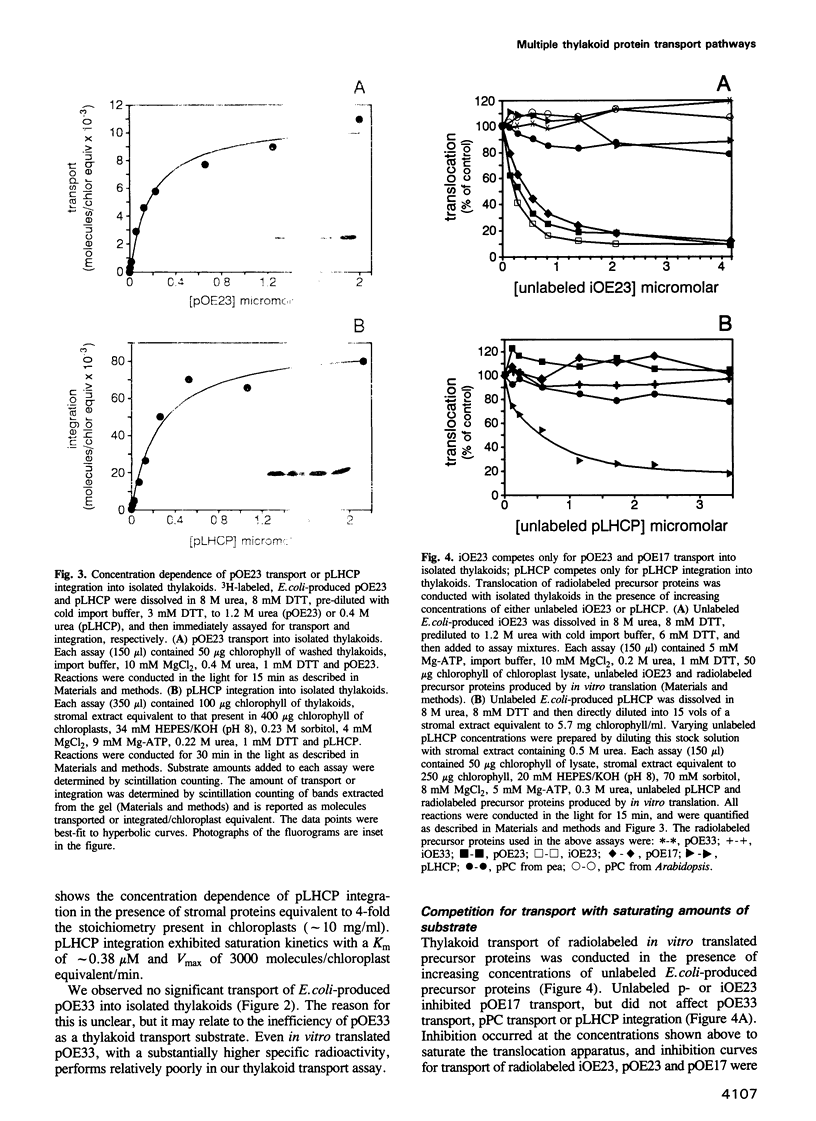
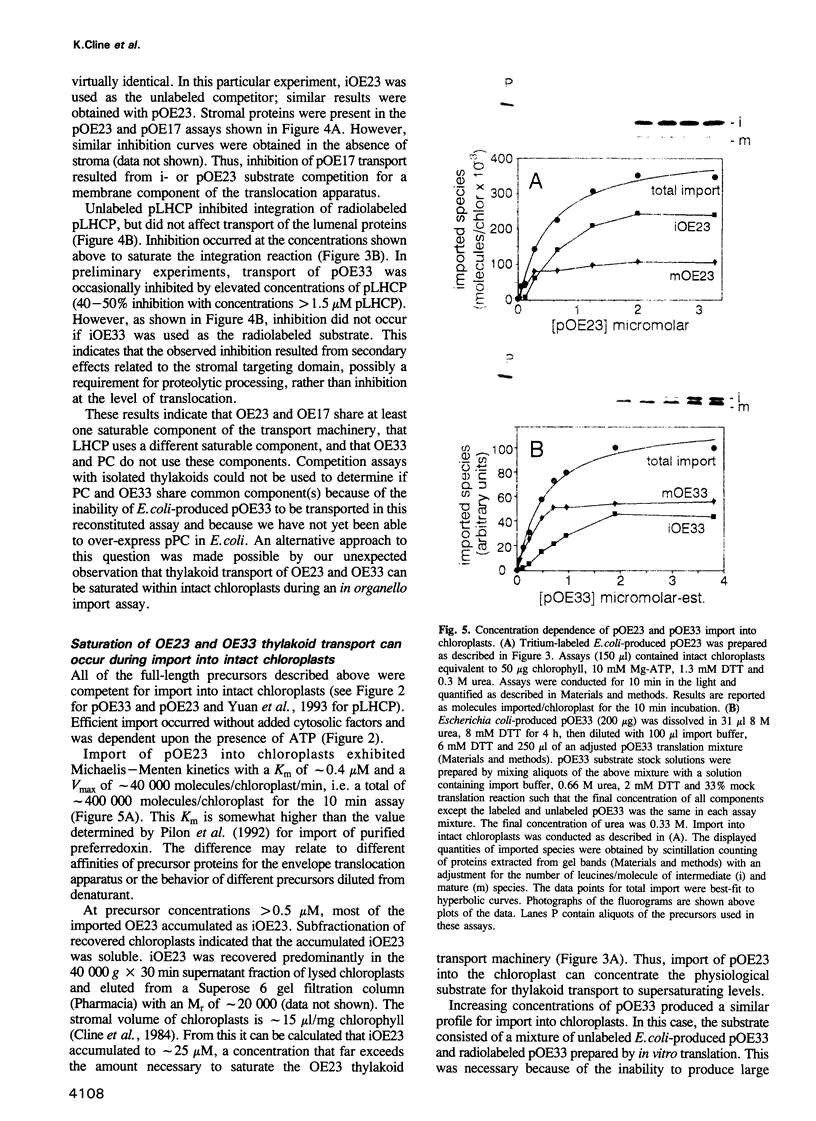
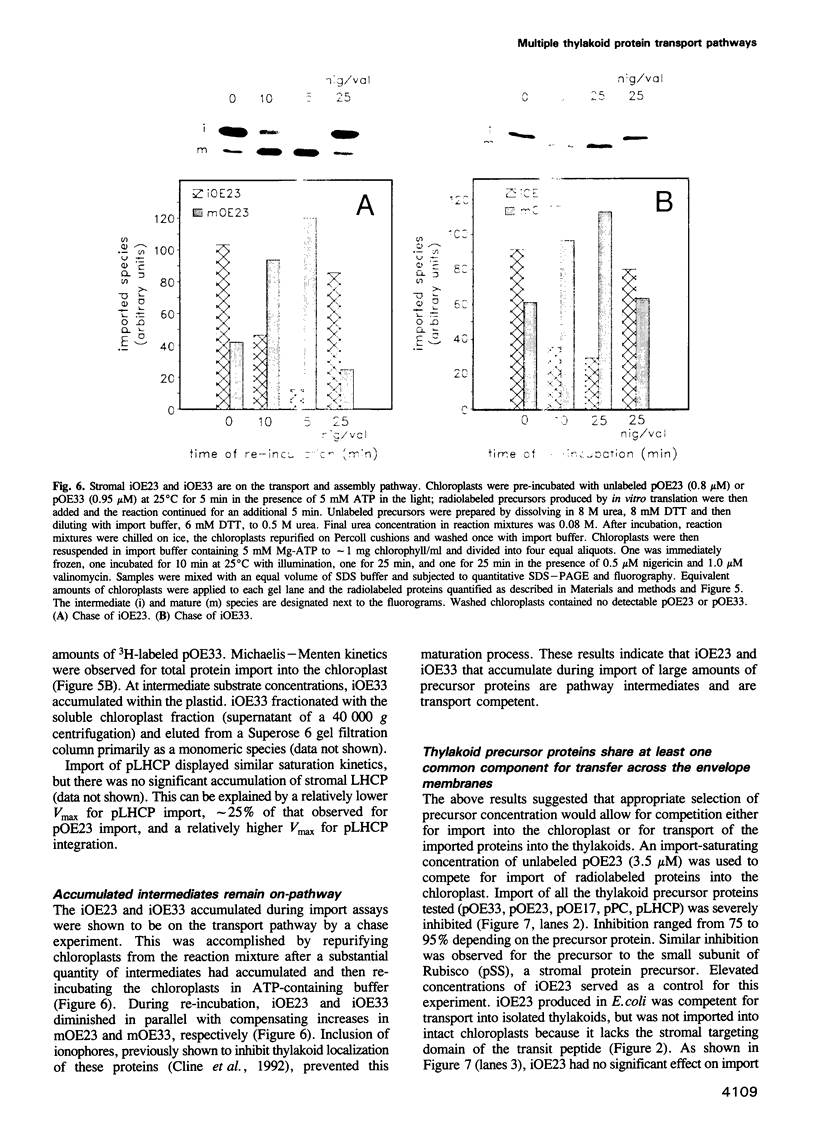
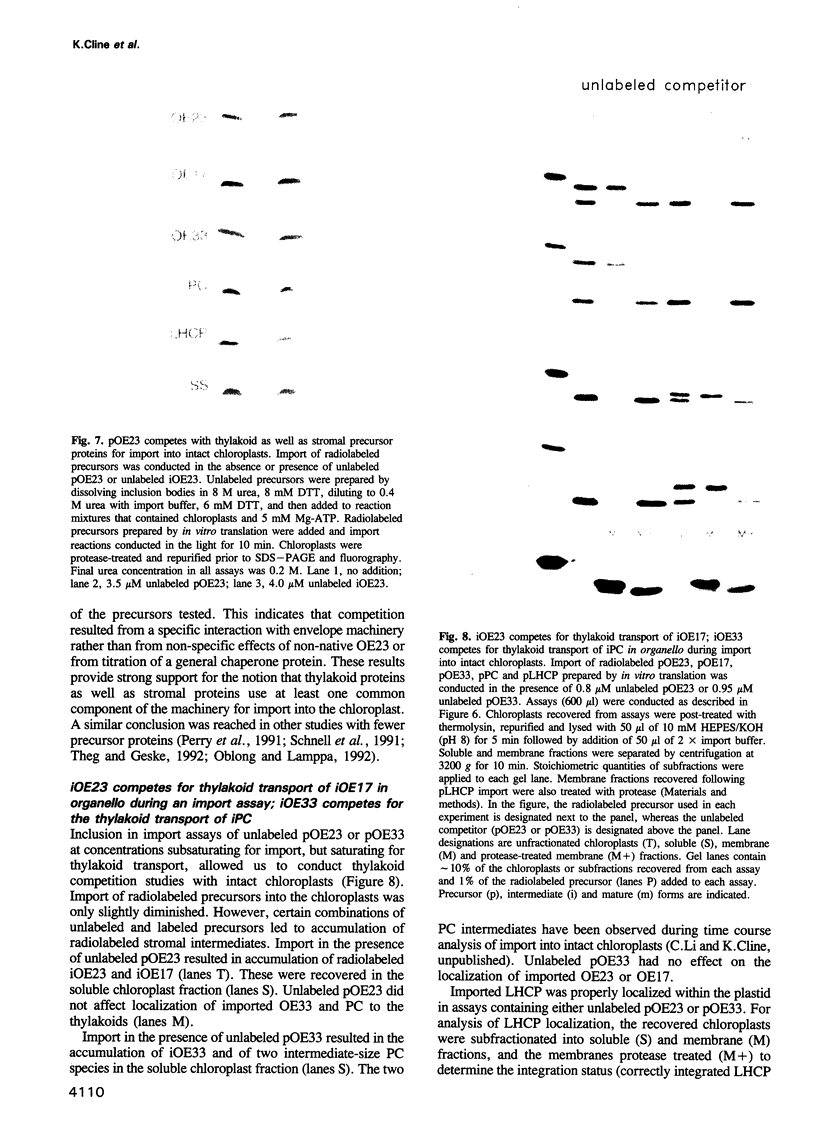
Abstract
Many thylakoid proteins are cytosolically synthesized and have to cross the two chloroplast envelope membranes as well as the thylakoid membrane en route to their functional locations. In order to investigate the localization pathways of these proteins, we over-expressed precursor proteins in Escherichia coli and used them in competition studies. Competition was conducted for import into the chloroplast and for transport into or across isolated thylakoids. We also developed a novel in organello method whereby competition for thylakoid transport occurred within intact chloroplasts. Import of all precursors into chloroplasts was similarly inhibited by saturating concentrations of the precursor to the OE23 protein. In contrast, competition for thylakoid transport revealed three distinct precursor specificity groups. Lumen-resident proteins OE23 and OE17 constitute one group, lumenal proteins plastocyanin and OE33 a second, and the membrane protein LHCP a third. The specificity determined by competition correlates with previously determined protein-specific energy requirements for thylakoid transport. Taken together, these results suggest that thylakoid precursor proteins are imported into chloroplasts on a common import apparatus, whereupon they enter one of several precursor-specific thylakoid transport pathways.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Smith S. M. Synthesis of the small subunit of ribulose-bisphosphate carboxylase from genes cloned into plasmids containing the SP6 promoter. Biochem J. 1986 Dec 15;240(3):709–715. doi: 10.1042/bj2400709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchincloss A. H., Alexander A., Kohorn B. D. Requirement for three membrane-spanning alpha-helices in the post-translational insertion of a thylakoid membrane protein. J Biol Chem. 1992 May 25;267(15):10439–10446. [PubMed] [Google Scholar]
- Bassham D. C., Bartling D., Mould R. M., Dunbar B., Weisbeek P., Herrmann R. G., Robinson C. Transport of proteins into chloroplasts. Delineation of envelope "transit" and thylakoid "transfer" signals within the pre-sequences of three imported thylakoid lumen proteins. J Biol Chem. 1991 Dec 15;266(35):23606–23610. [PubMed] [Google Scholar]
- Bauerle C., Keegstra K. Full-length plastocyanin precursor is translocated across isolated thylakoid membranes. J Biol Chem. 1991 Mar 25;266(9):5876–5883. [PubMed] [Google Scholar]
- Cline K., Ettinger W. F., Theg S. M. Protein-specific energy requirements for protein transport across or into thylakoid membranes. Two lumenal proteins are transported in the absence of ATP. J Biol Chem. 1992 Feb 5;267(4):2688–2696. [PubMed] [Google Scholar]
- Cline K., Fulsom D. R., Viitanen P. V. An imported thylakoid protein accumulates in the stroma when insertion into thylakoids is inhibited. J Biol Chem. 1989 Aug 25;264(24):14225–14232. [PubMed] [Google Scholar]
- Cline K. Import of proteins into chloroplasts. Membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J Biol Chem. 1986 Nov 5;261(31):14804–14810. [PubMed] [Google Scholar]
- Cline K. Light-Harvesting Chlorophyll a/b Protein : Membrane Insertion, Proteolytic Processing, Assembly into LHC II, and Localization to Appressed Membranes Occurs in Chloroplast Lysates. Plant Physiol. 1988 Apr;86(4):1120–1126. doi: 10.1104/pp.86.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Andrews J., Keegstra K. Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol. 1984 Jul;75(3):675–678. doi: 10.1104/pp.75.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. S., Cooper H. J., Smith M. A., Sims M. J., Parker D., Gewert D. Improved cloning efficiency of polymerase chain reaction (PCR) products after proteinase K digestion. Nucleic Acids Res. 1991 Jan 11;19(1):184–184. doi: 10.1093/nar/19.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douwe de Boer A., Weisbeek P. J. Chloroplast protein topogenesis: import, sorting and assembly. Biochim Biophys Acta. 1991 Nov 13;1071(3):221–253. doi: 10.1016/0304-4157(91)90015-o. [DOI] [PubMed] [Google Scholar]
- Driessen A. J. Bacterial protein translocation: kinetic and thermodynamic role of ATP and the protonmotive force. Trends Biochem Sci. 1992 Jun;17(6):219–223. doi: 10.1016/0968-0004(92)90381-i. [DOI] [PubMed] [Google Scholar]
- Halpin C., Elderfield P. D., James H. E., Zimmermann R., Dunbar B., Robinson C. The reaction specificities of the thylakoidal processing peptidase and Escherichia coli leader peptidase are identical. EMBO J. 1989 Dec 1;8(12):3917–3921. doi: 10.1002/j.1460-2075.1989.tb08572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
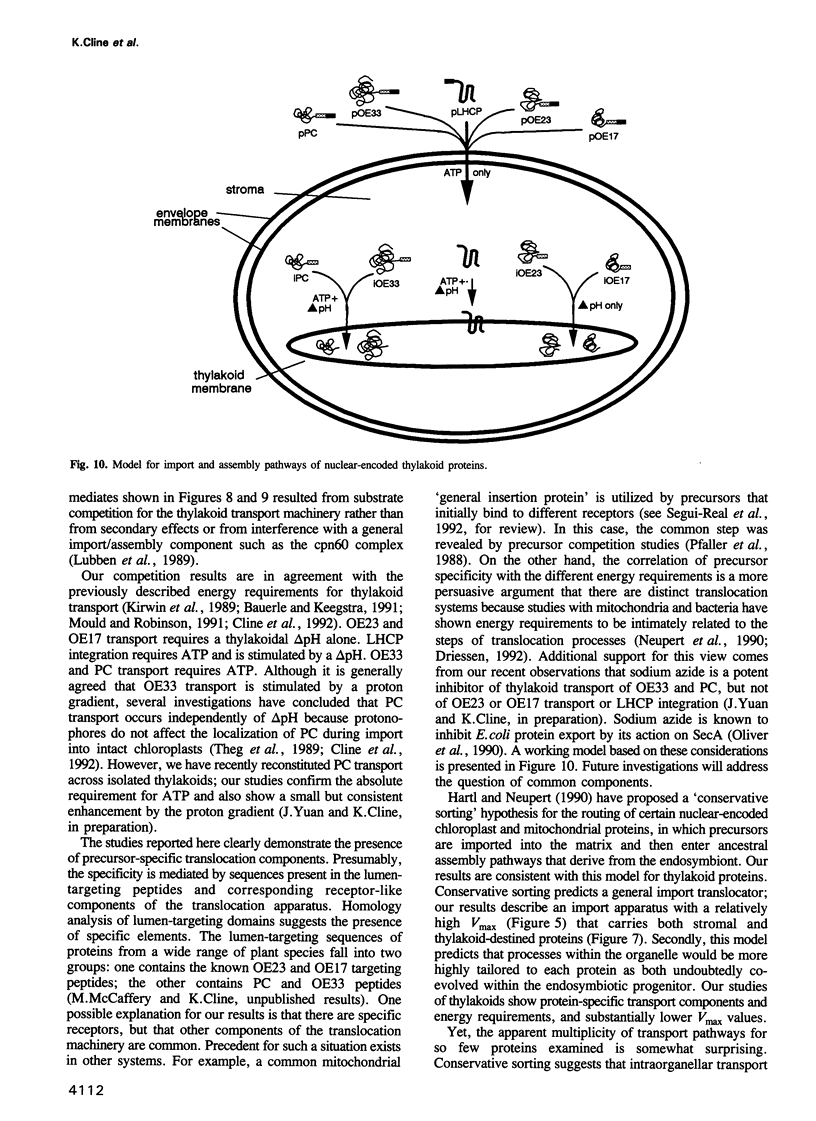
- Hartl F. U., Neupert W. Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science. 1990 Feb 23;247(4945):930–938. doi: 10.1126/science.2406905. [DOI] [PubMed] [Google Scholar]
- James H. E., Bartling D., Musgrove J. E., Kirwin P. M., Herrmann R. G., Robinson C. Transport of proteins into chloroplasts. Import and maturation of precursors to the 33-, 23-, and 16-kDa proteins of the photosynthetic oxygen-evolving complex. J Biol Chem. 1989 Nov 25;264(33):19573–19576. [PubMed] [Google Scholar]
- Kirwin P. M., Meadows J. W., Shackleton J. B., Musgrove J. E., Elderfield P. D., Mould R., Hay N. A., Robinson C. ATP-dependent import of a lumenal protein by isolated thylakoid vesicles. EMBO J. 1989 Aug;8(8):2251–2255. doi: 10.1002/j.1460-2075.1989.tb08349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamppa G. K. The chlorophyll a/b-binding protein inserts into the thylakoids independent of its cognate transit peptide. J Biol Chem. 1988 Oct 15;263(29):14996–14999. [PubMed] [Google Scholar]
- Lee H. J., Wilson I. B. Enzymic parameters: measurement of V and Km. Biochim Biophys Acta. 1971 Sep 22;242(3):519–522. doi: 10.1016/0005-2744(71)90144-6. [DOI] [PubMed] [Google Scholar]
- Lin K. H., Cheng S. Y. An efficient method to purify active eukaryotic proteins from the inclusion bodies in Escherichia coli. Biotechniques. 1991 Dec;11(6):748, 750, 752-3. [PubMed] [Google Scholar]
- Lubben T. H., Donaldson G. K., Viitanen P. V., Gatenby A. A. Several proteins imported into chloroplasts form stable complexes with the GroEL-related chloroplast molecular chaperone. Plant Cell. 1989 Dec;1(12):1223–1230. doi: 10.1105/tpc.1.12.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty D. R., Hattori T., Carson C. B., Vasil V., Lazar M., Vasil I. K. The Viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell. 1991 Sep 6;66(5):895–905. doi: 10.1016/0092-8674(91)90436-3. [DOI] [PubMed] [Google Scholar]
- Mould R. M., Robinson C. A proton gradient is required for the transport of two lumenal oxygen-evolving proteins across the thylakoid membrane. J Biol Chem. 1991 Jul 5;266(19):12189–12193. [PubMed] [Google Scholar]
- Mould R. M., Shackleton J. B., Robinson C. Transport of proteins into chloroplasts. Requirements for the efficient import of two lumenal oxygen-evolving complex proteins into isolated thylakoids. J Biol Chem. 1991 Sep 15;266(26):17286–17289. [PubMed] [Google Scholar]
- Neupert W., Hartl F. U., Craig E. A., Pfanner N. How do polypeptides cross the mitochondrial membranes? Cell. 1990 Nov 2;63(3):447–450. doi: 10.1016/0092-8674(90)90437-j. [DOI] [PubMed] [Google Scholar]
- Oblong J. E., Lamppa G. K. Precursor for the light-harvesting chlorophyll a/b-binding protein synthesized in Escherichia coli blocks import of the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. J Biol Chem. 1992 Jul 15;267(20):14328–14334. [PubMed] [Google Scholar]
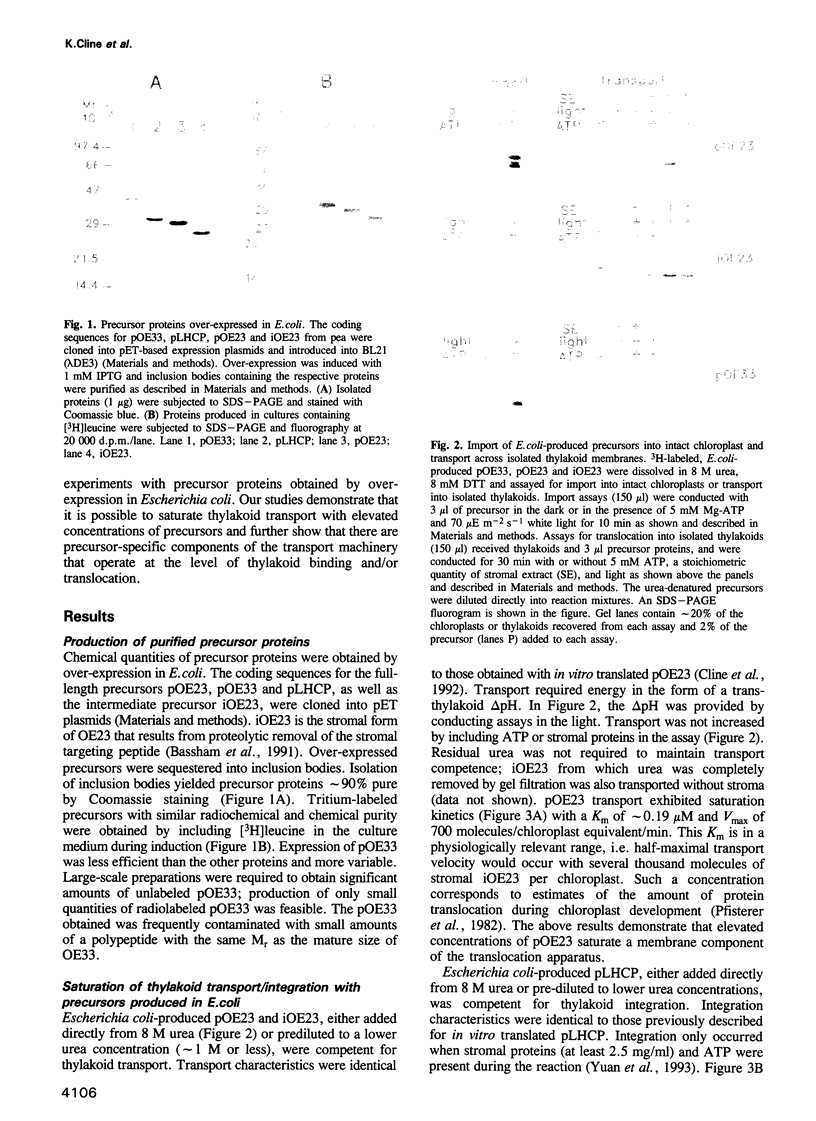
- Oliver D. B., Cabelli R. J., Dolan K. M., Jarosik G. P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payan L. A., Cline K. A stromal protein factor maintains the solubility and insertion competence of an imported thylakoid membrane protein. J Cell Biol. 1991 Feb;112(4):603–613. doi: 10.1083/jcb.112.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. E., Buvinger W. E., Bennett J., Keegstra K. Synthetic analogues of a transit peptide inhibit binding or translocation of chloroplastic precursor proteins. J Biol Chem. 1991 Jun 25;266(18):11882–11889. [PubMed] [Google Scholar]
- Pfaller R., Steger H. F., Rassow J., Pfanner N., Neupert W. Import pathways of precursor proteins into mitochondria: multiple receptor sites are followed by a common membrane insertion site. J Cell Biol. 1988 Dec;107(6 Pt 2):2483–2490. doi: 10.1083/jcb.107.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
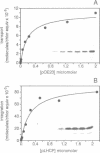
- Pfisterer J., Lachmann P., Kloppstech K. Transport of proteins into chloroplasts. Binding of nuclear-coded chloroplast proteins to the chloroplast envelope. Eur J Biochem. 1982 Aug;126(1):143–148. doi: 10.1111/j.1432-1033.1982.tb06758.x. [DOI] [PubMed] [Google Scholar]
- Pilon M., Weisbeek P. J., de Kruijff B. Kinetic analysis of translocation into isolated chloroplasts of the purified ferredoxin precursor. FEBS Lett. 1992 May 4;302(1):65–68. doi: 10.1016/0014-5793(92)80286-p. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. E., Cline K., Stephens L. C., Bacot K. O., Viitanen P. V. Early events in the import/assembly pathway of an integral thylakoid protein. Eur J Biochem. 1990 Nov 26;194(1):33–42. doi: 10.1111/j.1432-1033.1990.tb19423.x. [DOI] [PubMed] [Google Scholar]
- Schnell D. J., Blobel G., Pain D. Signal peptide analogs derived from two chloroplast precursors interact with the signal recognition system of the chloroplast envelope. J Biol Chem. 1991 Feb 15;266(5):3335–3342. [PubMed] [Google Scholar]
- Segui-Real B., Stuart R. A., Neupert W. Transport of proteins into the various subcompartments of mitochondria. FEBS Lett. 1992 Nov 16;313(1):2–7. doi: 10.1016/0014-5793(92)81171-h. [DOI] [PubMed] [Google Scholar]
- Smeekens S., Bauerle C., Hageman J., Keegstra K., Weisbeek P. The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell. 1986 Aug 1;46(3):365–375. doi: 10.1016/0092-8674(86)90657-4. [DOI] [PubMed] [Google Scholar]
- Theg S. M., Bauerle C., Olsen L. J., Selman B. R., Keegstra K. Internal ATP is the only energy requirement for the translocation of precursor proteins across chloroplastic membranes. J Biol Chem. 1989 Apr 25;264(12):6730–6736. [PubMed] [Google Scholar]
- Theg S. M., Geske F. J. Biophysical characterization of a transit peptide directing chloroplast protein import. Biochemistry. 1992 Jun 2;31(21):5053–5060. doi: 10.1021/bi00136a018. [DOI] [PubMed] [Google Scholar]
- Viitanen P. V., Doran E. R., Dunsmuir P. What is the role of the transit peptide in thylakoid integration of the light-harvesting chlorophyll a/b protein? J Biol Chem. 1988 Oct 15;263(29):15000–15007. [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]