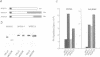Abstract
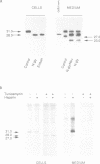
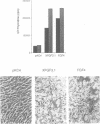
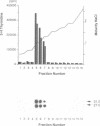
Fibroblast growth factor 3 (FGF3) was first identified as the product of a cellular oncogene activated by mouse mammary tumour virus but its normal role appears to be in the developing embryo. To gain further insights into its function, we have isolated sequences encoding the FGF3 homologue in Xenopus laevis, XFGF3. COS-1 cells transfected with XFGF3 cDNA express a 31 kDa product, p31, generated by signal peptide cleavage and Asn-linked glycosylation at the single consensus site. This product is secreted and becomes associated with the cell surface and extracellular matrix. Proteolytic cleavage of p31 in the extracellular compartment results in an amino-terminally truncated product, p27, that is also glycosylated. Both p31 and p27 bind quantitatively to heparin-Sepharose and can be displaced from the cell surface and extracellular matrix by soluble heparin. Conditioned medium containing these two proteins is capable of inducing transient morphological transformation of NIH3T3 cells and of stimulating DNA synthesis in quiescent C57MG and BALB/MK cells which express different isoforms of FGF receptors 1 and 2. Since XFGF3 behaves very differently from its mouse counterpart, we constructed chimeras in which amino-terminal sequences from XFGF3 were fused with carboxy-terminal sequences from mouse FGF3. Increasing the contribution from mouse FGF3 led to a more restricted host range for the chimeric ligand.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Amaya E., Musci T. J., Kirschner M. W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991 Jul 26;66(2):257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Bates B., Hardin J., Zhan X., Drickamer K., Goldfarb M. Biosynthesis of human fibroblast growth factor-5. Mol Cell Biol. 1991 Apr;11(4):1840–1845. doi: 10.1128/mcb.11.4.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottaro D. P., Rubin J. S., Ron D., Finch P. W., Florio C., Aaronson S. A. Characterization of the receptor for keratinocyte growth factor. Evidence for multiple fibroblast growth factor receptors. J Biol Chem. 1990 Aug 5;265(22):12767–12770. [PubMed] [Google Scholar]
- Brookes S., Smith R., Casey G., Dickson C., Peters G. Sequence organization of the human int-2 gene and its expression in teratocarcinoma cells. Oncogene. 1989 Apr;4(4):429–436. [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Dickson C., Deed R., Dixon M., Peters G. The structure and function of the int-2 oncogene. Prog Growth Factor Res. 1989;1(3):123–132. doi: 10.1016/0955-2235(89)90006-9. [DOI] [PubMed] [Google Scholar]
- Dixon M., Deed R., Acland P., Moore R., Whyte A., Peters G., Dickson C. Detection and characterization of the fibroblast growth factor-related oncoprotein INT-2. Mol Cell Biol. 1989 Nov;9(11):4896–4902. doi: 10.1128/mcb.9.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Kaufman R. J. Analysis of synthesis, processing, and secretion of proteins expressed in mammalian cells. Methods Enzymol. 1990;185:577–596. doi: 10.1016/0076-6879(90)85046-q. [DOI] [PubMed] [Google Scholar]
- Finch P. W., Rubin J. S., Miki T., Ron D., Aaronson S. A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989 Aug 18;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Friesel R., Brown S. A. Spatially restricted expression of fibroblast growth factor receptor-2 during Xenopus development. Development. 1992 Dec;116(4):1051–1058. doi: 10.1242/dev.116.4.1051. [DOI] [PubMed] [Google Scholar]
- Friesel R., Dawid I. B. cDNA cloning and developmental expression of fibroblast growth factor receptors from Xenopus laevis. Mol Cell Biol. 1991 May;11(5):2481–2488. doi: 10.1128/mcb.11.5.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M., Deed R., MacAllan D., Walther W., Dickson C., Peters G. Cell transformation by Int-2--a member of the fibroblast growth factor family. Oncogene. 1991 Jan;6(1):65–71. [PubMed] [Google Scholar]
- Goldfarb M. The fibroblast growth factor family. Cell Growth Differ. 1990 Sep;1(9):439–445. [PubMed] [Google Scholar]
- Isaacs H. V., Tannahill D., Slack J. M. Expression of a novel FGF in the Xenopus embryo. A new candidate inducing factor for mesoderm formation and anteroposterior specification. Development. 1992 Mar;114(3):711–720. doi: 10.1242/dev.114.3.711. [DOI] [PubMed] [Google Scholar]
- Kiefer P., Peters G., Dickson C. The Int-2/Fgf-3 oncogene product is secreted and associates with extracellular matrix: implications for cell transformation. Mol Cell Biol. 1991 Dec;11(12):5929–5936. doi: 10.1128/mcb.11.12.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Abraham J. A., Haaparanta T., Palisi T. M., Kirschner M. W. The presence of fibroblast growth factor in the frog egg: its role as a natural mesoderm inducer. Science. 1988 Nov 18;242(4881):1053–1056. doi: 10.1126/science.3194757. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Goddard J. M., Capecchi M. R. Mice homozygous for a targeted disruption of the proto-oncogene int-2 have developmental defects in the tail and inner ear. Development. 1993 Jan;117(1):13–28. doi: 10.1242/dev.117.1.13. [DOI] [PubMed] [Google Scholar]
- Miki T., Fleming T. P., Bottaro D. P., Rubin J. S., Ron D., Aaronson S. A. Expression cDNA cloning of the KGF receptor by creation of a transforming autocrine loop. Science. 1991 Jan 4;251(4989):72–75. doi: 10.1126/science.1846048. [DOI] [PubMed] [Google Scholar]
- Moore R., Casey G., Brookes S., Dixon M., Peters G., Dickson C. Sequence, topography and protein coding potential of mouse int-2: a putative oncogene activated by mouse mammary tumour virus. EMBO J. 1986 May;5(5):919–924. doi: 10.1002/j.1460-2075.1986.tb04304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990 Jun 25;18(12):3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. J., Lee F. S., Dickson C., Peters G., Pattengale P., Leder P. The int-2 gene product acts as an epithelial growth factor in transgenic mice. EMBO J. 1990 Mar;9(3):907–913. doi: 10.1002/j.1460-2075.1990.tb08188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musci T. J., Amaya E., Kirschner M. W. Regulation of the fibroblast growth factor receptor in early Xenopus embryos. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8365–8369. doi: 10.1073/pnas.87.21.8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswander L., Martin G. R. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992 Mar;114(3):755–768. doi: 10.1242/dev.114.3.755. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Cardiff R. D., Kuo A., Leder P. Int-2, an autocrine and/or ultra-short-range effector in transgenic mammary tissue transplants. J Natl Cancer Inst. 1992 Jun 3;84(11):887–892. doi: 10.1093/jnci/84.11.887. [DOI] [PubMed] [Google Scholar]
- Pappin D. J., Coull J. M., Köster H. Solid-phase sequence analysis of proteins electroblotted or spotted onto polyvinylidene difluoride membranes. Anal Biochem. 1990 May 15;187(1):10–19. doi: 10.1016/0003-2697(90)90410-b. [DOI] [PubMed] [Google Scholar]
- Paterno G. D., Gillespie L. L., Dixon M. S., Slack J. M., Heath J. K. Mesoderm-inducing properties of INT-2 and kFGF: two oncogene-encoded growth factors related to FGF. Development. 1989 May;106(1):79–83. doi: 10.1242/dev.106.1.79. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. S., Osada H., Finch P. W., Taylor W. G., Rudikoff S., Aaronson S. A. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989 Feb;86(3):802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta M., Burgess W., Givol D., Epstein J., Neiger N., Kaplow J., Crumley G., Dionne C., Jaye M., Schlessinger J. Receptor for acidic fibroblast growth factor is related to the tyrosine kinase encoded by the fms-like gene (FLG). Proc Natl Acad Sci U S A. 1989 Nov;86(22):8722–8726. doi: 10.1073/pnas.86.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Heath J. K., Godsave S. F. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987 Mar 12;326(6109):197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C. Mesoderm induction and mesoderm-inducing factors in early amphibian development. Development. 1989 Apr;105(4):665–677. doi: 10.1242/dev.105.4.665. [DOI] [PubMed] [Google Scholar]
- Stamp G., Fantl V., Poulsom R., Jamieson S., Smith R., Peters G., Dickson C. Nonuniform expression of a mouse mammary tumor virus-driven int-2/Fgf-3 transgene in pregnancy-responsive breast tumors. Cell Growth Differ. 1992 Dec;3(12):929–938. [PubMed] [Google Scholar]
- Tannahill D., Isaacs H. V., Close M. J., Peters G., Slack J. M. Developmental expression of the Xenopus int-2 (FGF-3) gene: activation by mesodermal and neural induction. Development. 1992 Jul;115(3):695–702. doi: 10.1242/dev.115.3.695. [DOI] [PubMed] [Google Scholar]
- Thompson J., Slack J. M. Over-expression of fibroblast growth factors in Xenopus embryos. Mech Dev. 1992 Sep;38(3):175–182. doi: 10.1016/0925-4773(92)90051-k. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Lasfargues E. Y., Sheffield J. B., Coutinho W. G. Murine mammary tumor virus (MuMTV) infection of an epithelial cell line established from C57BL/6 mouse mammary glands. Virology. 1978 Oct 1;90(1):12–22. doi: 10.1016/0042-6822(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Venesio T., Taverna D., Hynes N. E., Deed R., MacAllan D., Ciardiello F., Valverius E. M., Salomon D. S., Callahan R., Merlo G. The int-2 gene product acts as a growth factor and substitutes for basic fibroblast growth factor in promoting the differentiation of a normal mouse mammary epithelial cell line. Cell Growth Differ. 1992 Jan;3(1):63–71. [PubMed] [Google Scholar]
- Volk R., Köster M., Pöting A., Hartmann L., Knöchel W. An antisense transcript from the Xenopus laevis bFGF gene coding for an evolutionarily conserved 24 kd protein. EMBO J. 1989 Oct;8(10):2983–2988. doi: 10.1002/j.1460-2075.1989.tb08448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., McMahon A. P. Expression pattern of the FGF-related proto-oncogene int-2 suggests multiple roles in fetal development. Development. 1989 Jan;105(1):131–136. doi: 10.1242/dev.105.1.131. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Peters G., Dickson C., McMahon A. P. Expression of the FGF-related proto-oncogene int-2 during gastrulation and neurulation in the mouse. EMBO J. 1988 Mar;7(3):691–695. doi: 10.1002/j.1460-2075.1988.tb02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]