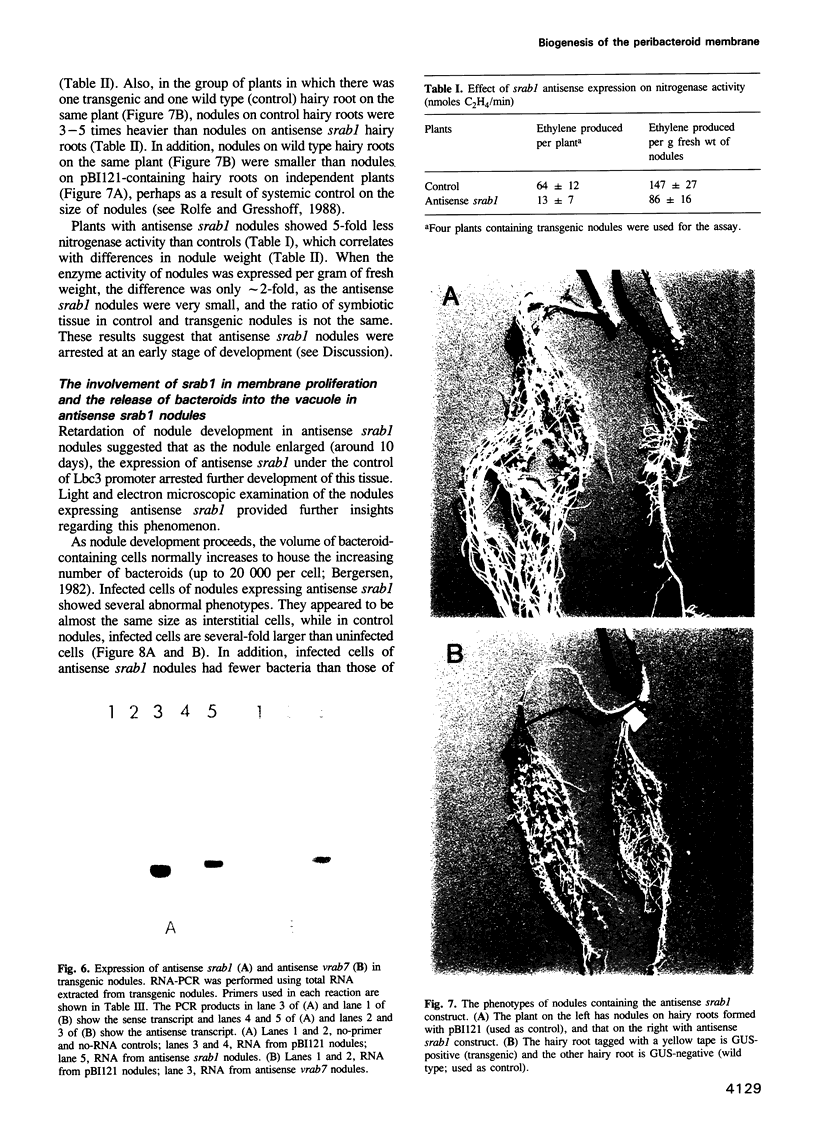
Abstract
The peribacteroid membrane (PBM) in legume root nodules is derived from plasma membrane following endocytosis of Rhizobium by fusion of newly synthesized vesicles. We studied the roles of plant Rab1p and Rab7p homologs, the small GTP-binding proteins involved in vesicular transport, in the biogenesis of the PBM. Three cDNAs encoding legume homologs of mammalian Rab1p and Rab7p were isolated from soybean (sRab1p, sRab7p) and Vigna aconitifolia (vRab7p). sRab1p was confirmed to be a functional counterpart of yeast Ypt1p (Rab1p) by complementation of a yeast ypt1-1 mutant. Both srab1 and vrab7 genes are induced during nodulation with the level of vrab7 mRNA being 12 times higher than that in root meristem and leaves. This induction directly correlates with membrane proliferation in nodules. Antisense constructs of srab1 and vrab7, under a nodule-specific promoter (leghemoglobin, Lbc3), were made in a binary vector and transgenic nodules were developed on soybean hairy roots obtained through Agrobacterium rhizogenes-mediated transformation. Both antisense srab1 and vrab7 nodules were smaller in size and showed lower nitrogenase activity than controls. The antisense srab1 nodules showed lack of expansion of infected cells, fewer bacteroids per cell and their frequent release into vacuoles. In contrast, antisense vrab7 expressing nodules showed accumulation of late endosomal structure and multivesicular bodies in the perinuclear region. These data suggest that both Rab1p and Rab7p are essential for the development of the PBM compartment in effective symbiosis.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
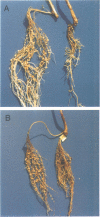
- Anuntalabhochai S., Terryn N., Van Montagu M., Inzé D. Molecular characterization of an Arabidopsis thaliana cDNA encoding a small GTP-binding protein, Rha1. Plant J. 1991 Sep;1(2):167–174. [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Brewin N. J. Development of the legume root nodule. Annu Rev Cell Biol. 1991;7:191–226. doi: 10.1146/annurev.cb.07.110191.001203. [DOI] [PubMed] [Google Scholar]
- Brisson N., Verma D. P. Soybean leghemoglobin gene family: normal, pseudo, and truncated genes. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4055–4059. doi: 10.1073/pnas.79.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C., Parton R. G., Mather I. H., Stunnenberg H., Simons K., Hoflack B., Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992 Sep 4;70(5):715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Delauney A. J., Verma D. P. A soybean gene encoding delta 1-pyrroline-5-carboxylate reductase was isolated by functional complementation in Escherichia coli and is found to be osmoregulated. Mol Gen Genet. 1990 May;221(3):299–305. doi: 10.1007/BF00259392. [DOI] [PubMed] [Google Scholar]
- Fisher R. F., Long S. R. Rhizobium--plant signal exchange. Nature. 1992 Jun 25;357(6380):655–660. doi: 10.1038/357655a0. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Donath C., Sander C. A yeast gene encoding a protein homologous to the human c-has/bas proto-oncogene product. Nature. 1983 Dec 15;306(5944):704–707. doi: 10.1038/306704a0. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Clague M. J. Regulation of intracellular membrane transport. Curr Opin Cell Biol. 1992 Aug;4(4):593–599. doi: 10.1016/0955-0674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Howell K. E. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990 Aug 10;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- Haubruck H., Prange R., Vorgias C., Gallwitz D. The ras-related mouse ypt1 protein can functionally replace the YPT1 gene product in yeast. EMBO J. 1989 May;8(5):1427–1432. doi: 10.1002/j.1460-2075.1989.tb03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfgen R., Willmitzer L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 1988 Oct 25;16(20):9877–9877. doi: 10.1093/nar/16.20.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N. G., Stein B., Suzuki H., Verma D. P. Expression of antisense nodulin-35 RNA in Vigna aconitifolia transgenic root nodules retards peroxisome development and affects nitrogen availability to the plant. Plant J. 1993 Apr;3(4):599–606. doi: 10.1046/j.1365-313x.1993.03040599.x. [DOI] [PubMed] [Google Scholar]
- Magee T., Newman C. The role of lipid anchors for small G proteins in membrane trafficking. Trends Cell Biol. 1992 Nov;2(11):318–323. doi: 10.1016/0962-8924(92)90172-j. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M., Lin V. K. Comparison of two different hybridization systems in northern transfer analysis. Biotechniques. 1989 Apr;7(4):331-2, 334. [PubMed] [Google Scholar]
- Manen J. F., Simon P., Van Slooten J. C., Osterås M., Frutiger S., Hughes G. J. A nodulin specifically expressed in senescent nodules of winged bean is a protease inhibitor. Plant Cell. 1991 Mar;3(3):259–270. doi: 10.1105/tpc.3.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga L. S., Diaz R., Stahl P. D. Regulatory role for GTP-binding proteins in endocytosis. Science. 1989 Jun 23;244(4911):1475–1477. doi: 10.1126/science.2499930. [DOI] [PubMed] [Google Scholar]
- Miao G. H., Hong Z., Verma D. P. Topology and phosphorylation of soybean nodulin-26, an intrinsic protein of the peribacteroid membrane. J Cell Biol. 1992 Jul;118(2):481–490. doi: 10.1083/jcb.118.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao G. H., Hong Z., Verma D. P. Two functional soybean genes encoding p34cdc2 protein kinases are regulated by different plant developmental pathways. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):943–947. doi: 10.1073/pnas.90.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar C. M., Prange R., Gallwitz D. A carboxyl-terminal cysteine residue is required for palmitic acid binding and biological activity of the ras-related yeast YPT1 protein. EMBO J. 1988 Apr;7(4):971–976. doi: 10.1002/j.1460-2075.1988.tb02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nap J. P., Bisseling T. Developmental biology of a plant-prokaryote symbiosis: the legume root nodule. Science. 1990 Nov 16;250(4983):948–954. doi: 10.1126/science.250.4983.948. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Zelechowska M., Foster V., Bergmann H., Verma D. P. Primary structure of the soybean nodulin-35 gene encoding uricase II localized in the peroxisomes of uninfected cells of nodules. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5040–5044. doi: 10.1073/pnas.82.15.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K., Diefenthal T., Vingron M., Sander C., Schell J. Molecular cloning and structural analysis of genes from Zea mays (L.) coding for members of the ras-related ypt gene family. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):787–791. doi: 10.1073/pnas.89.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. G., Lyttleton P. Coated and smooth vesicles in the biogenesis of cell walls, plasma membranes, infection threads and peribacteroid membranes in root hairs and nodules of white clover. J Cell Sci. 1982 Dec;58:63–78. doi: 10.1242/jcs.58.1.63. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Broach J. R. Cloning genes by complementation in yeast. Methods Enzymol. 1991;194:195–230. doi: 10.1016/0076-6879(91)94017-7. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Segev N., Botstein D. The ras-like yeast YPT1 gene is itself essential for growth, sporulation, and starvation response. Mol Cell Biol. 1987 Jul;7(7):2367–2377. doi: 10.1128/mcb.7.7.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev N. Mediation of the attachment or fusion step in vesicular transport by the GTP-binding Ypt1 protein. Science. 1991 Jun 14;252(5012):1553–1556. doi: 10.1126/science.1904626. [DOI] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Longtin D., Madrzak C., Verma D. P. Genetic locus in Rhizobium japonicum (fredii) affecting soybean root nodule differentiation. J Bacteriol. 1986 May;166(2):628–634. doi: 10.1128/jb.166.2.628-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale E. J., Bourne J. R., Khosravi-Far R., Der C. J., Balch W. E. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. 1992 Nov;119(4):749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Kazazian V., Zogbi V., Bal A. K. Isolation and characterization of the membrane envelope enclosing the bacteroids in soybean root nodules. J Cell Biol. 1978 Sep;78(3):919–936. doi: 10.1083/jcb.78.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma DPS. Signals in Root Nodule Organogenesis and Endocytosis of Rhizobium. Plant Cell. 1992 Apr;4(4):373–382. doi: 10.1105/tpc.4.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann H., Hengst L., Gallwitz D. Endocytosis in yeast: evidence for the involvement of a small GTP-binding protein (Ypt7p). Cell. 1992 Dec 24;71(7):1131–1142. doi: 10.1016/s0092-8674(05)80062-5. [DOI] [PubMed] [Google Scholar]
- van der Sluijs P., Hull M., Webster P., Mâle P., Goud B., Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992 Sep 4;70(5):729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]