Abstract
Acid reflux-induced heartburn and noncardiac chest pain are processed peripherally by sensory nerve endings in the wall of the esophagus, but the underlying mechanism is still unclear. This study aims to determine the effects of acid on esophageal vagal nociceptive afferent subtypes. Extracellular single-unit recordings were performed in guinea pig vagal nodose or jugular C fiber neurons by using ex vivo esophageal-vagal preparations with intact nerve endings in the esophagus. We recorded action potentials (AP) of esophageal nodose or jugular C fibers evoked by acid perfusion and compared esophageal distension-evoked AP before and after acid perfusion. Acid perfusion for 30 min (pH range 7.4 to 5.8) did not evoke AP in nodose C fibers but significantly decreased their responses to esophageal distension, which could be recovered after washing out acid for 90 min. In jugular C fibers, acid perfusion not only evoked AP but also inhibited their responses to esophageal distension, which were not recovered after washing out acid for 120 min. Lower concentration of capsaicin perfusion mimicked acid-induced effects in nodose and jugular C fibers. Pretreatment with TRPV1 antagonist AMG9810, but not acid-sensing ion channel (ASIC) inhibitor amiloride, significantly inhibited acid-induced effects in nodose and jugular C fiber. These results demonstrate that esophageal vagal nociceptive afferent nerve subtypes display distinctive responses to acid. Acid activates jugular, but not nodose, C fibers and inhibits both of their responses to esophageal distension. These effects are mediated mainly through TRPV1. This inhibitory effect is a novel finding and may contribute to esophageal sensory/motor dysfunction in acid reflux diseases.
Keywords: esophagus, vagal, C fiber, acid, TRPV1, ASICs
acid reflux in the esophagus can induce esophageal painful sensation such as heartburn and noncardiac chest pain. Proton pump inhibitor (PPI) therapy is often effective for symptom relief, but one-third of patients still have persistent symptoms during PPI therapy. A better understanding of the mechanisms of acid-evoked esophageal nociception will be helpful to develop an effective pain modulator to manage these symptoms in addition to PPIs. Esophageal sensation and nociception are generated from sensory afferent nerve endings in the wall of the esophagus and transmitted through both spinal (6, 15, 16) and vagal (13, 14, 23, 25) pathways to the central nervous system to induce painful sensation (11). Nociceptors are special subtypes of sensory afferents, which include C fibers and Aδ fibers and are able to respond to noxious chemical, mechanical, and thermal stimuli. In both somatic and visceral tissues, nociceptive C fiber activation is usually associated with painful sensations (10). Based on this, a key event triggering sensations such as heartburn and pain from the esophagus is likely to involve the activation of esophageal C fibers. More definitive statements in this regard cannot be made at this time since there is little known about the characteristics and activation/sensitization mechanisms of esophageal nociceptors. The most common noxious stimuli are usually from refluxed gastric contents such as acid and bile salts. These refluxates may directly activate esophageal nociceptors or induce local inflammation, which then sensitize esophageal nociceptors (11).
The response of sensory afferent to tissue acidosis depends on extracellular pH and requires different ion channels/receptors including acid-sensing ion channels (ASICs); transient receptor potential vanilloid-1 (TRPV1); ionotropic purinoceptors (P2X); several Na+, K+, and Ca2+ channels; and proton-sensing G protein-coupled receptors (7). The expression and function of these ion channels in visceral afferent nerve subtypes, such as C fiber and Aδ fiber, are different (5). Previous studies have demonstrated that infusion with acid at lower pH activates both esophageal spinal and vagal afferents (1, 13, 14, 16, 25). Presently, it is still less clear which subtype of afferents and which ion channel(s) are responsible for acid-induced sensory transduction in the esophagus.
Our previous studies demonstrated that esophageal vagal afferents have distinctive subtypes of nociceptive afferents, namely nodose and jugular C fibers, but not nodose Aδ fibers. They are able to respond to mechanical stimulation in the noxious range without saturation and can be activated by noxious chemical (e.g., capsaicin) stimulation (23). TRPV1 is a newly defined nonselective cationic ion channel. It is thermally sensitive and can be activated by capsaicin and proton (4, 10). Activation of TRPV1 by proton in the tissue requires much lower pH (usually pH < 6.0) than most of the other acid-responsive ion channels/receptors (7, 10). In the present study, we tested the hypothesis that, among esophageal vagal afferent subtypes, both nodose and jugular nociceptive C fibers respond to noxious acid stimulation, and such responses are mediated mainly through TRPV1.
METHODS
All experiments were approved by the Johns Hopkins Animal Care and Use Committee. Male Hartley guinea pigs (Hilltop Laboratory Animals, Scottsdale, PA) weighing 150–300 g were used.
Esophageal-vagal preparation.
Extracellular single unit recordings from nodose or jugular neurons were performed in ex vivo esophageal-vagal preparations with intact nerve endings in the esophagus according to our previous studies (22, 23). Briefly, guinea pigs were killed by CO2 inhalation and exsanguination, and the esophagus and trachea with intact bilateral extrinsic vagal innervation (including jugular and nodose ganglia) were dissected. The tissue was pinned in a small Sylgard-lined Perspex chamber filled with Krebs bicarbonate buffer [KBS, composed of (in mM) 118 NaCl, 5.4 KCl, 1.0 NaH2PO4, 1.2 MgSO4, 1.9 CaCl2, 25.0 NaHCO3, and 11.1 dextrose, and gassed with 95% O2-5% CO2, pH 7.4, 35°C]. The two compartments were separately superfused with KBS (pH 7.4, 35°C, 4–6 ml/min). Polyethylene tubing was inserted 3–5 mm into the cranial and caudal esophagus and secured for esophageal distension. Isobaric (constant pressure) distension of the esophagus was achieved by increasing intraluminal esophageal pressure to 10, 30, and 60 mmHg. The pressure was generated by a calibrated device utilizing fluid (KBS) columns.
Extracellular single unit recording ex vivo.
Extracellular recordings were performed via an aluminosilicate glass microelectrode pulled with a Flaming-Brown micropipette puller (Sutter Instrument, Novato, CA) and filled with 3 M sodium chloride (electrode resistance 2 MΩ). The electrode was placed into an electrode holder connected directly to the headstage (A-M Systems, Everett, WA). A return electrode of silver-silver chloride wire and ground silver-silver chloride pellet were placed in the perfusion fluid of the recording compartment. The recorded signal was amplified (Microelectrode AC amplifier 1800, A-M Systems) and filtered (low cutoff, 0.3 kHz; high cutoff, 1 kHz), and the resultant activity was displayed on an oscilloscope (TDS 340, Tektronix, Beaverton, OR) and a model TA240 chart recorder (Gould, Cleveland, OH). The data were stored and analyzed on a Macintosh computer using the software TheNerveOfIt (sampling frequency 33 kHz; PHOCIS, Baltimore, MD) and further processed with spreadsheet software (Microsoft Excel 2007).
The recording electrode was micromanipulated into the nodose or jugular ganglion (left or right). A distension-sensitive unit was identified when esophageal distension (with a rapid increase in intraluminal pressure to 60 mmHg for 5 s) evoked action potential discharge. The serosal surface of the esophagus was then searched with a stimulation electrode (pulse duration 1.5 ms, frequency 1 Hz) applied to the tissue. A mechanosensitive receptive field was located when the electronic stimulus evoked discharge of action potentials with waveforms identical to the action potentials evoked by distension. Conduction time was measured as the time between the stimulation pulse and the action potential (visualized by oscilloscope). Conduction velocity was calculated by dividing the length of the approximated nerve pathway by conduction time. Isobaric esophageal distension for 20 s with an intraluminal pressure of 10, 30, and 60 mmHg separated by at least 60 s was used to determine the distension pressure-nerve activity relationship of an esophageal afferent fiber. To assess the reproducibility of distension-evoked activation, this distension protocol was repeated after at least 5 min. The distension-evoked response was quantified as the peak frequency of action potentials discharged during the 20 s of distension from which the spontaneous activity (if present) was subtracted. The peak frequency (Hz) was defined as the maximal frequency of action potential discharge.
After recording the baseline spontaneous activity and mechanical excitability (esophageal distension under the pressure of 10, 30, and 60 mmHg) of esophageal vagal C fiber, the acidic KBS (pH 4.4) was perfused to the serosal surface of the esophagus for 30 min. The action potential discharges of esophageal nodose or jugular C fibers induced by acid were monitored continuously for 30 min and analyzed in 1-s bins (yielding the number of action potentials in each second, Hz). The esophageal distension evoked responses of these fibers were also detected at the end of acid perfusion. Then the acid was washed out with fresh KBS (pH = 7.4) for 120 min. Esophageal distension-evoked responses were recorded every 30 min during washing. At the end of recording, capsaicin (1 μM) was perfused to the serosal surface of the esophagus to define the fiber type. In a separate study, the effects of lower concentrations of capsaicin (0.1–0.2 μM) on esophageal afferents were investigated by the same protocol. We chose to perfuse acid through the serosal surface of the esophagus because the results from our previous study showed that intraesophageal acid (pH = 2–3) infusion for more than 30 min did not activate esophageal nodose and jugular C fibers, owing to the high resistance of esophageal epithelial barrier to acid in guinea pig (25).
To further determine the roles of TRPV1 and ASICs in acid-induced responses in vagal afferents, TRPV1 antagonist AMG9810 or ASIC inhibitor amiloride diluted in KBS (pH 7.4) was preperfused to the serosal surface of the esophagus for 30 min. Then in the presences of AMG9810 (1 μM) or amiloride (10 μM), acidic KBS (pH 4.4) was perfused to surface of the esophagus for another 30 min. The responses of esophageal vagal afferents were monitored continuously followed by esophageal distension at the end of chemical perfusion. The acid and the antagonist/inhibitor were washed out for 120 min, then capsaicin (1 μM) or allyl isothiocyanate (AITC; 300–400 μM) was perfused at the end of the recording. The pH values in the chamber were monitored every 30 min during the whole perfusion process.
The compounds used in the experiment included hydrochloric acid (HCl, adjusted with KBS to pH = 4.4), capsaicin and AITC (TRPV1 and TRPA1 agonists, usually activate C fiber), amiloride (an inhibitor of ASICs), and AMG9810 (TRPV1 antagonist) (all from Sigma-Aldrich, St. Louis, MO). The compounds were diluted in KBS to final concentration on the day of use.
Data analysis.
In extracellular study, we only analyzed the results from capsaicin- or AITC-responsive C fibers, which were confirmed by the end of each recording to indicate that the nerve terminals were exposed to chemical perfusion. The acid-evoked nerve response was quantified as the peak frequency of the action potential discharge within a 5-min period and averaged from six recording periods for total 30 min. The peak frequency (Hz) of the action potential discharges were presented as means ± SE and compared by paired t-test and one-way repeated-measure ANOVA. For all experiments, significance was defined as P < 0.05.
RESULTS
A total of 62 esophageal vagal afferent nerve fibers were recorded and analyzed in this study. The average conduction velocity of nodose C fibers was 0.84 ± 0.07 m/s (n = 31), and that of jugular C fibers was 0.96 ± 0.06 m/s (n = 31). We studied one afferent C fiber (either nodose or jugular) from each animal per day. So the total number of recorded C fibers (n) is equal to the number of animals (N) used in the experiments.
Based on our previous studies (22, 23), we defined an afferent fiber as C fiber based on three criteria: 1) conduction velocity less than 1.0–1.5 m/s, 2) response to mechanical stimulation in the noxious range without saturation, and 3) response to noxious chemical stimuli such as capsaicin or AITC.
In each experiment, the acidic KBS at pH 4.4 was perfused into the esophageal tissue compartment where pH value dropped from 7.4 initially to 5.84 (the lowest point) after 30-min perfusion. After washing out acid with fresh KBS for 30 min, the pH value in the tissue compartment was recovered to 7.37.
Acid activated esophageal jugular C fibers and inhibited their responses to esophageal distension.
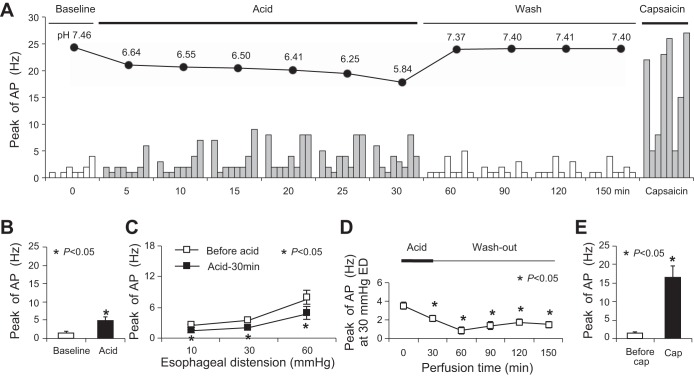
Acidic KBS significantly induced activation responses in most jugular C fibers (n = 6/8) (Fig. 1A, Table 1). The peak discharge rate of action potentials averaged 4.75 ± 1.11 Hz after acid perfusion (compared with baseline activity at 1.50 ± 0.42 Hz, P = 0.01, n = 8) (Fig. 1B). The activation responses persisted in the presence of acid and lasted for 15 min after washing out acid. The peak discharge rates returned back to 2.00 ± 0.57 Hz after washing out acid for 30 min (compared with baseline: P = 0.45, n = 8) (Fig. 1A).
Fig. 1.
Acid activated esophageal jugular C fibers and inhibited their response to esophageal distension (ED; n = 8). A: time courses of the pH values in perfused Krebs bicarbonate buffer (KBS) and the relevant acidic KBS-induced action potentials (AP) in each jugular C fibers. B: averaged data of acid perfusion evoked AP in jugular C fibers. C: esophageal distension evoked AP before and after 30-min perfusion with acid. D: time course of mechanical excitability changes (evoked by ED at 30 mmHg) in jugular C fibers after acid perfusion. E: averaged data on capsaicin (Cap)-evoked AP in jugular C fibers.
Table 1.
Acid-induced effects on esophageal jugular and nodose C fibers
| Acid | AMG9810+Acid | Amiloride+Acid | ||
|---|---|---|---|---|
| Activation response | ||||
| Jugular | Capsaicin (+) | 6/8 | 1/6 | 5/5 |
| Capsaicin (−) | 1/2 | 0/4 | ||
| Nodose | Capsaicin (+) | 1/9 | 2/6 | 0/5 |
| Capsaicin (−) | 0/3 | 0/1 | ||
| Decreased mechanical excitability | ||||
| Jugular | Capsaicin (+) | 8/8 | 0/6 | 3/5 |
| Capsaicin (−) | 1/2 | 4/4 | ||
| Nodose | Capsaicin (+) | 9/9 | 1/6 | 5/5 |
| Capsaicin (−) | 1/3 | 1/1 | ||
Values are number of fibers affected/total number of fibers.
Following acid perfusion for 30 min, the peak discharge rates evoked by esophageal distension significantly decreased in jugular C fibers. The peak discharge rates were 2.38 ± 0.26, 3.50 ± 0.38, and 7.63 ± 1.25 Hz at distension pressures of 10, 30, and 60 mmHg, respectively, before acid perfusion. These rates decreased to 1.38 ± 0.32, 2.13 ± 0.35, and 4.75 ± 1.15 Hz after acid perfusion for 30 min (before vs. after acid: P < 0.05, n = 8) (Fig. 1C).
The decreased mechanical excitability induced by acid in jugular C fibers was irreversible. The decrease of peak discharge rates evoked by esophageal distension persisted after washing out acid for 120 min. The peak discharge rates were 1.00 ± 0.27, 1.50 ± 0.33, and 1.88 ± 0.52 Hz at distension pressures of 10, 30, and 60 mmHg, respectively, after washing out acid for 120 min (compared with control: P < 0.05, n = 8). Figure 1D showed peak discharge rates evoked by esophageal distension with pressure of 30 mmHg at different time points. To determine whether these jugular C fibers were TRPV1-positive fibers, TRPV1 agonist capsaicin (1 μM) was perfused after washing out acid for 120 min. Capsaicin activated all studied jugular fibers (n = 8/8) (Table 1, Fig. 1A). The peak discharge rates were 16.38 ± 3.31 Hz after capsaicin perfusion (compared with baseline activities before capsaicin at 1.38 ± 0.42 Hz, P < 0.01, n = 8) (Fig. 1E), and such activation responses were followed by total abolishment to any other stimulation in almost all recorded C fibers.
Acid did not activate esophageal nodose C fibers but inhibited their responses to esophageal distension.
Acid perfusion in tissue compartment for 30 min did not evoke action potential discharges in most esophageal nodose C fibers (n = 8/9) (Table 1, Fig. 2A). The peak discharge rate of action potentials averaged 2.89 ± 1.18 Hz (n = 9) during 30-min perfusion with acid, which was not significantly above the baseline (1.67 ± 0.29 Hz, P = 0.36, n = 9) (Fig. 2B). After acid perfusion, their responses to esophageal distension were compared. The peak discharge rates evoked by esophageal distension were significantly decreased from 3.56 ± 0.87, 6.56 ± 1.38, and 10.00 ± 1.70 Hz (before acid perfusion) to 2.56 ± 0.77, 3.67 ± 1.18, and 4.78 ± 1.35 Hz (after 30-min acid perfusion) at distension pressures of 10, 30, and 60 mmHg, respectively (P < 0.05, n = 9) (Fig. 2C).
Fig. 2.
Acid did not activate esophageal nodose C fibers but inhibited their response to esophageal distension (n = 9). A: time courses of the pH values in perfused KBS and the relevant acidic KBS-induced AP in each nodose C fibers. B: averaged data of acid perfusion evoked AP in nodose C fibers. C: esophageal distension-evoked AP before and after 30-min perfusion with acid. D: time course of mechanical excitability changes (evoked by ED at 30 mmHg) in nodose C fibers after acid perfusion. E: averaged data on capsaicin-evoked AP in nodose C fibers.
Unlike jugular C fibers, decreased responses to esophageal distension in nodose C fibers were reversible. The peak discharge rates evoked by different esophageal distension pressures returned back to 3.67 ± 1.22, 5.44 ± 1.34, and 8.44 ± 2.14 Hz after washing out acid for 90 min (P > 0.05, compared with those before acid perfusion). Figure 2D showed the peak discharge rates evoked by esophageal distension at 30 mmHg at different time points. Capsaicin activated all nodose C fibers (n = 9/9) by the end of recordings (Table 1, Fig. 2A). The peak discharge rates were averaged 14.33 ± 2.48 Hz by capsaicin perfusion (compared with the activities before capsaicin perfusion at 1.33 ± 0.29 Hz, P = 0.001, n = 9) (Fig. 2E), and such activation responses were followed by total abolishment to any other stimulation in almost all recorded nodose C fibers.
TRPV1 agonist capsaicin, at lower concentration, mimicked acid-induced effects in esophageal jugular and nodose C fibers.
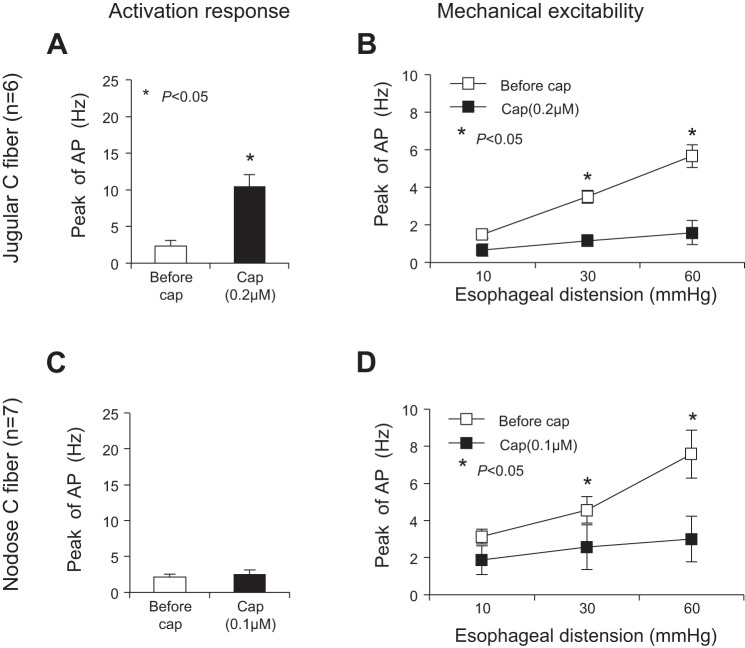
Similar to the effects induced by acid, capsaicin at a lower concentration also had distinctive effects on jugular and nodose C fibers. Capsaicin, at 0.2 μM but not 0.1 μM, evoked action potential discharges in jugular C fibers (n = 6). The peak discharge rate of action potentials averaged 10.05 ± 1.61 Hz after capsaicin perfusion (compared with baseline activity at 2.33 ± 0.8 Hz, P = 0.002, n = 6) (Fig. 3A). The peak discharge rates evoked by esophageal distension significantly decreased in jugular C fibers by capsaicin perfusion. The peak discharge rates were 1.5 ± 0.22, 3.50 ± 0.34, and 5.67 ± 0.61 Hz at distension pressures of 10, 30, and 60 mmHg before capsaicin. These rates decreased to 0.67 ± 0.33, 1.17 ± 0.31, and 1.60 ± 0.65 Hz after capsaicin perfusion (P < 0.05 at distention pressures of 30 and 60 mmHg compared with control) (Fig. 3B).
Fig. 3.
Capsaicin, at lower concentration, mimicked acid-induced effects in esophageal jugular and nodose C fibers. A and B: capsaicin (0.2 μM, 30 min) evoked AP in jugular C fibers and inhibited their response to esophageal distension (n = 6). C and D: capsaicin (0.1 μM, 30 min) did not evoke AP in nodose C fibers but inhibited their responses to esophageal distension (n = 7).
In nodose C fibers, capsaicin (0.1 μM) did not evoke action potential discharges (n = 5). The peak discharge rate of action potentials averaged 2.57 ± 0.57 Hz after capsaicin perfusion, which was not significantly changed (comparing with baseline activity at 2.14 ± 0.4 Hz, P = 0.078, n = 7) (Fig. 3C). After perfusion with capsaicin (0.1 μM) for 30 min, the mechanical excitabilities were decreased in these nodose C fibers. The peak discharge rates evoked by esophageal distension were significantly decreased from 3.14 ± 0.40, 4.57 ± 0.72, and 7.57 ± 1.29 Hz (before capsaicin perfusion) to 1.86 ± 0.77, 2.57 ± 1.21, and 3.00 ± 1.23 Hz (after capsaicin) at distension pressures of 10, 30, and 60 mmHg, respectively (P < 0.05, at distention pressures of 30 and 60 mmHg) (Fig. 3D).
Consistent with our previous studies (23), capsaicin at a high concentration (1 μM) first evoked action potential discharges and then abolished mechanical excitabilities in both jugular and nodose C fibers. The peak discharge rate in jugular fibers was 10.00 ± 2.70 Hz after capsaicin perfusion (compared with that before capsaicin at 1.33 ± 0.33 Hz, P < 0.05, n = 6). The peak discharge rate in nodose fibers was 13.70 ± 1.98 Hz (compared with that before capsaicin at 2.00 ± 0.38 Hz, P < 0.05, n = 7). After perfusion with capsaicin for 30 min, all jugular and nodose C fibers no longer responded to esophageal distension. All action potential discharges dropped to zero after capsaicin perfusion for 5–30 min.
TRPV1 antagonist AMG9810 inhibited acid-induced effects in esophageal jugular and nodose C fibers.
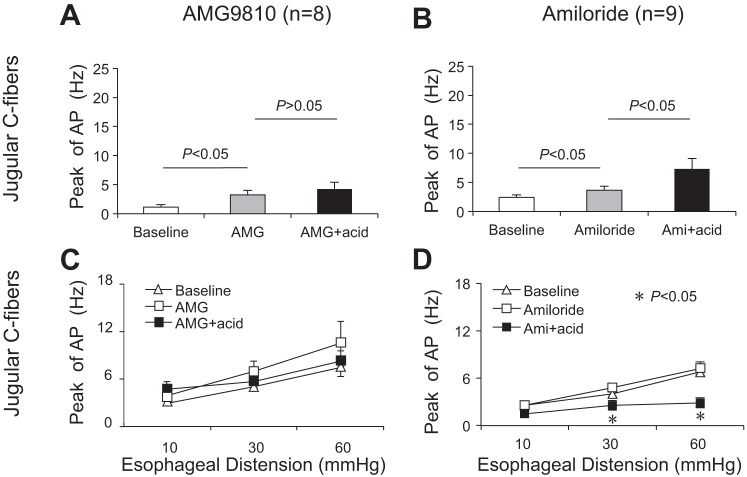
Since the lower concentration of capsaicin mimicked acid-induced effects in esophageal jugular and nodose C fibers, we further tested the hypothesis that TRPV1 antagonist could block these effects. Perfusion with TRPV1 receptor antagonist AMG9810 (1 μM for 30 min) itself evoked action potential discharges in most jugular C fibers (n = 7/8). The peak discharge rate averaged 3.25 ± 0.77 Hz after AMG9810 perfusion (compared with baseline at 1.13 ± 0.44 Hz, P = 0.006, n = 8). However, AMG9810 did not significantly change the mechanical excitabilities of these jugular C fibers. The peak discharge rates were 3.0 ± 0.46, 5.00 ± 0.50, and 7.38 ± 0.98 Hz before and 3.88 ± 0.61, 7.00 ± 1.20, and 10.50 ± 2.82 Hz after AMG9810 perfusion at esophageal distension pressures of 10, 30, and 60 mmHg, respectively (P > 0.05, n = 8).
Continually in the presence of AMG9810, acid perfused for 30 min did not activate most of these jugular C fibers (n = 6/8). The peak discharge rate was not significantly changed (before vs. after AMG9810+acid: 3.25 ± 0.77 vs. 4.25 ± 1.16 Hz, P = 0.155, n = 8) (Fig. 4A). Similarly, in the presence of AMG9810, acid perfusion did not decrease mechanical excitabilities in these jugular C fibers. The peak discharge rates were 3.88 ± 0.61, 7.00 ± 1.20, and 10.5 ± 2.82 Hz before and 4.75 ± 1.00, 5.75 ± 0.92, and 8.25 ± 1.31 Hz after AMG9810+acid perfusion for 30 min at esophageal distension pressures of 10, 30, and 60 mmHg, respectively (P > 0.05, n = 8) (Fig. 4C). This indicated that TRPV1 antagonist AMG9810 prevented acid-induced decrease of mechanical excitability in jugular C fiber. After washing out AMG9810+acid for 30 min, six of eight fibers were still not be able to respond to capsaicin (1 μM). All these six fibers were further proved to respond to TRPA1 agonist AITC (300–400 μM). Furthermore, after washing out capsaicin for another 60 min, four of these six fibers could be activated by capsaicin (1 μM) (Table 1).
Fig. 4.
AMG9810, but not amiloride (Ami), inhibited acid-induced effects in esophageal jugular C fibers. A: TRPV1 antagonist AMG9810 significantly inhibited acid-evoked AP in esophageal jugular C fibers (n = 8). B: acid-sensing ion channels (ASIC) inhibitor amiloride did not prevent acid-evoked AP in esophageal jugular C fibers (n = 9). C: AMG9810 significantly blocked acid-induced inhibition of mechanical excitability in esophageal jugular C fibers (n = 8). D: amiloride did not prevent acid-induced decrease of mechanical excitability in esophageal jugular C fibers (n = 9).
In nodose C fibers, AMG9810 (1 μM) perfusion in tissue compartment for 30 min also evoked action potential discharges in some of studied C fibers (n = 4/9). The peak discharge rate averaged 4.44 ± 0.93 Hz after AMG9810 perfusion (compared with baseline at 2.33 ± 0.44 Hz, P = 0.034, n = 9). AMG9810 slightly increased mechanical excitabilities in some of these nodose C fibers (n = 4–7 of 9 fibers) at different esophageal distension pressures, but overall mechanical excitability from all nine fibers did not significantly changed at distension pressures of 10 and 60 mmHg before and after AMG98010 perfusion (P = 0.15 and P = 0.19, n = 9). The peak discharge rate induced by esophageal distension at 30 mmHg increased from 5.33 ± 1.01 Hz to 8.78 ± 1.91 Hz before and after AMG perfusion for 30 min (P = 0.037, n = 9).
Since acid did not activate nodose C fibers as shown above, perfusion with AMG9810+acid did not change the peak discharge rates in most (6/9) nodose C fibers (AMG9810 vs. AMG9810+acid: 4.44 ± 0.93 vs. 6.33 ± 1.47 Hz, P = 0.051, n = 9) (Table 1). But pretreatment with AMG9810 inhibited acid-induced decrease of mechanical excitability in most of these nodose C fibers (7/9) (Table 1). The peak discharge rates were 5.44 ± 1.26, 8.78 ± 1.91, and 13.89 ± 3.16 Hz at distension pressures of 10, 30, and 60 mmHg after AMG9810 perfusion for 30 min. These peak discharge rates did not significantly changed after perfusion with AMG9810+acid for 30 min (5.33 ± 1.59, 9.33 ± 2.89, and 9.11 ± 1.77 Hz at distension pressures of 10, 30, and 60 mmHg, respectively, P > 0.05, n = 9) (Fig. 5A). After washing out AMG9810+acid for 30 min, the responses of nodose C fibers to TRPV1 agonist capsaicin were tested. Capsaicin (1 μM) evoked action potential discharges in six of nine nodose C fibers (Table 1). The other three fibers, which were negative to capsaicin, could be activated by AITC (300–400 μM).
Fig. 5.
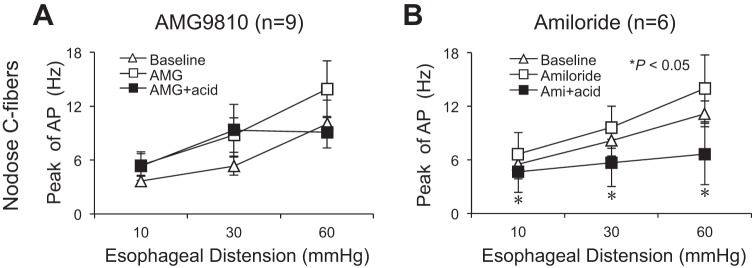
AMG9810, but not amiloride, inhibited acid-induced effect in esophageal nodose C fibers. A: TRPV1 antagonist AMG9810 significantly blocked acid-induced decrease of mechanical excitability in esophageal nodose C fibers (n = 9). B: ASIC inhibitor amiloride did not prevent acid-induced decrease of mechanical excitability in esophageal nodose C fibers (n = 6).
ASIC inhibitor amiloride did not prevent acid-induced effects in esophageal jugular and nodose C fibers.
To determine whether ASICs also contributed to acid-induced effects in esophageal afferent subtypes, the ASIC inhibitor amiloride was selected in the present study. Perfusion with amiloride for 30 min itself slightly evoked action potential discharges in jugular C fibers. The peak discharge rate increased from baseline of 2.44 ± 0.44 Hz to 3.67 ± 0.65 Hz (P = 0.005, n = 9). But perfusion with amiloride for 30 min did not change the mechanical excitability in jugular C fiber. Following perfusion with and continually in the presence of amiloride, acid still induced activation responses and decreased mechanical excitabilities in these jugular C fibers. The peak of action potential discharges increased from 3.67 ± 0.65 Hz to 7.33 ± 1.78 Hz before and after amiloride+acid perfusion (P = 0.049, n = 9) (Fig. 4B, Table 1). Esophageal distension-evoked action potential discharges decreased from 2.56 ± 0.29, 4.78 ± 0.55, and 7.22 ± 0.83 Hz to 1.44 ± 0.38, 2.56 ± 0.63, and 2.89 ± 0.65 Hz at distension pressures of 10, 30, and 60 mmHg, respectively (P < 0.05 at 30 and 60 mmHg, n = 9). After washing out amiloride+acid, four of nine fibers had no response to capsaicin (1 μM) but could be activated by AITC (300–400 μM) (Fig. 4D, Table 1).
In nodose C fiber, amiloride perfusion for 30 min did not induce action potential discharges (baseline vs. amiloride: 1.83 ± 0.31 vs. 3.67 ± 1.15, P = 0.15, n = 6). Esophageal distension-evoked action potential discharges did not significantly change (before vs. after amiloride: 5.50 ± 1.63, 8.17 ± 1.74, and 11.17 ± 1.45 Hz to 6.67 ± 2.39, 9.67 ± 2.36, and 14.00 ± 3.73 Hz at distension pressures of 10, 30, and 60 mmHg, respectively; P > 0.05, n = 6). Following perfusion with and continually in the presence of amiloride, acid perfusion significantly decreased mechanical excitabilities in all studied nodose C fibers. The peak discharge rates decreased from 6.67 ± 2.39, 9.67 ± 2.36, and 14.00 ± 3.73 Hz to 4.67 ± 2.30, 5.67 ± 2.64, and 6.67 ± 3.44 Hz after acid perfusion (P < 0.05, n = 6). After washing out amiloride and acid, one of six fibers did not respond to capsaicin (1 μM) but did respond to AITC (300–400 μM) (Fig. 5B, Table 1).
DISCUSSION
Acid-induced sensory transduction in visceral organ and tissue involves different ion channels and receptors in sensory nerve endings, depending on the acidity in the extracellular matrix (7). Refluxed acid in the esophagus can stimulate both spinal and vagal afferents and induces painful sensation such as heartburn (11). Previous studies have demonstrated that noxious acid stimulation activates only part of esophageal vagal (1, 13, 14) or spinal (16) afferent nerves. This possibly indicates that only certain subtypes of afferent nerves participate in acid-evoked sensory transduction. Vagal sensory afferents in the esophagus (and other visceral organs) are derived from two distinct embryonic tissues: cranial placodes and neural crest, which have different neurotrophin regulation during development. Placode-derived sensory nerves innervating the visceral are exclusively supplied by vagal nerves and have their neuronal cell bodies in vagal nodose ganglia. Neurocrest-derived sensory neurons are located in both vagal jugular (supranodose) ganglia and spinal dorsal root ganglia (DRG) (5). Our previous studies have shown that among esophageal vagal afferents, only subtypes of nodose C fibers and jugular C fibers can discriminate innoxious and noxious esophageal mechanical and chemical (capsaicin) stimuli, and have distinctive profiles of ion channel expression and function (22, 23). In this regard, we hypothesized that esophageal vagal afferent nociceptive subtypes distinctively responded to lower pH acid stimulation. Our data supported this hypothesis and demonstrated that lower pH acid perfusion activates esophageal jugular, but not nodose, C fibers. Acid perfusion inhibits both of their responses to esophageal distension. These effects can be mimicked by lower concentration of capsaicin and prevented by TRPV1 antagonist AMG9810. An elegant study (1) recently demonstrated that gastroesophageal luminal acidification transiently increased the resting firing frequency in mouse vagal nodose neurons but didn't alter their responses to mechanical distension. Furthermore, TRPV1 knockout mice displayed significant reduction in response to distension. We think several factors may contribute to these different observations between their findings and our present results, such as recording methods, species of animals, afferent nerve subtypes, and the way to study TRPV1 antagonism.
Both TRPV1 and ASICs play important roles in proton-induced excitation and sensitization of nociceptors (4, 10, 17, 20, 21); however, their capabilities to respond to proton are different. In general, ASICs are involved in acute and mild pH decrease-induced ischemic pain in skeletal or cardiac muscles, whereas TRPV1 contributes predominantly to persistent and lower pH acid-evoked responses in cutaneous and visceral nociceptors (10). The roles of TRPV1 in sensory transduction and transmission in the gastrointestinal tract have been well described. TRPV1 plays a prominent role in acid-induced activation and sensitization of sensory afferents in the esophagus (1, 6, 13, 14), stomach (19), and colon (3, 9, 18). Certain subtypes of vagal ganglion neurons extensively express TRPV1, which are believed to mediate nociceptive sensation from the upper gut (24, 26). In esophageal vagal afferent, TRPV1 contributed much more than ASIC3 in acid-induced responses (1), and such effects could be blocked by TRPV1 antagonist AMG9810 (14). Our current data was consistent with the results from these studies and further clarified that two subtypes of esophageal vagal nociceptive afferents, nodose and jugular C fibers, distinctively responded to acid stimulation, and their responses were mediated mainly through a TRPV1-dependent mechanism. Our data showed that lower pH acid perfusion activated jugular C fibers but not nodose C fibers. Our previous studies (23) and the present data demonstrated that the TRPV1 agonist capsaicin evoked more action potential discharges in jugular C fibers than nodose C fibers in the esophagus. This indicates that activation responses to TRPV1 agonist between these two nociceptive afferent subtypes are different. This may account for the observation in the present study that acid at pH of 7.4 to 5.8 only evoked activation responses in jugular but not nodose C fibers.
Even though TRPV1 is less likely encoding mechanical stimulation directly in vagal afferents (2), TRPV1 knockout mice show decreased responses to gastroesophageal distension in vagal afferent (1) and to colon-rectal distension in colonic afferent (9). This suggested that TRPV1 might still indirectly contribute to mechanical sensory transduction in visceral afferents. Activation of TRPV1 with capsaicin in nociceptor was usually followed by a refractory period, during which the nociceptor is resistant to capsaicin itself and other stimuli, including mechanical stimulation. Such refractory state may or may not be recovered depending on the dose and duration of initial capsaicin application (4). Our previous studies showed that capsaicin perfusion, at the concentration that inducing activation, usually led to such refractory state to both mechanical and chemical stimuli in esophageal nodose and jugular C fibers (23). Our data in the present study demonstrated that acid perfusion inhibited mechanical excitabilities in both nodose and jugular C fibers, and these inhibitory effects could be recovered in nodose but not jugular C fibers after washing out acid in the tissue. Both activation and inhibition effects of acid were mimicked by lower concentration of capsaicin and blocked by TRPV1 antagonist AMG9810, supporting the hypothesis that TRPV1 plays an important role in acid-evoked responses in esophageal vagal afferent subtypes.
ASICs are expressed in both DRG and nodose neurons (8, 12) and participate in mechanical distension and acid perfusion-induced sensory transduction in esophageal vagal (nodose) afferents (1, 12), gastric nodose and DRG neurons (19), and colon afferent fibers (9) and colonic DRG neurons (18). However, their contributions are much less obvious than TRPV1 and may only play minor roles in acid-induced sensory transduction in these visceral afferents (1, 9). Consistent with the results from these previous studies, our data in the present study did not favor a role of ASICs in acid-sensing process in esophageal nodose and jugular C fibers. First, ASICs usually are rapidly inactivating, and require rapid acid exposure to investigate (9, 10). In the present study, it took ∼20–30 min for the pH to drop from 7.4 to 5.8 in the tissue compartment after perfusion with noxious acidic KBS at a pH value of 4.4. This experimental design may have therefore reduced the apparent influence of these channels. Second, ASICs sense tissue acidity usually with pH ranging from 7.4 to 6.0 (10, 20, 21), which is higher than acidic KBS with pH of 5.8 in the present study. Third, pretreatment with ASIC inhibitor amiloride does not prevent acid-evoked effects. It is noteworthy that, owing to the limitations of this experimental design and the nonspecific inhibition effect of amiloride, the involvements of ASIC subtypes in acid-sensing and acid-induced changes in mechanical excitability in these vagal afferent subtypes deserve further exploration.
In summary, the present study demonstrates that esophageal vagal nociceptive afferent subtypes display distinctive responses to acid. Noxious acid perfusion activates esophageal jugular but not nodose C fibers and inhibits both of their responses to esophageal distension. These effects are mediated mainly through TRPV1 in these nociceptive C fiber subtypes. This finding adds our knowledge on the mechanism of acid-evoked responses in esophageal nociceptive afferents and may help to further elucidate the mechanism of acid-induced sensory/motor dysfunction in healthy and inflamed esophagus.
GRANTS
This study is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK087991 and the Blaustein Pain Research Fund (S. Yu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.Y. and S.Y. conception and design of research; X.Y., Y.H., and S.Y. performed experiments; X.Y., Y.H., and S.Y. analyzed data; X.Y., Y.H., and S.Y. interpreted results of experiments; X.Y. and S.Y. prepared figures; X.Y. and S.Y. drafted manuscript; X.Y. and S.Y. edited and revised manuscript; X.Y., Y.H., and S.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Bradley J. Undem and Nicholas C. Zachos (Department of Medicine, Johns Hopkins University) for insightful comments on the study.
REFERENCES
- 1.Bielefeldt K, Davis BM. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am J Physiol Gastrointest Liver Physiol 294: G130–G138, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bielefeldt K, Zhong F, Koerber HR, Davis BM. Phenotypic characterization of gastric sensory neurons in mice. Am J Physiol Gastrointest Liver Physiol 291: G987–G997, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Brierley SM, Carter R, Jones W, 3rd, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol 567: 267–281, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24: 487–517, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Christianson JA, Bielefeldt K, Altier C, Cenac N, Davis BM, Gebhart GF, High KW, Kollarik M, Randich A, Undem B, Vergnolle N. Development, plasticity and modulation of visceral afferents. Brain Res Rev 60: 171–86, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrington AM, Brierley SM, Isaacs NJ, Young RL, Ashley Blackshaw L. Identifying spinal sensory pathways activated by noxious esophageal acid. Neurogastroenterol Motil 25: e660–e668, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Holzer P. Acid sensing by visceral afferent neurones. Acta Physiol (Oxf) 201: 63–75, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes PA, Brierley SM, Young RL, Blackshaw LA. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J Comp Neurol 500: 863–875, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25: 10981–10989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol 29: 355–384, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Page AJ, Blackshaw LA. Roles of gastro-oesophageal afferents in the mechanisms and symptoms of reflux disease. Hand Exp Pharmacol 194: 227–257, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 54: 1408–1415, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol 87: 2095–2103, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Peles S, Medda BK, Zhang Z, Banerjee B, Lehmann A, Shaker R, Sengupta JN. Differential effects of transient receptor vanilloid one (TRPV1) antagonists in acid-induced excitation of esophageal vagal afferent fibers of rats. Neuroscience 161: 515–525, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin C, Chandler MJ, Jou CJ, Foreman RD. Responses and afferent pathways of C1–C2 spinal neurons to cervical and thoracic esophageal stimulation in rats. J Neurophysiol 91: 2227–2235, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Qin C, Farber JP, Foreman RD. Intraesophageal chemicals enhance responsiveness of upper thoracic spinal neurons to mechanical stimulation of esophagus in rats. Am J Physiol Gastrointest Liver Physiol 294: G708–G716, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Smith ES, Omerbašić D, Lechner SG, Anirudhan G, Lapatsina L, Lewin GR. The molecular basis of acid insensitivity in the African naked mole-rat. Science 334: 1557–1560, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Sugiura T, Bielefeldt K, Gebhart GF. Mouse colon sensory neurons detect extracellular acidosis via TRPV1. Am J Physiol Cell Physiol 292: C1768–C1774, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci 25: 2617–2627, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest 110: 1185–1190, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci 14: 461–471, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu S, Ouyang A. TRPA1 in bradykinin-induced mechanical hypersensitivity of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 296: G255–G265, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea pig oesophagus. J Physiol 563: 831–842, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Jones S, Brody K, Costa M, Brookes SJ. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol Gastrointest Liver Physiol 286: G983–G991, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Liu Z, Heldsinger A, Owyang C, Yu S. Intraluminal acid activates esophageal nodose C fibers after mast cell activation. Am J Physiol Gastrointest Liver Physiol 306: G200–G207, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol Gastrointest Liver Physiol 298: G212–G221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]