Abstract
Background
In past decade, the treatment of gastric adenocarcinoma has evolved as a result of the publication of two seminal randomized controlled trials. We aimed to examine treatment trends for resectable gastric cancer (stage I–III) using the National Cancer Data Base (NCDB). Our hypothesis was that the use of chemotherapy and chemoradiotherapy in addition to surgery for the treatment of gastric adenocarcinoma has increased from 2000 to 2009.
Methods
Patients diagnosed with stage I–III gastric adenocarcinoma between 2000 and 2009 were selected from the NCDB Hospital Comparison Benchmark Reports. Attention was paid to the initial treatment regimen and data on hospital setting were collected and analyzed. The Cochran–Armitage test for trend was used to assess changes in treatment over time.
Results
A total of 50,778 patients with stage I–III gastric adenocarcinoma were included in the analysis. Between 2000 and 2009, the use of surgery alone decreased significantly across all three stages at both teaching hospitals and community hospitals (p < 0.0001 for all cases). In the same period, the use of chemotherapy in addition to surgery increased significantly across all three stages and at both hospital settings (p < 0.0001 for all cases). Surgery plus chemoradiotherapy increased for stage I–III disease at community hospitals (p < 0.05 for all) but only increased significantly for stage II disease at teaching hospitals (p < 0.01). Incidentally, nonsurgical treatment increased across all three stages at both hospital settings (p < 0.001 for all cases).
Conclusions
Data from the NCDB from 2000 to 2009 demonstrate that there has been an increasing use of chemotherapy in addition to surgery for resectable gastric cancer.
In 2012, approximately 21,000 cases of gastric cancer were diagnosed. Resection provides the only chance for cure. Outcomes have improved, with the 5-year survival rate doubling between 1975 and 2007 from 15 to 27 %.1 The basis of this change is multifactorial, including modifications in surgical technique and improvements in chemotherapy and radiotherapy. However, the ideal combination of treatment modalities remains controversial.
Several trials in the past decade have shown the benefit of additional therapies. A US Intergroup Trial demonstrated increased survival time among patients receiving adjuvant chemoradiotherapy versus observation after resection.2 The British MAGIC trial further showed the benefit of perioperative chemotherapy, demonstrating an improved 5-year survival compared to observation after surgery.3 The results of these trials have led the United Kingdom and parts of Europe to adopt the perioperative chemotherapy regimen, while the United States continues to primarily use adjuvant chemoradiotherapy.4,5
Little is known about how widely these protocols have been adopted since the publications acknowledging their efficacy. Using the National Cancer Data Base (NCDB), we evaluated trends in the surgical treatment of gastric adenocarcinoma at American cancer centers between 2000 and 2009.
METHODS
The NCDB is a national oncology outcomes database for over 1,500 Commission on Cancer-accredited cancer programs. The NCDB is a joint program of the American College of Surgeon’s Commission on Cancer and the American Cancer Society. Institutional review board approval was received from our institution to access the database, which was accessed on March 15, 2013.
Patients diagnosed with stage I–III gastric adenocarcinoma between 2000 and 2009 were selected from the NCDB Hospital Comparison Benchmark Reports. Histologic subtypes listed under “gastric adenocarcinoma” included intestinal type adenocarcinoma, signet ring cell carcinoma, and adenocarcinoma not otherwise specified. A combined American Joint Committee on Cancer (AJCC) Stage Group is used in the NCDB Benchmark Reports; this includes the pathological stage group where documented, augmented by the clinical stage group where pathological stage is not recorded. The 5th edition of the AJCC cancer staging manual was used for cases diagnosed between 2000 and 2002, and the 6th edition of the AJCC cancer staging manual was used for cases diagnosed between 2003 and 2009.6,7
Attention was paid to the initial treatment regimen. The NCDB does not identify the timing of therapies received in addition to surgery. Surgery plus radiotherapy and chemotherapy is referred to interchangeably as “chemoradiotherapy.” We designated “nonsurgical therapy” to include chemotherapy only, chemoradiotherapy only, or no first-course treatment. The NCDB categorizes patients as receiving “other specified therapy” when the first course of treatment does not fall under any of the above-mentioned categories.
We defined private insurance as including managed care or any private insurance. Patient comorbidities are recorded in the NCDB according to the Charlson comorbidity score using the Deyo method of mapping administrative codes.8 The Charlson comorbidity score was available for the period 2003–2009; patients in the period 2000–2002 had their score listed as “not applicable.”
Data regarding hospital setting were analyzed. The NCDB defined teaching/research hospitals as facilities associated with university medical schools or are designated as National Cancer Institute Comprehensive Cancer Care programs. Teaching/research hospitals make up 18 % of the reporting hospitals and account for 33 % of cases in the NCDB. The NCDB defines community cancer centers as facilities that diagnose or treat 100–649 cancer cases annually and comprehensive community cancer centers as facilities that treat 650 or more cancer cases annually. In this study, we considered community and comprehensive community cancer centers together; we refer to them generally as “community hospitals.” Community hospitals make up 74 % of all reporting hospitals and account for 63 % of cases in the NCDB. Hospitals that do not fulfill these criteria were categorized as “other” and make up the remaining portion of reporting hospitals in the NCDB.
We compared treatment trends from 2000 to 2009. The Cochran-Armitage test for trend was used to assess changes in treatment over time. Percentage change in the use of a treatment modality is reported as the absolute change between 2009 and 2000 unless otherwise noted. Patient demographics were compared between the two hospital settings. Pearson’s chi-square test was used to compare categorical variables. The level of statistical significance was set to p < 0.05. All reported p values are two-tailed. Statistical analysis was done using JMP Pro 10.0 (Cary, NC) and GraphPad Prism 6 (La Jolla, CA).
RESULTS
Demographics
A total of 50,778 patients with stage I–III gastric adenocarcinoma were included in the study. Patient demographics at community and teaching hospitals are shown in Table 1. With the exception of cancer stage, all demographics varied significantly between community and teaching hospitals (p < 0.0001 for all). Notable differences included an older population with a higher portion of patients with a Charlson Comorbidity Score of 2 or greater receiving treatment at community hospitals.
TABLE 1.
Patient demographics
| Characteristic | Community hospital (n = 30,593) |
Teaching hospital (n = 20,185) |
||
|---|---|---|---|---|
| n | % | n | % | |
| Age* | ||||
| <50 years | 2,565 | 8.38 | 2,199 | 10.89 |
| 50–59 years | 4,231 | 13.83 | 3,515 | 17.41 |
| 60–69 years | 6,958 | 22.74 | 5,030 | 24.92 |
| 70–79 years | 9,549 | 31.21 | 5,857 | 29.02 |
| 80–89 years | 6,361 | 20.79 | 3,211 | 15.91 |
| 90 years and over | 929 | 3.04 | 373 | 1.85 |
| Female gender* | 10,988 | 35.92 | 6,776 | 33.57 |
| Race* | ||||
| White | 21,667 | 70.82 | 13,551 | 67.13 |
| Nonwhite | 8,926 | 29.18 | 6,634 | 32.87 |
| Insurance status* | ||||
| Not insured | 722 | 2.36 | 670 | 3.32 |
| Private insurance | 9,441 | 30.86 | 6,868 | 34.03 |
| Government | 19,886 | 65.00 | 11,442 | 56.69 |
| Insurance status unknown | 544 | 1.78 | 1,205 | 5.97 |
| Stage | ||||
| I | 12,308 | 40.23 | 8,198 | 40.61 |
| II | 7,574 | 24.76 | 4,862 | 24.09 |
| III | 10,711 | 35.01 | 7,125 | 35.30 |
| Histology* | ||||
| Adenocarcinoma, NOS | 22,204 | 72.58 | 13,878 | 68.75 |
| Intestinal type adenocarcinoma | 2,560 | 8.37 | 1,987 | 9.84 |
| Signet ring cell carcinoma | 5,829 | 19.05 | 4,320 | 21.40 |
| Charlson comorbidity score* | ||||
| 0 | 14,244 | 46.56 | 10,346 | 51.26 |
| 1 | 4,870 | 15.92 | 3,065 | 15.18 |
| ≥2 | 1,947 | 6.36 | 1,031 | 5.11 |
| NA (before 2003) | 9,532 | 31.16 | 5,743 | 28.45 |
NOS not otherwise specified, NA not applicable
Significant difference between community and teaching hospitals by chi-square test
Surgery as Treatment of Gastric Adenocarcinoma
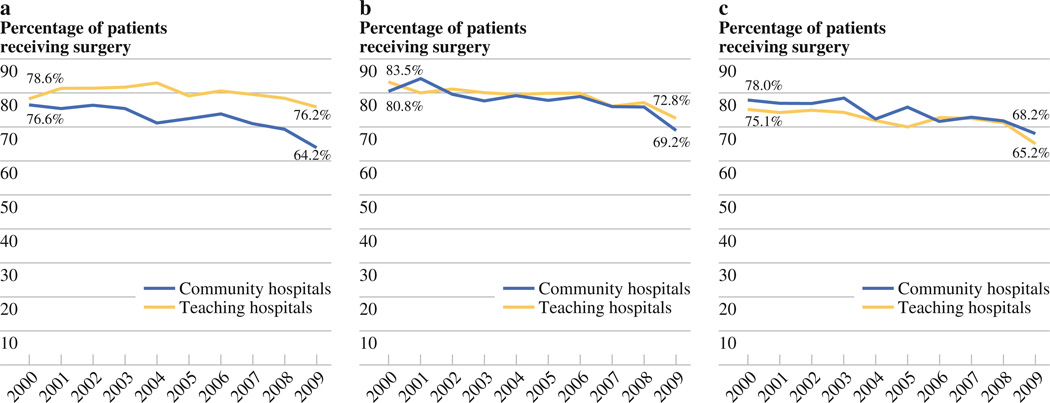
From 2000 to 2009, the use of surgery as a component of treatment of stage I–III disease decreased significantly at both community hospitals (p < 0.0001 for all) and teaching hospitals during the study period (p < 0.01 for all). In 2009, 64.2 % of stage I patients at community hospitals received surgery compared to 76.2 % at teaching hospitals (p < 0.0001) (Fig. 1a). In that same year, 69.2 % of stage II patients received surgery at community hospitals compared to 72.8 % at teaching hospitals (p = 0.15) (Fig. 1b). Of all stage III patients in 2009, 68.2 % received surgery at community hospitals compared to 65.2 % at teaching hospitals (p = 0.2) (Fig. 1c).
FIG. 1.
a Surgical resection rates for stage I gastric adenocarcinoma, 2000–2009. b Surgical resection rates for stage II gastric adenocarcinoma, 2000–2009. c Surgical resection rates of stage III gastric adenocarcinoma, 2000–2009
Use of Surgery Alone
The use of surgery alone decreased significantly for stage I–III disease at both community and teaching hospitals from 2000 to 2009 (p < 0.0001 for all). Among patients with stage I disease who underwent surgery in 2009 at both community and teaching hospitals, 72.4 % did not receive additional therapy. This represents a 10.7 % decrease in the use of surgery alone for stage I disease at community hospitals since 2000 and a 7.6 % decrease at teaching hospitals. Among stage II patients who underwent surgery in 2009, 39.2 % received surgery alone at community hospitals, a 17.6 % decrease since 2000. In 570 S. Raigani et al. comparison, 28.6 % of patients underwent surgery alone at teaching hospitals, a decrease of 24.3 % since 2000. Among stage III patients who received surgery in 2009, 34.0 % received surgery alone at community hospitals compared to 28.6 % at teaching hospitals, representing a 12.5 and 19.5 % decrease, respectively. In 2009, there was no significant difference in utilization of surgery alone for treatment of stage I and III patients when comparing community to teaching hospitals. However, stage II patients received surgery alone more frequently at community hospitals compared to teaching hospitals in 2009 (p = 0.014).
Use of Surgery Plus Chemotherapy
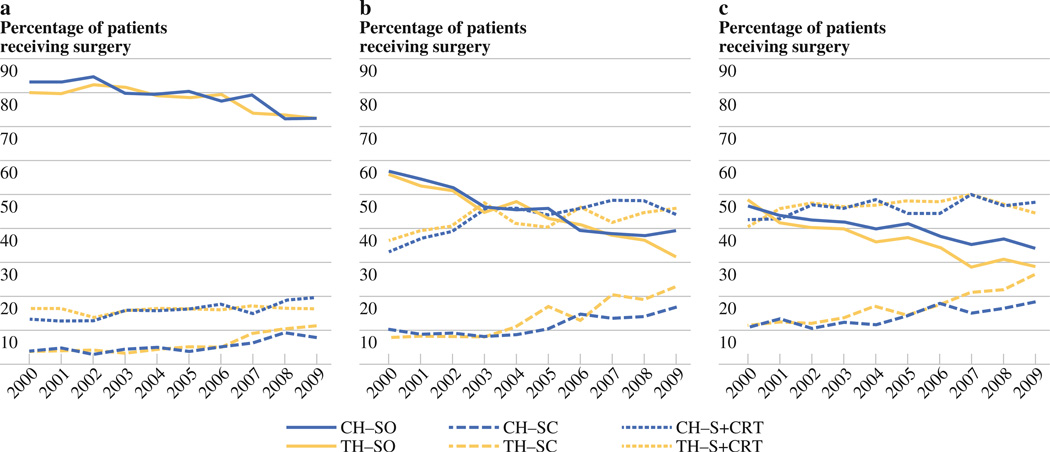
The use of chemotherapy in addition to surgery increased significantly for stage I–III disease at both community and teaching hospitals during the study period (p < 0.0001 for all). Of all stage I patients who underwent surgery at community hospitals in 2009, 7.9 % received additional chemotherapy, a 4.1 % increase from 2000. Likewise, of stage I patients who underwent surgery at teaching hospitals in 2009, 11.5 % received additional chemotherapy, a 7.8 % increase since 2000 (Fig. 2a). Of stage II patients who underwent surgery at community hospitals in 2009, 16.8 % received surgery plus chemotherapy, a 6.5 % increase. Likewise, 22.8 % of stage II patients at teaching hospitals received surgery plus chemotherapy in 2009, a 15.0 % increase since 2000 (Fig. 2b). Similar increases were seen for stage III disease, with 18.4 % of community hospitals patients receiving surgery plus chemotherapy in 2009 (7.4 % increase) compared to 26.8 % of teaching hospitals patients (15.5 % increase) (Fig. 2c). In 2009, patients with stage I–III disease received chemotherapy in addition to surgery more frequently at teaching hospitals compared to community hospitals (p < 0.05 for all three).
FIG. 2.
a Surgical treatment of stage I gastric adenocarcinoma. CH community hospitals, TH teaching hospitals, SO surgery only, SC surgery plus chemotherapy, S+CRT surgery plus chemoradiotherapy. b Surgical treatment of stage II gastric adenocarcinoma. c Surgical treatment of stage III gastric adenocarcinoma
Use of Surgery, Radiotherapy, and Chemotherapy
The use of surgery in combination with chemoradiotherapy (S+CRT) increased for stage I–III disease at community hospitals during the study period (p < 0.05 for all). Of all stage I patients who underwent surgery at community hospitals in 2009, 19.7 % received S+CRT, a 6.7 % increase since 2000 (Fig. 2a). Likewise, of stage II patients who received surgery at community hospitals, 44.1 % received S+CRT in 2009 (11.1 % increase) (Fig. 2b). Of stage III patients who received surgery at community hospitals, 47.6 % received S+CRT in 2009 (5.1 % increase) (Fig. 2c). Although the fraction of stage I–III patients at teaching hospitals who received S+CRT was comparable to that of community hospitals, only stage II disease saw a significant increase in the use of S+CRT (p < 0.01). Of stage II patients who underwent surgery at teaching hospitals in 2009, 45.8 % received S+CRT, a 9.3 % increase since 2000. In 2009, the rate of surgery in combination with chemoradiotherapy for treatment of stage I–III disease did not differ significantly between community and teaching hospitals.
Use of Nonsurgical Therapy
The use of nonsurgical therapy (NST) for stages I–III gastric adenocarcinoma increased significantly at both community and teaching hospitals during the study period (p < 0.001 for all). A total of 31.3 % of stage I patients at community hospitals received NST in 2009, a 12.5 % increase from 2000 compared to 20.0 % of stage I patients at teaching hospitals (3.2 % increase). The use of NST for stage II disease nearly doubled during the study period at both community and teaching hospitals with approximately 23 % receiving NST in 2009. Similarly, there was an approximate 11 % increase in NST for stage III disease at both hospitals, with 27.8 % of patients at community hospitals and 31.4 % at teaching hospitals receiving NST in 2009. When comparing the rate of NST for gastric adenocarcinoma between the two hospital settings, there was a significant difference for stage I disease (p < 0.0001), but NST was used at comparable rates for stage II and III disease at the two hospital settings.
DISCUSSION
Our study aimed to define trends in the treatment approach to resectable gastric adenocarcinoma in the United States. Using the NCDB, we found that there was a significant increase in the use of surgery plus chemotherapy as well as a significant decrease in the use of surgery alone for stage I–III disease. The use of surgery plus chemoradiotherapy continued to increase at community hospitals. However, the use of NST for treatment of resectable gastric adenocarcinoma increased.
Previous publications have demonstrated improved patient survival with the addition of adjuvant or neoadjuvant therapy compared to observation after surgery.2,3,9,10 Given the evidence in favor of therapy in additional to surgery, it would be expected that the use of surgery alone for treatment of resectable gastric adenocarcinoma is declining, with the exception of patients with stage IA disease, who were not included in either the Intergroup or MAGIC trial. Sherman et al.11 demonstrated a decreasing use of surgery alone from 64.3 to 39.0 % for stage IB–III disease between 1998 and 2007.Our study corroborates these findings by demonstrating that the rate of surgery alone has decreased from 56.4 to 35.8 % for stage II disease, and from 47.2 to 31.8 % for stage III disease between 2000 and 2009. Although surgery alone for stage I disease has decreased significantly since 2000, 72.4 % of these patients received no additional therapy in 2009. However, the number of stage IA versus stage IB patients is not evaluable in our data set.
After several small phase 3 trials that demonstrated improved survival with radiotherapy, the publication of the Intergroup 0016 trial changed the treatment approach to patients with resectable gastric adenocarcinoma.12–14 Sherman et al. found that less than 30 % of patients with stage IB–III gastric adenocarcinoma received postoperative adjuvant therapy in 1998 and 1999, increasing to approximately 40 % after 2001.11 Using the Surveillance, Epidemiology, and End Results database, Coburn et al.15 reported that 14.6 % of patients with resected gastric adenocarcinoma received adjuvant radiotherapy before the Intergroup 0016 trial compared to 30.4%in the 3 years after publication. Our study further verifies these findings, showing that the use of S+CRT increased significantly since 2000 for stage I–III disease treated at community hospitals. On the other hand, although a comparable portion of teaching hospitals patients received S+CRT, we found that the use of S+CRT increased only for stage II patients in this cohort. We report that of all patients in our study who underwent surgery in 2009, approximately 18 % of stage I and 45 % of stage II and III patients received S+CRT.
Cunningham and colleagues demonstrated improved overall survival with perioperative chemotherapy.3 Sherman et al. demonstrated an increased use of neoadjuvant therapy, from 5.9 % in 1998 to 20.0 % in 2007.11 Because the NCDB data accessed for the current study does not distinguish between adjuvant and neoadjuvant therapy, the designation of surgery plus chemotherapy is most appropriate in this comparison. In our study, the use of surgery plus chemotherapy increased significantly for stage I–III disease at both teaching and community hospitals between 2000 and 2009. Moreover, teaching hospitals utilized surgery plus chemotherapy more often than community hospitals for all three stages of disease. However, patients who received chemotherapy alone represent a minority proportion of patients, potentially as a result of slow implementation of MAGIC trial results or a preference for the use of radiotherapy as a component of therapy. In 2000, we found that among patients who received any therapy in addition to surgery, only 21 % received chemotherapy (stage I–III). However, by 2009, this portion had increased to a range of 30–35 %, indicating a shift in treatment paradigms. Recent trials continue to validate the efficacy of neoadjuvant and perioperative chemotherapy and have likely contributed to the increased use of chemotherapy in addition to surgery among American clinicians.16,17
The current study also demonstrates the more troubling finding that the use of NST for resectable gastric adenocarcinoma is increasing. The use of NST increased significantly at both community and teaching hospitals between 2000 and 2009. There was at least an 11 % increase in NST at both hospital settings, with the exception of stage I patients at teaching hospitals, which showed only a 3.2 % increase. Potential explanations for this finding are an aging patient population with increased comorbidities who are not suitable for surgery, as well as prolonged toxicity from preoperative therapy. The reasons for the increase in nonsurgical treatment could not be further explored using this data set.
This study is subject to several limitations. First, the NCDB does not distinguish between adjuvant or neoadjuvant treatments that patients receive or specify specific treatment protocols for chemotherapy or radiotherapy. As a result, generalizing conclusions from this study may result in small inaccuracies. However, given the large number of patients in the study, the overwhelming power of the study limits this factor. Second, the NCDB does not distinguish between stage IA or IB gastric adenocarcinoma, and as a result both patient populations were included in our analysis. Moreover, the database does not specify the extent of lymph node resection or lymph node involvement. These factors directly affect the treatment that is selected for each patient, and as a result, our data provide a less specific overview of current treatment trends.
In conclusion, the treatment of resectable gastric adenocarcinoma is continually changing in the United States. An increasing proportion of patients are receiving chemotherapy or chemoradiotherapy in addition to surgery with a concomitant decline in the utilization of surgery alone. However, there appears to be an increasing utilization of NST for resectable gastric adenocarcinoma.
ACKNOWLEDGMENT
SR would like to thank the Case Western Reserve University School of Medicine for providing support with the Dean’s Summer Research Award for first-year medical students.
Footnotes
DISCLOSURE The authors declare no conflict of interest.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Okines A, Verheij M, Allum W, Cunningham D, Cervantes A ESMO Guidelines Working Group. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:50–54. doi: 10.1093/annonc/mdq164. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network Guidelines in Oncology. [Accessed 1 Apr 2013];Gastric cancer, version 2.2013. http://www.nccn.org. [Google Scholar]
- 6.Fleming ID, Cooper JS, Henson DE, Hutter RV, et al., editors. AJCC cancer staging manual. 5th ed. New York: Lippincott-Raven; 1997. [Google Scholar]
- 7.Greene FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 8.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 9.Dent DM, Werner ID, Novis B, Cheverton P, Brice P. Prospective randomized trial of combined oncological therapy for gastric carcinoma. Cancer. 1979;44:385. doi: 10.1002/1097-0142(197908)44:2<385::aid-cncr2820440203>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Moertel CG, Childs DS, O’Fallon JR, Holbrook MA, Schutt AJ, Reitemeier RJ. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol. 1984;2:1249. doi: 10.1200/JCO.1984.2.11.1249. [DOI] [PubMed] [Google Scholar]
- 11.Sherman KL, Merkow RP, Bilimoria KY, et al. Treatment trends and predictors of adjuvant and neoadjuvant therapy for gastric adenocarcinoma in the United States. Ann Surg Oncol. 2013;20:362–370. doi: 10.1245/s10434-012-2552-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZX, Gu XZ, Yin WB, Huang GJ, Zhang DW, Zhang RG. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)—report on 370 patients. Int J Radiat Oncol Biol Phys. 1998;42:929–934. doi: 10.1016/s0360-3016(98)00280-6. [DOI] [PubMed] [Google Scholar]
- 13.Moertel CG, Childs DS, O’Fallon JR, Holbrook MA, Schutt AJ, Reitemeier RJ. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol. 1984;2:1249–1254. doi: 10.1200/JCO.1984.2.11.1249. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi M, Abe M. Intra-operative radiotherapy for carcinoma of the stomach. Eur J Surg Oncol. 1986;12:247–250. [PubMed] [Google Scholar]
- 15.Coburn NG, Guller U, Baxter NN, et al. Adjuvant therapy for resected gastric cancer—rapid, yet incomplete adoption following results of Intergroup 0116 trial. Int J Radiat Oncol Biol Phys. 2008;70:1073–1080. doi: 10.1016/j.ijrobp.2007.07.2378. [DOI] [PubMed] [Google Scholar]
- 16.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210. doi: 10.1200/JCO.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]