Abstract
The biologic drugs bevacizumab and ranibizumab have revolutionized treatment of diabetic macular edema and macular degeneration, leading causes of blindness. Ophthalmologic use of these drugs has increased, now accounting for roughly one-sixth of the Medicare Part B drug budget. Ranibizumab and bevacizumab have similar efficacy and potentially minor differences in adverse event rates, but at $2,023 per dose, ranibizumab costs forty times more than bevacizumab. Using modeling methods, we predict ten-year (2010–2020) population-level costs and health benefits of using bevacizumab and ranibizumab. Our results show that if all patients were treated with the less-expensive bevacizumab instead of current usage patterns, Medicare Part B, patients, and the health care system would save $18 billion, $4.6 billion, and $29 billion, respectively. Altering patterns of use with these therapies by encouraging bevacizumab use and hastening approval of biosimilar therapies would dramatically reduce spending without substantially affecting patient outcomes.
Policy makers are looking for easy ways to substantially reduce Medicare spending without adversely affecting patient health.1 Biologic therapy for neovascular age-related macular degeneration and clinically significant diabetic macular edema is costly and thus an area worth reviewing for potential cost savings.2–5 Clinically significant diabetic macular edema and neovascular age-related macular degeneration are leading causes of blindness and over two million US patients currently have these diseases.6,7 Until recently, therapeutic options to restore vision in patients with these conditions were limited. However, with the recent advent of anti–vascular endothelial growth factor (anti-VEGF) agents, many patients’ vision can be restored.8–10 These medications target bleeding and swelling in the retina by causing regression of abnormal blood vessels associated with these conditions. Such therapies belong to the category of biologic drugs, which comprise large complex molecules manufactured within living cells. These drugs can often be costly to develop and manufacture.
Genentech, a division of Roche (Basel, Switzerland), manufactures the two most common anti-VEGF drugs, bevacizumab (Avastin) and ranibizumab (Lucentis). Ranibizumab has US Food and Drug Administration (FDA) approval for use in patients with neovascular age-related macular degeneration and clinically significant diabetic macular edema. Bevacizumab is FDA approved for treating certain forms of systemic cancers, but is frequently used off-label to treat these two ocular diseases. Some ophthalmologists prefer bevacizumab because of cost: Ranibizumab costs $2,023 per dose, whereas bevacizumab costs about $55 per dose.5 These costs multiply as patients require as many as twelve injections annually to maintain improvements in vision.
In head-to-head government-funded trials involving patients with neovascular age-related macular degeneration, bevacizumab had similar efficacy to ranibizumab.11,12 Trials with patients who have clinically significant diabetic macular edema are ongoing, but a recent meta-analysis found no significant differences in efficacy or safety between bevacizumab and ranibizumab.13 Genentech maintains that it has not sought FDA approval for bevacizumab for ocular conditions because ranibizumab was already designed for those conditions.14
The head-to-head trials and the meta-analysis were inadequately powered to detect small differences in safety. However, studies of patients administered bevacizumab in systemic chemotherapeutic doses 150 times the concentration of the targeted ocular injections have shown higher rates of arteriothrombotic events (stroke, myocardial infarction, vascular death) and venous thrombotic events the risk for these events may also be higher with bevacizumab than with ranibizumab in the lower ophthalmologic doses. Another concern is possible adverse events resulting from potential contamination during compounding (the apportioning of 100 to 400mg vials into 1.25mg vials for ophthalmologic use), which only bevacizumab requires. In the head-to-head trials for macular degeneration, arteriothrombotic events and death rates did not differ between the agents. Rates between the agents did differ, however, for patients having one or more of any type of serious adverse events in one trial (31.7 percent for ranibizumab, 39.9 percent for bevacizumab; p = 0.004),12 although not in the other trial (26 percent versus 27 percent).11 Over all, the scientific literature suggests similar efficacy between the drugs, but ranibizumab may have a slightly better safety profile. According to cost-effectiveness studies, bevacizumab confers greater value than ranibizumab does for both ocular conditions.15–18
The information now available on comparative effectiveness and safety allows for more complete comparative analyses to be conducted on the drugs’ health and financial effects. In 2010 the Medicare Part B spending on ranibizumab and bevacizumab totaled $2 billion, approximately one-sixth of the entire Medicare Part B budget.5 The need for analyses of the comparative health and financial effects gains urgency given the rising incidence of these ocular diseases. By 2020, it is projected that nearly three million people will experience visual impairment from neovascular age-related macular degeneration,7,19,20 and another two million from diabetic macular edema.21 As the Medicare-eligible population continues to expand, identifying potential savings in Medicare’s budget while maintaining high-quality care will be critical.
The debate over the high cost of ranibizumab gained national attention in April when the Centers for Medicare and Medicaid Services released a data set of payments to over 880,000 health care providers who collectively received $77 billion in 2012 under the Medicare Part B fee-for-service program. The data release sparked considerable coverage by media organizations, with many noting that ophthalmologists were among the physicians receiving the highest reimbursements from Medicare, attributed to their use of ranibizumab. The press coverage frequently cited the cost differential with bevacizumab as an example of what appeared to be unnecessarily excessive Medicare spending.
Study Data And Methods
Using modeling methods, we predict ten-year population-level costs and health benefits of using bevacizumab and ranibizumab. First, we forecast the incidence and prevalence of each disease from 2010 to 2020. We then model increasing adoption of anti-VEGF therapies. Finally, we use Markov models of diabetic macular edema and neovascular age-related macular degeneration progression to predict overall costs and quality-adjusted life-years (QALYs) for each disease (Appendix Exhibit A1).22
Incidence And Prevalence Estimates
For diabetic macular edema, we first project future incidence and prevalence rates of diabetes mellitus using age-specific Census population forecasts,23 with estimates of diabetes prevalence by age.24 We combine the diabetes prevalence projections with prevalence estimated for clinically significant diabetic macular edema among people with diabetes using published data.6
For neovascular age-related macular degeneration, we combined age-specific Census population forecasts with prevalence estimates for this disease by age.7 In sensitivity analyses, we consider slightly lower prevalence of neovascular age-related macular degeneration by age, as suggested by other studies.25–27
Treatment Estimates
Therapeutic use of anti-VEGF agents for these two conditions is relatively new. Ranibizumab received FDA approval for neovascular age-related macular degeneration in 2006 and for clinically significant diabetic macular edema in 2010. Bevacizumab has been used off-label since 2005. Adoption of both therapies has been increasing.28,29
The logistic curve–s-shaped with an initial exponential growth phase that reaches an asymptotic maximum–is frequently used to model technology adoption.30 Growth predicted by using the logistic curve matched reasonably well with historical data on the fraction of patients with neovascular age-related macular degeneration receiving anti-VEGF therapies28,29 (Appendix Exhibit A2).22 Since adoption of anti-VEGF therapies is lower for diabetic macular edema than for neovascular age-related macular degeneration (because FDA approval occurred later), we applied the growth pattern observed for neovascular age-related macular degeneration to diabetic macular edema. In our simulation, at peak adoption 75 percent of patients with these diseases are given anti-VEGF therapies on initial diagnosis. Adoption assumptions are varied in sensitivity analyses.
Market Share
Currently, approximately two-thirds of patients undergoing anti-VEGF therapy for neovascular age-related macular degeneration receive bevacizumab; the rest, ranibizumab.29 We applied those same proportions to future anti-VEGF therapy users. We also examine the potential costs and health outcomes if either therapy held a larger fraction of the market.
Drug Costs
For neovascular age-related macular degeneration, anti-VEGF users received 6.3–11.7 injections annually.12 For diabetic macular edema, we assumed that all patients received nine injections annually.31 In sensitivity analysis (Appendix Exhibit A3),22 we examined lower numbers of annual injections (3.6–4.5), as observed in a Medicare-claims analysis of neovascular age-related macular degeneration,29 and as many as twelve injections annually, as some patients routinely receive.
Per injection, bevacizumab costs $55 (including compounding cost) and ranibizumab costs $2,023, according to average Medicare contractor payments.5 Based on a study of discontinuation of anti-VEGF therapy for neovascular age-related macular degeneration29 (and because anti-VEGF therapies’ effectiveness and potential retinal effects are uncertain in the long term),32 we assumed that 70 percent of the patients receiving anti-VEGF therapy for incident macular degeneration discontinued treatment after one year, and 30 percent continued use indefinitely. We assumed that patient costs (in the form of copayments and the portion of premiums for supplemental insurance that accounts for anti-VEGF therapy) are 20 percent of overall costs, and Medicare covers the remaining 80 percent.
Related Diseases: Costs, Health Effects
Although studies suggest similar efficacy between the drugs, those analyses were underpowered to detect small differences for uncommon safety events. One of these agents could conceivably have slightly better vision and safety outcomes than the other, affecting costs and population health outcomes. To model long-term disease-related costs and health effects, we created Markov models capturing worsening or improving vision, as measured by Snellen visual acuity scores, and the proportions of patients experiencing severe systemic effects previously linked to these two therapies–specifically, myocardial infarction, cerebrovascular accident, venous thromboembolism, and death. Anti-VEGF treatment incurs costs but improves vision. Long-term cumulative costs and QALYs are calculated according to patient age. We used data from the Comparison of AMD Treatments Trials12 for the effectiveness and side-effect rates for treatment of neovascular age-related macular degeneration with each agent. For diabetic macular edema, we assumed equal clinical effectiveness between the agents, following the results of the meta-analysis.13 (The related cost-effectiveness studies provide further details of the models.)15,16
We compared long-term spending on ophthalmologic use of anti-VEGF drugs to the overall Medicare Part B budget using projections from the Centers for Medicare and Medicaid Services (CMS) Office of the Actuary on Medicare prescription-drug spending33 and assumed a constant Part B fraction of that spending.
We considered the perspective of CMS and examined the therapies’ costs to patients (copayments and insurance premiums) and the health care system. We assessed costs over a ten-year time horizon and discounted future costs by 3 percent annually as is recommended for health economic analyses.34
Model Validation
Although models cannot completely represent reality35 and model validation is never completely possible,36 the model compares favorably with current estimates and models of prevalence and spending. We compared our model estimates of diabetes mellitus, neovascular age-related macular degeneration, and clinically significant diabetic macular edema with published estimates for 2010, 2020, and 2030.21,25,37,38 We also compared 2010 spending estimates from the model with recent CMS-reported spending on both agents.4,5
Sensitivity Analyses
Biosimilars are subsequent versions of biologic products. Because these medications are more complex than small molecule drugs, how biosimilar medications should receive FDA approval is debatable.2 Although the Affordable Care Act (ACA) included the Biologics Price Competition and Innovation Act of 2009 to create an abbreviated licensure pathway for biosimilar drugs, key aspects of the pathway are still unsettled39 and other barriers (such as patents and exclusivity) remain before US patients can access less-expensive biosimilars. Therefore, although Genentech’s patents on ranibizumab will expire in 2017–2020,4,40 when biosimilars may become available is uncertain. To understand the potential impact of biosimilars, we varied the biosimilars’ date of entry in the anti-VEGF market as well as adoption rates. We also varied the generic product’s price between the current bevacizumab cost and 25 percent off the price of ranibizumab, similar to European pricing.39
Adverse Event Rates
The myocardial infarction, cerebrovascular accident, and venous thromboembolism rates observed in the Comparison of AMD Treatments Trials were similar for bevacizumab and ranibizumab (1.2–1.7 percent versus 0.5–1.5 percent).12 Although these rates did not differ significantly, small differences were difficult to detect with the trials’ sample size because of the rarity of the events. Because uncertainty remains on the anti-VEGF therapies’ relative safety and the possibility of arteriothrombotic and venous thrombotic events is particularly a concern, we conducted sensitivity analyses to examine how changes to adverse event estimates could affect overall conclusions.
Bevacizumab Reimbursement
One potential disincentive to prescribe bevacizumab is the drug’s relatively low Part B revenue, compared with ranibizumab. Because anti-VEGF agents are purchased and injected by physicians, Medicare reimburses the provider directly at the average sales price of the medication plus 6 percent. The Department of Health and Human Services’ (HHS) Office of Inspector General reported that physicians’ profit margin per ranibizumab vial was $95–approximately three times that of bevacizumab ($29).5 Increasing provider reimbursement for bevacizumab could reduce providers’ economic incentive to prescribe the more expensive drug. We examine how increased reimbursement for bevacizumab and substitution of bevacizumab for ranibizumab market share may affect overall Part B spending on these drugs.
Limitations
Our model does not assess people with simultaneous clinically significant diabetic macular edema and neovascular age-related macular degeneration. However, these diseases rarely occur concurrently, and if so, they would likely still be treated with the same anti-VEGF therapies, making this a minor error.
Study Results
Our model projects rates of diabetes mellitus, clinically significant diabetic macular edema, and neovascular age-related macular degeneration similar to previously reported estimates.21,25,37,38 Overall spending in 2010 on bevacizumab and ranibizumab as predicted by our model is similar to data reported by the HHS Office of Inspector General, helping to validate the model4,5 (Appendix Exhibit A7).22
With current practice patterns (for example, two-thirds of patients receive bevacizumab, one-third ranibizumab), CMS will spend $20 billion and patients will spend $5 billion on bevacizumab and ranibizumab over the next decade. If all patients immediately switched to bevacizumab and continued using it over the ten-year period, CMS spending on these drugs would drop to about $1.7 billion (savings, $18 billion) over the decade-long period, and patients would spend $420 million (savings, $4.6 billion). If all patients switched to the FDA-approved drug, ranibizumab, CMS and patient spending would increase to $57 billion and $14 billion, respectively (Exhibit 1).
EXHIBIT 1.
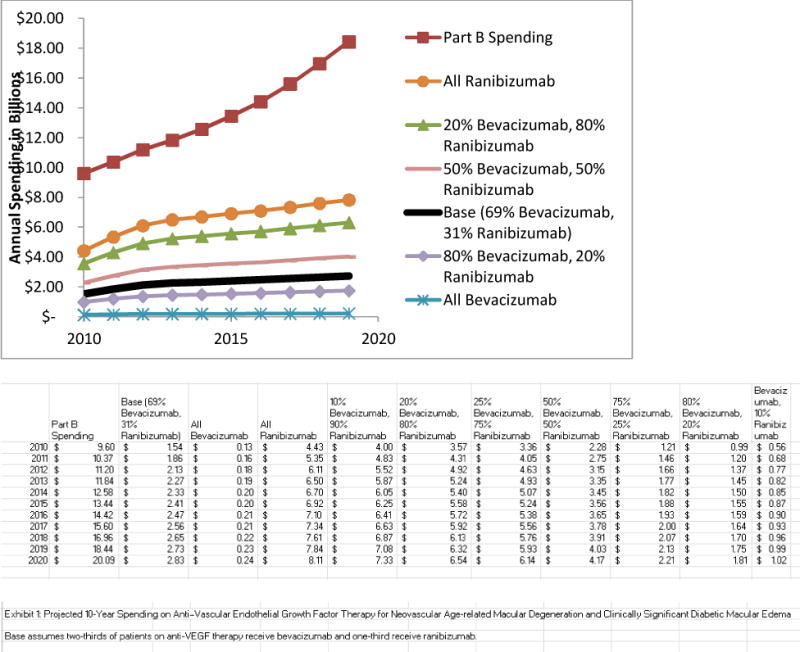
Projected Ten-Year (2010–2020) Spending On Anti–Vascular Endothelial Growth Factor Therapy For Neovascular Age-Related Macular Degeneration And Clinically Significant Diabetic Macular Edema
SOURCES Authors’ analysis. aSee Note 29 in text.
NOTE Base assumes two-thirds of patients on anti-VEGF therapy receive bevacizumab and one-third receive ranibizumab, which is consistent with current usage.a
Including costs for patients younger than sixty-five and other health care expenses (for example, managing adverse events from anti-VEGF therapy), the difference in total spending by the health care system between all patients (with either condition) receiving bevacizumab instead of current use patterns is $29 billion over ten years ($23 billion in anti-VEGF drug costs for CMS and patients, $6 billion in other health system expenses). Using ranibizumab may confer a minor health benefit because of potentially small side-effect differences between the drugs (0–0.04 QALYs per person, depending on age and ocular condition). However, because ranibizumab’s costs are considerably higher (range is $18,000–$145,000 per person treated, depending on age and ocular condition), overall medical costs would increase by approximately $2 million for every QALY gained if ranibizumab were used instead of bevacizumab.
Spending on ranibizumab was roughly one-sixth of the entire 2010 Part B drug budget. However, if all patients with these two ocular conditions used bevacizumab, the spending amount would constitute about 2 percent of the Part B budget. In contrast, if all patients used ranibizumab, spending could consume approximately 40 percent of the budget (Exhibit 1).
Sensitivity Analyses
Prevalence of Neovascular Age-Related Macular Degeneration
Because of uncertainty in prevalence projections for neovascular age-related macular degeneration, we explored alternative assumptions. We scaled our neovascular age-related macular degeneration estimate down by 20 percent to more closely match projections by Rein and colleagues25 (Appendix Exhibit A4).22 Under these assumptions, the difference in Part B spending between all patients using bevacizumab versus baseline patterns of anti-VEGF use would be $16 billion discounted over ten years, and the total difference in patient spending would be $4 billion.
Delayed Release Of Biosimilars
Each one-year delay in the availability of low-cost biosimilar anti-VEGF therapy for ophthalmologic use could cost as much as $1.4 billion to CMS and $340 million to patients, depending on the biosimilar’s price, year of entry, and rate of adoption (Appendix Exhibit A3).22
Reimbursement Rates
For about every 5 percent increase in providers switching from ranibizumab to bevacizumab, CMS would save $1 billion. If reimbursement for bevacizumab were increased to $121 per injection, to match the profit margin of ranibizumab, 8 percent of ranibizumab prescriptions would have to change to bevacizumab for the total spending on anti-VEGF therapies to remain budget neutral (Exhibit 2). If the reimbursement were raised beyond that, an increasing number of ranibizumab prescriptions would have to switch to bevacizumab for the program to remain budget neutral. Any switching beyond that would save costs for CMS.
EXHIBIT 2.
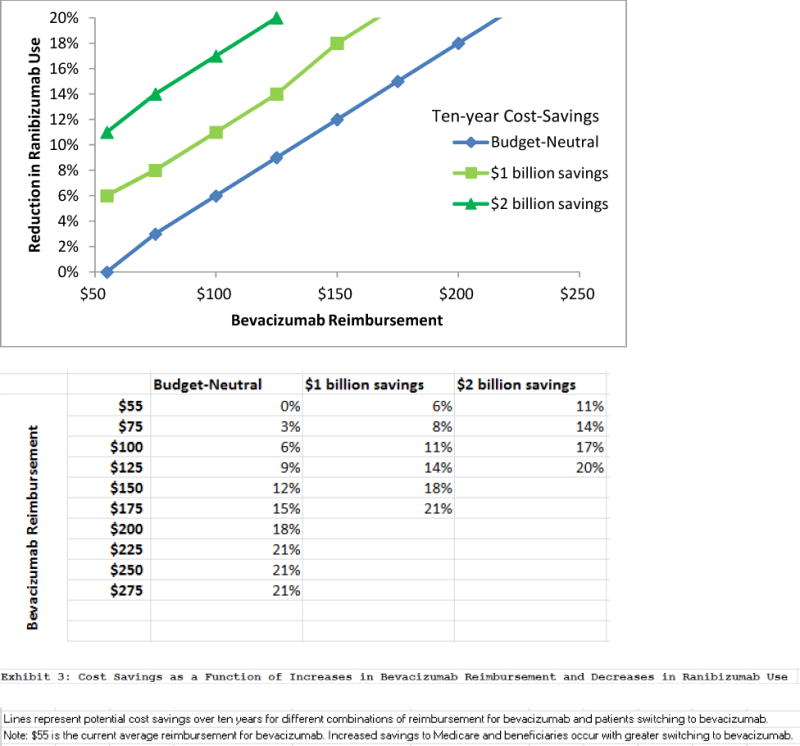
Cost Savings As A Function Of Increased Bevacizumab Reimbursement And Decreased Ranibizumab Use
SOURCES Authors’ analysis. aSee Note 5 in text.
NOTES Lines represent potential cost savings over ten years for different combinations of reimbursement for bevacizumab and patients switching to bevacizumab. $55 is the current average reimbursement for bevacizumab.a Savings to Medicare and beneficiaries increase with greater switching to bevacizumab.
Bevacizumab-Associated Risk For Arteriothrombotic Events
Given lingering concerns about bevacizumab’s potentially elevated rates of systemic arteriothrombotic and venous thrombotic complications,3 we explored how the results might change if bevacizumab had higher event rates than those reported in clinical trials. If the event rate were five times as high as those observed in the Comparison of AMD Treatments Trials12 and Diabetic Retinopathy Clinical Research Network trials41–which seems highly unlikely–patients would lose 1.2 million QALYs using bevacizumab instead of ranibizumab. However, using bevacizumab instead of ranibizumab would still produce ten-year savings of more than $46 billion to CMS and almost $12 billion to patients in overall medical expenses.
Discussion
At a time of widespread concern about Medicare spending, we find that $18 billion could be reduced from the Medicare Part B budget and $4.6 billion in patient copayments, and supplemental insurance costs could be eliminated with little impact to patient health by switching from a particular expensive drug to a similar but much cheaper agent with comparable effectiveness. The magnitude of this single change rivals the $14 billion that the Congressional Budget Office estimated would be saved over ten years by the controversial Independent Payment Advisory Board, which has authority to change the Medicare program if spending exceeds a target growth rate.42
Patients also could pocket considerable savings from this switch. Among people with no supplemental insurance who live on the average Social Security retirement benefit, monthly ranibizumab copayments could exceed 30 percent of their income, compared with less than 1 percent with use of bevacizumab.43 On the basis of current practice patterns, in which one-third of patients with diabetic macular edema or neovascular age-related macular degeneration receive ranibizumab, we project that patients will spend almost $5 billion in copayments and supplemental insurance premiums over ten years. If all patients with these conditions switched to bevacizumab, the collective savings could be $4.6 billion.
Yet, some uncertainty surrounds the safety of these two anti-VEGF therapies.3 Unfortunately, the Comparison of AMD Treatments Trials and the Inhibit VEGF in Age-related choroidal Neovascularisation (IVAN) trial lacked the power to detect small, significant differences in severe side effects (such as cerebrovascular accident, myocardial infarction, and venous thromboembolism) between the therapies. The capacity to identify such small differences may require larger clinical trials, whose time and expense could be questionable. Our study quantifies the difference in Medicare spending between the two drugs but does not precisely identify the value of clinical trials on these therapies.
Concerns have been raised about physicians prescribing medications off-label with little scientific support.44 However, good evidence exists regarding bevacizumab’s noninferior efficacy to ranibizumab as ophthalmologic therapy.11,12 The Office of Inspector General for HHS recommended that CMS increase the use of bevacizumab to control Part B expenditures.4 Genentech, which sells both agents, lacks financial incentive to seek FDA approval for ophthalmologic use of the cheaper agent, bevacizumab.
Our study also raises questions about biologic drug competition. The price of ranibizumab may eventually drop in response to generic competition. However, patent and exclusivity rights provide legal barriers to biologic drug competition. Genentech currently has patent rights for ranibizumab that expire in 2017 and 2020 along with exclusivity rights through 2018. However, a recent case involving the biologic drug Enbrel, in which the manufacturer was afforded seventeen years of additional patent protection, illustrates the still-uncertain environment surrounding patents.45
In addition to legal barriers, technical challenges to producing similar biologic drugs may delay the entry of generic competition.2 The safety and efficacy of biologic drugs are closely tied to their specific process of manufacture, which is, in turn, protected by several overlapping patents. This may result in delayed generic entry, biosimilars that have little price advantage over brand drugs, and reduced overall generic competition.46 Perhaps most important, while the Biologics Price Competition and Innovation Act portion of the ACA was intended to encourage companies to develop biosimilars by easing the FDA approval process for these drugs, details of the FDA approval process have been slow to develop and remain unclear.39 Changes to the legal or technological landscape allowing for faster entry of biosimilars could potentially mean tens of billions of dollars in CMS savings. Each additional year of delay could cost CMS as much as $1.4 billion for these anti-VEGF therapies alone.
The existence of two pharmaceutical products with similar efficacy but vastly different costs may encourage the use of novel payment models to encourage use of the less-expensive drug. Medicare faces challenges to implementing novel payment models, as it is legally obligated to pay 106 percent of the average sales prices of pharmaceuticals. However, it could examine creative policies to make bevacizumab more attractive than ranibizumab to prescribe. Medicare could, for instance, more than double the reimbursement for bevacizumab to make the margins in dollars to ophthalmologists for prescribing bevacizumab and ranibizumab equivalent. If more than 8 percent of ranibizumab prescriptions were switched to bevacizumab, Medicare Part B would save money even at this increased reimbursement rate for bevacizumab.
This analysis takes the perspective of the payer, patients, and the health system. However, savings to the payers and patients are lost revenue and profits to the pharmaceutical manufacturer. Thus, the benefits of switching from ranibizumab to bevacizumab may have offsetting losses to others in society (for example, loss in share prices and dividends to pharmaceutical company shareholders). This analysis highlights powerful pressures and incentives that different parties (private payers, Medicare, patients, and pharmaceutical manufacturers) may have in the debate over ranibizumab versus bevacizumab for ophthalmologic use.
The manufacturer faces an interesting dilemma. Genentech makes more revenue if patients use ranibizumab instead of bevacizumab. It has no incentive to conduct a trial that may show that bevacizumab is similar to ranibizumab. But, it would be difficult for the company to restrict access to bevacizumab for ophthalmologic use, without also restricting access to bevacizumab in the lucrative cancer-therapy market, estimated at $2.5 billion in the United States.47 Genentech attempted to restrict sales to third-party compounding pharmacies but was thwarted by strong pressure from ophthalmologists and compounding pharmacies.48 Currently it strongly encourages use of ranibizumab over bevacizumab, yet about two-thirds of patients use bevacizumab. The company reported conducting eighteen clinical trials and spending $1.4 billion to develop ranibizumab.49 Encouraging use of bevacizumab would blunt Genentech’s profit incentives to sell ranibizumab or may discourage the development of similar products. Those changed incentives, which we did not evaluate in our study, are a trade-off worth considering.
Conclusion
A lesson from this study for US policy makers is that anti-VEGF therapy for ophthalmologic use is an easy target for cost savings. Changing future use patterns to favor bevacizumab over ranibizumab could eliminate $18 billion from the Medicare Part B budget and $4.6 billion in patient costs. However, achieving these savings would require overcoming substantial roadblocks. Concerns remain about uncommon systemic side effects associated with use of these medications. A future scientific study adequately powered to detect whether side-effect rates differ between these two anti-VEGF agents could be valuable.
The prospects for providing incentives to ophthalmologists to prescribe bevacizumab instead of ranibizumab are far from assured, because the 106 percent reimbursement rule for pharmaceuticals makes it difficult for CMS to encourage the use of a product with similar safety and efficacy but a much lower price. Ultimately, novel solutions, such as increasing reimbursement for less-expensive drugs or changing regulations regarding biosimilars and encouraging appropriate off-label use, may help CMS to realize substantial savings.
Supplementary Material
Acknowledgments
This research was supported by a National Eye Institute K23 Mentored Clinician Scientist Award (No. 1K23EY019511-01).
Notes
- 1.Hussey PS, Eibner C, Ridgely MS, McGlynn EA. Controlling U.S. health care spending–separating promising from unpromising approaches. N Engl J Med. 2009;361(22):2109–11. doi: 10.1056/NEJMp0910315. [DOI] [PubMed] [Google Scholar]
- 2.Pollack A. Costly drugs known as biologics prompt exclusivity debate. New York Times. 2009 Jul 21; [Google Scholar]
- 3.Schmucker C, Ehlken C, Agostini HT, Antes G, Ruecker G, Lelgemann M, et al. A safety review and meta-analyses of bevacizumab and ranibizumab: off-label versus goldstandard. PLoS One. 2012;7(8):e42701. doi: 10.1371/journal.pone.0042701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Office of Inspector General, Department of Health and Human Services. Review of Medicare part b Avastin and Lucentis treatments for age-related macular degeneration. Washington (DC): HHS; 2011. Sep 6, [Google Scholar]
- 5.Office of Inspector General, Department of Health and Human Services. Medicare payments for drugs used to treat wet age related macular degeneration. Washington (DC): HHS; 2012. Apr 20, [Google Scholar]
- 6.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010;304(6):649–56. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman DS, O’Colmain BJ, Muñoz B, Tomany SC, McCarty C, deJong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 9.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258–67. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 12.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–98. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virgili G, Parravano M, Menchini F, Brunetti M. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database Syst Rev. 2012;12:CD007419. doi: 10.1002/14651858.CD007419.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Whoriskey P, Keating D. An effective eye drug is available for $50. But many doctors choose a $2,000 alternative. Washington Post. 2013 Dec 7; Business. [Google Scholar]
- 15.Stein JD, Newman-Casey PA, Kim DD, Nwanyanwu KH, Johnson MW, Hutton DW. Cost-effectiveness of various interventions for newly diagnosed diabetic macular edema. Ophthalmology. 2013;120(9):1835–42. doi: 10.1016/j.ophtha.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein JD, Newman-Casey PA, Mrinalini T, Lee PP, Hutton DW. Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration. Ophthalmology. 2014;121(4):936–45. doi: 10.1016/j.ophtha.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raftery J, Clegg A, Jones J, Tan SC, Lotery A. Ranibizumab (Lucentis) versus bevacizumab (Avastin): modelling cost effectiveness. Br J Ophthalmol. 2007;91(9):1244–6. doi: 10.1136/bjo.2007.116616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel JJ, Mendes MA, Bounthavong M, Christopher ML, Boggie D, Morreale AP. Cost-utility analysis of bevacizumab versus ranibizumab in neovascular age-related macular degeneration using a Markov model. J Eval Clin Pract. 2012;18(2):247–55. doi: 10.1111/j.1365-2753.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 19.National Advisory Eye Council. Vision research, a national plan, 1994–1998. Bethesda (MD): HHS; 1993. [Google Scholar]
- 20.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 21.Saaddine JB, Honeycutt AA, Narayan KM, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005–2050. Arch Ophthalmol. 2008;126(12):1740–7. doi: 10.1001/archopht.126.12.1740. [DOI] [PubMed] [Google Scholar]
- 22.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 23.US Census Bureau. 2012 national population projections [Internet] Washington (DC): Census Bureau; 2012. [cited 2014 Apr 9]. Available from: http://www.census.gov/population/projections/data/national/2012.html. [Google Scholar]
- 24.Centers for Disease Control and Prevention. Percentage of civilian, noninstitutionalized population with diagnosed diabetes, by age, United States, 1980–2011 [Internet] Atlanta (GA): CDC; 2013. [cited 2013 Apr 4]. Available from: http://www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm. [Google Scholar]
- 25.Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J, et al. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127(4):533–40. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- 26.Varma R, Fraser-Bell S, Tan S, Klein R, Azen SP, Los Angeles Latino Eye Study Group Prevalence of age-related macular degeneration in Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111(7):1288–97. doi: 10.1016/j.ophtha.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Klein R, Klein BE, Knudtson MD, Wong TY, Cotch MF, Liu K, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006;113(3):373–80. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Stein JD, Hanrahan BW, Comer GM, Sloan FA. Diffusion of technologies for the care of older adults with exudative age-related macular degeneration. Am J Ophthalmol. 2013;155(4):688–96. doi: 10.1016/j.ajo.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curtis LH, Hammill BG, Qualls LG, DiMartino LD, Wang F, Schulman KA, et al. Treatment patterns for neovascular age-related macular degeneration: analysis of 284 380 medicare beneficiaries. Am J Ophthalmol. 2012;153(6):1116–24. doi: 10.1016/j.ajo.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Ruttan VW. What happened to technology adoption-diffusion research? Sociologia Ruralis. 1996;36(1):51–73. [Google Scholar]
- 31.Diabetic Retinopathy Clinical Research Network. Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077 e1035. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grunwald JE, Daniel E, Huang J, Ying GS, Maguire MD, Toth CA, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150–61. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Office of the Actuary, Centers for Medicare and Medicaid Services. National health expenditure projections 2011–2021 [Internet] Baltimore (MD): CMS; 2011. [cited 2013 May 1]. Available from: http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/Proj2011PDF.pdf. [Google Scholar]
- 34.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York (NY): Oxford University Press; 1996. [Google Scholar]
- 35.Diez Roux AV. Complex systems thinking and current impasses in health disparities research. Am J Public Health. 2011;101(9):1627–34. doi: 10.2105/AJPH.2011.300149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sterman JD. Business dynamics: systems thinking and modeling for a complex world. Boston (MA): McGraw-Hill; 2000. [Google Scholar]
- 37.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokota F, Thompson KM. Value of information literature analysis: a review of applications in health risk management. Med Decis Making. 2004;24(3):287–98. doi: 10.1177/0272989X04263157. [DOI] [PubMed] [Google Scholar]
- 39.Megerlin F, Lopert R, Taymor K, Trouvin JH. Biosimilars and the European experience: implications for the United States. Health Aff (Millwood) 2013;32(10):1803–10. doi: 10.1377/hlthaff.2009.0196. [DOI] [PubMed] [Google Scholar]
- 40.United States Patent and Trademark Office. Patent terms extended under 35 USC sect 156 [Internet] Alexandria (VA): USPTO; 2012. [cited 2013 May 1]. Available from: http://www.uspto.gov/patents/resources/terms/156.jsp. [Google Scholar]
- 41.Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL, 3rd, Friedman SM, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609–14. doi: 10.1016/j.ophtha.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elmendorf DW. CBO’s analysis of the major health care legislation enacted in March 2010. Washington (DC): Congressional Budget Office; 2011. [Google Scholar]
- 43.Social Security Administration. Monthly statistical snapshot, March 2014 [Internet] Washington (DC): Social Security Administration; 2014. [cited 2014 April 16]. Available from: http://www.ssa.gov/policy/docs/quickfacts/stat_snapshot/ [Google Scholar]
- 44.Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021–6. doi: 10.1001/archinte.166.9.1021. [DOI] [PubMed] [Google Scholar]
- 45.Harrison C. Enbrel patent surfaces. Nat Biotechnol. 2012;30(2):123. doi: 10.1038/nbt0212-123. [DOI] [PubMed] [Google Scholar]
- 46.Analysis Group. Biologics, biosimilars, and generics [Internet] Boston (MA): Analysis Group; 2010. [cited 2013 Apr 23]. Available from: http://www.analysisgroup.com/anticipating_biosimilar_challenge.aspx. [Google Scholar]
- 47.Drugs.com. Avastin sales data [Internet] 2013 [cited 2014 Jan 29]. Available from: http://www.drugs.com/stats/avastin.
- 48.Mantone J. Genentech, eye docs make peace on Avastin [blog on the Internet] 2007 [cited 2014 Jan 31]. Available from: http://blogs.wsj.com/health/2007/12/20/genentech-eye-docs-make-peace-on-avastin/
- 49.US Senate Special Committee on Aging. A prescription for savings: reducing drug costs to Medicare, hearing, July 21, 2011 (Serial 112–7) Washington (DC): US Government Printing Office; 2011. Jul 21, [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


