Abstract
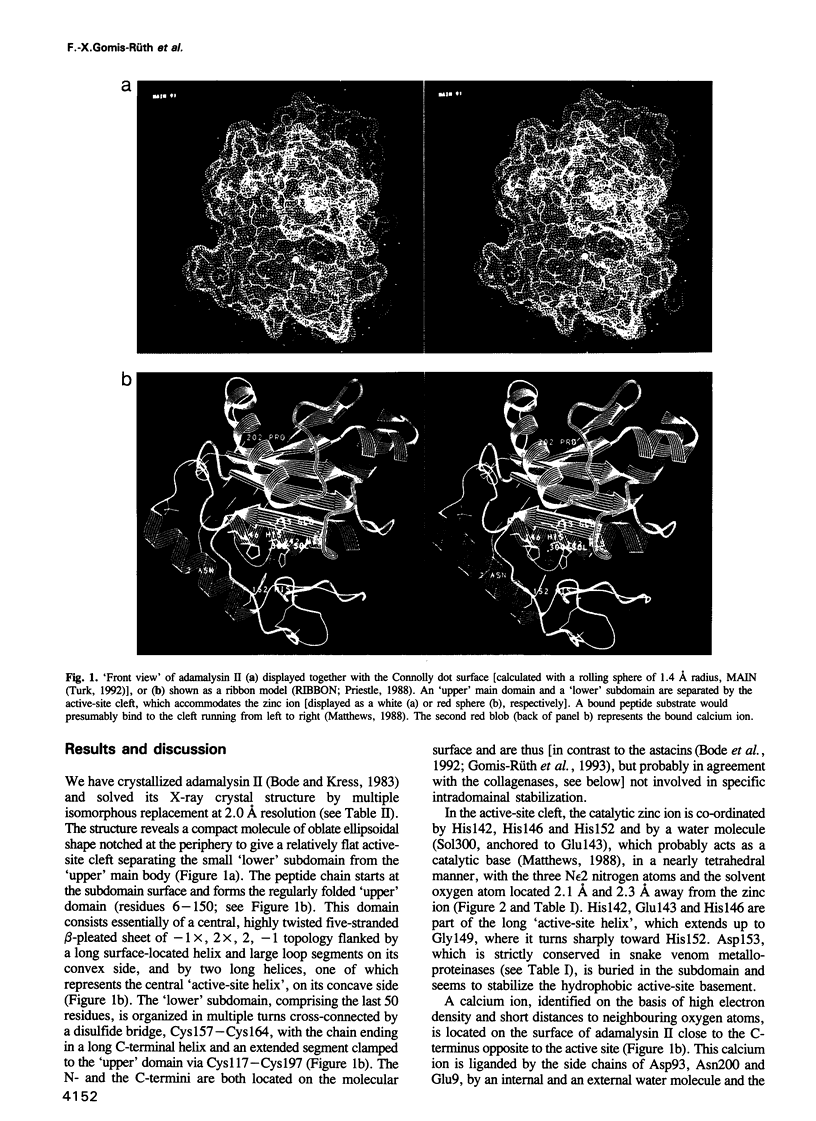
Adamalysin II, a 24 kDa zinc endopeptidase from the snake venom of Crotalus adamanteus, is a member of a large family of metalloproteinases isolated as small proteinases or proteolytic domains of mosaic haemorrhagic proteins from various snake venoms. Homologous domains have recently been detected in multimodular mammalian reproductive tract proteins. The 2.0 A crystal structure of adamalysin II reveals an ellipsoidal molecule with a shallow active-site cleft separating a relatively irregularly folded subdomain from the calcium-binding main molecular body composed of a five-stranded beta-sheet and four alpha-helices. The folding of the peptide fragment containing the zinc-binding motif HExxHxxGxxH bears only a distant resemblance to thermolysin, but is identical to that found in astacin, with the three histidines and a water molecule (linked to the glutamic acid) likewise constituting the zinc ligand; adamalysin II lacks a fifth (tyrosine) zinc ligand, however, leaving its zinc ion tetrahedrally co-ordinated. Furthermore, adamalysin II and astacin share an identical active-site basement formed by a common Metturn. Due to their virtually identical active-site environment and similar folding topology, the snake venom metalloproteinases (hitherto called adamalysins) and the astacins (and presumably also the matrix metalloproteinases/mammalian collagenases and the Serratia proteinase-like large bacterial proteinases) might be grouped into a common superfamily with distinct differences from the thermolysin family.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au L. C., Huang Y. B., Huang T. F., Teh G. W., Lin H. H., Choo K. B. A common precursor for a putative hemorrhagic protein and rhodostomin, a platelet aggregation inhibitor of the venom of Calloselasma rhodostoma: molecular cloning and sequence analysis. Biochem Biophys Res Commun. 1991 Dec 16;181(2):585–593. doi: 10.1016/0006-291x(91)91230-a. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Blobel C. P., White J. M. Structure, function and evolutionary relationship of proteins containing a disintegrin domain. Curr Opin Cell Biol. 1992 Oct;4(5):760–765. doi: 10.1016/0955-0674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- Blobel C. P., Wolfsberg T. G., Turck C. W., Myles D. G., Primakoff P., White J. M. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature. 1992 Mar 19;356(6366):248–252. doi: 10.1038/356248a0. [DOI] [PubMed] [Google Scholar]
- Bode W., Gomis-Rüth F. X., Huber R., Zwilling R., Stöcker W. Structure of astacin and implications for activation of astacins and zinc-ligation of collagenases. Nature. 1992 Jul 9;358(6382):164–167. doi: 10.1038/358164a0. [DOI] [PubMed] [Google Scholar]
- Fox J. W., Campbell R., Beggerly L., Bjarnason J. B. Substrate specificities and inhibition of two hemorrhagic zinc proteases Ht-c and Ht-d from Crotalus atrox venom. Eur J Biochem. 1986 Apr 1;156(1):65–72. doi: 10.1111/j.1432-1033.1986.tb09549.x. [DOI] [PubMed] [Google Scholar]
- Gomis-Rüth F. X., Stöcker W., Huber R., Zwilling R., Bode W. Refined 1.8 A X-ray crystal structure of astacin, a zinc-endopeptidase from the crayfish Astacus astacus L. Structure determination, refinement, molecular structure and comparison with thermolysin. J Mol Biol. 1993 Feb 20;229(4):945–968. doi: 10.1006/jmbi.1993.1098. [DOI] [PubMed] [Google Scholar]
- Hite L. A., Shannon J. D., Bjarnason J. B., Fox J. W. Sequence of a cDNA clone encoding the zinc metalloproteinase hemorrhagic toxin e from Crotalus atrox: evidence for signal, zymogen, and disintegrin-like structures. Biochemistry. 1992 Jul 14;31(27):6203–6211. doi: 10.1021/bi00142a005. [DOI] [PubMed] [Google Scholar]
- Holland D. R., Tronrud D. E., Pley H. W., Flaherty K. M., Stark W., Jansonius J. N., McKay D. B., Matthews B. W. Structural comparison suggests that thermolysin and related neutral proteases undergo hinge-bending motion during catalysis. Biochemistry. 1992 Nov 24;31(46):11310–11316. doi: 10.1021/bi00161a008. [DOI] [PubMed] [Google Scholar]
- Holmes M. A., Matthews B. W. Structure of thermolysin refined at 1.6 A resolution. J Mol Biol. 1982 Oct 5;160(4):623–639. doi: 10.1016/0022-2836(82)90319-9. [DOI] [PubMed] [Google Scholar]
- Jiang W., Bond J. S. Families of metalloendopeptidases and their relationships. FEBS Lett. 1992 Nov 9;312(2-3):110–114. doi: 10.1016/0014-5793(92)80916-5. [DOI] [PubMed] [Google Scholar]
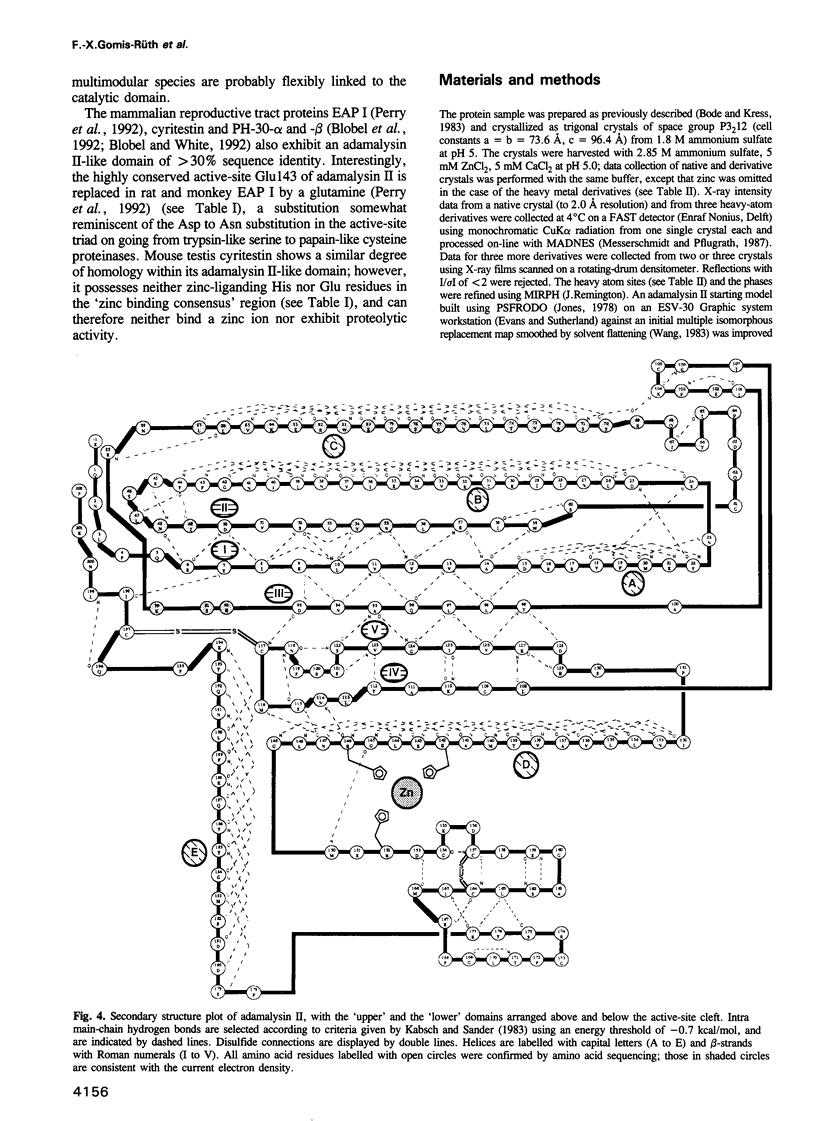
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Kini R. M., Evans H. J. Structural domains in venom proteins: evidence that metalloproteinases and nonenzymatic platelet aggregation inhibitors (disintegrins) from snake venoms are derived by proteolysis from a common precursor. Toxicon. 1992 Mar;30(3):265–293. doi: 10.1016/0041-0101(92)90869-7. [DOI] [PubMed] [Google Scholar]
- Kress L. F., Catanese J. J. Identification of the cleavage sites resulting from enzymatic inactivation of human antithrombin III by Crotalus adamanteus proteinase II in the the presence and absence of heparin. Biochemistry. 1981 Dec 22;20(26):7432–7438. doi: 10.1021/bi00529a017. [DOI] [PubMed] [Google Scholar]
- Kress L. F. Inactivation of human plasma serine proteinase inhibitors (serpins) by limited proteolysis of the reactive site loop with snake venom and bacterial metalloproteinases. J Cell Biochem. 1986;32(1):51–58. doi: 10.1002/jcb.240320106. [DOI] [PubMed] [Google Scholar]
- Kurecki T., Laskowski M., Sr, Kress L. F. Purification and some properties of two proteinases from Crotalus adamanteus venom that inactivate human alpha 1-proteinase inhibitor. J Biol Chem. 1978 Nov 25;253(22):8340–8345. [PubMed] [Google Scholar]
- Miyata T., Takeya H., Ozeki Y., Arakawa M., Tokunaga F., Iwanaga S., Omori-Satoh T. Primary structure of hemorrhagic protein, HR2a, isolated from the venom of Trimeresurus flavoviridis. J Biochem. 1989 May;105(5):847–853. doi: 10.1093/oxfordjournals.jbchem.a122756. [DOI] [PubMed] [Google Scholar]
- Murphy G. J., Murphy G., Reynolds J. J. The origin of matrix metalloproteinases and their familial relationships. FEBS Lett. 1991 Sep 2;289(1):4–7. doi: 10.1016/0014-5793(91)80895-a. [DOI] [PubMed] [Google Scholar]
- Murphy G., Docherty A. J. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992 Aug;7(2):120–125. doi: 10.1165/ajrcmb/7.2.120. [DOI] [PubMed] [Google Scholar]
- Nakahama K., Yoshimura K., Marumoto R., Kikuchi M., Lee I. S., Hase T., Matsubara H. Cloning and sequencing of Serratia protease gene. Nucleic Acids Res. 1986 Jul 25;14(14):5843–5855. doi: 10.1093/nar/14.14.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper M. P., Jacobson M. A. Sequence of a cDNA encoding the platelet aggregation inhibitor trigramin. Nucleic Acids Res. 1990 Jul 25;18(14):4255–4255. doi: 10.1093/nar/18.14.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine M. J., Desmond H. P., Theakston R. D., Crampton J. M. Purification, cloning, and molecular characterization of a high molecular weight hemorrhagic metalloprotease, jararhagin, from Bothrops jararaca venom. Insights into the disintegrin gene family. J Biol Chem. 1992 Nov 15;267(32):22869–22876. [PubMed] [Google Scholar]
- Perry A. C., Jones R., Barker P. J., Hall L. A mammalian epididymal protein with remarkable sequence similarity to snake venom haemorrhagic peptides. Biochem J. 1992 Sep 15;286(Pt 3):671–675. doi: 10.1042/bj2860671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph A., Chamberlain S. H., Chu H. L., Retzios A. D., Markland F. S., Jr, Masiarz F. R. Amino acid sequence of fibrolase, a direct-acting fibrinolytic enzyme from Agkistrodon contortrix contortrix venom. Protein Sci. 1992 May;1(5):590–600. doi: 10.1002/pro.5560010505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings N. D., Barrett A. J. Evolutionary families of peptidases. Biochem J. 1993 Feb 15;290(Pt 1):205–218. doi: 10.1042/bj2900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Argos P. Exploring structural homology of proteins. J Mol Biol. 1976 Jul 25;105(1):75–95. doi: 10.1016/0022-2836(76)90195-9. [DOI] [PubMed] [Google Scholar]
- Sanchez E. F., Diniz C. R., Richardson M. The complete amino acid sequence of the haemorrhagic factor LHFII, a metalloproteinase isolated from the venom of the bushmaster snake (Lachesis muta muta). FEBS Lett. 1991 Apr 22;282(1):178–182. doi: 10.1016/0014-5793(91)80472-f. [DOI] [PubMed] [Google Scholar]
- Shannon J. D., Baramova E. N., Bjarnason J. B., Fox J. W. Amino acid sequence of a Crotalus atrox venom metalloproteinase which cleaves type IV collagen and gelatin. J Biol Chem. 1989 Jul 15;264(20):11575–11583. [PubMed] [Google Scholar]
- Takeya H., Arakawa M., Miyata T., Iwanaga S., Omori-Satoh T. Primary structure of H2-proteinase, a non-hemorrhagic metalloproteinase, isolated from the venom of the habu snake, Trimeresurus flavoviridis. J Biochem. 1989 Jul;106(1):151–157. doi: 10.1093/oxfordjournals.jbchem.a122805. [DOI] [PubMed] [Google Scholar]
- Takeya H., Nishida S., Miyata T., Kawada S., Saisaka Y., Morita T., Iwanaga S. Coagulation factor X activating enzyme from Russell's viper venom (RVV-X). A novel metalloproteinase with disintegrin (platelet aggregation inhibitor)-like and C-type lectin-like domains. J Biol Chem. 1992 Jul 15;267(20):14109–14117. [PubMed] [Google Scholar]
- Takeya H., Oda K., Miyata T., Omori-Satoh T., Iwanaga S. The complete amino acid sequence of the high molecular mass hemorrhagic protein HR1B isolated from the venom of Trimeresurus flavoviridis. J Biol Chem. 1990 Sep 25;265(27):16068–16073. [PubMed] [Google Scholar]
- Takeya H., Onikura A., Nikai T., Sugihara H., Iwanaga S. Primary structure of a hemorrhagic metalloproteinase, HT-2, isolated from the venom of Crotalus ruber ruber. J Biochem. 1990 Nov;108(5):711–719. doi: 10.1093/oxfordjournals.jbchem.a123270. [DOI] [PubMed] [Google Scholar]
- Titani K., Torff H. J., Hormel S., Kumar S., Walsh K. A., Rödl J., Neurath H., Zwilling R. Amino acid sequence of a unique protease from the crayfish Astacus fluviatilis. Biochemistry. 1987 Jan 13;26(1):222–226. doi: 10.1021/bi00375a029. [DOI] [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]