Abstract
Introduction:
Native nephrectomy in patients with autosomal dominant polycystic kidney disease (ADPKD) is performed on a case-by-case basis. We determine if pre-transplant maximal kidney length (MKL) can be used to predict ultimate nephrectomy status.
Methods:
We performed a retrospective review of ADPKD patients who underwent renal transplantation at our centre between January 2000 and December 2012. Pre-transplant measurements of MKL alone, MKL adjusted for height (HtMKL), weight (WtMKL) and body mass index (BMI-MKL) were each assessed for their predictive ability via a receiver operating characteristic (ROC) curve analysis.
Results:
In total, 84 patients met our inclusion criteria, of which 17 (20.2%) underwent native nephrectomy. An MKL ROC curve analysis revealed an area under the curve (AUC) of 0.867 (95% confidence interval [CI] 0.775–0.931; p < 0.001). An optimal cutoff criterion of >21.5 cm revealed a sensitivity of 94.1% (95% CI 71.3–99.9) and specificity of 70.1% (95% CI 57.7–80.7) for eventual nephrectomy. The AUC of HtMKL, WtMKL and BMI-MKL ROC curves did not differ significantly from MKL alone. HtMKL improved specificity, but not overall test performance. The determination of the cut-off MKL may be influenced by the single-centre retrospective nature of this analysis, as well as the fact that renal size was determined by ultrasound and not computerized tomography or magnetic resonance imaging.
Conclusion:
MKL in patients with ADPKD is associated with the eventual need for nephrectomy and may be a useful clinical tool to risk stratify these patients and therefore guide patient conversations to a decision to leave the native kidneys in situ.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary disorder of the kidneys, with a worldwide prevalence ranging from 1:400 to 1:1000.1 It is primarily characterized by the gradual development of cysts within the kidney, which over time result in renal parenchymal loss and the development of end-stage renal disease (ESRD) in a substantial proportion of sufferers.2,3 Although the rate of cystic growth and accompanying kidney enlargement varies between individuals, there is a positive trend over time.4,5 Increases in total kidney volume (TKV) precede and reliably predict declining glomerular function,6,7 such that by the time that they develop ESRD most patients likely have markedly enlarged kidneys.7
Many patients suffer symptomatic sequelae, such as cyst hemorrhage, flank pain, recurrent infections, nephrolithiasis, and symptoms of mass effect (i.e., early satiety, nausea and vomiting, and abdominal discomfort), from their enlarged kidneys.8,9 Consequently, patients with ADPKD are often required to undergo native nephrectomy when these symptoms become intractable or when, in the course of preparing for renal transplantation, the native kidneys are found to impinge upon the true pelvis and preclude the placement of a donor allograft.9–11 Additionally, native nephrectomy may be undertaken in the presence of suspected malignancy; RCC is 2 to 3 times more likely in the ADPKD-ESRD population than in the ESRD population without ADPKD.12 Although the indications for native nephrectomy may be related to kidney size, the decision to proceed with native nephrectomy is often undertaken on an individual basis, without specific reference to kidney size measurements.
The goal of our study was to determine if pre-transplant maximal kidney length (MKL) – an objective size criterion readily available in a clinical setting – can predict eventual nephrectomy status in ADPKD patients undergoing renal transplantation. Furthermore, we evaluate whether augmenting this measure by adjusting for height (HtMKL), weight (WtMKL) or BMI (BMI-MKL), could enhance our ability to predict eventual nephrectomy status. Specifically, we sought to determine if the need for future native nephrectomy could be confidently ruled in or ruled out at the time of transplant assessment.
Methods
This is a single-centre, cohort study conducted at the London Health Sciences Centre, London, Canada. Institutional Ethics approval was obtained (REB #11–117).
Data collection
We identified all adult ADPKD patients who underwent renal transplantation at our institution between January 1, 2000 and December 31, 2012. Chart review was undertaken to determine patient demographic information, transplantation characteristics, nephrectomy status, timing and indication, pre-transplant MKL, date of first dialysis and total follow-up time. MKL was obtained from pre-transplant ultrasonographic investigations, as well as from computed tomography (CT) scans in rare instances where the former were unavailable (n = 2). HtMKL, WtMKL and BMI-MKL were calculated from the data collected.
Statistical analysis
The variables of interest – demographics, donor type, time on dialysis, MKL and associated measures, timing of MKL measurements and follow-up time – were compared across the Nephrectomy and No-nephrectomy groups using the two-tailed Mann-Whitney U test (ordinal data) and Fisher’s Exact test (nominal data). Statistical software was used to construct receiver operator characteristic (ROC) curves for the continuous variables of interest (MKL, HtMKL, WtMKL, BMI-MKL) as predictors of the binary outcome of Nephrectomy and the No-nephrectomy groups. The point with the highest Youden index on each ROC curve was used to determine optimal cut-off values. The McNemar Chi-Squared Test was used to compare the sensitivities and specificities of optimal cut-off points on separate ROC curves. The Holm-Bonferonni correction method13 was applied to maintain the family-wise error rate at α = 0.05. All p values retaining their significance post-correction are indicated throughout the paper with an asterisk (*). All statistical calculations were performed with the IBM SPSS 20 and MedCalc 12 statistical packages.
Results
A total of 102 patients with ADPKD underwent renal transplantation at our institution between January 1, 2000 and December 31, 2012. In all, 84 patients (82.4%) met all of our eligibility criteria. The remaining 18 patients (17.6%) were excluded from further analysis due to missing or incomplete records (n = 13), remote native nephrectomy prior to the study period (n = 3), or ongoing consideration for post-transplant native nephrectomy at the time of data collection (n = 2).
Nephrectomy incidence, timing and indication
A total of 17 patients (20.2%) underwent native nephrectomy during the study period; 14 of these patients underwent a single operation with either bilateral or unilateral kidney removal, while 3 underwent staged procedures, for a total of 20 separate procedures. The most common indication for native nephrectomy was solely symptomatic in nature (n = 11), followed by allograft space concerns with or without associated symptoms (n = 7), and the presence of suspicious complex cysts (n = 2). Symptoms in our cohort included flank pain, abdominal discomfort, early satiety, nausea and vomiting, bleeding cysts and recurrent infection.
Comparison between Nephrectomy and No-nephrectomy groups
No significant differences were found between the 2 groups with respect to demographic characteristics, time spent on dialysis, donor sources, or median follow-up post-transplant (Table 1). The median MKL of the Nephrectomy group (27.0 cm; interquartile range [IQR] 24.6–35.1) was significantly greater than that of the No-nephrectomy group (19.7 cm; IQR 18.0–23.0), (p < 0.001*). HtMKL, WtMKL and BMI-MKL revealed a similar relationship between the groups. MKL of the native kidneys was measured a median of 459 days (IQR 318–637) prior to transplantation, with no significant difference noted between the groups. Ultrasonographic investigations were used to determine MKL in 82 (97.7%) patients; CT scans were used in the remaining 2 (2.3%).
Table 1.
Demographic and donor characteristics, time on dialysis, kidney size and associated measures, and follow-up time, between the Nephrectomy and No-nephrectomy groups
| Parameter |
N (%)/Median (IQR)
|
p value | |
|---|---|---|---|
| Nephrectomy | AUS | ||
| Age (yr) | 50 (41–57) | 54 (IQR 47 – 62) | 0.104 |
| Sex | 6.4 | 6.4 | 0.91 |
| Male | 33 (49%) | 11 (65%) | 0.289 |
| Female | 34 (51%) | 6 (35%) | 1.00 |
| BMI (kg/m2) | 27.0 (24.3–29.8) | 26.9 (IQR 23.8–29.7) | 0.947 |
| Time spent on dialysis (days before transplant) | 424 (31–745) | 507 (IQR 186–1094) | 0.289 |
| Donor type | 11 | 4 | 0.40 |
| LD | 7 (18%) | 12 (41%) | 0.121 |
| NDD | 8 (69%) | 46 (47%) | 0.71 |
| DCD | 2 (13%) | 9 (12%) | 0.48 |
| Kidney size | 5 | 2 | 0.71 |
| MKL (cm) | 27.0 (IQR 24.6–35.1) | 19.7 (IQR 18.0–23.0) | <0.001* |
| HtMKL (cm/m) | 16.8 (IQR 14.3–18.4) | 11.6 (IQR 10.3–13.1) | <0.001* |
| WtMKL (cm/kg) | 0.37 (IQR 0.29–0.43) | 0.25 (IQR 0.22–0.31) | <0.001* |
| BMI-MKL (cm/kg/m2) | 1.0 (IQR 0.91–1.30) | 0.73 (IQR 0.63–0.86) | <0.001* |
| Time of kidney size measurements (days before transplant) | 471 (IQR 135–1140) | 446 (IQR 320–633) | 0.819 |
| Follow-up post-transplant (days) | 1627 (IQR 625–2786) | 1828 (IQR 728–2727) | 0.776 |
BMI: body mass index; LD: living donor; NDD: neurologic determination of death, DCD: donation after cardiac death, IQR: inter-quartile range; MKL: maximal kidney length; HtMKL: height MKL; WtMKL: weight MKL.
p values retaining their significance post-Holm-Bonferonni correction.
Predictive value of MKL and associated measures
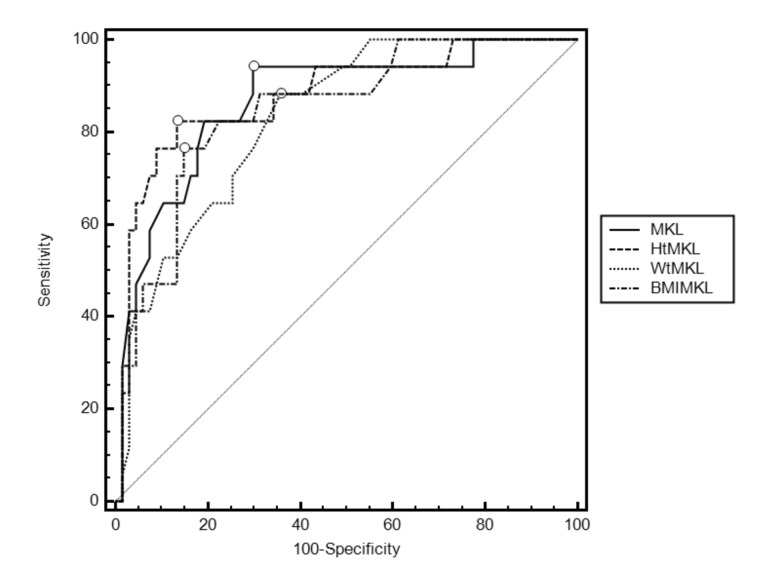
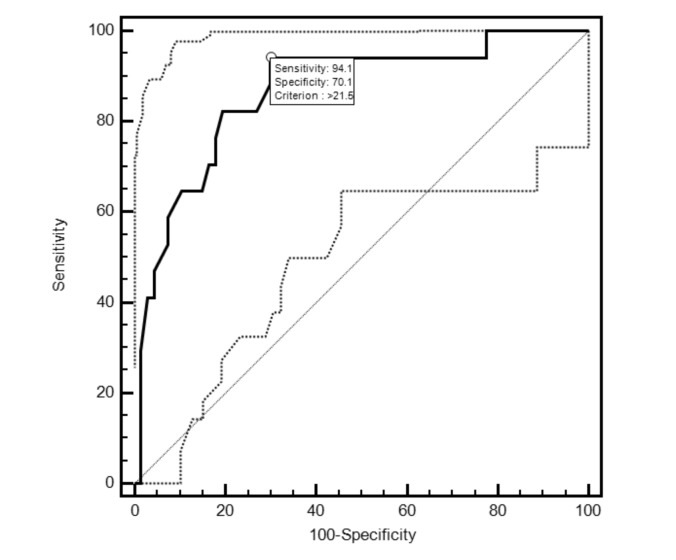
ROC curves constructed for the MKL, HtMKL, WtMKL and BMI-MKL variables revealed no significant differences between the respective areas under the curve (AUC) (Fig. 1). The optimal cut-off point on the MKL ROC curve was >21.5 cm, which corresponds to a sensitivity of 94.1% (95% CI 71.3–99.9) and a specificity of 70.1% (95% CI 57.7–80.7) for the eventual nephrectomized state. Figure 2 illustrates the MKL ROC curve with its optimal cut-off point and 95% confidence intervals. The HtMKL ROC curve yielded an optimal cut-off point of >13.9 cm/m, which corresponds to a sensitivity of 82.4% (95% CI 56.6–96.2) and a specificity of 86.6% (95% CI 76.9–93.7) for the eventual nephrectomized state. Further analysis revealed that the 16.6% increase in specificity offered by the optimal cut-off point on the HtMKL curve was statistically significant (p < 0.001*). The corresponding 11.7% decrease in sensitivity on the HtMKL curve did not reach significance (p = 0.5).
Fig. 1.
Receiver operating characteristic (ROC) curves for maximal kidney length (MKL) and associated measures, as predictors of eventual nephrectomized state; optimal cut-off points are indicated with a circle on each curve. Areas under the curve (AUC) as follows: MKL 0.867 (95% CI 0.775–0.931; p < 0.001*); Height MKL 0.878 (95% CI 0.788–0.939; p < 0.001*); Weight MKL 0.831 (95% CI 0.734–0.904; p < 0.001*); Body mass index MKL 0.847 (95% CI 0.752–0. 915; p < 0.001*). AUC did not differ significantly in pair-wise comparisons [data not shown]. All p values indicated with a * retained their significance post-Holm-Bonferonni correction.
Fig. 2.
Receiver operating characteristic (ROC) curve with 95% CI for maximal kidney length (MKL), as a predictor of eventual nephrectomized state; optimal cut-off point with associated values is indicated. Area under the curve (AUC) is 0.867 (95% CI 0.775–0.931; p < 0.001*). For a cut-off point of >21.5 cm, the sensitivity is 94.1% (95% CI 71.3–99.9), specificity is 70.1% (95% CI 57.7–80.7), positive likelihood ratio 3.15 (95% CI 2.14–4.64), negative likelihood ratio 0.08 (95% CI 0.01–0.57). All p values indicated with a * retained their significance post-Holm-Bonferonni correction.
Discussion
Native nephrectomies performed on our cohort of patients were undertaken in similar frequencies prior to, concurrent with, or post-transplantation, indicating the lack of any particular timing preference at our institution during the study period. Most nephrectomies were performed for symptomatic reasons alone (55%); abdominal pain and cyst hemorrhage were the most frequently cited complaints, in keeping with the literature.2
Overall, our results reveal that pre-transplant MKL is a strong predictor of eventual nephrectomy status, with an ROC AUC of 0.867 and an optimal sensitivity of 94.1% and specificity of 70.1% for the eventual nephrectomized state, corresponding to a cut-off criterion of >21.5 cm. This would appear to suggest that, in a clinical setting, native nephrectomy may not be required for a substantial number of patients whose MKL is less than 21.5 cm. In fact, only 1 patient in our cohort with an MKL <21.5 cm underwent native nephrectomy, secondary to the presence of a suspected malignancy and in the absence of any other symptoms.
Although the natural history of ADPKD involves a general increase in kidney size over time, recent data suggest that native kidneys may, in fact, be subject to a significant (up to 40%) decrease in overall volume post-transplantation, even in the absence of a significant mammalian target of rapamycin (mTOR) inhibitor.14,15 Although a concomitant increase in liver volume was noted by the study in question,14 a decrease in the native kidney volume may nonetheless substantially decrease the risk of symptom based renal complications. This decrease may render durable our efforts to rule out the need for native nephrectomy early in the transplant assessment process since, if native kidneys can be expected to shrink post-transplantation, a pre-transplant determination of MKL is likely to represent a lifetime maximum for a patient’s overall kidney size.
Of note, the optimal-cut off point on the HtMKL ROC curve of >13.9 cm/m, with a sensitivity of 82.4% and a specificity of 86.6% for the eventual nephrectomized state, significantly enhanced specificity (+16.5%) as compared to MKL alone. The corresponding decrease in sensitivity (−11.7%) did not reach statistical significance; however, it is likely that our cohort was simply underpowered to determine even moderate differences in sensitivity, since this measure depends upon the relative performance of the two tests with respect to our [small] Nephrectomy group (n = 17). While the increase in specificity offered by the HtMKL measure represents an improvement over MKL alone, it should be noted that the overall performance of the test was not improved, as evidenced by respective ROC AUCs that did not significantly differ (Fig. 1). Furthermore, the high threshold required to proceed with native nephrectomy casts doubt on the utility of this measurement to contribute anything of substance to the decision-making process over MKL alone. While a pre-transplant kidney size measurement may be used to reassure a patient that they are unlikely to ever require a native nephrectomy, it is more difficult to envision such a measurement being used as a rationale to proceed with native nephrectomy in the absence of clinical symptoms, the latter of which are already sufficient to prompt a consideration of native nephrectomy. Thus, an increase in specificity for the eventual nephrectomy state may be of limited clinical utility, especially if it comes at the potential expense of sensitivity. Nevertheless, it is plausible that in the setting of a more accurate measure of kidney size, such as total kidney volume (TKV), a measure adjusting for height (htTVK) may further enhance the specificity for eventual nephrectomy state to the point of yielding some clinical utility. However, at present such measures are not routinely used in clinical settings.3
The limitations of our study include its single-centre retrospective design. The former is noteworthy because the threshold to perform a native nephrectomy has been influenced by the patient population and practice patterns at our centre. To validate our findings, a multicentre analysis would be of use to increase the number of events (nephrectomy) and remove surgical bias. A further shortcoming of our study is the use of a limited measurement of kidney size (MKL) in lieu of measurements, such as TKV, that are known to be more robust. While we acknowledge this disadvantage, MKL was chosen because it was the simplest and most consistently recorded measure at our institution. However, it is worth noting that while there is considerable variability between sonographic measurements, kidney length is the most reproducible measurement using this imaging technique, insofar as measurements of the kidney are concerned.16 Nevertheless, future studies using an estimate of TKV may well realize an enhanced predictive power given that these are generally estimated on the basis of magnetic resonance imaging or CT scans where measurements are considerably more reliable.
Conclusion
Our data suggest that pre-transplant measurements of MKL can predict eventual nephrectomy status. To our knowledge, we are the first to quantitatively describe this relationship. In a clinical setting, MKL may be used to confidently rule out the need for future native nephrectomy in a subset of patients, and may thus guide patient conversations to the effect of lending more weight to the decision to leave the native kidneys in situ.
Footnotes
Competing interests: Dr. Cristea, Dr. Yanko, Dr. Felbel, Dr. House, Dr. Sener and Dr. Luke declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:332–42. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 2.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–68. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman TI. Polycystic kidney disease: A 2011 update. Curr Opin Nephrol Hypertens. 2012;21:189–94. doi: 10.1097/MNH.0b013e32835011a7. [DOI] [PubMed] [Google Scholar]
- 4.Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–30. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 5.Grantham JJ, Cook LT, Torres VE, et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;73:108–16. doi: 10.1038/sj.ki.5002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tokiwa S, Muto S, China T, et al. The relationship between renal volume and renal function in autosomal dominant polycystic kidney disease. Clin Exp Nephrol. 2011;15:539–45. doi: 10.1007/s10157-011-0428-y. [DOI] [PubMed] [Google Scholar]
- 7.Chapman AB, Bost JE, Torres VE, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–86. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam A, Perrone RD. Management of ESRD in patients with autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:164–72. doi: 10.1053/j.ackd.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Wagner MD, Prather JC, Barry JM. Selective, concurrent bilateral nephrectomies at renal transplantation for autosomal dominant polycystic kidney disease. J Urol. 2007;177:2250–4. doi: 10.1016/j.juro.2007.01.146. discussion 2254. [DOI] [PubMed] [Google Scholar]
- 10.Fuller TF, Brennan TV, Feng S, et al. End stage polycystic kidney disease: Indications and timing of native nephrectomy relative to kidney transplantation. J Urol. 2005;174:2284–8. doi: 10.1097/01.ju.0000181208.06507.aa. [DOI] [PubMed] [Google Scholar]
- 11.Cohen D, Timsit MO, Chretien Y, et al. Place of nephrectomy in patients with autosomal dominant polycystic kidney disease waiting for renal transplantation. Prog Urol. 2008;18:642–9. doi: 10.1016/j.purol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Hajj P, Ferlicot S, Massoud W, et al. Prevalence of renal cell carcinoma in patients with autosomal dominant polycystic kidney disease and chronic renal failure. Urology. 2009;74:631–4. doi: 10.1016/j.urology.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 13.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65. [Google Scholar]
- 14.Yamamoto T, Watarai Y, Kobayashi T, et al. Kidney volume changes in patients with autosomal dominant polycystic kidney disease after renal transplantation. Transplantation. 2012;93:794–8. doi: 10.1097/TP.0b013e318246f910. [DOI] [PubMed] [Google Scholar]
- 15.Goldfarb DA. Re: Kidney volume changes in patients with autosomal dominant polycystic kidney disease after renal transplantation. J Urol. 2012;188:2268. doi: 10.1016/j.juro.2012.07.074. [DOI] [PubMed] [Google Scholar]
- 16.O’Neill WC, Robbin ML, Bae KT, et al. Sonographic assessment of the severity and progression of autosomal dominant polycystic kidney disease: the Consortium of Renal Imaging Studies in Polycystic Kidney Disease (CRISP) Am J Kidney Dis. 2005;46:1058–64. doi: 10.1053/j.ajkd.2005.08.026. [DOI] [PubMed] [Google Scholar]