Abstract
We aimed to model esophageal bolus transit based on esophageal pressure topography (EPT) landmarks, concurrent intrabolus pressure (IBP), and esophageal diameter as defined with fluoroscopy. Ten healthy subjects were studied with high-resolution impedance manometry and videofluoroscopy. Data from four 5-ml barium swallows (2 upright, 2 supine) in each subject were analyzed. EPT landmarks were utilized to divide bolus transit into four phases: phase I, upper esophageal sphincter (UES) opening; phase II, UES closure to the transition zone (TZ); phase III, TZ to contractile deceleration point (CDP); and phase IV, CDP to completion of bolus emptying. IBP and esophageal diameter were analyzed to define functional differences among phases. IBP exhibited distinct changes during the four phases of bolus transit. Phase I was associated with filling via passive dilatation of the esophagus and IBP reflective of intrathoracic pressure. Phase II was associated with auxotonic relaxation and compartmentalization of the bolus distal to the TZ. During phase III, IBP exhibited a slow increase with loss of volume related to peristalsis (auxotonic contraction) and passive dilatation in the distal esophagus. Phase IV was associated with the highest IBP and exhibited isometric contraction during periods of nonemptying and auxotonic contraction during emptying. IBP may be used as a marker of esophageal wall state during the four phases of esophageal bolus transit. Thus abnormalities in IBP may identify subtypes of esophageal disease attributable to abnormal distensibility or neuromuscular dysfunction.
Keywords: impedance, manometry, bolus transit, esophageal emptying, fluoroscopy
the introduction of high-resolution manometry (HRM) and esophageal pressure topography (EPT) have increased our appreciation of the complexities of the swallow mechanism. Initial studies performed by Clouse and Staiano (3) recognized that peristalsis was not a seamless wave of propagation and instead was a coordinated sequence of contraction involving four distinct segments separated by pressure troughs. That work suggested that morphological and functional differences existed among the segments in terms of neural control and structure.
The increased axial resolution and accurate depiction of the space-time continuum of contractility inherent with HRM also facilitated the identification of distinct manometric landmarks with distinct functional and structural significance. The first pressure trough, also known as the transition zone (TZ), demarcates the region in which a shift in tissue morphology from striated to smooth muscle occurs, along with conversion from central to peripheral control of peristalsis (5, 6, 13). Analyzing seminal work from Behar and Biancani (1), one also appreciates that spastic motor disorders of the smooth muscle esophagus selectively involve the region distal to this manometric transition zone, making it an important landmark in EPT analysis (2). A second transition point along the wavefront, the contractile deceleration point (CDP) occurs at the distal margin of the third topographic esophageal segment identifiable by profound slowing of the wavefront (16). Simultaneous fluoroscopy and endoscopic clipping of the squamocolumnar junction suggest that this landmark represents the time at which propagation decelerates and the peristaltic contraction converges with the proximal aspect of the relaxed, effaced, and elongated lower esophageal sphincter (LES) (11). Morphologically, this represents the phrenic ampulla, and emptying during this phase is not associated with peristaltic stripping but occurs via a distinct mechanism that is more akin to a distended balloon returning to its zero state in conjunction with a shift from inhibitory to excitatory tone of the LES in conjunction with esophageal reelongation.
Although much of the emphasis of EPT to date has focused on the complexity of the contractile wave, there has been little emphasis on the role of the mechanical aspects of the esophageal wall that allow it to accommodate the bolus during esophageal filling and subsequently propel the bolus during emptying. Distensibility of the esophageal wall is modulated by its intrinsic elastic properties and the neurogenic influences that modulate excitation and inhibition. This delicate interplay is essential in promoting normal bolus transit, and intrabolus pressure (IBP) represents an important surrogate of these interactions. However, IBP is heterogeneous along the swallow and would be better conceptualized based on EPT landmarks that define periods of accommodation, compartmentalization, or emptying. Thus we hypothesize that a detailed assessment of IBP during each EPT-defined phase of bolus transit may provide important insight into the interaction between contractile activity and esophageal wall distensibility. Hence we developed a model of bolus transit that defined four distinct functional phases based on EPT landmarks with the objective of characterizing IBP within these phases. Additionally, we sought to describe the functional morphological correlates of these phases using concurrent fluoroscopy to determine the characteristic esophageal wall state during each phase.
METHODS
Subjects
Ten healthy asymptomatic subjects (4 men, mean age 28 yr, range 21–47) underwent concurrent high-resolution impedance manometry (HRIM) and videofluoroscopy to assess the relationship between EPT landmarks and functional anatomic correlates defined by fluoroscopy. Volunteers were recruited by advertisement or word of mouth and had no history of gastrointestinal symptoms or surgery. The study protocol was approved by the Northwestern University Institutional Review Board and informed consent was obtained from each subject.
Study Protocol
HRIM studies were done after at least a 6-h fast. The HRIM catheter utilized was a 4.2-mm outer diameter solid-state assembly with 36 circumferential pressure sensors at 1-cm intervals and also incorporated 18 impedance segments at 2-cm intervals (Given Imaging, Los Angeles, CA). Transducers were calibrated at 0 and 300 mmHg with use of externally applied pressure. The HRIM assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with about three intragastric pressure sensors. The HRIM protocol included two supine 5-ml swallows and two 5-ml upright (standing) swallows by using barium during simultaneous fluoroscopy to minimize radiation exposure. The barium was mixed with normal saline at a ratio of 1:1 to preserve image quality and promote an adequate impedance change during swallowing. During barium swallows, fluoroscopic imaging and HRIM data were simultaneously displayed on a computer screen and stored on the hard drive for further analysis.
Data Analysis
Landmarks for the four phases of bolus transit.
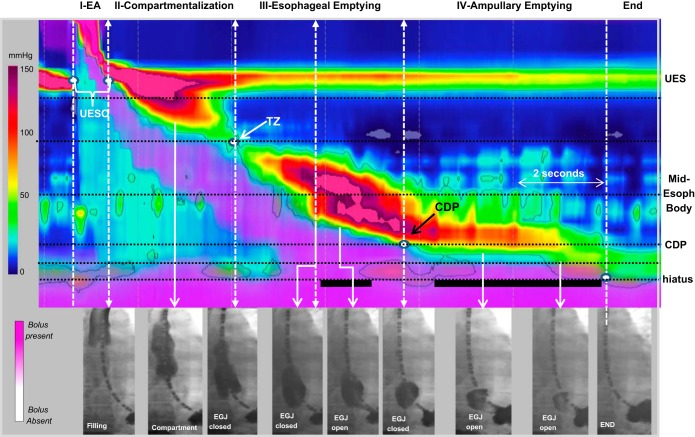
Pressure and anatomy measurements were made based on EPT landmarks and divided into four distinct esophageal phases of bolus transit using horizontal (axial location) and vertical (time) landmarks to define phase domains (Fig. 1). Phase I (accommodation) represented the time period during which the upper esophageal sphincter (UES) was relaxed and open and the bolus was propelled into the esophagus by the oropharyngeal swallow mechanism. Phase II (compartmentalization) was the time period during which the UES was closed and the contraction was propagating to the TZ. Phase III (esophageal emptying) represented the time period during which the contractile wavefront was propagating to the CDP, and phase IV (ampullary emptying) represented the time period from the CDP to the completion of bolus transit. Additionally, horizontal landmarks were utilized to demarcate axial movement of the measurement domains to avoid intersection with the UES and LES. Four horizontal landmarks were utilized to demarcate the zones of interest for measurement: 1) 1 cm distal to the inferior border of the UES, 2) the CDP, 3) the diaphragmatic hiatus, and 4) the midpoint between the hiatus and CDP to define the ampulla. The zones of interest were also defined by borders created by the propagating wavefront demarcated by the 30 mmHg isobaric contour. In instances of weak peristalsis or with a break in the 30 mmHg wavefront, a tangential line skirting the fragmented contraction was utilized. In instances of failed peristalsis, a 12-s time window was utilized as the default and phases III and IV were considered as merged into phase III.
Fig. 1.
The 4 phases of esophageal bolus transit. The phases of bolus transit are presented on an esophageal pressure topography plot (EPT) with combined impedance topography in a single supine swallow using simultaneous fluoroscopic images at various time points along the swallow. The EPT plot uses standardized color scale range for pressure, and the isobaric contour (solid black line) circumscribes all pressure values above 30 mmHg. The impedance utilizes an impedance topography scale to delineate liquid (pink shading). The white dashed lines represent the temporal landmarks for the 4 phases based on landmarks [upper esophageal sphincter (UES) opening (UESO); transition zone (TZ); contractile deceleration point (CDP)] from the start and end of the swallow. The horizontal dotted black lines represent the measurement domains for the IBP derived at the nadir impedance value for each phase and also the locations for measuring the pressure-diameter relationship. In phase I (esophageal accommodation, EA) the esophagus is filling, and at the end of phase I, the bolus has not reached the distal esophagus. The lumen is filled with air in the distal region. During phase II the bolus is compartmentalized between the UES and esophagogastric junction (EGJ), which remains closed. Phase III is associated with peristalsis, and esophageal emptying may begin during phase III if a preferential pressure gradient is obtained when the EGJ is open. Flow through the EGJ is noted by the solid black horizontal lines localized at the crural diaphragm. Phase IV represents the time period after peristalsis when emptying occurs via ampullary emptying, which is a distinct mechanism from peristalsis.
Measurements of IBP.
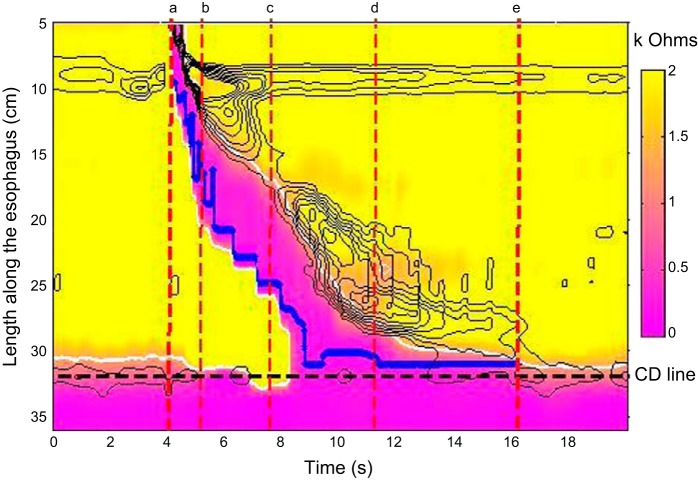
IBP measurements during the four phases of bolus transit were made by plotting the nadir impedance value as a marker of liquid presence (9) and determining the pressure at that time during each phase of transit (14) (Fig. 2). The median IBP and the slope of the IBP during each phase were calculated. The IBP slope was calculated as a simple linear change in pressure over time. These were referenced to intragastric pressure to characterize the esophagogastric pressure difference.
Fig. 2.
Methodology utilized to measure intrabolus pressure (IBP) during the 4 phases of bolus transit. The impedance data are plotted by impedance topography to highlight liquid presence (scale 0–2.0 kΩ). The pressure data are depicted with isobaric contour lines (black lines) and the time points for defining the various phases are highlighted by the red dashed lines [phase I (a–b); phase II (b–c); phase III (c–d), and phase IV (d–e)]. The nadir impedance (solid blue line) was plotted by using the UES, the crural diaphragm (CD), and the 30 mmHg isobaric contour as the boundaries to contain the measurement within the bolus. Pressure at the nadir impedance was used through each phase as the measure of IBP and this was referenced to simultaneous gastric pressure to highlight the pressure difference that would promote bolus transport into the stomach.
Anatomical measurements.
Video images from barium swallows were reviewed by two investigators (J. E. Pandolfino and H. Imam) using ManoView software (Eso 3.0, Given Imaging, Los Angeles, CA). Time points during which flow occurred through the esophagogastric junction (EGJ) were noted and the duration of flow occurring during each phase was calculated. The corresponding diameters (in mm) at the midpoint between the TZ and CDP, at the CDP, at the ampulla, and at the hiatal canal during phases I, II, and III were measured with the impedance ring spacing as the reference. The simultaneous pressure was measured at these locations to establish the pressure-diameter relationship at each region of interest.
Statistical Analysis
All results were expressed as median with interquartile range. Wilcoxon rank sum and Kruskal-Wallis tests were utilized to compare groups, and statistical significance was accepted when P value < 0.05.
RESULTS
Functional Correlates of the Four Phases of Bolus Transit
The duration of emptying for each phase and the corresponding percent of overall emptying evident from fluoroscopy is presented in Table 1. The duration was least in phase I and numerically greatest in phase IV; however, this was not statistically significant. Flow through the EGJ did not occur until the onset of phase III and the percentage of flow time was different between the upright and supine positions. The majority of trans-EGJ flow in the supine position occurred during phase IV. In contrast, trans-EGJ flow duration was evenly split between phases III and IV in the upright position.
Table 1.
Durations of bolus transit phases
| Phase I | Phase II | Phase III | Phase IV | |
|---|---|---|---|---|
| Duration, supine, s | 0.9 (0.8–1.1) | 2.7 (2.5–2.9) | 3.2 (2.7–3.9) | 4.3 (3.4–5.2) |
| Duration upright, s | 0.8 (0.7–0.9) | 2.6 (2.3–2.9) | 3.2 (3.0–3.7) | 3.9 (3.4–5.1) |
| Percent of overall trans-EGJ flow time, supine | 0 (0–0) | 0 (0–0) | 14.8 (4.1–28.0)* | 86.1 (64.7–98.2)* |
| Percent of overall trans-EGJ flow time, upright | 0 (0–0) | 0 (0–0) | 52.6 (46.7–77.7) | 49.1 (36.1–76.4) |
P < 0.05 vs. upright position during the same phase.
Intrabolus Pressure During the Four Phases of Bolus Transit
The median pressure and slope of the pressure change during each phase of bolus transit are presented in Table 2. Median IBP was significantly greater during phases I, II, and IV in the supine position compared with the upright position. The slope of IBP pressure change was close to zero during phases I and II in both the supine and upright position, suggesting that filling of the esophagus was occurring via auxotonic/isotonic relaxation or minimal passive dilatation. The slopes of the pressure change during phases III and IV were significantly greater than during phases I and II, but not significantly different from each other.
Table 2.
Comparison of E-G pressure difference by nadir impedance and rate of pressure change (slope of the IBP curve) during each phase of bolus transit measured at the locus of nadir impedance values
| Supine |
Upright |
|||||||
|---|---|---|---|---|---|---|---|---|
| Phase I | Phase II | Phase III | Phase IV | Phase I | Phase II | Phase III | Phase IV | |
| E-G pressure difference, mmHg | −1.7* (−5.9 to −0.2) | 0.8* (−1.9 to 3.1) | 2.5 (0.2 to 5.2) | 10.0* (9.1 to 12.0) | −6.9 (−7.3 to 5.9) | −4.9 (−0.2 to −3.7) | 0.1 (−2.9 to 3.4) | 2.2 (1.3 to 5.8) |
| Rate of pressure change, mmHg/s | 0.08* (0.05 to 0.15) | −0.7 (−1.15 to 0.03) | 1.6 (1.4 to 3.2) | 5.7 (3.6 to 6.1) | 0.04 (0.01 to 0.08) | 0.06 (−0.4 to 1.4) | 1.5 (0.8 to 2.4) | 2.1 (1.3 to 2.9) |
Values are medians (interquartile range).
E-G pressure difference, esophagogastric pressure difference; IBP, intrabolus pressure.
P < 0.05 vs. upright position during the same phase.
Correlation Between IBP and Radial Diameter
The maximal esophageal diameter was significantly greater at the end of phase II and at the end of phase III compared with the end of phase I despite there being no significant difference in the overall IBP at each time point. Assessing the relationship of pressure-diameter at the EPT landmarks suggested that esophageal distensibility was greatest during phase II (Table 3).
Table 3.
Comparison of maximal esophageal diameter and concurrent mean pressures at the landmark measurement times. Distensibility, approximated by diameter/pressure, was greatest during phase II
| Pressure, mmHg | Diameter, mm | Diameter/Pressure, mm/mmHg | |
|---|---|---|---|
| End of Phase I | 10.0(6.7–14.5) | 19.8 (15.0–25.0) | 2.1 (1.3–3.0) |
| End of Phase II | 8.9 (6.4–12.1) | 28.3 (23.5–30)* | 3.5 (2.2–4.2)*† |
| End of phase III | 12.9 (11.0–15.4) | 27.5 (25.0–30.4)* | 2.1 (1.7–2.4) |
P < 0.05 vs. upper esophageal sphincter;
P < 0.05 vs. contractile deceleration point (CDP).
The mechanical state at three different axial positions (midesophagus, CDP, and midampulla) was assessed by measuring the relationship between the slope of change for the diameter and pressure during each phase of bolus transit (Table 4) (Fig. 3). Evident in Table 4, phase I was associated with passive dilatation with air and liquid as evidenced by the increase in IBP and impedance changes consistent with air preceding liquid. Phase II was associated with auxotonic relaxation as there was a decrease in IBP with a concomitant increase in diameter (note data from supine, midesophagus; upright, midesophagus, CD, and ampulla). During phase III, the midesophagus exhibited auxotonic contraction attributable to circular muscle contraction decreasing the luminal diameter and increasing IBP. In contrast, at the corresponding time, the more distal esophagus and ampulla initially exhibited passive dilatation with an increase in diameter, eventually transitioning to isometric contraction when the diameter limits were reached and IBP continued to increase. Phase IV was associated with isometric contraction when the diaphragm was contracting (diameter did not change, but IBP rose). This phase eventually transitioned to auxotonic contraction when IBP continued to increase and the diameter decreased associated with restoration of tone in the LES. IBP also decreased during periods of emptying consistent with decompression.
Table 4.
Mechanical state of the esophageal wall at 3 locations: mid-smooth muscle esophagus, CDP location, and midpoint of the ampulla during Phase IV
| Phase I | Phase II | Phase III | Phase IV | |
|---|---|---|---|---|
| Supine | ||||
| Midesophagus | ||||
| Pressure slope | 2.6 (−2.1 to 7.5) | −1.3 (−2 to −0.8) | 3.5 (0.3 to 7.2) | CM contraction |
| Diameter slope | Air Filled | 0.125 (−0.02 to 0.4) | −0.1 (−0.99 to −0.0) | Closed |
| Mechanical State | Auxotonic Relaxation | Auxotonic Contraction | ||
| CDP | ||||
| Pressure slope | 0.8 (0 to 1.6) | 0.12 (−0.7 to 1.2) | 2.8 (1.4 to 7.1) | CM contraction |
| Diameter slope | Closed | Air | 0.4 (−0.2 to 0.6) | Closed |
| Mechanical State | Passive Dilatation | |||
| Ampulla | ||||
| Pressure slope | Sphincter | −0.7 (−3.1 to 0.7) | 2.2 (1.5 to 3.3) | 4.1 (−2.2 to 16.6) |
| Diameter slope | Closed | Closed | 0.3 (0.02 to 0.6) | −0.4 (−0.7 to 0) |
| Mechanical State | Passive Dilatation | Auxotonic Contraction, Isometric Contraction | ||
| Upright | ||||
| Midesophagus | ||||
| Pressure slope | 3.81 (3.1 to 7.0) | −0.71 (−1.9 to −0.2) | 2.3 (0.25 to 4.4) | CM contraction |
| Diameter slope | Air Filled | 0.1 (0 to 0.32) | −0.25 (−0.9 to −0.1) | Closed |
| Mechanical State | Auxotonic Relaxation | Auxotonic Contraction | ||
| CDP | ||||
| Pressure slope | 3.7 (1.7 to 6.6) | −0.78 (−1.5 to 0.5) | 1.1 (0.7 to 2.6) | CM contraction |
| Diameter slope | Closed | 0.17 (0.1 to 0.36) | 0.0 (−0.09 to 0.1) | Closed |
| Mechanical State | Auxotonic Relaxation | Isometric Contraction, Passive Dilatation | ||
| Ampulla | ||||
| Pressure slope | Sphincter | −0.95 (−2.7 to 0.7) | 1.8 (0.89 to 2.8) | 5.6 (1.6 to 15.6) |
| Diameter slope | Closed | 0.27 (0.1 to 0.5) | 0.0 (−0.25 to 0.3) | −1.25 (−1.6 to 0.05) |
| Mechanical State | Auxotonic Relaxation | Isometric Contraction, Passive Dilatation | Auxotonic Contraction, Isometric Contraction | |
All measurements were made when no flow was occurring to focus on the mechanical state of the wall and not the response to emptying. Data are presented as median (interquartile range). Pressure slope (mmHg/s), Diameter slope (mm/s). CM, circular muscle, Passive Dilatation [increase in pressure and diameter], Isometric Contraction [no to minimal change in diameter associated with an increase in pressure], Isometric relaxation [no to minimal change in diameter with a decrease in pressure], Auxotonic Contraction [decrease in diameter and an increase in pressure], Auxotonic relaxation [increase in diameter with a decrease in pressure.
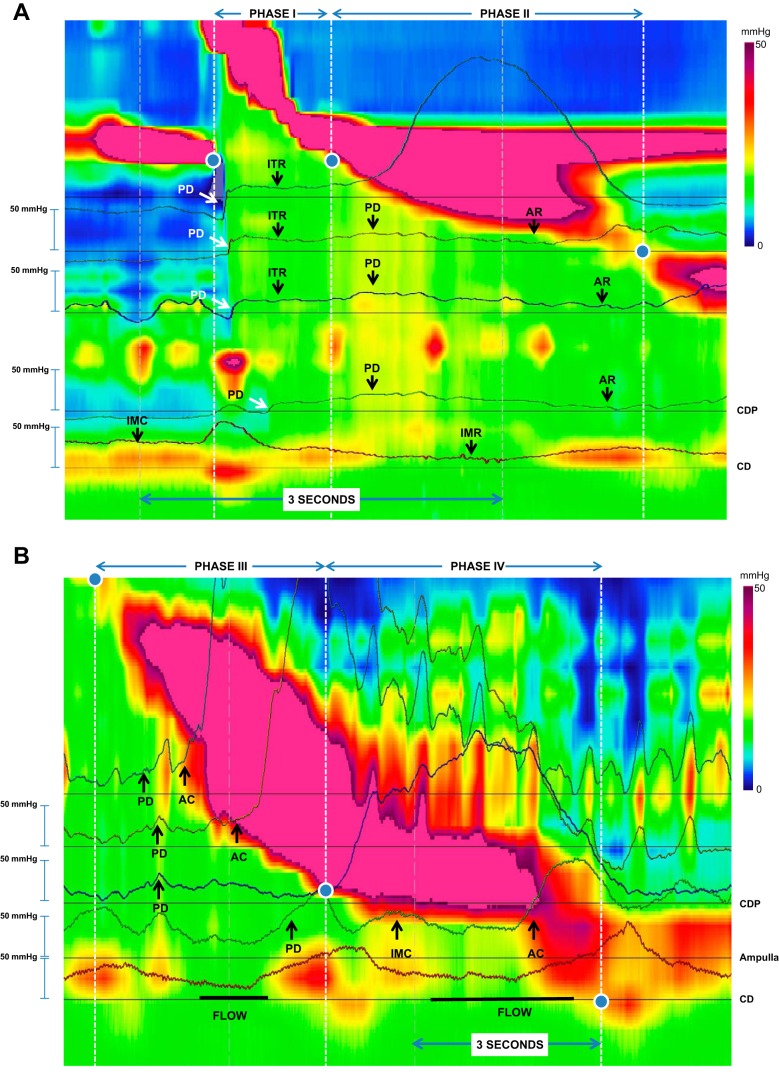
Fig. 3.
Changes in IBP during the 4 phases of bolus transit. Esophageal pressure topography plots with simultaneous pressure tracings at axial positions along the esophageal body superimposed. The color scale and tracings utilize a range of 0–50 mmHg and the swallow is separated into two parts to better detail the changes over time (A, phases I and II; B, phases III and IV). Additionally, the period of flow of barium on fluoroscopy are noted by the thick horizontal lines below the CD on B. In A, phase I is associated with an abrupt increase in pressure opening up and filling the esophagus, consistent with passive dilatation (PD). The pressure flattens during this phase, yet the esophagus continues to fill and the diameter is increasing and this is consistent with isotonic relaxation (ITR). Once the UES closes, phase II begins with a slight increase in pressure and diameter consistent with PD. Eventually, the wall state shifts to auxotonic relaxation (AR) where the pressure decreases as a function of esophageal body wall relaxation due to the deglutitive inhibition. The EGJ converts from a state of isometric contraction to a state of isometric relaxation, but the lumen remains closed at this point. During phase III (B), the esophageal wall converts from a state of auxotonic contraction (AC) near the contracting wavefront and PD in the distal esophagus when the esophagus is not emptying. During phase IV, there is isometric contraction (IMC) increasing the pressure during crural contraction to facilitate emptying and AC when the lower esophageal sphincter/ampulla is collapsing due to the return of sphincter tone. Emptying is associated with a drop in pressure as the pressurized lumen is decompressed.
DISCUSSION
In this study, we present a model for describing the dynamics of bolus transit through the esophagus using EPT landmarks to define four distinct phases. To date, most of the focus on esophageal motility has been directed at peristaltic contractility, largely ignoring the mechanical state of the esophageal wall during the nonperistaltic periods. Using well-defined EPT landmarks, we characterized four phases of bolus transit, differentiated by unique features of esophageal morphology and IBP. We hypothesize that this approach may improve our understanding of dysphagia pathogenesis because it classifies IBP measurements into functional components that serve to inform the mechanical state of the esophageal wall.
Swallowing has conventionally been studied in terms of functional phases: the oropharyngeal swallow, esophageal peristalsis, and LES relaxation. Our proposed model is in line with this approach but has moved toward analyzing the interplay between peristalsis and esophageal wall distensibility during bolus transit, using IBP as a surrogate. The work is an extension of an experimental model that assessed neurogenic and myogenic activity in albino rabbit intestine (4). Costa et al. (4) described 12 possible mechanical states of the gut wall and utilized a pressure-diameter relationship to deduce these states during intestinal activity. Using combined videofluoroscopy and HRIM, we sought to determine whether well-defined EPT landmarks could distinguish physiologically distinct phases of esophageal bolus transit. The impetus for this approach came from our interest in defining pressurization patterns in the esophagus and the realization that these patterns have clinical significance. For instance, panesophageal pressurization in achalasia provides important functional information demonstrating some degree of intact esophageal contractility and a better prognosis compared with a flaccid esophagus (15). Similarly, we were interested in the early pressurization patterns exhibited in eosinophilic esophagitis as these changes may be a marker of reduced wall compliance related to fibrosis and inflammation (17).
Hence, we integrated the previous segmental model of deglutitive motility conceived by Clouse and Staiano (3) with concepts exploring the mechanical states of the luminal wall proposed by Costa et al. (4). Localization of UES relaxation and the intersection of the pharyngeal wavefront with the UES are easily determined on high-resolution manometry and represent the borders of phase I. During this first phase (accommodation), the bolus is rapidly propelled into the esophagus (Fig. 3A). However, one caveat for this model is the assumption that UES relaxation and opening are normal, since this may otherwise reduce filling of the esophageal body during phase I and falsely lower filling pressure. The IBP in the esophagus during accommodation is modulated by neurogenic, myogenic, and mechanical properties of the esophageal wall. According to our observations, IBP during this phase was negative, consistent with intrathoracic pressure. During phase I, bolus transit generates a minimal increase in pressure associated with rapid esophageal dilatation. Thus esophageal filling during this stage is predominantly via passive dilatation. It is possible that abnormalities in mechanical properties of the esophageal wall associated with reduced compliance related to esophageal wall fibrosis, hypertrophy, or edema (12, 17) would shift the slope of the pressure-diameter curve toward a more stressed state. We speculate that symptoms associated with these disorders could potentially be perceived in the oropharynx as an obstruction to oropharyngeal transit despite the fact that the abnormality is within the body of the esophagus.
Phase II (compartmentalization) is the time period between UES closure compartmentalization of the bolus distal to the TZ by the propagating first segment. There is no bolus emptying during this phase because the primary mechanical state of the LES during phase II is isometric relaxation. Esophageal IBP actually decreases during this phase while the lumen undergoes a reduction in axial length and an increase in diameter (Fig. 3A, Table 4). Thus the mechanical state of the distal esophagus during phase II is most consistent with auxotonic relaxation related to deglutitive inhibition. Disorders of inhibition or reduced esophageal wall compliance would create a scenario in which the distal esophagus could not accommodate the bolus. Based on Boyle's law, a rise in IBP would occur because luminal volume is not increasing this would likely shift the mechanical state during phase II from auxotonic relaxation to isometric contraction. That scenario could potentially lead to poor bolus transit with a greater potential for retrograde flow, diverticulum formation, and chest pain through stretch or tension mediated afferent mechanoreceptors.
Phase III (esophageal emptying) is the time during which smooth muscle peristalsis occurs and the associated decrease in luminal volume during peristaltic propagation is accompanied by a concomitant increase in IBP that, again, should follow Boyle's law. A caveat to this relationship is that esophageal emptying may begin once the IBP is sufficient to open the EGJ and this will make an assessment of wall mechanics difficult because the pressure changes will be modified by the degree of emptying. The mechanical state of the esophageal body is complex during this phase of bolus transit as it is sequentially changing from an open lumen into a closed lumen via the contracting circular muscle. The esophageal compartment distal to the propagating contraction continues to maintain the pressure-diameter relationship through passive dilatation or isometric pressure increase as evidenced by an increase in IBP and esophageal emptying once it is sufficient to open the EGJ (Fig. 3B, Table 4). This interplay between the mechanical state of the esophagus and EGJ opening is crucial to bolus transit, and abnormalities of either the esophageal wall or EGJ during this phase can be associated with altered wall tension and a predisposition to esophageal wall strain and dilatation. Obstruction during this phase has been shown to be associated with changes in contractile vigor of the various smooth muscle segments (2, 7, 18).
The fourth phase of bolus transit (ampullary emptying) extends from the CDP to the end of the swallow. The CDP is an important pressure topography landmark because it represents the end of the nonsphincteric esophagus and is anatomically in close proximity with the muscular contractile A-ring or proximal aspect of the esophageal vestibule (10, 11, 16). Although the IBP was significantly lower during phases I, II, and IV in the upright swallows, it was most obvious in phase IV. This was likely due to a combination of the effect of gravity and differences in emptying patterns between the two positions. We found that most emptying during supine swallows occurred during phase IV and approximately half of emptying during upright swallows was restricted to phase IV. Emptying during this point is not peristaltic but is associated with a conversion from passive dilatation of the sphincter to isometric contraction during crural contraction and auxotonic contraction during emptying when the IBP is increasing in the context of diminishing volume. The IBP during phase IV is greater than that encountered during emptying in phase III and an elevation of IBP and a prolongation of this phase may suggest an obstruction associated with complications related to fundoplication or restrictive bariatric procedures.
A potential limitation of the present model is in the landmarks chosen to delineate the four phases. However, these landmarks were carefully chosen based on previous work. The UES landmarks used for the first phase mirror the oral and pharyngeal stages of swallowing and provides a logical method to assess how the esophagus reacts to this phase of swallowing. Both the transition zone and the CDP have been shown to have physiological significance in terms of peristaltic activity (3) and esophageal emptying (11); thus they differentiate distinct morphology of bolus transit, which justifies using these landmarks.
Another limitation of this study is the lack of intramural ultrasound data as the mechanical wall state will be highly dependent on muscle wall thickening and longitudinal muscle contraction. Previous work by the San Diego group have shown that longitudinal muscle function may modify pressurization patterns in achalasia (8) and future studies should include this measurement to better refine the role of longitudinal muscle in wall distensibility. Finally, the measurement of IBP alone may not be sufficient to assess the mechanical state as measurement of luminal geometry is required to fully elucidate this information. Although we validated our study with concurrent fluoroscopy, we do not advocate this as a routine and believe that our measurements should be sufficient. Our attempts at utilizing the impedance technique in the study by Costa et al. (4) suffered from the fact that the esophagus is not a closed lumen and fills with air and liquid and thus opening dimensions may be more difficult to measure accurately. One could use relative changes in diameter since these have been available on most of the software packages represented as a spatial variation of the impedance inverse to obtain information on changes in diameter. However, techniques such as high-resolution impedance planimetry [Functional Lumen Imaging Probe (FLIP)] may still be required to accurately measure diameter.
In summary, esophageal bolus transit can be conceptualized as four distinct phases with specific morphology and function that can be defined based on IBP during each phase. The IBP during each phase will change based on the mechanical state of the esophageal wall and thus abnormalities in both the median pressure and slope of pressure change can be utilized to highlight abnormal function. We hypothesize that esophageal diseases such as eosinophilic esophagitis, achalasia, and postsurgical dysphagia will manifest with distinct abnormalities in specific phases. Additionally, we hypothesize that nonobstructive dysphagia in patients with nonspecific or borderline motor abnormalities will benefit from a more careful analysis of IBP changes and time duration during each phase as this approach could potentially identify subtle obstruction or anatomical abnormalities at the EGJ.
GRANTS
This work was supported R01 DK079902 (J. E. Pandolfino) from the National Institutes of Health.
DISCLOSURES
John E. Pandolfino discloses consulting and educational associations with Given Imaging. There are no relevant competing financial and other interests exist for other authors (Z. Lin, B. Yim, A. Gawron, H. Imam, P. J. Kahrilas).
AUTHOR CONTRIBUTIONS
Z.L., B.Y., A.G., H.I., and J.E.P. analyzed data; Z.L., B.Y., H.I., P.J.K., and J.E.P. interpreted results of experiments; Z.L. and J.E.P. prepared figures; Z.L., P.J.K., and J.E.P. edited and revised manuscript; Z.L., B.Y., A.G., H.I., P.J.K., and J.E.P. approved final version of manuscript; A.G. and J.E.P. performed experiments; P.J.K. and J.E.P. conception and design of research; J.E.P. drafted manuscript.
REFERENCES
- 1.Behar J, Biancani P. Pathogenesis of simultaneous esophageal contractions in patients with motility disorders. Gastroenterology 105: 111–118, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil 24, Suppl 1: 57–65, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clouse RE, Staiano A. Topography of normal and high-amplitude esophageal peristalsis. Am J Physiol Gastrointest Liver Physiol 265: G1098–G1107, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Costa M, Wiklendt L, Arkwright JW, Spencer NJ, Omari T, Brookes SJ, Dinning PG. An experimental method to identify neurogenic and myogenic active mechanical states of intestinal motility. Front Syst Neurosci 7: 7, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh SK, Janiak P, Fox M, Schwizer W, Hebbard GS, Brasseur JG. Physiology of the oesophageal transition zone in the presence of chronic bolus retention: studies using concurrent high resolution manometry and digital fluoroscopy. Neurogastroenterol Motil 20: 750–759, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Ghosh SK, Janiak P, Schwizer W, Hebbard GS, Brasseur JG. Physiology of the esophageal pressure transition zone: separate contraction waves above and below. Am J Physiol Gastrointest Liver Physiol 290: G568–G576, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Gyawali CP, Kushnir VM. High-resolution manometric characteristics help differentiate types of distal esophageal obstruction in patients with peristalsis. Neurogastroenterol Motil 23: 502-e197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong SJ, Bhargava V, Jiang Y, Denboer D, Mittal RK. A unique esophageal motor pattern that involves longitudinal muscles is responsible for emptying in achalasia esophagus. Gastroenterology 139: 102–111, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imam H, Marrero F, Shay S. Impedance nadir values correlate with barium bolus amount. Dis Esophagus 25: 600–607, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Kahrilas PJ, Lin S, Spiess AE, Brasseur JG, Joehl RJ, Manka M. Impact of fundoplication on bolus transit across esophagogastric junction. Am J Physiol Gastrointest Liver Physiol 275: G1386–G1393, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Kwiatek MA, Nicodeme F, Pandolfino JE, Kahrilas PJ. Pressure morphology of the relaxed lower esophageal sphincter: the formation and collapse of the phrenic ampulla. Am J Physiol Gastrointest Liver Physiol 302: G389–G396, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittal RK, Kassab G, Puckett JL, Liu J. Hypertrophy of the muscularis propria of the lower esophageal sphincter and the body of the esophagus in patients with primary motility disorders of the esophagus. Am J Gastroenterol 98: 1705–1712, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Narawane NM, Bhatia SJ, Mistry FP, Abraham P, Dherai AJ. Manometric mapping of normal esophagus and definition of the transition zone. Indian J Gastroenterol 17: 55–57, 1998 [PubMed] [Google Scholar]
- 14.Nguyen NQ, Holloway RH, Smout AJ, Omari TI. Automated impedance-manometry analysis detects esophageal motor dysfunction in patients who have non-obstructive dysphagia with normal manometry. Neurogastroenterol Motil 25: 238–245, e164, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology 135: 1526–1533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandolfino JE, Leslie E, Luger D, Mitchell B, Kwiatek MA, Kahrilas PJ. The contractile deceleration point: an important physiologic landmark on oesophageal pressure topography. Neurogastroenterol Motil 22: 395–400, e90, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roman S, Hirano I, Kwiatek MA, Gonsalves N, Chen J, Kahrilas PJ, Pandolfino JE. Manometric features of eosinophilic esophagitis in esophageal pressure topography. Neurogastroenterol Motil 23: 208–214, e111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut 49: 145–151, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]