to rapidly assess TALEN activity, embryonic microinjection and downstream screening steps to identify target site mutations.
Keywords: Aedes aegypti, TALEN, gene editing, TALE effector, mosquito, meganuclease
1. Introduction
Aedes aegypti (Ae. aegypti) mosquitoes are vectors of several medically important viruses, such as yellow fever virus and the dengue viruses (Severson et al., 2004). Global increases in both the distribution of Ae. aegypti and dengue virus epidemics have been observed in the last 50 years (Yacoub et al., 2013). Dengue hemorrhagic fever, the more severe form of dengue, is also increasing (Back and Lundkvist, 2013). In total, more than 2.5 billion people, mostly in tropical and subtropical regions, are potentially at risk for dengue (Guzman et al., 2010).
The genome sequence for Ae. aegypti was published in 2007 (Nene et al., 2007). While substantial transcriptomic data is available for this mosquito in response to a range of stimuli such as pathogen infection (Bonizzoni et al., 2012), insecticides/pollutants (David et al., 2010), and bloodfeeding (Dissanayake et al., 2010, Bonizzoni et al., 2011), as well as in various tissues/developmental stages (Biedler et al., 2012, Akbari et al., 2013, Price et al., 2011), little direct experimental evidence supports the function of most genes. TALENs could be used for targeted gene disruption as a means to study dsDNA break repair, gene function and to develop a possible mechanism for gene drive. Increasing knowledge about vectors at a genetic level may help provide valuable information for population control by fueling an understanding of insecticide response, innate immunity, genome evolution, and genetic manipulation of mosquitoes (Blair et al., 2000, Severson et al., 2004).
The development of Transcription Activator-Like Effector Nucleases (TALENs). TALENs and other site specific nucleases (Gaj et al., 2013, Sun and Zhao, 2013) have dramatically altered the landscape for reverse genetics-based applications. These enzymes have been successfully used to modify a number of model and non-model organisms by generating small insertions and deletions that result from endogenous DNA repair of double-strand breaks in the genome at specific target DNA sequences. A number of insect genomes have been successfully targeted, including the model organism Drosophila melanogaster (Liu et al., 2012), the silkworm Bombyx mori (Sajwan et al., 2012, Ma et al., 2012), the cricket Gryllus bimaculatus (Watanabe et al., 2012) and the malaria mosquito Anopheles gambiae (Smidler et al., 2013). In this paper we provide a step-by-step protocol for targeted gene editing of the Ae. aegypti genome based on recent findings indicating useful frequencies of TALEN-mediated genome editing in this disease vector (Aryan et al., 2013a).
2. Materials
2.1. Plasmid construction
2.1.1. TALEN constructs
PUb promoter-based expression vector (Anderson et al., 2010)
Assembled TALEN
Restriction enzymes (NEB, Ipswich, MA)
Primer pairs (250 μM)
Platinum Pfx (Invitrogen, Carlsbad, CA)
MgSO4 (Invitrogen, Carlsbad, CA)
10 mM solution of mixed deoxynucleotides (Promega, Madison, WI)
10X PCR buffer (Invitrogen, Carlsbad, CA)
PCR cycler (Bio-Rad, Hercules, CA)
Loading buffer
DNA ladder (NEB, Ipswich, MA)
Endo-free Maxi-prep kit (Qiagen, Valencia, CA)
Endo-free Midi kit (Machery-Nagel, Bethlehem, PA)
2.1.2. Single-Strand Annealing (SSA) assay
Target site oligonucleotides
Firefly luciferase based SSA reporter plasmid (Aryan et al., 2013a)
Dual-Luciferase reporter assay kit (Promega, Madison, WI)
GloMax Multi+ Microplate Multimode instrument (Promega, Madison, WI)
2.2. Mosquito mutagenesis
2.2.1. Mosquito rearing
Fish food (Tetra, Madison, WI)
12″×12″×12″ Colony cage (Bioquip, CA)
Defibrinated sheep blood (Colorado Serum Company, Denver, CO)
10% sucrose
2.2.2. Manufacture and beveling of needles
Glass microcapillaries (Kwik-Fil, Sarasota, FL)
Sutter P-2000 Micropipette puller (Novato, CA, USA)
Sutter BV-10 Microelectrode Beveller (Novato, CA, USA)
2.2.3. Microinjection of embryos
Flash light aspirator (Bioquip, CA)
Tweezers (Dumont #5 Inox 11cm)
Filter paper (3MM Whatman, PA)
Coverslip (Thermo Fisher, MA)
Double-sided tape (Scotch, St. Paul, MN)
Halocarbon oil 27 (Sigma, St. Louis, MO)
Leica micromanipulator (Buffalo Grove, IL, USA)
FemtoJet microinjector (Eppendorf, Westbury, NY, USA)
1X injection buffer, [5 mM KCl and 0.1 mM NaH2PO4 (pH 6.8)](Coates et al., 1998)
2.3. PCR and mutational analysis
Gene-specific primers
Phire Animal Tissue DirectPCR kit (Thermo Scientific, Lafayette, CO)
PCR cycler (Bio-Rad, CA)
NucleoSpin Gel and PCR clean-up kit (Machery-Nagel, Bethlehem, PA)
3. Methods
3.1. Overview
The goal of this paper is to provide methods for targeted gene disruption in the mosquito Ae. aegypti. Despite complete sequencing of the Ae. aegypti genome, the functions of most encoded genes remain unknown. Thus, we have adapted techniques developed to generate transgenic mosquitoes (Jasinskiene et al., 2007), in order to achieve targeted gene disruption with TALENs (Aryan et al., 2013a). The ability to directly and specifically disrupt a gene of interest will be vital to the future study of mosquito biology, behavior and pathogen transmission.
Using embryonic microinjection to introduce exogenous plasmid DNA into the developing germline of Ae. aegypti embryos, we first tested the ability of a pair of TALENs to recognize their target site in a highly sensitive luciferase-based reporter assay, capable of detecting single-strand annealing (SSA)-based repair of TALEN-induced double-stranded DNA (dsDNA) breaks. TALENs that display sufficient editing activity in this assay are considered good candidates for germline-based experiments. Our results indicate that TALEN-based disruption of Ae. aegypti genes can be a highly efficient process, with rates of gene editing between 20–40% (Aryan et al., 2013a). Though this protocol was developed using an easily scored physical marker, TALEN mutagenesis efficiencies were sufficiently robust as to allow detection of mutant alleles through a PCR/sequencing approach, similar to methods recently described in reports using Zinc-Finger Nucleases (ZFN) to modify the Ae. aegypti genome (DeGennaro et al., 2013, Liesch et al., 2013). Thus, a next step could be using TALEN-based enzymes to stimulate the knock-in of a transgene (Liesch et al., 2013), or the incorporation of a single-stranded oligonucleotide through homologous recombination.
3.2. Target site selection
Site-specific gene editing is the near complete realization of reverse genetics in non-model organisms. However, there are some theoretical limitations to the genomic sequences that can be potentially targeted with TALENs. TALENs designed to target highly repetitive sequences will introduce numerous double-stranded DNA breaks, potentially leading to chromosomal instability. Targeting unique portions of the genome is likely to be simpler. The number of TALE repeats can be manipulated to help achieve this, as more repeats increase the number of specific interactions necessary for DNA binding. Binding of TALEs to off-target sites may decrease survival rates and increase sterility in the resulting progeny. However, unlike vertebrate systems, where costs per animal can be substantial, the mosquito’s short generation time permits rapid production and manipulation. Thus, it is not absolutely necessary that the sequences targeted are unique, as this can be sorted out after the fact with a PCR-based strategy. This is an important point, as the Ae. aegypti genome contains a large number of recently duplicated genes and/or pseudogenes, many of which are interesting targets for TALEN mutagenesis.
Towards the development of a standardized methodology, an initial TALEN pair was assembled targeting the Ae. aegypti kmo gene. One of few surviving physical mutant strains from a formerly extensive collection (Craig and Hickey, 1967), this strain contains a mutation in this gene, allowing us to perform complementation-based assays to quickly identify new alleles (Wendell et al., 2000). Loss of KMO activity is associated with loss of eye pigmentation, substantially simplifying the downstream screening steps. A gene corresponding to the full length Ae. aegypti kmo was annotated as AAEL008879 on supercontig 1.354 of the current genome assembly (AaegL1). Another gene, AAEL012757, located on supercontig 1.736, shared the first 5 exons (of 7) and was 98.3% identical to the full length kmo gene at the nucleotide level. As this second gene model was incomplete, we reasoned that it may represent a pseudogene. We later determined that loss of gene AAEL0088979 was sufficient to disrupt eye pigmentation, confirming that AAEL012757 is indeed non-functional (Aryan et al., 2013a). An initial ~300 bp amplicon at our selected target site included both genomic locations, while a second ~700bp amplicon, where one primer was placed in a more divergent intron, succeeded in amplifying only the true kmo gene. Thus, we propose the following steps for selecting target sequences in the Ae. aegypti genome:
Choose gene/sequence of interest.
Perform tblastx search using the translated ORF of the target protein, and/or blastn search using the target against the current genome assembly (contigs/scaffolds). Identify any regions that are identical or near identical to your target gene. Highly repetitive sequences should be avoided, but the presence of one or a few highly homologous sequences may not in and of itself be an obstacle.
Design and test PCR primers to amplify your target region. As long as primers can be designed from a more divergent flanking region to obtain specific amplicons, it may even be beneficial to target a sequence with multiple occurrences. For example, a single TALEN pair may be designed to target all four genes of a multi-gene family, as long as four unique amplicons could be obtained during the screening step.
Verify target region is free from SNPs. This is likely the most critical step in the TALEN design process, due to the high specificity of TALE binding and the abundance of SNPs in various lab and field strains of Ae. aegypti. For this step, it is best to pool DNA extracted from a large number of individuals (n=50–100) to be sure of capturing an approximately representative allele frequency. Perform PCR using each target pair of primers, and sequence the product directly. Fixed differences from the genomic reference are acceptable, as long as they are incorporated into the TALE design, while polymorphic nucleotides should be marked and avoided.
Use your favorite TALE design software [For example E-TALEN (Heigwer et al., 2013) or MojoHand (Neff et al., 2013)] to select the precise location of TALE binding within the target region. If the goal is to generate frameshift mutations in an ORF, then attention should be paid to several other factors such as the presence of alternative start codons, alternative promoters or splice variants which may confound experimental outcomes. TALENs can be assembled directly using published methods (Kim et al., 2009, Kim et al., 1996, Bitinaite et al., 1998) or can be purchased through a number of commercial vendors.
3.3. Validation of TALEN activity in early embryos
3.3.1. Promoter-driven TALEN expression
Heritable gene editing relies on the expression of active nucleases in the developing germ tissue of an organism. In mosquitoes, active ZFN, Homing endonucleases (HE), or TALEN nucleases have been generated through the injection of purified messenger RNA (DeGennaro et al., 2013, Liesch et al., 2013), transcriptional activity from a set of integrated transgenes (Smidler et al., 2013), or through transient expression from an injected DNA plasmid (Aryan et al., 2013a, Aryan et al., 2013b). In order to determine the best promoter for expression of TALENs in Ae. aegypti, we used the dual-luciferase assay system to compare the transcriptional capacity of four different promoters (D. pseudoobscura hsp82, baculovirus IE1 and Ae. aegypti UbL40 and PUb) in early Ae. aegypti embryos. As the expression of luciferase by the PUb promoter was more than 1000X greater than any other promoter at early timepoints (2–4hr), and 10–100X greater at later times (12–72hr) (Aryan et al., 2013b), we chose a PUb-based vector for expression of TALEN constructs. All of the TALEN ORFs were cloned in a manner that retained the original context surrounding the translational start of the PUb gene, including the 5′UTR and corresponding intron (Anderson et al., 2010). To generate a plasmid vector for rapid insertion of TALEN constructs (referred to as pSLfa-PUb MCS) the EGFP gene was excised from pSLfa-PUb-EGFP-SV40 (Anderson et al., 2010) with NcoI/NotI and replaced with an MCS (annealed oligonucleotides). Assembled TALEN constructs were ligated into pSLfa-PUb/MCS to create pSLfa-PUb-TALEN-KMO (R/L). Initially, DNA for each of the PUb-TALEN plasmids was prepared using the Qiagen Endo-free Maxi-prep kit (Qiagen, Valencia, CA); DNA free of bacterial endotoxins has historically been of sufficient purity for mosquito transgenic experiments. However, during our experiments, we discovered that the Machery-Nagel endo-free midi kit is quicker, has a simpler protocol, and is less expensive than the Qiagen Endo-free Maxi-prep kit. Also, a smaller volume of overnight culture (100ml) is required using the Machery-Nagel endo-free midi kit, in comparison to the culture volume (250ml) required for the Qiagen Endo-free Maxi-prep kit. Both kits produced similar rates of gene editing in Ae. aegypti mosquitoes (Aryan et al., 2013a). Other endo-free DNA preparation methods also may produce DNA of acceptable quality.
Our initial experiments used in vitro generated RNA, rather than DNA-based plasmid expression. While we did detect significant SSA activity and heritable gene editing, as described below, in both cases TALEN activity was substantially lower using mRNA than that observed during DNA plasmid-based expression (data not shown). Thus, while subcloning a set of TALENs into a mosquito-specific expression plasmid may add an extra step, it may be accompanied by a concomitant increase in the probability of success. While we acknowledge that purified RNA may also be acceptable, we prefer DNA-based expression due to ease of manipulation after some initial cloning steps.
3.3.2. Single-Strand Annealing test assay
TALEN constructs can vary widely in their ability to generate DNA breaks at different target sites. This variability is not well understood, but may be influenced by both TALEN architecture and chromosomal structure at the target site, including bound protein complexes. Thus, it is critical to validate each new TALEN pair in a transient assay (24hr) prior to initiating germline-based editing experiments, which often take 2–3 months. This section describes a single-stranded annealing (SSA)-based assay optimized for evaluating new TALEN constructs in Ae. aegypti embryos.
Step1: Clone target site (including both TALE binding regions and spacer) into the spacer region of the plasmid pUb-SSA-FFluc (Aryan et al., 2013a)
This places the target site in between two direct repeats corresponding to the first 300 bp of the firefly luciferase ORF. Successful double-strand DNA break induction can be repaired via the single-stranded annealing pathway [reviewed in (Smith et al., 2000)], resulting in collapse of the direct repeats and restoration of the firefly luciferase ORF (Aryan et al., 2013a, Aryan et al., 2013b). Functionally, the activity of the TALEN is proportional to the observed increase in firefly luciferase activity. As SSA-based repair is efficient even when direct repeats are spaced by hundreds or thousands of nucleotides (Porteus and Carroll, 2005), multiple target sites can be assembled into a single SSA vector using gene synthesis technology. For example (Fig. 1A), we have assembled a single sequence containing 5 homing endonuclease sites and 3 TALEN target sites (Aryan et al., 2013a, Aryan et al., 2013b). A multiple cloning site containing 4 unique restriction enzyme sites can be used to insert the synthetic fragment (Fig. 1A). Thus, a large cohort of TALEN constructs can be evaluated with a single SSA test vector.
Figure 1. Rapid evaluation of TALEN activity using an SSA-based test vector.
(A) Design and concept of the SSA test vector. TALEN target sites are placed into the multiple cloning site (BglII-PstI-PacI-XhoI), interspersed with at least 5 termination codons in each reading frame to prevent read-through of luciferase. TALEN activity results in a double-stranded DNA break (lightning bolt symbol). Homology between direct repeats guides the SSA-based repair process resulting in the production of functional luciferase. (B) Determination of optimal TALEN expression vector concentration in SSA assay in Ae. aegypti early embryos. Each point represents the ratio of Firefly luciferase (FF-luc) to Renilla luciferase (R-luc) in ~100 Ae. aegypti embryos 24 hr post injection. Letters represent statistically distinct groups (ANOVA, Kruskal-Wallis test, p=0.0027; Dunn’s Multiple Comparison Post test). Horizontal lines indicate the mean, error bars represent S.E.M.
Step2: Inject purified expression vectors encoding TALENs, SSA test construct and normalization control
For transgenic insertion experiments, we usually inject a maximum DNA concentration of ~800 ng/μl; at higher DNA concentrations the viscosity of the solution begins to exceed the limits of the microcapillary, and the needle clogs. In order to identify the optimal concentration of TALEN expression plasmids [pSLfa-PUb-TAL-KMO (R/L)] for gene editing experiments, TALEN plasmids (25 ng/μl–200 ng/μl) were coinjected with SSA test plasmid and pGL3-hsp82-REN control plasmids into Ae. aegypti embryos (Fig. 1B). Activation of the SSA construct was proportional to the amount of TALEN plasmid injected and reached a plateau at ~100 ng/μl, suggesting for maximum sensitivity TALEN expression plasmid concentration should be 100–200 ng/μl. Typical DNA concentrations for each plasmid are listed in Table 1. See section 3.4 for a detailed description of the microinjection procedure.
Table 1.
DNA concentrations for SSA-based transient TALEN assay
| Plasmid | Role | Concentration |
|---|---|---|
| pPUb-TALEN (R) | TALEN | 100–200 ng/μl |
| pPUb-TALEN (L) | TALEN | 100–200 ng/μl |
| pPUb-SSA-FFluc | SSA reporter | 200 ng/μl |
| pKhsp82-REN | Normalization control | 200 ng/μl |
Step 3: Age injected embryos 24–48 hr, lyse and measure luciferase activity
The PUb promoter is highly active in early embryos, and substantial TALEN protein is expected to be produced within hours of microinjection. Expression is robust throughout development, thus embryos can be harvested when convenient, typically at 24hr, and stored at −80C until processing. Embryos should be homogenized in the lysis buffer provided by the manufacturer (Promega, Madison, WI). Activity of both firefly luciferase (FF-luc) and Renilla luciferase (R-luc) are determined by using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) with a GloMax Multi+ Microplate Multimode instrument according to the manufacturer’s protocol. Typically, we will perform6 replicates of 100–120 embryos for each new TALEN to ensure sufficient sample for statistical comparisons.
3.4. Mosquitoes mutagenesis
3.4.1. Mosquito rearing
Ae. aegypti mosquitoes were reared at 28°C and 50–60% humidity with a photoperiod of 10 hours dark and 14 hours light (Adelman et al., 2008). Larvae were fed with pulverized fish food in 4 liters of reverse osmosis purified water until pupation. Larvae were hatched and reared at a density of approximately 300 per pan. At higher densities, larval competition results in smaller bodied adults. We have observed that these smaller adults also produce smaller eggs, making the injection process more difficult. Thus, larval density should be manipulated to produce the largest adults possible. During larval rearing, the water should be exchanged at the first signs of cloudiness resulting from excessive fungal or bacterial growth. Overfeeding should be avoided. Pans should be checked for pupae each day, which should be picked and placed in a colony cage (Bioquip, CA). Adult mosquitoes were maintained on 10% sucrose and blood-fed using artificial membrane feeders and defibrinated sheep blood (Colorado Serum Company, Denver, CO). For convenience, we recommend performing bloodfeeds on Thursday/Friday, so that the mosquitoes are ready to lay eggs for microinjections the entire following week.
3.4.2. Manufacture and beveling of needles
For embryo microinjection, microcapillary needles (CAT# 1B100-4, Kwik-Fil, Sarasota, FL) were manufactured using a Sutter P-2000 Micropipette puller (Novato, CA, USA) (Heat=270, FIL=3, VEL=37, DEL=250, PUL=140). The Sutter BV-10 Microelectrode Beveller was used to bevel the needles to an angle of ~20°. After beveling, each needle was examined under magnification to ensure the opening of the needle was suitable. We usually bevel a large cohort of needles ahead of time (20–40), check them under the microscope (100×–200× total magnification) to ensure they are not too big or broken (Fig. 2), and then store them. Needles are placed into large (150×15mm) petri dishes containing a strip of clay or putty to immobilize them and to prevent either end of the needle from contacting the surface of the dish or each other. Dishes are sealed with parafilm to prevent dust from clogging the finished needles. Any dust in the room or on the bench can clog the needle, therefore cleaning the room and bench frequently is recommended.
Figure 2. Optimal needle shape for Ae. aegypti embryonic microinjection.
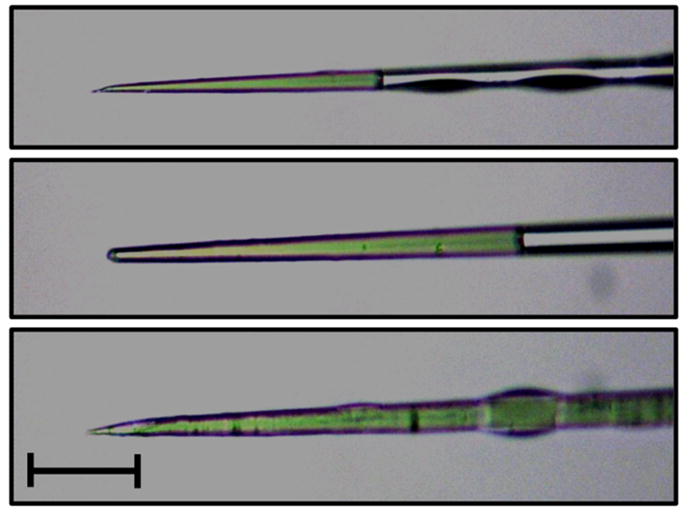
Photographs of a high quality needle with optimally beveled tip (top), a broken/blunt needle (middle), and a needle with appropriate bevel but with a bore size that is too large (bottom). Needles are filled with green food coloring to increase contrast. Scale bar indicates 50 μM.
3.4.3. Microinjection of embryos
Step 1: Collect eggs
Three to four days after blood feeding (typically Monday if the mosquitoes were fed the previous Thurs/Fri), ~20 gravid female mosquitoes should be placed in a 50 ml conical tube that had been prepared with a piece of water-saturated cotton at the bottom and a damp piece of filter paper covering the cotton. A flash light aspirator (Bioquip, CA) can be used to transfer mosquitoes from the cage to the tube, with the stoppered tube left in the dark. After one hour, the mosquitoes should be removed and the filter paper extracted. Typically, there are sufficient eggs to produce 2–3 lines as described below.
Step 2: Generate line (n=~120)
Approximately 100 to 120 gray to darkish-gray embryos (Fig. 3) should be transferred to a new piece of filter paper using tweezers (Dumont #5 Inox 11cm). Embryos are arranged in a line on damp filter paper (3MM Whatman, PA) under a dissecting microscope. All embryos should be oriented in the same direction to allow injection of the posterior pole. To facilitate efficient embryo transfer to a coverslip (Thermo Fisher, MA), the filter paper should be dried using extra filter paper pieces. Transfer of the embryos involves inverting a coverslip that had been prepared with double-sided tape (Scotch, St. Paul, MN) and gently pressing it onto the embryos. The posterior ends of the embryos should be very close, but not at, the edge of the double sided tape.
Figure 3. Ae. aegypti embryos for microinjection.

Immediately after oviposition, Ae. aegypti embryos are white and too soft to be injected without clogging the microcapillary needle. After ~45 minutes they begin to darken, and by 2 hrs have become too rigid to inject. The preferred stages for injection, as well as anterior (A) and posterior (P) poles, are indicated.
Step 3. Desiccation
Embryos require slight desiccation prior to needle puncture to relieve internal pressure, which would otherwise cause the injected embryo to burst. Embryos should be desiccated at room temperature and observed carefully to assess the ‘dimpling’ indicative of the appropriate level of desiccation. This time varies depending on the room temperature and humidity, but is roughly 20–60 sec. When the appropriate level of desiccation is reached (Fig. 4), embryos should be covered with halocarbon oil 27 (Sigma, St. Louis, MO) to prevent over-desiccation. At this point, the coverslip containing the embryos is placed on a stack of glass slides on a compound microscope stage. To prevent the coverslip from moving around on the slide, a small drop of water should be placed first, allowing the surface tension to immobilize the coverslip.
Figure 4. Desiccation of Ae. aegypti embryos prior to microinjection.
Time lapse photos of an Ae. aegypti embryo after transfer from filter paper to the coverslip. The optimal time for desiccation depends on the humidity and temperature of the manipulation room, but can be identified by the first appearance of dimpling on the embryos (white arrows).
Step 4. Fill needle
At this point, the needle can be backfilled with ~2 μl of injection mix [300 ng/μl pPUb-TAL EN (R) and 300 ng/μl pPUb-TALEN (L) in 1X injection buffer] by using a microloader tip (CAT# 5242956.003, Eppendorf, Westbury, NY, USA). We perform all microinjections using a Leica micromanipulator (Buffalo Grove, IL, USA) and a FemtoJet microinjector (Eppendorf, Westbury, NY, USA) under 100× magnification (10× eyepiece and 10× objective of the compound microscope). We recommend having a 20× objective on hand as well, as it is sometimes easier to evaluate the quality of microcapillary needles at 200× magnification. Injection mixes should be kept on ice when not in use and typically need to be re-centrifuged almost every 2 hours to prevent fine debris from clogging the needle.
Step 5. Injection
Once inserted into the layer of oil covering the line of embryos, DNA solution should be observed to flow at a slow but steady rate out of the needle. Fast flow indicates that the needle bore size is too large, or that the pressure is set too high. Failure to observe any injection solution leaving the needle could indicate that bore size is too small, the viscosity of the DNA solution is too high, the pressure is too low, or that the needle is clogged with debris. After making any adjustments as needed to the pressure, and replacing any low quality needles, embryos should be injected into the posterior pole by using the stage controls to move the embryos on to the needle tip. If needles become continually clogged, this is a sign that the viscosity of the solution is too high. In this case the injection mix should be re-generated from the stock plasmids, taking care to centrifuge each at high speed (13,000g) prior to pipetting, with quantification repeated to be sure the total DNA plasmid concentration is in the acceptable range (≤800 ng/μl). Excessive blocking of the needle by yolk protein from the injected embryos is a sign that the embryos are too young (Fig. 3, white-gray). In contrast, there will typically be a few embryos that aged to the point where they have become too hard to inject without breaking the needle tip (Fig. 3, black). To avoid including these in downstream steps, gently push them backwards using the needle, making it easy to identify and avoid transferring the over aged embryos to filter paper in the subsequent step. Likewise, some embryos may move unexpectedly, or otherwise not be in an acceptable position for injection. These should also be moved back from the main line or injected and intentionally burst. Ideally, the entire injection procedure should be completed within 3–5 minutes, though when multiple needle changes are required more time is typically required (10–15 min). At longer times, mortality due to the halocarbon oil starts to become an issue.
Step 6. Transfer back to filter paper and remove oil
Immediately following injection, halocarbon oil should be drained off by touching the coverslip to a dry piece of filter paper. Using fine tweezers (Dumont #5 Inox 11cm) embryos are transferred from the cover slip to a piece of wet filter paper (3MM Whatman, PA) and placed in a plastic beaker with wet cotton.
Step 7. Return to insectary
When all injections for the day have been completed, beakers are covered with piece of parafilm and kept under insectary conditions. After 5 days, each paper containing injected embryos should be placed (embryo-side down) in water in a plastic rearing pan with a small amount of ground fish food. The pans should be checked every day for at least 2 weeks after injection (injected embryos hatch over a long time due to variable delays in development) to pick the pupae. We separate G0 survivor pupae into single vials (Genesee Scientific Corporation, San Diego, CA); emergent adults are collected each day and transferred into male-only or female-only cages. As an initial sign of TALEN activity, G0 females can be screened for somatic editing. With our initial TALEN pair targeting the kmo gene, we were able to perform this screening visually, through cell autonomous disruption in eye pigment formation. We observed that ~15% of G0 females displayed obvious disruptions in eye pigmentation, indicating TALEN-mediated gene editing of both copies of the kmo gene was occurring at high frequency (Fig. 5). Where phenotypes are not obvious, this initial measure of somatic activity can be performed through PCR-based approaches (Bassett et al., 2013) or through next-generation sequencing (DeGennaro et al., 2013). Regardless of how somatic activity is determined, downstream experiments should be focused on those individuals displaying the greatest level of somatic activity.
Figure 5. Somatic TALEN editing of Ae. aegypti injected G0 individuals.

White-light photographs of wild-type (A) or TALEN-edited (B–D) G0 individuals that had been injected with a TALEN pair targeting the Ae. aegypti kmo gene.
3.5. Complementation with known mutant alleles
In some instances, it may be possible to observe a phenotypic change with mutagenesis of just a single chromosome. This may be in cases where the additive effects of both gene copies are essential, where only one copy exists (Y-chromosome), or where existing mutant alleles are available. In these cases, new mutant alleles can be readily identified phenotypically in the 1st generation following injection (G1). We performed this experiment using the khw strain of Ae. aegypti, which is expected to fail to complement any new mutations in the kmo gene. Pools of 20–25 individual G0 females were mated to 15–20 males of the complementary strain prior to bloodfeeding and egg collection. G0 males were anesthetized under CO2 and mated individually to 5 virgin females for two to three days, at which point they were combined into families. After the first bloodmeal, G1 larvae were screened for failure to complement. When TALEN activity is high, G0 females can be placed directly into single rearing tubes and allowed to deposit eggs individually. This is our preferred method, as screening of G1 progeny for failure to complement, as well as direct determination of TALEN expression on mosquito fertility can be performed more precisely. The bodies of both G0 founder females and any G1 positive individuals were snap frozen and used for PCR once progeny had been obtained.
3.6. Identifying novel TALEN-induced deletions through PCR and sequencing
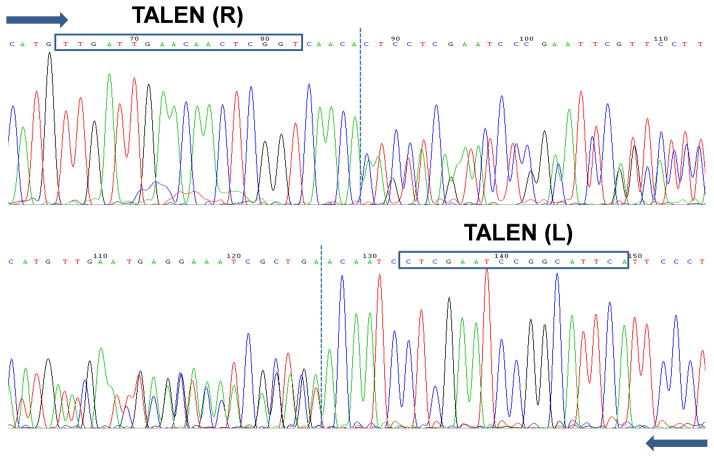
To identify TALEN-induced deletions at the recognition site in the absence of a physical marker, PCR primers were used to amplify a 707 bp product. Using the Phire Animal Tissue Direct PCR kit (Thermo Scientific, Lafayette, CO), an adult leg was placed directly in the master mix to be used as template as described by the manufacturer. We note that the absolute size of the amplicon is not critical, and in this case was dictated by the presence of the confounding kmo pseudogene. As stated above, amplicons should be sized to generate an identifiably unique product. All amplicons were purified using the NucleoSpin Gel and PCR clean-up kit and sequenced directly in both directions using the same primers used during the PCR step. When only a single allele is disrupted, sequence quality will be high up until the deletion site, at which point each nucleotide position will be represented by two different bases (Fig. 6). More commonly, two intact alleles will yield a high quality sequence throughout. Given an editing rate similar to the kmo TALEN pair, sequencing 10 PCR amplicons (10 G1 individuals) from each of 10 G0 female founders (100 amplicons total) would have been sufficient to identify approximately 3 unique deletions, with multiple mutant individuals for each (Aryan et al., 2013a). This corresponds to a single 96-well PCR plate/cleanup and with sequencing could be completed in just a few days.
Figure 6. PCR identification of single allele disruptions.
Chromatograms obtained from sequenced PCR amplicons with one intact and one disrupted kmo allele at the TALEN target site. TALE binding regions are indicated. Block arrows indicate the direction of sequencing. Dotted lines indicate the transition from single to double peaks.
4. Summary
Ae. aegypti is the most important vector of arboviruses worldwide, and is a model for other mosquito vectors of disease agents. A sequenced genome (Nene et al., 2007), detailed gene expression data (Akbari et al., 2013), and the ability to perform genetic manipulations such as transgene insertion (Jasinskiene et al., 1998, Coates et al., 1998) and site-specific recombination (Nimmo et al., 2006) lend this mosquito to advanced studies of genetic factors involved in critical mosquito behaviors such as pathogen transmission, host seeking and bloodfeeding. The ability to generate targeted gene knockouts using the protocol we have developed should greatly accelerate our understanding of these phenotypes, and suggest new methods of controlling disease transmission.
We emphasize that the protocol presented here represents a starting point, as it is likely that the efficiency of TALEN-based editing experiments can likely be further improved. For example, the design of TALEN pairs centered on a native restriction site would allow the development of an RFLP assay able to identify single allele disruptions in a dominant fashion (Bedell et al., 2012, Liu et al., 2012, Zhang et al., 2013). Though this would reduce the number of TALEN pairs per gene, for strict loss-of-function experiments the number of valid targets within each gene would still be expected to be substantial. Alternatively, the incorporation of single-stranded DNA oligonucleotides into the DSB site could be used to introduce dominant-acting restriction sites (Bedell et al., 2012). The CEL I-based mismatch-recognition enzyme, Surveyor nuclease, has also been used to detect TALE-based editing events (Watanabe et al., 2012, Carlson et al., 2012). In our experiments, CEL I-based approaches were unsuccessful due to false positives resulting from the presence of the kmo pseudogene (data not shown). We note that with some minor modifications, the protocol described here could very easily be adapted to the CRISPR/CAS9 system, which has been highly successful in a number of organisms including D. melanogaster (Bassett et al., 2013, Gaj et al., 2013, Mali et al., 2013, Carroll, 2013). Finally, complete knock-in experiments using Zinc-Finger Nucleases have recently been reported for Ae. aegypti (Liesch et al., 2013), albeit at low frequency (<1%). Knock-in experiments allow detection and tracking of mutant alleles and would substantially decrease the time, effort and cost of TALEN knockout experiments; increasing the efficiency of this strategy is of the highest priority.
Highlights.
A protocol for TALEN-based editing of the Aedes aegypti genome has been developed.
Transient SSA reporter assay can identify active TALENs for germline experiments.
Somatic TALEN editing is common and is a good indicator for germline editing.
PCR followed by sequencing can identify single allele disruptions in G1 progeny.
Acknowledgments
The project described was supported by grant number [AI085091 and AI099843] from NIAID and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ADELMAN ZN, ANDERSON MA, MORAZZANI EM, MYLES KM. A transgenic sensor strain for monitoring the RNAi pathway in the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol. 2008;38:705–13. doi: 10.1016/j.ibmb.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKBARI OS, ANTOSHECHKIN I, AMRHEIN H, WILLIAMS B, DILORETO R, SANDLER J, HAY BA. The developmental transcriptome of the mosquito Aedes aegypti, an invasive species and major arbovirus vector. G3 (Bethesda) 2013;3:1493–509. doi: 10.1534/g3.113.006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON MA, GROSS TL, MYLES KM, ADELMAN ZN. Validation of novel promoter sequences derived from two endogenous ubiquitin genes in transgenic Aedes aegypti. Insect Mol Biol. 2010;19:441–9. doi: 10.1111/j.1365-2583.2010.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARYAN A, ANDERSON MA, MYLES KM, ADELMAN ZN. TALEN-based gene disruption in the dengue vector Aedes aegypti. PLoS One. 2013a;8:e60082. doi: 10.1371/journal.pone.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARYAN A, ANDERSON MAE, MYLES KM, ADELMAN ZN. Germline excision of transgenes in Aedes aegypti by homing endonucleases. Sci Rep. 2013b;3 doi: 10.1038/srep01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACK AT, LUNDKVIST A. Dengue viruses - an overview. Infect Ecol Epidemiol. 2013;3 doi: 10.3402/iee.v3i0.19839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASSETT ANDREWR, TIBBIT C, PONTING CHRISP, LIU JL. Highly Efficient Targeted Mutagenesis of Drosophila with the CRISPR/Cas9 System. Cell Reports. 2013;4:220–228. doi: 10.1016/j.celrep.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEDELL VM, WANG Y, CAMPBELL JM, POSHUSTA TL, STARKER CG, KRUG RG, 2ND, TAN W, PENHEITER SGMAAC, LEUNG AY, FAHRENKRUG SC, CARLSON DF, VOYTAS DF, CLARK KJ, ESSNER JJ, EKKER SC. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491:114–8. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIEDLER JK, HU W, TAE H, TU Z. Identification of early zygotic genes in the yellow fever mosquito Aedes aegypti and discovery of a motif involved in early zygotic genome activation. PLoS One. 2012;7:e33933. doi: 10.1371/journal.pone.0033933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITINAITE J, WAH DA, AGGARWAL AK, SCHILDKRAUT I. FokI dimerization is required for DNA cleavage. Proceedings of the National Academy of Sciences. 1998;95:10570–10575. doi: 10.1073/pnas.95.18.10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAIR CD, ADELMAN ZN, OLSON KE. Molecular strategies for interrupting arthropod-borne virus transmission by mosquitoes. Clin Microbiol Rev. 2000;13:651–61. doi: 10.1128/cmr.13.4.651-661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONIZZONI M, DUNN WA, CAMPBELL CL, OLSON KE, DIMON MT, MARINOTTI O, JAMES AA. RNA-seq analyses of blood-induced changes in gene expression in the mosquito vector species, Aedes aegypti. BMC Genomics. 2011;12:82. doi: 10.1186/1471-2164-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONIZZONI M, DUNN WA, CAMPBELL CL, OLSON KE, MARINOTTI O, JAMES AA. Complex modulation of the Aedes aegypti transcriptome in response to dengue virus infection. PLoS One. 2012;7:e50512. doi: 10.1371/journal.pone.0050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSON DF, TAN W, LILLICO SG, STVERAKOVA D, PROUDFOOT C, CHRISTIAN M, VOYTAS DF, LONG CR, WHITELAW CB, FAHRENKRUG SC. Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci U S A. 2012;109:17382–7. doi: 10.1073/pnas.1211446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL D. Staying on target with CRISPR-Cas. Nat Biotechnol. 2013;31:807–9. doi: 10.1038/nbt.2684. [DOI] [PubMed] [Google Scholar]
- COATES CJ, JASINSKIENE N, MIYASHIRO L, JAMES AA. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proceedings of the National Academy of Sciences. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAIG GB, JR, HICKEY WA. Genetics of Aedes aegypti. In: WRIGHT JWRP, editor. Genetics of Insect Vectors of Disease. New York: Elsevier; 1967. pp. 67–131. [Google Scholar]
- DAVID JP, COISSAC E, MELODELIMA C, POUPARDIN R, RIAZ MA, CHANDOR-PROUST A, REYNAUD S. Transcriptome response to pollutants and insecticides in the dengue vector Aedes aegypti using next-generation sequencing technology. BMC Genomics. 2010;11:216. doi: 10.1186/1471-2164-11-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEGENNARO M, MCBRIDE CS, SEEHOLZER L, NAKAGAWA T, DENNIS EJ, GOLDMAN C, JASINSKIENE N, JAMES AA, VOSSHALL LB. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498:487–91. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISSANAYAKE SN, RIBEIRO JM, WANG MH, DUNN WA, YAN G, JAMES AA, MARINOTTI O. aeGEPUCI: a database of gene expression in the dengue vector mosquito, Aedes aegypti. BMC Res Notes. 2010;3:248. doi: 10.1186/1756-0500-3-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAJ T, GERSBACH CA, BARBAS CF., 3RD ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUZMAN MG, HALSTEAD SB, ARTSOB H, BUCHY P, FARRAR J, GUBLER DJ, HUNSPERGER E, KROEGER A, MARGOLIS HS, MARTINEZ E, NATHAN MB, PELEGRINO JL, SIMMONS C, YOKSAN S, PEELING RW. Dengue: a continuing global threat. Nat Rev Micro. 2010 doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIGWER F, KERR G, WALTHER N, GLAESER K, PELZ O, BREINIG M, BOUTROS M. E-TALEN: a web tool to design TALENs for genome engineering. Nucleic Acids Research. 2013 doi: 10.1093/nar/gkt789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JASINSKIENE N, COATES CJ, BENEDICT MQ, CORNEL AJ, RAFFERTY CS, JAMES AA, COLLINS FH. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proc Natl Acad Sci U S A. 1998;95:3743–7. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JASINSKIENE N, JUHN J, JAMES AA. Microinjection of A. aegypti embryos to obtain transgenic mosquitoes. J Vis Exp. 2007;219 doi: 10.3791/219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM HJ, LEE HJ, KIM H, CHO SW, KIM JS. Targeted genome editing in human cells with zinc finger nucleases constructed via modular assembly. Genome Research. 2009;19:1279–1288. doi: 10.1101/gr.089417.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM YG, CHA J, CHANDRASEGARAN S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proceedings of the National Academy of Sciences. 1996;93:1156–1160. doi: 10.1073/pnas.93.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIESCH J, BELLANI LL, VOSSHALL LB. Functional and Genetic Characterization of Neuropeptide Y-Like Receptors in Aedes aegypti. PLoS Negl Trop Dis. 2013;7:e2486. doi: 10.1371/journal.pntd.0002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU J, LI C, YU Z, HUANG P, WU H, WEI C, ZHU N, SHEN Y, CHEN Y, ZHANG B, DENG WM, JIAO R. Efficient and specific modifications of the Drosophila genome by means of an easy TALEN strategy. J Genet Genomics. 2012;39:209–15. doi: 10.1016/j.jgg.2012.04.003. [DOI] [PubMed] [Google Scholar]
- MA S, ZHANG S, WANG F, LIU Y, XU H, LIU C, LIN Y, ZHAO P, XIA Q. Highly efficient and specific genome editing in silkworm using custom TALENs. PLoS One. 2012;7:e45035. doi: 10.1371/journal.pone.0045035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALI P, ESVELT KM, CHURCH GM. Cas9 as a versatile tool for engineering biology. Nat Methods. 2013;10:957–63. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEFF KL, ARGUE DP, MA AC, LEE HB, CLARK KJ, EKKER SC. Mojo Hand, a TALEN design tool for genome editing applications. BMC Bioinformatics. 2013;14:1. doi: 10.1186/1471-2105-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NENE V, WORTMAN JR, LAWSON D, HAAS B, KODIRA C, TU Z, LOFTUS B, XI Z, MEGY K, GRABHERR M, REN Q, ZDOBNOV EM, LOBO NF, CAMPBELL KS, BROWN SE, BONALDO MF, ZHU J, SINKINS SP, HOGENKAMP DG, AMEDEO P, ARENSBURGER P, ATKINSON PW, BIDWELL S, BIEDLER J, BIRNEY E, BRUGGNER RV, COSTAS J, COY MR, CRABTREE J, CRAWFORD M, DEBRUYN B, DECAPRIO D, EIGLMEIER K, EISENSTADT E, EL-DORRY H, GELBART WM, GOMES SL, HAMMOND M, HANNICK LI, HOGAN JR, HOLMES MH, JAFFE D, JOHNSTON JS, KENNEDY RC, KOO H, KRAVITZ S, KRIVENTSEVA EV, KULP D, LABUTTI K, LEE E, LI S, LOVIN DD, MAO C, MAUCELI E, MENCK CFM, MILLER JR, MONTGOMERY P, MORI A, NASCIMENTO AL, NAVEIRA HF, NUSBAUM C, O’LEARY S, ORVIS J, PERTEA M, QUESNEVILLE H, REIDENBACH KR, ROGERS YH, ROTH CW, SCHNEIDER JR, SCHATZ M, SHUMWAY M, STANKE M, STINSON EO, TUBIO JMC, VANZEE JP, VERJOVSKI-ALMEIDA S, WERNER D, WHITE O, WYDER S, ZENG Q, ZHAO Q, ZHAO Y, HILL CA, RAIKHEL AS, SOARES MB, KNUDSON DL, LEE NH, GALAGAN J, SALZBERG SL, PAULSEN IT, DIMOPOULOS G, COLLINS FH, BIRREN B, FRASER-LIGGETT CM, SEVERSON DW. Genome Sequence of Aedes aegypti, a Major Arbovirus Vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMMO DD, ALPHEY L, MEREDITH JM, EGGLESTON P. High efficiency site-specific genetic engineering of the mosquito genome. Insect Mol Biol. 2006;15:129–36. doi: 10.1111/j.1365-2583.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTEUS MH, CARROLL D. Gene targeting using zinc finger nucleases. Nat Biotech. 2005;23:967–973. doi: 10.1038/nbt1125. [DOI] [PubMed] [Google Scholar]
- PRICE DP, NAGARAJAN V, CHURBANOV A, HOUDE P, MILLIGAN B, DRAKE LL, GUSTAFSON JE, HANSEN IA. The fat body transcriptomes of the yellow fever mosquito Aedes aegypti, pre- and post-blood meal. PLoS One. 2011;6:e22573. doi: 10.1371/journal.pone.0022573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAJWAN S, TAKASU Y, TAMURA T, UCHINO K, SEZUTSU H, ZUROVEC M. Efficient disruption of endogenous Bombyx gene by TAL effector nucleases. Insect Biochem Mol Biol. 2012 doi: 10.1016/j.ibmb.2012.10.011. [DOI] [PubMed] [Google Scholar]
- SEVERSON DW, KNUDSON DL, SOARES MB, LOFTUS BJ. Aedes aegypti genomics. Insect Biochemistry and Molecular Biology. 2004;34:715–721. doi: 10.1016/j.ibmb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- SMIDLER AL, TERENZI O, SOICHOT J, LEVASHINA EA, MAROIS E. Targeted Mutagenesis in the Malaria Mosquito Using TALE Nucleases. PLoS ONE. 2013;8:e74511. doi: 10.1371/journal.pone.0074511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH J, BIBIKOVA M, WHITBY FG, REDDY AR, CHANDRASEGARAN S, CARROLL D. Requirements for double-strand cleavage by chimeric restriction enzymes with zinc finger DNA-recognition domains. Nucleic Acids Res. 2000;28:3361–9. doi: 10.1093/nar/28.17.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN N, ZHAO H. Transcription activator-like effector nucleases (TALENs): a highly efficient and versatile tool for genome editing. Biotechnol Bioeng. 2013;110:1811–21. doi: 10.1002/bit.24890. [DOI] [PubMed] [Google Scholar]
- WATANABE T, OCHIAI H, SAKUMA T, HORCH HW, HAMAGUCHI N, NAKAMURA T, BANDO T, OHUCHI H, YAMAMOTO T, NOJI S, MITO T. Non-transgenic genome modifications in a hemimetabolous insect using zinc-finger and TAL effector nucleases. Nat Commun. 2012;3:1017. doi: 10.1038/ncomms2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENDELL MD, WILSON TG, HIGGS S, BLACK WC. Chemical and gamma-ray mutagenesis of the white gene in Aedes aegypti. Insect Mol Biol. 2000;9:119–25. doi: 10.1046/j.1365-2583.2000.00166.x. [DOI] [PubMed] [Google Scholar]
- YACOUB S, MONGKOLSAPAYA J, SCREATON G. The pathogenesis of dengue. Curr Opin Infect Dis. 2013;26:284–9. doi: 10.1097/QCO.0b013e32835fb938. [DOI] [PubMed] [Google Scholar]
- ZHANG Y, ZHANG F, LI X, BALLER JA, QI Y, STARKER CG, BOGDANOVE AJ, VOYTAS DF. Transcription activator-like effector nucleases enable efficient plant genome engineering. Plant Physiol. 2013;161:20–7. doi: 10.1104/pp.112.205179. [DOI] [PMC free article] [PubMed] [Google Scholar]