Abstract
Benign prostatic hyperplasia (BPH) is considered a frequent cause of bladder outlet obstruction (BOO) and lower urinary tract symptoms. This review addresses the bladder response to BOO and focuses on the alterations and biochemical adaptability of the bladder wall in the presence of hypoxia. A literature review of published articles has been performed, including both in vivo and in vitro studies on human and animal tissue.
Introduction
Benign prostatic hyperplasia (BPH) accompanied by bladder outlet obstruction (BOO) affects 30% of men over 60.1 It is a common disorder of the male urogenital tract and clinically presents with lower urinary tract symptoms (LUTS), such as hesitancy, straining, weak urine flow, frequency, nocturia and urgency.2 BPH-related symptomatology is attributed to obstructed outflow (BOO), which results from either prostate enlargement (static component) and/or increased α-adrenergic activity at the level of bladder neck and prostatic urethra (dynamic component).3,4
BOO can also be associated with a variety of morphological, contractile and biochemical changes within the bladder in both experimental and clinical studies. The symptomatic presentation of the disease is related largely to these degenerative changes that occur in the bladder. In general, the bladder modifies its structure to compensate the increased resistance to flow, while significant hypoxia ensues because of the high resistance to flow and consequent high intravesical pressure.
The present review addresses current data on the response of the bladder to BOO, particularly focusing on the bladder wall alterations and biochemical adaptability in the presence of hypoxia.
Methods
For this study, we used in vivo and in vitro studies on human tissue and animal model experiments to estimate the consequences of outlet obstruction on the bladder wall. A search of the PubMed, Scopus and Web of Science databases using the terms “prostatic hyperplasia,” “urinary bladder neck obstruction,” “urinary bladder” and “prostate” was conducted. The research focused on articles describing the alterations within the bladder wall induced by BOO.
The study population were human and animal models with BOO. Two authors independently screened the titles and abstracts of the articles identified from the search for relevance. Outcome parameters were the morphological and biochemical changes occurring within the bladder wall of subjects with outlet obstruction, associated with the development of LUTS. The research was limited to the period from 1980 to 2013. Only English peer-reviewed studies were included. If some articles were republished by the same authors, we included only their newest version in the reviewing process. The same process was followed in case of similar articles written by different groups.
We excluded manuscripts that were irrelevant to the objective of this review, as well as those published only in abstract form. Any disagreement was resolved by discussion and final decision was based on a consensus. In the end, 64 manuscripts were included (Fig. 1).
Fig 1.
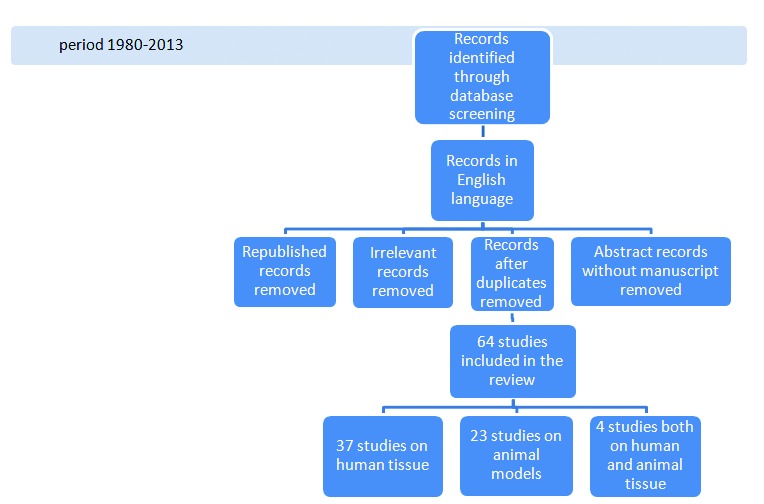
Flow diagram of the reviewing process.
Results
Structural changes in the bladder wall
The urinary bladder often responds to BOO with hypertrophy, accompanied by an augmentation of connective tissue components and replacement of proteins of the contractile apparatus of the smooth muscle cell, with their non-muscle (embryonic) isoforms, such as non-muscle myosin heavy chain (MHC), a-isoform of tropomyosyn, calponin, β- and γ-actin.5–10 Detrusor muscle myosin is a type II myosin composed of two pairs of heavy (MHC) and light (MLC) chains. Two MHC isoforms have been identified in the detrusor muscle, namely isoform 1 (204 kDa) and isoform 2 (200 kDa). In an animal study, the relative amount of MHC isoform 2 decreased during obstruction-induced bladder wall hypertrophy; however, the overall concentration of myosin in the smooth muscle cells increased, whereas the concentration of actin was unchanged. These alterations were normalized after removal of the obstruction, suggesting that the turnover of contractile and cytoskeletal proteins is fast and can be regulated in response to changes in the increased functional demands in smooth muscle, due to the high urethral resistance during BOO.8
High bladder pressure induces adaptive changes in the bladder structure, which, in the long term, are visible as muscle enlargement and collagen deposition.11–13 The increase in connective tissue between muscle fibres and muscle bundles significantly decreases bladder elasticity and therefore bladder compliance.14 In a recent study, Metcalfe and colleagues demonstrated that, in partially obstructed rat bladders, the progression to fibrosis is typically accompanied by increased levels of epidermal growth factor (EGF), insulin-like growth factor 1 (IGF-1) and connective tissue growth factor (CTGF).15
Obstruction-induced smooth muscle remodelling and hypertrophy are compensatory responses aimed to produce the increased force required to expel urine against the obstruction. These compensatory changes are associated with altered expression of contractile proteins and various signalling and regulatory proteins, such as calmodulin, Rho-activated kinase and caveolins.16–20 At the cellular level, muscarinic receptor-mediated bladder contraction involves two main pathways, voltage-operated Ca++ channels and a Rho-kinase. While the opening of the sensitive Ca++ channels allows for a marked elevation of intracellular Ca++ concentrations resulting in contraction, the Rho-kinase constitutes a main pathway, which enhances detrusor Ca++ sensitization necessary for the detrusor muscle to maintain contraction.16 The expression of Rho-kinase is abnormally upregulated in the hypertrophied urinary bladder, resulting probably in alterations of muscle relaxation in the prostate, bladder neck and urethra in the voiding phase, as well as detrusor overactivity in the storage phase.20 Animal studies indicate that Rho-kinase inhibitors may have a role as future treatments for bladder dysfunction.17 Phosphodiesterase-5 inhibitors (PDE5-Is) induce down-regulation of Rho-kinase activity and this could probably explain their significant efficacy in patients with BOO.21
As a result of the structural changes of the bladder wall occurring during BOO, the bladder function can remain relatively normal as the hypertrophying detrusor initially counteracts the progressive increase in urethral resistance. The limited changes in micturition pressure and flow characteristics that occur during the compensated function are not usually disabling enough to motivate patients to seek medical attention. During the progress of the disease, mainly due to the ischemia influence, bladder function will begin to deteriorate and finally the bladder will become decompensated.
Adjustment to bladder wall ischemia
BOO and the ensuing muscle hypertrophy and collagen deposition within the bladder wall result in a significant decrease in detrusor blood flow and hence impaired oxygen diffusion to the tissues.22 Focal hypoxia occurs early in the initial response, activating angiogenic regulators which induce the construction of new blood vessels within the interstitial spaces around the muscle bundles.22–27 The expression of angiostatic factors, such as Endostatin XV, due to the constantly increased collagen deposition and subsequent fibrosis, results in inhibition of further angiogenesis, decreasing blood flow, continuing ischemia injury and gradual progression to end-stage decompensation.28–30
During the compensation phase, an increased expression of hypoxia inducible factor-a (HIF-a) is observed (Fig. 2a).31 HIF-a stimulates angiogenesis at a rate that enables vascular density and blood flow to increase relative to the increase in bladder mass.22 Moreover, HIF-a also increases glucose uptake and availability for glycolysis and stimulates transcription of a wide range of gene products, some of which alter the metabolism of the cell to enable it to survive better in a hypoxic environment.32 In a study in rats, Ghafar and colleagues showed that BOO resulted in a significant increase in HIF-a, vascular endothelial growth factor (VEGF) and angiopoietin-1, suggesting that oxygen perfusion of the hypertrophied muscle bundle is affected.32 It has been proposed that during obstruction, the detrusor reduces its own oxygen supply by producing pressures that compress the small blood vessels.33,34 Prevention of bladder wall ischemia may potentially lead to a lesser degree of detrusor hypertrophy and hence bladder dysfunction; new treatment approaches towards this direction are currently being studied.35
Fig. 2a.

Hypoxia and increased expression of hypoxia inducible factor-a (HIF-a) are observed during the progression of BPH. BPH: benign prostate hyperplasia; BOO: bladder outlet obstruction; HIF-a: hypoxia-induced factor a; ↑: increased; ↓: reduced.
These findings were recently confirmed in humans by Mcnab and colleagues, by using new imaging technologies.36 The authors performed a study in which the changes in tissue oxygenation and hemodynamics of 14 asymptomatic and 6 subjects with LUTS, were monitored using near-infrared spectroscopy (NIRS). The results indicated an overall reduction in the detrusor blood volume and the availability of oxygenated hemoglobin over the course of voiding in patients with LUTS compared to asymptomatic subjects. Their results are in agreement with the study by Farag and colleagues.37 Moreover, the significantly reduced detrusor blood flow in both full and empty bladder states was also demonstrated by Doppler ultrasonography in patients with BOO.38
Contrary to these findings, previous publications reported either inconsistent variations in blood flow during BOO39 or increased blood flow with increasing volume and pressure;40 however, these studies were either experimental (not performed on human bladders) or reporting results from early and/or very mild phases of obstruction.
It has been established that the obstructed bladder commonly exhibits evidence of decreased aerobic metabolism, increased anaerobic metabolism and reduced high-energy phosphate content.41 As a result to hypoxia, obstructed bladders appear hypervascular22 and partially denervated42 and exhibit alterations in the mitochondrial structure and function43–45 and the glycogen content.46,47 Obstruction also induces protein oxidation in the detrusor smooth muscle;48 lactic acid, due to the anaerobic metabolism, accumulates causing contractile dysfunction.49 These findings suggest that ischemia and hypoxia are responsible for the development of bladder dysfunction in BOO.24,25 However, it is not known whether this is mediated directly through an effect on the detrusor smooth muscle50 or as a result of neuronal loss and subsequent smooth muscle changes.51 Animal studies in decompensated bladders showed that the severity of contractile dysfunction was correlated with the magnitude of the decrease in blood flow to the muscle compartment, supporting the hypothesis that ischemia is an etiological factor for bladder decompensation after BOO.29
Mitochondria-ATP-glucose metabolism
Mitochondrial enzyme activity is crucial in the energy production and contractility of detrusor muscle52 and it has been shown to increase with the severity of partial BOO in the male.45 As the obstruction progresses, increased oxidative stress in the detrusor muscle occurs, leading to a significantly higher incidence and proportion of mitochondrial DNA deletions.44 Electron microscopy evaluation of the obstructed rabbit bladder showed that mitochondria within detrusor muscle cells become progressively more swollen. Six weeks post-obstruction, similarly swollen mitochondria are also present in other cell types within the bladder wall, such as fibroblasts, Schwann cells, endothelium and perivascular smooth muscle. These findings of mitochondrial damage have been interpreted as evidence of ischemic damage of the bladder wall as a consequence of outflow obstruction.53 Similar mitochondrial damage has been noted in human detrusor smooth muscle cells in biopsy samples removed from patients with bladder outflow obstruction.14
Currently, many investigators consider mitochondrial alteration a crucial factor in voiding dysfunction and hypothesize that severe and irreversible mitochondrial damage, marked by disruption of outer membrane, could explain the frequent persistence of symptoms after removing the BOO in men.54
Furthermore, during obstruction, mitochondrial enzyme activity subsides, leading to impaired oxidative metabolism, as evidenced by specific decreases in the activity of citrate synthase (CS), malate dehydrogenase and cytochrome oxidase.55 ATP provides most of the cellular energy required to maintain cell function.56 Adequate cytosolic ATP concentration is maintained by anaerobic metabolism of glucose to pyruvate and subsequent oxidative metabolism of pyruvate to CO2 and H2O within the mitochondria through the tricarboxylic acid (TCA) cycle and respiratory chain pathway.57 CS is the rate-limiting enzyme of the TCA cycle, which provides substrates for the respiratory chain. A reduction in respiratory chain substrates would lead to decreased oxidative phosphorylation (i.e., decreased ATP synthesis). Bladder biopsies from men with significant obstructive symptoms, secondary to BPH, have demonstrated a marked decrease in CS activity compared to bladder samples isolated from unobstructed men of the same age.58
There is also evidence of reduced aerobic and increased anaerobic metabolism in obstructed bladders. Partial obstruction of the rabbit bladder induces a shift from aerobic to anaerobic metabolism, as evidenced by the shift in glucose metabolism from CO2 to lactic acid generation. Similarly, there is a marked decrease in the metabolism of pyruvate to CO2.59
Glycogen content
It has been demonstrated that during obstruction the bladder muscle reduces its own oxygen supply by producing pressures that compress the small blood vessels.33,34 This prompts parts of the muscle to function anaerobically and glycogen may be used as an alternative energy supplier.47 In chronic ischemic periods, the bladder may adapt by increasing the amount of glycogen stored in muscles cells. In an animal model, de Jong and colleagues showed that glycogen deposition in the bladder wall is directly related to bladder function during obstruction;46 the strongest glycogen deposition was found in bladders with the highest pressures, lowest compliance and highest contractility. At first, little deposition occurred close to the serosal side of the detrusor layer and, later on, the strongest accumulation appeared throughout the whole detrusor layer up to the urothelium. The authors concluded that glycogen content is a clear marker of the severity of the functional changes of the urinary bladder during obstruction and they claimed that analyzing glycogen deposits may give insight in the severity of bladder damage and contribute to an accurate prognosis of bladder function.46 de Jong and colleagues also state that glycogen concentration could be used to recognize a bladder in the decompensation phase. From a clinical point of view, the development of fibre-optic based Raman spectroscopy for in vivo tissue analysis could be used to identify glycogen content and replace the invasive urodynamic pressure-flow study to diagnose BOO in men.60
Denervation
The urinary bladder stores urine for most of the day, a process facilitated by β-adrenergic receptor-mediated detrusor relaxation (predominately β3-subtype) and α1-adrenergic receptor-mediated contraction of the bladder neck.61 In the bladder, β-adrenergic receptors predominate over α-adrenergic receptors and the response of the normal detrusor to the neurotransmitter norepinephrine is relaxation.61 Physiological voiding is caused by detrusor contraction, induced by muscarinic receptor (preferentially M3) stimulation by the endogenous neurotransmitter acetylcholine.62
The occurrence of storage (irritative) symptoms upon BOO, clearly shows that the obstruction impairs voiding and storage function. However, the relief of BOO does not always resolve storage symptoms and this may be due to the persistence of the BOO-induced alterations in the bladder wall, even after the removal of obstruction. Such alterations may occur in the innervation level.
Several human bladder studies showed a significant loss of innervation (denervation) associated with obstructive dysfunction secondary to BPH.42 Neurones are known to be very sensitive to hypoxic damage, with grey matter more easily damaged than white.63 Denervation may arise because of damage to postganglionic parasympathetic neurones within the bladder wall and this damage may be caused by the transient bladder ischemia that occurs during obstructed micturition.64 Moreover, the partial bladder denervation during BOO may appear more on the cholinergic than on the sympathetic side of the system, as this form is more dominant in the bladder.65 A 56% reduction in the number of acetylcholine-positive nerves was demonstrated in bladder biopsies obtained from obstructed compared to non-obstructed men.66 Counts of nerve profiles confirmed the reduced density of autonomic innervation and not merely a decrease in the concentration of AchE.
The consistently reported relative denervation of the enlarged bladder can at least partly explain the reduced contractile responses to field stimulation under conditions of severe and/or long lasting BOO, due to a reduced availability of contractile transmitter.65 Postsynaptic nerve loss causes the neurogenic contractile dysfunction that results in decreased emptying, increased residual volume and chronic distension characteristic of early decompensation.
In any case, none of these findings can explain the bladder overactivity typically associated with BOO, as all would favour underactivity of the detrusor. The storage symptoms observed in patients with BOO could theoretically be explained by a decreased β-adrenergic input. In this context, new treatment strategies currently target β3-adrenoceptors to reduce storage symptoms.67 Although there are concerns regarding the impact of these drugs in the detrusor contraction of patients with BOO, in a recent study, the β3-adrenoceptor agonist mirabegron was evaluated and found to be safe.68
Conclusion
Bladder dysfunction secondary to BPH is a major affliction of the aging male. Bladder modifies its structure in order to compensate the increased resistance to flow. As a result there is a significant decrease in detrusor blood flow, especially in the late period of the obstruction. During the obstruction and hypoxia period, there are 6 major alterations of bladder wall morphology and detrusor biochemistry (Fig. 2b):
Muscle enlargement and collagen deposition;
Mitochondrial DNA deletions, mitochondrial damage and reduced mitochondrial substrate (e.g., glucose) utilization;
Decreased mitochondrial enzyme activity leading to decreased oxidative metabolism and ATP synthesis;
Reduced aerobic and increased anaerobic metabolism leading to lactic acid accumulation;
Glycogen deposition, as an alternative energy supplier; and
Reduced cholinergic nerve density and denervation.
Fig. 2b.
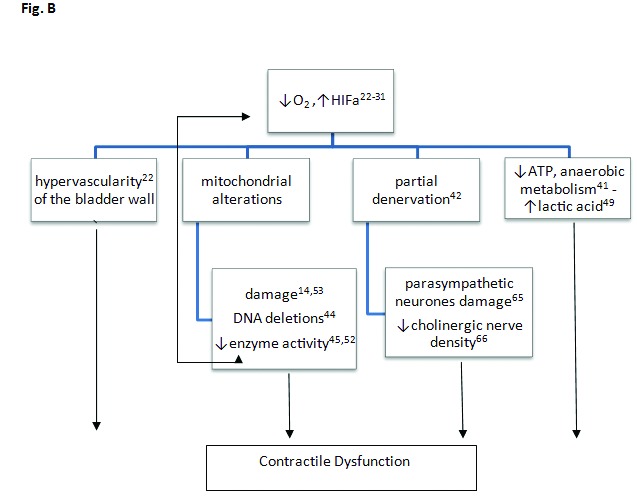
Obstruction-induced alterations occur within the urinary bladder resulting in contractile dysfunction. ATP: adenosine triphosphate; HIF-a: hypoxia induced factor a, ↑:increased, ↓:reduced.
In the case of long-lasting BOO, mitochondrial damage may become irreversible and this could explain the persistence of symptoms after relief of BOO in men.
Footnotes
Competing interests: Dr. Komninos and Dr. Mitsogiannis all declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Boyle P, Robertson C, Mazzetta C, et al. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int. 2003;92:409–14. doi: 10.1046/j.1464-410X.2003.04369.x. [DOI] [PubMed] [Google Scholar]
- 2.Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: Report from the standardization sub-committee of the international continence society. Neurourol Urodynamics. 2002;21:167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 3.Andersson KE. Storage and voiding symptoms: Pathophysiologic aspects. Urology. 2003;62:3–10. doi: 10.1016/j.urology.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Lepor H. Nonoperative management of benign prostatic hyperplasia. J Urol. 1989;141:1283–9. doi: 10.1016/s0022-5347(17)41282-1. [DOI] [PubMed] [Google Scholar]
- 5.Backhaus BO, Kaefer M, Haberstroh KM, et al. Alterations in the molecular determinants of bladder compliance at hydrostatic pressures less than 40 cm. H2O. J Urol. 2002;168:2600–4. doi: 10.1016/S0022-5347(05)64226-7. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi O. Response of bladder smooth muscle cells to obstruction: Signal transduction and the role of mechanosensors. Urology. 2004;63:11–6. doi: 10.1016/j.urology.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Burkhard FC, Lemack GE, Zimmen PE, et al. Contractile protein expression in bladder smooth muscle is a marker of phenotypic modulation after outlet obstruction in the rabbit model. J Urol. 2001;165:963–7. doi: 10.1016/S0022-5347(05)66585-8. [DOI] [PubMed] [Google Scholar]
- 8.Malmqvist U, Arner A, Uvelius B. Contractile and cytoskeletal proteins in smooth muscle during hypertrophy and its reversal. Am J Physiol. 1991;260:C1085–93. doi: 10.1152/ajpcell.1991.260.5.C1085. [DOI] [PubMed] [Google Scholar]
- 9.Sjuve R, Haase H, Ekblad E, et al. Increased expression of non-muscle myosin heavy chain-B in connective tissue cells of hypertrophic rat urinary bladder. Cell Tissue Res. 2001;304:271–8. doi: 10.1007/s004410000262. [DOI] [PubMed] [Google Scholar]
- 10.Mannikarottu AS, Disanto ME, Zderic SA, et al. Altered expression of thin filament-associated proteins in hypertrophied urinary bladder smooth muscle. Neurourol Urodyn. 2006;25:78–88. doi: 10.1002/nau.20121. [DOI] [PubMed] [Google Scholar]
- 11.Lee SD, Akbal C, Jung C. Intravesical pressure induces hyperplasia and hypertrophy of human bladder smooth muscle cells mediated by muscarinic receptors. J Paediatric Urol. 2006;2:271–6. doi: 10.1016/j.jpurol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Deveaud CM, Macarak EJ, Kucich U, et al. Molecular analysis of collagens in bladder fibrosis. J Urol. 1998;160:1518–27. doi: 10.1016/S0022-5347(01)62606-5. [DOI] [PubMed] [Google Scholar]
- 13.Levin RM, Wein AJ, Butyan R, et al. Update on bladder smooth-muscle physiology. World J Urol. 1994;12:226–32. doi: 10.1007/BF00191201. [DOI] [PubMed] [Google Scholar]
- 14.Levin RM, Haugaard N, O’Connor L, et al. Obstructive response of human bladder to BPH vs. rabbit bladder response to partial outlet obstruction: A direct comparison. Neurourol Urodyn. 2000;19:609–29. doi: 10.1002/1520-6777(2000)19:5<609::AID-NAU7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 15.Metcalfe PD, Wang J, Jiao H, et al. Bladder outlet obstruction: Progression from inflammation to fibrosis. BJU Int. 2010;106:1686–94. doi: 10.1111/j.1464-410X.2010.09445.x. [DOI] [PubMed] [Google Scholar]
- 16.Bing W, Chang S, Hypolite JA, et al. Obstruction-induced changes in urinary bladder smooth muscle contractility: A role for Rho kinase. Am J Physiol Renal Physiol. 2003;285:F990–7. doi: 10.1152/ajprenal.00378.2002. [DOI] [PubMed] [Google Scholar]
- 17.Peters SL, Schmidt M, Michel MC. Rho-kinase: A target for treating urinary bladder dysfunction? Trends Pharmacol Science. 2006;27:492–7. doi: 10.1016/j.tips.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Polyák E, Boopathi E, Mohanan S, et al. Alterations in caveolin expression and ultrastructure after bladder smooth muscle hypertrophy. J Urol. 2009;182:2497–503. doi: 10.1016/j.juro.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Boopathi E, Gomes CM, Goldfarb R, et al. Transcriptional repression of Caveolin-1 (CAV1) gene expression by GATA-6 in bladder smooth muscle hypertrophy in mice and human beings. Am J Pathol. 2011;178:2236–51. doi: 10.1016/j.ajpath.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christ GJ, Andersson KE. Rho-kinase and effects of Rho-kinase inhibition on the lower urinary tract. Neurourol Urodyn. 2007;26:948–54. doi: 10.1002/nau.20475. [DOI] [PubMed] [Google Scholar]
- 21.Anderson KE, de Groat WC, McVary KT, et al. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: Pathophysiology and mechanisms of action. Neurourol Urodyn. 2011;30:292–301. doi: 10.1002/nau.20999. [DOI] [PubMed] [Google Scholar]
- 22.Chichester P, Lieb J, Levin SS, et al. Vascular response of the rabbit bladder to short term partial outlet obstruction. Mol Cell Biochem. 2000;208:19–26. doi: 10.1023/a:1007061729615. [DOI] [PubMed] [Google Scholar]
- 23.Greenland JE, Brading AF. The effect of bladder out flow obstruction on detrusor blood flow changes during the voiding cycle in conscious pigs. J Urol. 2001;165:245–8. doi: 10.1097/00005392-200101000-00072. [DOI] [PubMed] [Google Scholar]
- 24.Elbadawi A, Meyer S, Regnier CH. Role of ischemia in structural changes in the rabbit detrusor following partial bladder outlet obstruction: A working hypothesis and a biomechanical/structural model proposal. Neurourol Urodyn. 1989;8:151–62. doi: 10.1002/nau.1930080207. [DOI] [Google Scholar]
- 25.Macnab A, Stothers L, Shadgan B. Monitoring detrusor oxygenation and hemodynamics noninvasively during dysfunctional voiding. Adv Urol. 2012 doi: 10.1155/2012/676303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: The multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44:637–49. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Rosen RC, Giuliano F, Carson CC. Sexual dysfunction and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) Eur Urol. 2005;47:824–37. doi: 10.1016/j.eururo.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki T, Larsson H, Tisi D, et al. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J Mol Biol. 2000;301:1179–90. doi: 10.1006/jmbi.2000.3996. [DOI] [PubMed] [Google Scholar]
- 29.Schroder A, Chichester P, Kogan BA, et al. Effect of chronic bladder outlet obstruction on the blood flow of the rabbit urinary bladder. J Urol. 2001;165:640–6. doi: 10.1097/00005392-200102000-00087. [DOI] [PubMed] [Google Scholar]
- 30.Levin RM, O’Connor LJ, Leggett RE, et al. Focal hypoxia of the obstructed bladder wall correlates intermediate decompensation. Neurourol Urodyn. 2003;22:156–63. doi: 10.1002/nau.10076. [DOI] [PubMed] [Google Scholar]
- 31.Koritsiadis G, Stravodimos K, Koutalellis G, et al. Immunohistochemical estimation of hypoxia in human obstructed bladder and correlation with clinical variables. BJU Int. 2008;102:328–32. doi: 10.1111/j.1464-410X.2008.07593.x. [DOI] [PubMed] [Google Scholar]
- 32.Ghafar MA, Anastasiadis AG, Olsson LE, et al. Hypoxia and an angiogenic response in the partially obstructed rat bladder. Lab Invest. 2002;82:903–9. doi: 10.1097/01.LAB.0000021135.87203.92. [DOI] [PubMed] [Google Scholar]
- 33.Greenland JE, Hvistendahl JJ, Andersen H, et al. The effect of bladder outlet obstruction on tissue oxygen tension and blood flow in the pig bladder. BJU Int. 2000;85:1109–14. doi: 10.1046/j.1464-410x.2000.00611.x. [DOI] [PubMed] [Google Scholar]
- 34.Azadzoi KM, Pontari M, Vlachiotis J, et al. Canine bladder blood flow and oxygenation: Changes induced by filling, contraction and outlet obstruction. J Urol. 1996;155:1459–65. doi: 10.1016/S0022-5347(01)66307-9. [DOI] [PubMed] [Google Scholar]
- 35.Nomiya M, Burmeister DM, Sawada N, et al. Prophylactic effect of tadalafil on bladder function in a rat model of chronic bladder ischemia. J Urol. 2013;189:754–61. doi: 10.1016/j.juro.2012.07.141. [DOI] [PubMed] [Google Scholar]
- 36.Macnab AJ, Shadgan B, Stothers L, et al. Ambulant monitoring of bladder oxygenation and hemodynamics using wireless near-infrared spectroscopy. Can Urol Assoc J. 2013;7:E98–104. doi: 10.5489/cuaj.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farag FF, Meletiadis J, Saleem MD, et al. Near-infrared spectroscopy of the urinary bladder during voiding in men with low urinary tract symptoms: A preliminary study. Biomed Res Int. 2013;2013:452857. doi: 10.1155/2013/452857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belenky A, Abarbanel Y, Cohen M, et al. Detrusor resistive index evaluated by Doppler ultrasonography as a potential indicator of bladder outlet obstruction. Urology. 2003;62:647–50. doi: 10.1016/S0090-4295(03)00510-7. [DOI] [PubMed] [Google Scholar]
- 39.Kroyer K, Bulow J, Nielsen SL, et al. Urinary blood flow. I. Comparison of clearance of locally injected 99mtechnetium pertechnate and radioactive microsphere technique in dogs. Urol Res. 1990;18:223–6. doi: 10.1007/BF00295852. [DOI] [PubMed] [Google Scholar]
- 40.Kershen RT, Azadzoi KM, Siroky MB. Blood flow, pressure and compliance in the male human bladder. J Urol. 2002;168:121–5. doi: 10.1016/S0022-5347(05)64843-4. [DOI] [PubMed] [Google Scholar]
- 41.Lin AT, Chen MT, Yang CH, et al. Blood flow of the urinary bladder: Effects of outlet obstruction and correlation with bioenergetic metabolism. Neurourol Urodyn. 1995;14:285–92. doi: 10.1002/nau.1930140309. [DOI] [PubMed] [Google Scholar]
- 42.Chapple CR, Milner P, Moss HE, et al. Loss of sensory neuropeptides in the obstructed human bladder. Br J Urol. 1992;70:373–81. doi: 10.1111/j.1464-410X.1992.tb15791.x. [DOI] [PubMed] [Google Scholar]
- 43.Damaser MS, Haugaard N, Uvelius B. Partial obstruction of the rat urinary bladder: Effects on mitochondria and mitochondrial glucose metabolism in detrusor smooth muscle cells. Neurourol Urodyn. 1997;16:601–7. doi: 10.1002/(SICI)1520-6777(1997)16:6<601::AID-NAU9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 44.Lu SH, Chang LS, Yang AH, et al. Mitochondrial DNA deletion of the human detrusor after partial bladder outlet obstruction-correlation with urodynamic analysis. Urology. 2000;55:603–7. doi: 10.1016/S0090-4295(99)00609-3. [DOI] [PubMed] [Google Scholar]
- 45.Lu SH, Wei YH, Chang LS, et al. Morphological and morphometric analysis of human detrusor mitochondria with urodynamic correlation after partial outlet obstruction. J Urol. 2000;163:225–9. doi: 10.1016/S0022-5347(05)68011-1. [DOI] [PubMed] [Google Scholar]
- 46.de Jong BWD, Wolffenbuttel KP, Scheepe JR, et al. The detrusor glycogen content of a de-obstructed bladder reflects the functional history of that bladder during PBOO. Neurourol Urodyn. 2008;27:454–60. doi: 10.1002/nau.20567. [DOI] [PubMed] [Google Scholar]
- 47.Pessina F, Solito R, Maestrini D, et al. Effect of anoxia-glucopenia and resuperfusion on intrinsic nerves of mammalian detrusor smooth muscle: Importance of glucose metabolism. Neurourol Urodyn. 2005;24:389–96. doi: 10.1002/nau.20094. [DOI] [PubMed] [Google Scholar]
- 48.Siflinger-Birnboim A, Levin RM, Hass MA. Partial outlet obstruction of the rabbit urinary bladder induces selective protein oxidation. Neurourol Urodyn. 2008;27:532–9. doi: 10.1002/nau.20557. [DOI] [PubMed] [Google Scholar]
- 49.Lin ATL, Shiao MS, Chen CJ, et al. Energetics of detrusor contraction: Effects of outlet obstruction. Neurourol Urodyn. 1992;11:605–14. doi: 10.1002/nau.1930110604. [DOI] [Google Scholar]
- 50.Zhao Y, Levin SS, Wein AJ, et al. Correlation of ischemia/reperfusion or partial outlet obstruction-induced spectrin proteolysis by calpain with contractile dysfunction in rabbit bladder. Urology. 1997;49:293–300. doi: 10.1016/S0090-4295(96)00452-9. [DOI] [PubMed] [Google Scholar]
- 51.Yokoyama O, Kawaguchi K, Hisazumi H. Denervation super sensitivity of the detrusor muscle due to bladder over distension, with special reference to the relationship between super sensitivity, and changes in the connective tissue. Hinyokika Kiyo. 1985;31:2127–34. [PubMed] [Google Scholar]
- 52.Lin AT, Chen KK, Yang CH, et al. Effects of outlet obstruction and its reversal on mitochondrial enzyme activity in rabbit urinary bladders. J Urol. 1998;160:2258–62. doi: 10.1016/S0022-5347(01)62306-1. [DOI] [PubMed] [Google Scholar]
- 53.Gosling JA, Kung LS, Dixon JS, et al. Correlation between the structure and function of the rabbit urinary bladder following partial outlet obstruction. J Urol. 2000;163:1349–56. doi: 10.1016/S0022-5347(05)67776-2. [DOI] [PubMed] [Google Scholar]
- 54.Mirone V, Imbimbo C, Longo N, et al. The detrusor muscle: An innocent victim of bladder outlet obstruction. Eur Urol. 2007;51:57–66. doi: 10.1016/j.eururo.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 55.Hsu TH-S, Levin RM, Wein AJ, et al. Alterations of mitochondrial oxidative metabolism in rabbit urinary bladder after partial outlet obstruction. Mol Cell Biochem. 1994;141:47–55. doi: 10.1007/BF00935590. [DOI] [PubMed] [Google Scholar]
- 56.Campbell JD, Agubosim S, Paul RJ. Compartmentation of metabolism and function in vascular smooth muscle: Quantitation of Na-pump activity and aerobic glycolysis. FASEB J. 1988;2:A755. [PubMed] [Google Scholar]
- 57.Siegman MJ, Butler TM, Mooers SU, et al. Chemical energetics of force development, force maintenance, and relaxation in mammalian smooth muscle. J Gen Physiol. 1980;76:609–29. doi: 10.1085/jgp.76.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levin RM, Haugaard N, Mogavero L, et al. Biochemical evaluation of obstructive bladder dysfunction in men secondary to BPH: A preliminary report. Urology. 1999;53:446–50. doi: 10.1016/S0090-4295(98)00497-X. [DOI] [PubMed] [Google Scholar]
- 59.Kato K, Lin ATL, Wein AJ, et al. Effect of outlet obstruction on glucose metabolism of the rabbit urinary bladder. J Urol. 1990;143:844–7. doi: 10.1016/s0022-5347(17)40114-5. [DOI] [PubMed] [Google Scholar]
- 60.Hanchanale VS, Rao AR, Das S. Raman spectroscopy and its urological applications. Indian J Urol. 2008;24:444–50. doi: 10.4103/0970-1591.39550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Michel MC, Vrydag W. α1-, α2- and β-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006;147:S88–119. doi: 10.1038/sj.bjp.0706619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andersson KE. Detrusor myocyte activity and afferent signaling. Neurourol Urodyn. 2010;29:97–106. doi: 10.1002/nau.20784. [DOI] [PubMed] [Google Scholar]
- 63.Haddad GG, Jiang C. O2 deprivation in the central nervous system: On mechanisms of neuronal response, differential sensitivity and injury. Prog Neurobiol. 1993;40:277–318. doi: 10.1016/0301-0082(93)90014-J. [DOI] [PubMed] [Google Scholar]
- 64.Geloso DA, Levin RM. Effect of partial outlet obstruction on the myogenic response to field stimulation. Gen Pharmacol. 1998;31:291–5. doi: 10.1016/S0306-3623(97)00437-0. [DOI] [PubMed] [Google Scholar]
- 65.Michel MC, Barendrecht MM. Physiological and pathological regulation of the autonomic control of urinary bladder contractility. Pharmacology & Therapeutics. 2008;117:297–312. doi: 10.1016/j.pharmthera.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Cumming JA, Chisholm GD. Changes in detrusor innervation with relief of outflow tract obstruction. Br J Urol. 1992;69:7–11. doi: 10.1111/j.1464-410X.1992.tb15448.x. [DOI] [PubMed] [Google Scholar]
- 67.Otsuki H, Kosaka T, Nakamura K, et al. β3-adrenoceptor agonist mirabegron is effective for overactive bladder that is unresponsive to antimuscarinic treatment or is related to benign prostatic hyperplasia in men. Int Urol Nephrol. 2013;45:53–60. doi: 10.1007/s11255-012-0343-5. [DOI] [PubMed] [Google Scholar]
- 68.Nitti V, Rosenberg S, Mitcheson HD, et al. Urodynamics and safety of the β3-adrenoceptor agonist Mirabegron in males with lower urinary tract symptoms and bladder outlet obstruction. J Urol. 2013;190:1320–7. doi: 10.1016/j.juro.2013.05.062. [DOI] [PubMed] [Google Scholar]


