Abstract
Introduction:
We evaluated the accuracy of current guidelines by analyzing bone scan results and clinical parameters of patients with prostate cancer to determine the optimal guideline for predicting bone metastasis.
Methods:
We retrospectively analyzed patients who were diagnosed with prostate cancer and who underwent a bone scan. Bone metastasis was confirmed by bone scan results with clinical and radiological follow-up. Serum prostate-specific antigen, Gleason score, percent of positive biopsy core, clinical staging and bone scan results were analyzed. We analyzed diagnostic performance in predicting bone metastasis of the guidelines of the European Association of Urology (EAU), American Urological Association (AUA), and the National Comprehensive Cancer Network (NCCN) guidelines as well as Briganti’s classification and regression tree (CART). We also compared the percent of positive biopsy core between patients with and without bone metastases.
Results:
A total 167 of 806 patients had bone metastases. Receiver operating curve analysis revealed that the AUA and EAU guidelines were better for detecting bone metastases than were Briganti’s CART and NCCN. No significant difference was observed between AUA and EAU guidelines. Patients with bone metastases had a higher percent positive core than did patients without metastasis (the cut-off value >55.6).
Conclusion:
The EAU and AUA guidelines showed better results than did Briganti’s CART and NCCN for predicting bone metastasis in the enrolled patients. A bone scan is strongly recommended for patients who have a higher percent positive core and who meet the EAU and AUA guidelines.
Introduction
A bone scan is recommended for prostate cancer staging to identify possible bone metastasis.1–5 However, a recent cross-sectional study showed poor adherence to imaging guidelines,6 although applying a bone scan according to guidelines showed a better result.7 Inappropriate bone scans of patients at low risk of bone metastasis8 and underuse of bone scans in patients with high risk9 have also been reported. Additionally, current Western guidelines should be applied to Asian patients with caution.10,11
Therefore, we evaluated the accuracy of the current guidelines by analyzing bone scan results and clinical parameters of patients with prostate cancer to determine the optimal guideline for predicting metastasis. Additionally, we examined the value of adding the percentage of positive biopsy core to predict bone metastasis.
Methods
Patient selection
This clinical retrospective observational study was carried out in accordance with the Declaration of Helsinki. We enrolled all patients at a single hospital with prostate cancer confirmed by transrectal ultrasonographic prostate biopsy from 2009 to 2011. All patients underwent a bone scan at staging. Bone metastasis was confirmed by bone scan findings with clinical and radiological follow-up of 2 to 4 years.
Data collection
Clinical, pathological and radiological data were collected. Clinical data, such as serum prostate-specific antigen (PSA) level, Gleason score (GS), percent of the positive biopsy core, and clinical staging by magnetic resonance imaging (MRI) were analyzed.
Bone scan
Tc-99m Hydroxymethylene diphosphonate (650 MBq) was injected intravenously, and scanning was performed 3 hours later. Planar images were obtained using a large field-of-view dual-head gamma camera (Millennium VG, GE, Milwaukee, WI). The scan was read by qualified nuclear physicians.
Confirmation of bone metastasis
Bone metastasis was confirmed by both bone scan findings and clinical follow-up. Clinical follow-up included bone scan and computed tomography (CT) or MRI for suspicious lesions.
Guidelines for recommending a bone scan
We examined the European Association of Urology (EAU) guidelines,3 American Urological Association (AUA) PSA best practice policy,1 the guidelines of the National Comprehensive Cancer Network (NCCN),4 and the classification and regression tree (CART) by Briganti and colleagues.2
According to the EAU guidelines, a bone scan may not be indicated in asymptomatic patients with a well or moderately differentiated tumour if the serum PSA level is <20 ng/mL. Based on the AUA guidelines, a bone scan is unnecessary with localized disease when the serum PSA level is <20 ng/mL and there is no clinical evidence of bone metastasis. A bone scan is appropriate according to the NCCN guidelines for symptomatic patients and/or those with life expectancy >5 years when they have any of the following: T1 disease with PSA >20 ng/mL or T2 disease with PSA >10 ng/mL, GS ≥8, or T3 or T4 or symptomatic disease. According to Briganti’s CART, a bone scan should be considered only for patients with a GS >7 or serum PSA level >10 ng/mL with a palpable tumour (cT2/T3).
Analysis
We analyzed whether a bone scan should be recommended for each patient by comparing the results with each guideline. We compared the level of percent positive biopsy core between patients with and without a bone metastasis, and identified the cut-off value for positive biopsy core percentage for predicting bone metastasis using receiver operating curve (ROC) analyses. The McNemar test was used to compare sensitivity and specificity. Medcalc version 11.3.1.0 statistical software (MedCalc Software, Mariakerke, Belgium) was used.
Results
Patients with bone metastasis
A total of 806 patients were enrolled, and all of them underwent a bone scan. Among them, 167 (20%) had bone metastasis at staging (Table 1). Patients with bone metastasis were older, had higher PSA levels, and had a higher percent positive biopsy core compared to those without metastasis.
Table 1.
Patient characteristics
| Patients with bone metastasis (n = 167) | Patients without bone metastasis (n = 639) | p value | |
|---|---|---|---|
| Age (mean ± SD) | 73.31 ± 6.92 | 71.76 ± 7.21 | 0.013 |
| PSA (ng/mL) (mean ± SD) | 252.87 ± 345.67 | 39.41 ± 94.14 | <0.001 |
| PSA (ng/mL), no. | |||
| 0–4.0 | 1 | 38 | |
| 4.01–10.0 | 24 | 261 | |
| 10.01–20.0 | 22 | 138 | |
| >20 | 120 | 202 | |
| Gleason score, no. | |||
| ≤7 | 66 | 466 | <0.001 |
| >7 | 101 | 173 | |
| Percent of positive core (mean ± SD) | 67.57 ± 32.07 | 46.74 ± 29.05 | <0.0001 |
SD: standard deviation; PSA: prostate-specific antigen.
The number of patients for whom a bone scan was recommended by each of the guidelines was as follows: 409 (50.7%) patients by the EAU guidelines, 409 (50.7%) by the AUA guidelines, 531 (65.9%) by Briganti’s CART, and 523 (64.9%) by the NCCN guidelines (61 patients were not available to be classified by the NCCN guidelines).
Comparison among guideline results
The EAU and AUA guidelines showed 100% agreement in recommending a bone scan. However, the McNemar test showed that the bone scan recommendations differed significantly in other comparisons (EAU vs. Briganti’s CART, EAU vs. NCCN, Briganti’s CART vs. AUA, Briganti’s CART vs. NCCN, and AUA vs. NCCN; all p < 0.0005).
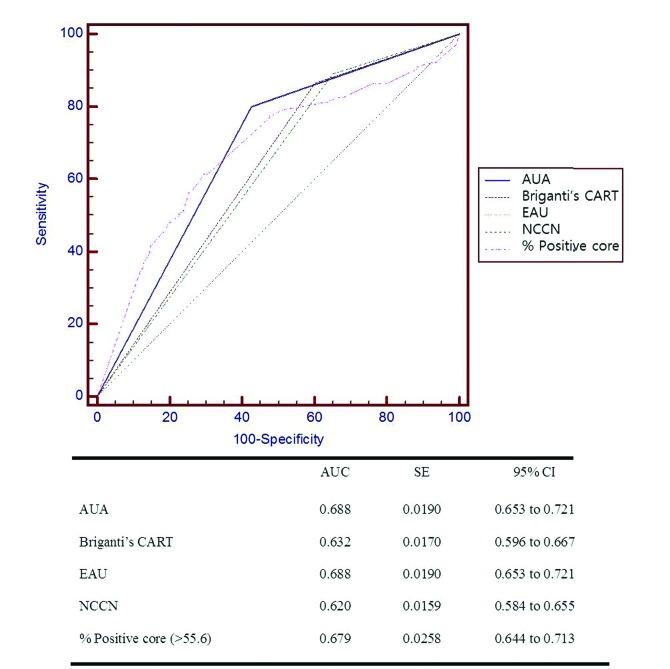
Pairwise comparisons of the ROC curves showed that the AUA and EAU guidelines had larger areas under the curve than did the other guidelines (Fig. 1). No significant difference was detected between the AUA and EAU guidelines. Among the 806 patients, 16 (2%) had bone metastasis even though none of guidelines recommended a bone scan.
Fig. 1.
Receiver operating characteristic (ROC) curve analyses (AUA, Briganti’s CART, EAU, NCC, percent positive core). Note: Pairwise comparison of ROC curves shows significant result with p < 0.05 in AUA vs. Briganti’s CART, AUA vs. NCCN, Briganti’s CART vs. EAU, Briganti’s CART vs. percent positive core, EAU vs. NCCN, NCCN vs. percent positive core. AUA: American Urological Association; EAU: European Association of Urology; NCCN: National Comprehensive Cancer Network; Briganti’s CART: Briganti’s classification and regression tree.
Cut-off value of percent of positive biopsy core for bone metastasis
Patients with bone metastases had a higher percent of positive biopsy core than did those without a metastasis (67.6 ± 32.1 vs. 46.7 ± 29.1, p < 0.0001). The cut-off value for percent positive biopsy core was >55.6 by ROC analysis, with sensitivity of 62.9% and specificity of 70.7% (Table 2). A combination of a cut-off value of 55.6% positive core and each guideline showed lower specificity and compatible sensitivity than did each guideline alone (Table 2).
Table 2.
Results of each guidelines and the combinations with percent positive core (>55.6)
| Sensitivity (%) | Specificity (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|
| EAU3 | 80.84 | 57.12 | 91.38 | 62.03 |
| AUA1 | 80.84 | 57.12 | 91.94 | 62.03 |
| NCCN4 | 89.10 | 34.80 | 92.34 | 46.17 |
| Briganti’s CART2 | 87.43 | 39.75 | 92.36 | 49.62 |
| % Positive core (>55.6) | 62.87 | 70.74 | 87.90 |
| Combination with % Positive core (>55.6) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sensitivity (%) | p* | Specificity (%) | p* | NPV (%) | Accuracy (%) | |
| EAU | 82.04 | 0.5 | 49.77 | <0.0001 | 91.38 | 56.45 |
| AUA | 82.04 | 0.5 | 49.77 | <0.0001 | 91.38 | 56.45 |
| NCCN | 89.74 | ¥ | 32.26 | 0.0001 | 92.23 | 44.30 |
| Briganti’s CART | 88.62 | 0.5 | 35.99 | <0.0001 | 92.37 | 46.90 |
p value: each guideline versus the combination of each guideline and percent positive core (>55.6).
Could not perform the test; AUA: American Urological Association; EAU: European Association of Urology; NCCN: National Comprehensive Cancer Network; Briganti’s CART: Briganti’s classification and regression tree; NPV: negative predictive value
Discussion
Prior studies have compared current guidelines in terms of their recommendation of a bone scan for prostate cancer staging.12,13 In 2012, De Nunzio and colleagues13 compared Briganti’s CART to the EAU guidelines in 313 patients with prostate cancer (6.4% had bone metastasis). They reported that Briganti’s CART was significantly more accurate than the EAU guideline for predicting bone metastasis using ROC analyses.13 However, in our study, the EAU and AUA guidelines showed a better result than the Briganti’s CART (Fig. 1). De Nunzio and colleagues, however, used the 2008 EAU guidelines,14 whereas we used those published in 2011.3 However, details related to bone scan recommendations for staging were the same.3,14 Ito and colleagues12 analyzed 508 patients (3.5% had bone metastasis) and reported that the EAU guidelines showed the best accuracy compared with those of the Japanese Urological Association, NCCN, AUA/ American Joint Committee on Cancer, and the American College of Radiology.12 Similar to our study, the EAU guideline results offered the best prediction, but those authors reported that the EAU guidelines were better than the AUA guidelines.
Due to the high rate of false-positive bone scans, we radiologically and clinically confirmed bone metastasis in our study. We followed patients for 2 to 4 years and used CT or MRI in some patients. The false-positive rate was higher in our study (20%) than in previous studies,12,13 perhaps due to the higher percentage of patients with bone metastasis in our study. However, other studies have reported even higher percentages of bone metastasis, including studies by Al-Ghazo and colleagues15 (98 patients, 39.7% had bone metastasis), Lai and colleagues16 (116 patients, 29.3% with metastasis), Kosuda and colleagues17 (1294 patients, 22% with metastasis), and Lee and colleagues11 (579 patients, 14.3% with metastasis). Therefore, the percentage of bone metastasis in our study was not unusually high.
Several studies have suggested that a bone scan can be omitted for prostate cancer staging if the serum PSA level and GS satisfy the following: PSA ≤20 ng/mL and GS <8;15 PSA <20 ng/mL and GS <8;18 PSA ≤10;16 PSA ≤10 or Gleason grade ≤2 or GS ≤6;17 and PSA ≤20 and GS ≤6.19 However, other reports have suggested that some patients with low PSA and GS had bone metastasis.20 Zaman and colleagues10 identified Asian patients with PSA ≤20 and GS <8 who had bone metastasis and suggested that we should be cautious when adopting Western guidelines for bone scans to an Asian population. The incidence and mortality rate of prostate cancer are lower in Asians than in northern European and Caucasian Americans.16 Lai and colleagues16 suggested that guidelines for Asians might differ from those for Caucasians. Lee and colleagues11 showed the need for a bone scan in patients with PSA values of 10 to 20 ng/mL and suggested that new guidelines might be needed for Asians. In our study of Korean patients, 16 (2% of total patients) had bone metastases, even though none of the 4 guidelines recommended a bone scan for them. Racial differences between studies might be the cause of discrepancies in the results between studies that applied these guidelines. Further studies including those looking at racial differences in each guideline are needed.
In 2009, Ritenour and colleagues21 suggested that the PSA threshold should be adjusted according to the GS for recommending a bone scan in newly diagnosed patients with prostate cancer; they recommended a bone scan for GS ≤7 and PSA >30 ng/mL and for GS ≥8 to 10 and PSA >10 ng/mL.21 In our study, we determined whether the percent of positive biopsy core could enhance the predictability of bone metastasis. However, combining the percent of positive biopsy core with the guidelines resulted in comparable sensitivity, but lower specificity (Table 2).
Our study has several limitations. We did not confirm bone metastasis by biopsy. Although the clinical follow-up included a bone scan and CT or MRI for suspicious lesions, the possibility of false-positive or false-negative results existed. Additionally, patients with a negative bone scan were not followed. Even though the specificity of a bone scan is high, the possibility of false-negative results existed.
Conclusion
Based on our study of Korean patients, the EAU and AUA guidelines showed better results than did Briganti’s CART and the NCCN guidelines for predicting bone metastasis. A bone scan is strongly recommended in patients who have a higher percent positive biopsy core, as per the EAU and AUA guidelines.
Footnotes
Competing interests: Dr. Chong, Dr. I. Hwang, Dr. Ha, Dr. S. Yu, Dr. E. Hwang, Dr. H. Yu, Dr. Kim, Dr. Jung, Dr. Kang, Dr. Kwon, and Dr. Park all declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009;182:2232–41. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 2.Briganti A, Passoni N, Ferrari M, et al. When to perform bone scan in patients with newly diagnosed prostate cancer: External validation of the currently available guidelines and proposal of a novel risk stratification tool. Eur Urol. 2010;57:551–8. doi: 10.1016/j.eururo.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Clinical recommendations. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed July 31, 2014.
- 5.Hwang EC, Kim JS, Kim SO, et al. Accuracy and factors affecting the outcome of multi-detector computerized tomography urography for bladder tumors in the clinical setting. Korean J Urol. 2011;52:13–8. doi: 10.4111/kju.2011.52.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makarov DV, Desai RA, Yu JB, et al. The population level prevalence and correlates of appropriate and inappropriate imaging to stage incident prostate cancer in the medicare population. J Urol. 2012;187:97–102. doi: 10.1016/j.juro.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 7.Cortes Romera M, Talavera Rubio MP, Garcia Vicente AM, et al. Are bone scintigraphy examinations requested in oncologic patients according to established indications? Rev Esp Med Nucl. 2007;26:286–93. [PubMed] [Google Scholar]
- 8.Lavery HJ, Brajtbord JS, Levinson AW, et al. Unnecessary imaging for the staging of low-risk prostate cancer is common. Urology. 2011;77:274–8. doi: 10.1016/j.urology.2010.07.491. [DOI] [PubMed] [Google Scholar]
- 9.Falchook AD, Salloum RG, Hendrix LH, et al. Use of bone scan during initial prostate cancer workup, downstream procedures, and associated medicare costs. Int J Radiat Oncol Biol Phys. 2014;89:243–8. doi: 10.1016/j.ijrobp.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaman MU, Fatima N, Sajjad Z. Metastasis on bone scan with low prostate specific antigen (≤20 ng/ ml) and Gleason’s score (<8) in newly diagnosed Pakistani males with prostate cancer: Should we follow Western guidelines? Asian Pac J Cancer Prev. 2011;12:1529–32. [PubMed] [Google Scholar]
- 11.Lee SH, Chung MS, Park KK, et al. Is it suitable to eliminate bone scan for prostate cancer patients with PSA ≤ 20 ng/mL? World J Urol. 2012;30:265–9. doi: 10.1007/s00345-011-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito Y, Kikuchi E, Maeda T, et al. Bone scan in patients with newly diagnosed prostate cancer; to perform or not?: External validation of the currently available guidelines for Japanese patients. J Urol. 2013;189:e901. doi: 10.1016/j.juro.2013.02.2106. [DOI] [Google Scholar]
- 13.De Nunzio C, Leonardo C, Franco G, et al. When to perform bone scan in patients with newly diagnosed prostate cancer: External validation of a novel risk stratification tool. World J Urol. 2012;31:365–9. doi: 10.1007/s00345-012-0880-7. [DOI] [PubMed] [Google Scholar]
- 14.Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Al-Ghazo MA, Ghalayini IF, Al-Azab RS, et al. Do all patients with newly diagnosed prostate cancer need staging radionuclide bone scan? A retrospective study. Int Braz J Urol. 2010;36:685–91. doi: 10.1590/s1677-55382010000600006. discussion 691–2. http://dx.doi.org/IBJUv36n6a5. [DOI] [PubMed] [Google Scholar]
- 16.Lai MH, Luk WH, Chan JC. Predicting bone scan findings using sPSA in patients newly diagnosed of prostate cancer: Feasibility in Asian population. Urol Oncol. 2011;29:275–9. doi: 10.1016/j.urolonc.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Kosuda S, Yoshimura I, Aizawa T, et al. Can initial prostate specific antigen determinations eliminate the need for bone scans in patients with newly diagnosed prostate carcinoma? A multicenter retrospective study in Japan. Cancer. 2002;94:964–72. doi: 10.1002/cncr.10340. [DOI] [PubMed] [Google Scholar]
- 18.McArthur C, McLaughlin G, Meddings RN. Changing the referral criteria for bone scan in newly diagnosed prostate cancer patients. Br J Radiol. 2012;85:390–4. doi: 10.1259/bjr/79184355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka N, Fujimoto K, Shinkai T, et al. Bone scan can be spared in asymptomatic prostate cancer patients with PSA of ≤20 ng/ml and Gleason score of ≤6 at the initial stage of diagnosis. Jpn J Clin Oncol. 2011;41:1209–13. doi: 10.1093/jjco/hyr118. [DOI] [PubMed] [Google Scholar]
- 20.Sanjaya IP, Mochtar CA, Umbas R. Correlation between low Gleason score and prostate specific antigen levels with incidence of bone metastases in prostate cancer patients: When to omit bone scans? Asian Pac J Cancer Prev. 2013;14:4973–6. doi: 10.7314/APJCP.2013.14.9.4973. [DOI] [PubMed] [Google Scholar]
- 21.Ritenour CW, Abbott JT, Goodman M, et al. The utilization of Gleason grade as the primary criterion for ordering nuclear bone scan in newly diagnosed prostate cancer patients. ScientificWorldJournal. 2009;9:1040–5. doi: 10.1100/tsw.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]