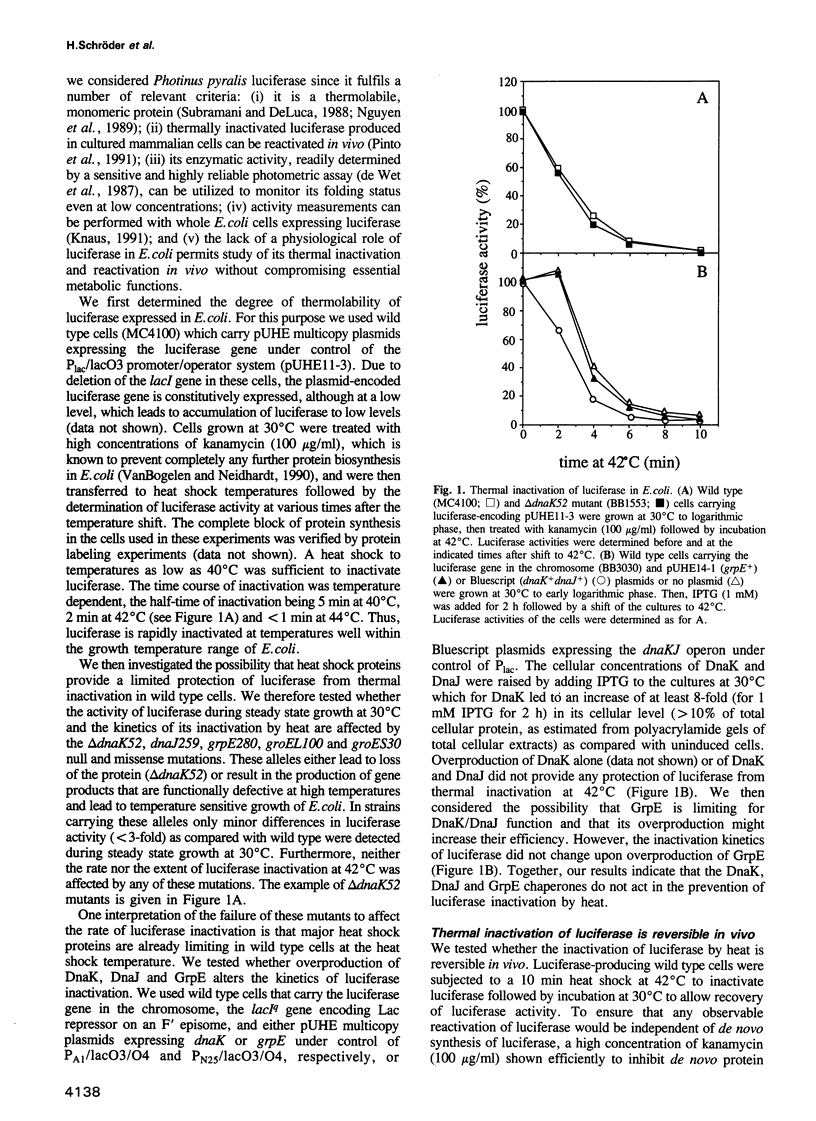
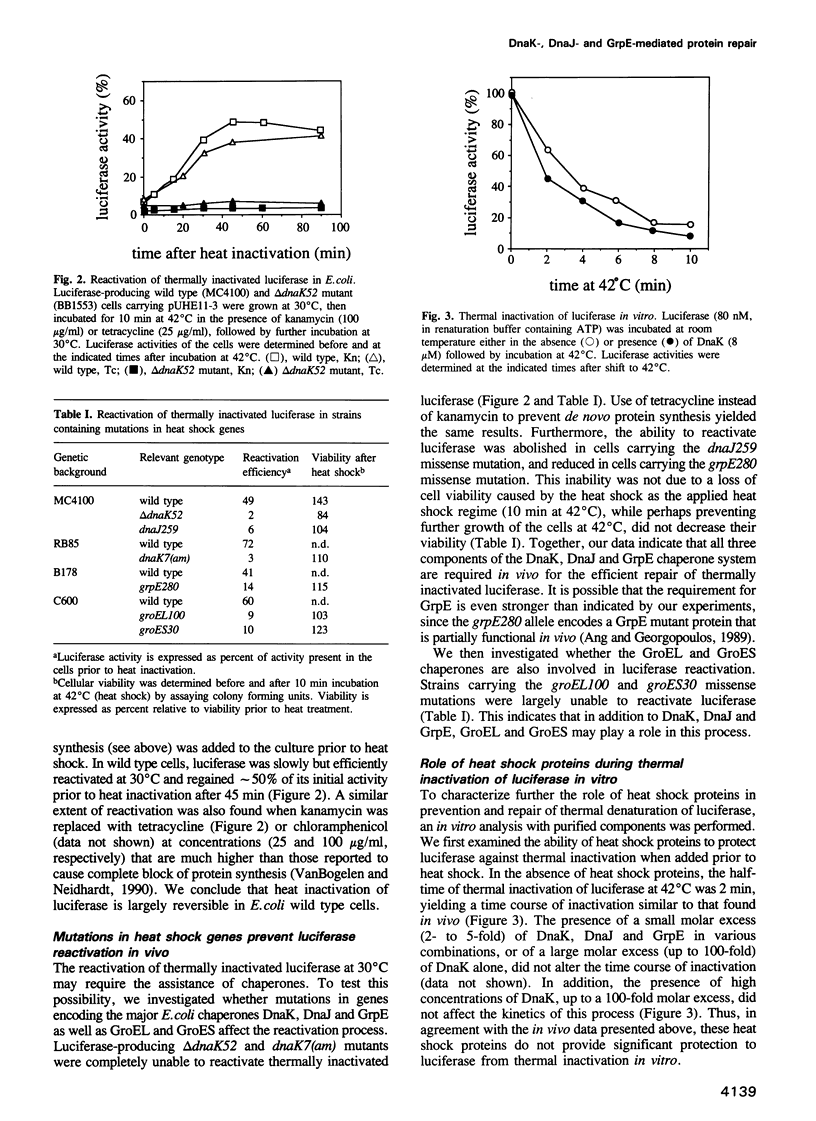
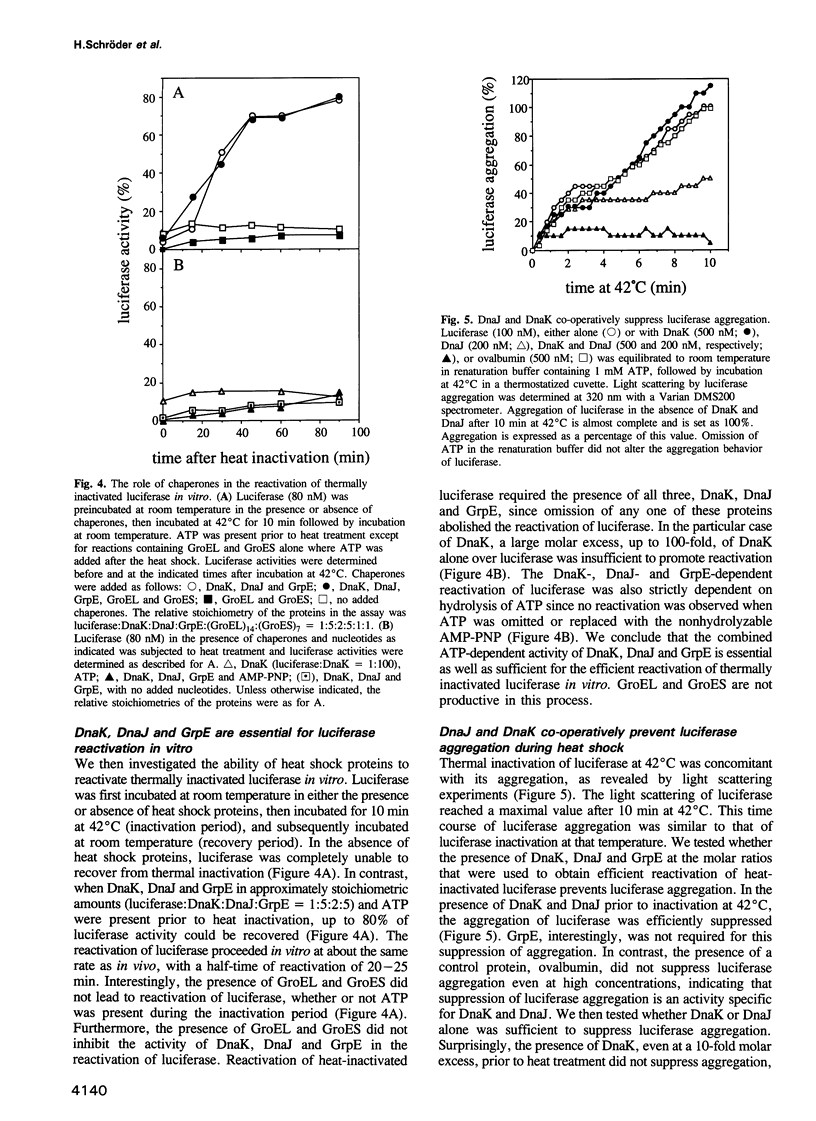
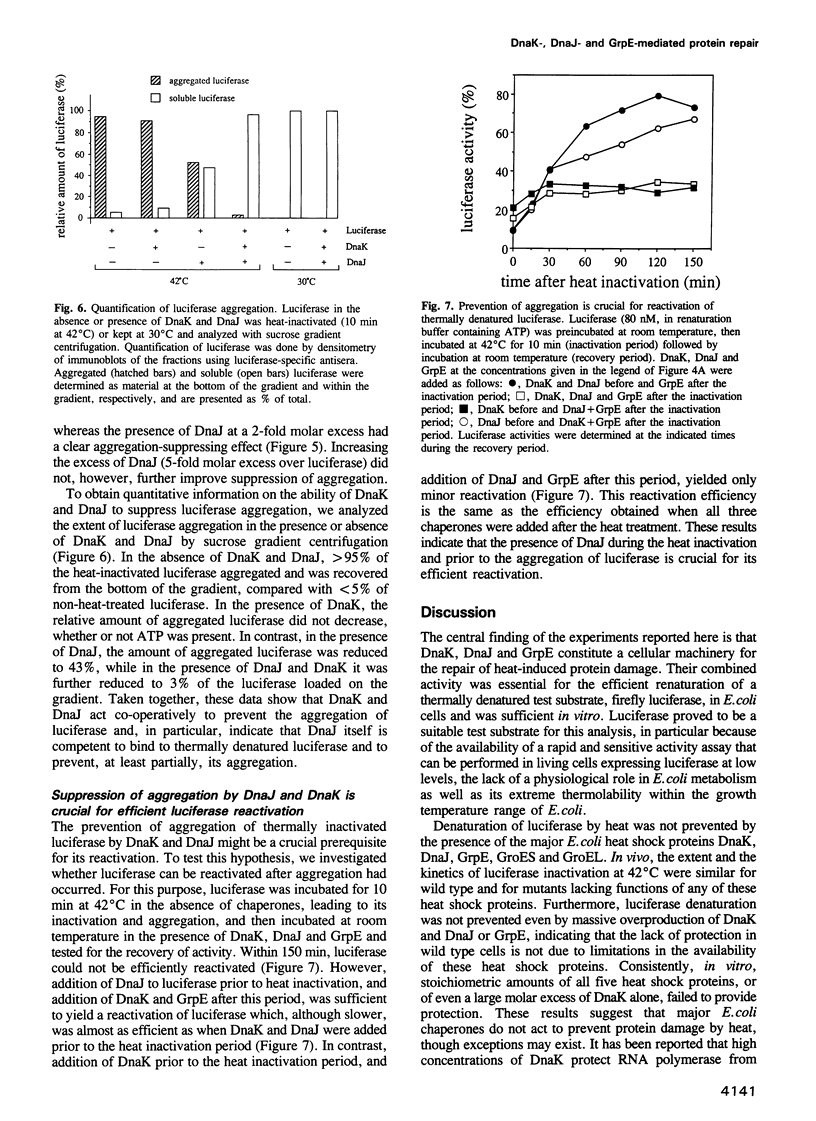
Abstract
Members of the conserved Hsp70 chaperone family are assumed to constitute a main cellular system for the prevention and the amelioration of stress-induced protein damage, though little direct evidence exists for this function. We investigated the roles of the DnaK (Hsp70), DnaJ and GrpE chaperones of Escherichia coli in prevention and repair of thermally induced protein damage using firefly luciferase as a test substrate. In vivo, luciferase was rapidly inactivated at 42 degrees C, but was efficiently reactivated to 50% of its initial activity during subsequent incubation at 30 degrees C. DnaK, DnaJ and GrpE did not prevent luciferase inactivation, but were essential for its reactivation. In vitro, reactivation of heat-inactivated luciferase to 80% of its initial activity required the combined activity of DnaK, DnaJ and GrpE as well as ATP, but not GroEL and GroES. DnaJ associated with denatured luciferase, targeted DnaK to the substrate and co-operated with DnaK to prevent luciferase aggregation at 42 degrees C, an activity that was required for subsequent reactivation. The protein repair function of DnaK, GrpE and, in particular, DnaJ is likely to be part of the role of these proteins in regulation of the heat shock response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang D., Georgopoulos C. The heat-shock-regulated grpE gene of Escherichia coli is required for bacterial growth at all temperatures but is dispensable in certain mutant backgrounds. J Bacteriol. 1989 May;171(5):2748–2755. doi: 10.1128/jb.171.5.2748-2755.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P., Sander C., Valencia A., Bukau B. A module of the DnaJ heat shock proteins found in malaria parasites. Trends Biochem Sci. 1992 Apr;17(4):129–129. doi: 10.1016/0968-0004(92)90319-5. [DOI] [PubMed] [Google Scholar]
- Bukau B. Regulation of the Escherichia coli heat-shock response. Mol Microbiol. 1993 Aug;9(4):671–680. doi: 10.1111/j.1365-2958.1993.tb01727.x. [DOI] [PubMed] [Google Scholar]
- Bukau B., Walker G. C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicate roles for heat shock protein in normal metabolism. J Bacteriol. 1989 May;171(5):2337–2346. doi: 10.1128/jb.171.5.2337-2346.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B., Walker G. C. Delta dnaK52 mutants of Escherichia coli have defects in chromosome segregation and plasmid maintenance at normal growth temperatures. J Bacteriol. 1989 Nov;171(11):6030–6038. doi: 10.1128/jb.171.11.6030-6038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B., Walker G. C. Mutations altering heat shock specific subunit of RNA polymerase suppress major cellular defects of E. coli mutants lacking the DnaK chaperone. EMBO J. 1990 Dec;9(12):4027–4036. doi: 10.1002/j.1460-2075.1990.tb07624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Clarke C. F., Cheng K., Frey A. B., Stein R., Hinds P. W., Levine A. J. Purification of complexes of nuclear oncogene p53 with rat and Escherichia coli heat shock proteins: in vitro dissociation of hsc70 and dnaK from murine p53 by ATP. Mol Cell Biol. 1988 Mar;8(3):1206–1215. doi: 10.1128/mcb.8.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Gross C. A. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991 Apr;16(4):135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- Cyr D. M., Lu X., Douglas M. G. Regulation of Hsp70 function by a eukaryotic DnaJ homolog. J Biol Chem. 1992 Oct 15;267(29):20927–20931. [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Feldheim D., Rothblatt J., Schekman R. Topology and functional domains of Sec63p, an endoplasmic reticulum membrane protein required for secretory protein translocation. Mol Cell Biol. 1992 Jul;12(7):3288–3296. doi: 10.1128/mcb.12.7.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Gaitanaris G. A., Papavassiliou A. G., Rubock P., Silverstein S. J., Gottesman M. E. Renaturation of denatured lambda repressor requires heat shock proteins. Cell. 1990 Jun 15;61(6):1013–1020. doi: 10.1016/0092-8674(90)90066-n. [DOI] [PubMed] [Google Scholar]
- Gamer J., Bujard H., Bukau B. Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor sigma 32. Cell. 1992 May 29;69(5):833–842. doi: 10.1016/0092-8674(92)90294-m. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Martin J., Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453. [DOI] [PubMed] [Google Scholar]
- Hellebust H., Uhlén M., Enfors S. O. Interaction between heat shock protein DnaK and recombinant staphylococcal protein A. J Bacteriol. 1990 Sep;172(9):5030–5034. doi: 10.1128/jb.172.9.5030-5034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itikawa H., Ryu J. Isolation and characterization of a temperature-sensitive dnaK mutant of Escherichia coli B. J Bacteriol. 1979 May;138(2):339–344. doi: 10.1128/jb.138.2.339-344.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Liberek K., Galitski T. P., Zylicz M., Georgopoulos C. The DnaK chaperone modulates the heat shock response of Escherichia coli by binding to the sigma 32 transcription factor. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3516–3520. doi: 10.1073/pnas.89.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberek K., Marszalek J., Ang D., Georgopoulos C., Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberek K., Osipiuk J., Zylicz M., Ang D., Skorko J., Georgopoulos C. Physical interactions between bacteriophage and Escherichia coli proteins required for initiation of lambda DNA replication. J Biol Chem. 1990 Feb 25;265(6):3022–3029. [PubMed] [Google Scholar]
- Martin J., Langer T., Boteva R., Schramel A., Horwich A. L., Hartl F. U. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature. 1991 Jul 4;352(6330):36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- McCarty J. S., Walker G. C. DnaK as a thermometer: threonine-199 is site of autophosphorylation and is critical for ATPase activity. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9513–9517. doi: 10.1073/pnas.88.21.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V. T., Morange M., Bensaude O. Protein denaturation during heat shock and related stress. Escherichia coli beta-galactosidase and Photinus pyralis luciferase inactivation in mouse cells. J Biol Chem. 1989 Jun 25;264(18):10487–10492. [PubMed] [Google Scholar]
- Pinto M., Morange M., Bensaude O. Denaturation of proteins during heat shock. In vivo recovery of solubility and activity of reporter enzymes. J Biol Chem. 1991 Jul 25;266(21):13941–13946. [PubMed] [Google Scholar]
- Saito H., Uchida H. Organization and expression of the dnaJ and dnaK genes of Escherichia coli K12. Mol Gen Genet. 1978 Aug 4;164(1):1–8. doi: 10.1007/BF00267592. [DOI] [PubMed] [Google Scholar]
- Sherman MYu, Goldberg A. L. Involvement of the chaperonin dnaK in the rapid degradation of a mutant protein in Escherichia coli. EMBO J. 1992 Jan;11(1):71–77. doi: 10.1002/j.1460-2075.1992.tb05029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly S., Fu C. F., Dalie B., Redfield B., Coleman T., Brot N., Weissbach H. Antibody to sigma 32 cross-reacts with DnaK: association of DnaK protein with Escherichia coli RNA polymerase. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5497–5501. doi: 10.1073/pnas.85.15.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra D., Georgopoulos C., Zylicz M. The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell. 1990 Sep 7;62(5):939–944. doi: 10.1016/0092-8674(90)90268-j. [DOI] [PubMed] [Google Scholar]
- Straus D. B., Walter W. A., Gross C. A. Escherichia coli heat shock gene mutants are defective in proteolysis. Genes Dev. 1988 Dec;2(12B):1851–1858. doi: 10.1101/gad.2.12b.1851. [DOI] [PubMed] [Google Scholar]
- Straus D., Walter W., Gross C. A. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of sigma 32. Genes Dev. 1990 Dec;4(12A):2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- Stueber D., Bujard H. Transcription from efficient promoters can interfere with plasmid replication and diminish expression of plasmid specified genes. EMBO J. 1982;1(11):1399–1404. doi: 10.1002/j.1460-2075.1982.tb01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine M., Feiss M., Stuart J., Yochem J. A new host gene (groPC) necessary for lambda DNA replication. Mol Gen Genet. 1977 Feb 28;151(1):27–34. doi: 10.1007/BF00446909. [DOI] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Neidhardt F. C. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen P. V., Lubben T. H., Reed J., Goloubinoff P., O'Keefe D. P., Lorimer G. H. Chaperonin-facilitated refolding of ribulosebisphosphate carboxylase and ATP hydrolysis by chaperonin 60 (groEL) are K+ dependent. Biochemistry. 1990 Jun 19;29(24):5665–5671. doi: 10.1021/bi00476a003. [DOI] [PubMed] [Google Scholar]
- Wickner S. H. Three Escherichia coli heat shock proteins are required for P1 plasmid DNA replication: formation of an active complex between E. coli DnaJ protein and the P1 initiator protein. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2690–2694. doi: 10.1073/pnas.87.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilstra-Ryalls J., Fayet O., Georgopoulos C. The universally conserved GroE (Hsp60) chaperonins. Annu Rev Microbiol. 1991;45:301–325. doi: 10.1146/annurev.mi.45.100191.001505. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]



