Abstract
The gastric ligaments are superficial cord-like structures, located on the lesser curvature of the stomach, that extend from the pylorus to the esophagus. These ligaments have been documented in a wide variety of mammalian species, including humans, but their composition and ontogeny is unexplored. Here, we demonstrate that, during ontogeny, the gastric ligaments are first visible as extensions from a C-shaped domain of Gata3-expressing cells that surround the future pylorus; this domain will later give rise to the pyloric outer longitudinal muscle (OLM). The open ends of the C are located ventrally, and, beginning at embryonic day (E) 13.5, the ligaments grow anteriorly from these points. Whereas most other ligaments of the stomach are neurovascular in nature, the gastric ligaments are composed of smooth muscle cells that mature between E14.5 and E16.5. The gastric ligaments coexpress the transcription factors Gata3, Nkx2–5, and Sox9, and germline loss of Gata3 or conditional deletion of Nkx2–5 abrogates Sox9 expression and impairs gastric ligament smooth muscle development; similar phenotypes were previously seen in the OLM. In accord with this molecular contiguity between the OLM and gastric ligaments, three-dimensional image reconstruction highlights physical contiguity between these smooth muscle structures, suggesting that they may work together as a unit to control flexure of the pyloric region, a function similar to the ligament of Treitz at the duodenojejunal junction. These findings may have implications for our understanding of normal pyloric sphincter function, as well as the common human congenital pathology idiopathic hypertrophic pyloric stenosis.
Keywords: idiopathic hypertrophic pyloric stenosis, ligament of treitz, Sox9
the intra-abdominal organs are loosely connected by a set of peritoneal ligaments, many of which arise as folds of peritoneum and may contain nerves and/or blood vessels. These structures are often named for the organs and/or anatomic structures that they connect and include the gastrosplenic, hepatogastric, hepatoduodenal, and splenorenal ligaments. In the upper abdomen, two other intra-abdominal ligaments have been described: the ligament of Treitz (otherwise known as the suspensory ligament of the duodenum) and the gastric ligaments (2, 9).
Unlike the peritoneal ligaments, the ligament of Treitz is a muscular structure; it contains a thin bundle of smooth muscle that connects the duodenum (most commonly at the duodenojejunal junction) to the abdominal aorta between the celiac and superior mesenteric arteries (2). At the duodenum, the smooth muscle of the ligament of Treitz integrates into both the outer longitudinal (OLM) and inner circular (ICM) smooth muscle layers; at the opposite end, the ligament of Treitz attaches to the aorta via a dense collection of connective tissue fibers. Contraction of the muscle fibers within the ligament of Treitz alters the angle of the duodenojejunal flexure, facilitating passage of food from the duodenum to jejunum. The nascent ligament of Treitz first appears in early fetal development as a band of loose connective tissue (i.e., superior retention band) (2, 3, 11). Later, smooth muscle cells populate the band and form the muscle of the ligament of Treitz.
The gastric ligaments are a pair of cord-like structures located superficially to the lesser curvature of the stomach that extend from the pylorus to the connective tissue surrounding the esophagus (9). The presence of these ligaments has been documented in a variety of mammalian species, including humans (9). Previous studies in our laboratory established that, in the mouse at embryonic days (E) 14.5 and 18.5, the gastric ligaments appear to connect to a group of cells at the pylorus that coexpress Gata3, Nkx2–5, and Sox9 (5, 10). Here, we document the development of these ligaments during mouse embryogenesis and examine their composition, molecular signature, and relationship to the pyloric OLM. At E12.5, a C-shaped domain of Gata3-expressing cells encircles the pyloric region, with the open portion of the “C” located ventrally. During subsequent development, this domain of Gata3 expression extends bilaterally along the ventral stomach, from the open ends of the C, to form the gastric ligaments. At E16.5, the gastric ligaments contain minimal neurovascular tissue; instead, they are composed primarily of smooth muscle cells. Molecularly, the pyloric transcription factors Gata3 and Nkx2–5 are coexpressed in the nascent gastric ligaments, and a subset of these cells also coexpress Sox9. Gata3 and Nkx2–5 are required for proper maturation of the gastric ligaments, as well as for Sox9 expression, and germline loss of Gata3 expression affects not only the pyloric constriction (10) but the overall shape of the antrum and pylorus. The cellular and molecular similarities between the gastric ligaments and pyloric OLM, together with the physical continuity of these structures [confirmed in three-dimensional (3-D) image reconstructions], suggest that they form a contiguous muscular entity that, similar to the ligament of Treitz at the duodenojejunal junction, may control the flexure of the pyloric region.
MATERIALS AND METHODS
Mice.
All animal experiments were approved by and carried out in accordance with the policies of the University of Michigan University Committee of Use and Care of Animals and Unit for Laboratory Animal Medicine. C57BL/6J (“wild-type”; WT) and CAGGCre-ER (1) mice were obtained from Charles River Laboratories (Wilmington, MA) and The Jackson Laboratory (004682; Bar Harbor, ME), respectively. Gata3lacZ/+ and Nkx2–5fl/+ mice have been described previously (10, 12).
Gata3 null embryos were generated via intercrosses of Gata3lacZ/+ animals; timed-pregnant dams were treated with α- and β-adrenergic agonists administered in the drinking water beginning at E7.5 to rescue Gata3 null pups from early embryonic lethality (4, 7, 10). For conditional Nkx2–5 deletion, Nkx2–5flox/+ mice were crossed to CAGGCre-ER animals and subsequently mated to homozygosity for the conditional allele; once-daily intraperitoneal injections of tamoxifen (T5648, 3 mg in 150 μl of corn oil; Sigma-Aldrich, St. Louis, MO) were administered to timed-pregnant dams beginning 2 days before embryo harvest (1, 10). Genotyping was performed using standard PCR methods, as described previously (10).
Histology and immunofluorescence.
Embryos retrieved from timed-pregnant dams were dissected on ice in 1× PBS. Tissue was fixed overnight at 4°C in 4% paraformaldehyde, embedded in paraffin, and sectioned at 5 μm. For hematoxylin and eosin staining, sections were deparaffinized in xylene, rehydrated through decreasing alcohol concentrations, stained with hematoxylin, incubated in bluing solution, counterstained with eosin, dehydrated through increasing alcohol concentrations, and equilibrated with xylene. Glass coverslips were mounted with Permount (SP15–100; Fisher Scientific, Waltham, MA).
For immunofluorescence, sections were deparaffinized using xylene, rehydrated in a decreasing alcohol gradient, and boiled in Antigen Unmasking Solution, Citric Acid Based (H-3300; Vector Laboratories, Burlingame, CA) for 10 min. Preblock was performed with 10% animal serum/0.01% Triton X-100 in 1× PBS (“blocking solution”) for 30 min before primary antibody incubation in diluted blocking solution. Sections were washed in 1× PBS before secondary antibody incubation in diluted blocking solution. If necessary for signal amplification, sections were washed again in 1× PBS before tertiary antibody incubation in diluted blocking solution. α-Smooth muscle actin (α-SMA) immunofluorescence was performed by incubating sections with Cy3-conjugated primary antibody (see below for details) at room temperature for 30 min, either independently after antigen retrieval or during incubation with secondary or tertiary antibodies. Glass coverslips were mounted with ProLong Gold Antifade Reagent with DAPI (P-36931; Life Technologies, Carlsbad, CA).
Primary antibodies used were as follows: Cy3-conjugated, mouse monoclonal to α-SMA (C6198, 1:500; Sigma-Aldrich); goat polyclonal to NKX2–5 (sc-8697, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal to GATA3 (sc-268, 1:50; Santa Cruz Biotechnology); rabbit polyclonal to SOX9 (AB5535, 1:200; Millipore, Darmstadt, Germany); rabbit polyclonal to peripherin (AB1530, 1:200; Millipore); and mouse monoclonal to CD31 (557355, 1:1,000; BD Pharmingen, San Jose, CA). Secondary antibodies used were as follows: biotinylated horse anti-mouse IgG (BA-2000, 1:200; Vector Laboratories); 488 donkey anti-rabbit IgG (A-21208, 1:100; Life Technologies); and APC-conjugated donkey anti-goat IgG (703-136-155, 1:100; Jackson ImmunoResearch, West Grove, PA). Tertiary antibodies used were as follows: Cy3/FITC/Cy5-conjugated mouse monoclonal to biotin (200-162-211/200-092-211/200-172-211, 1:200; Jackson ImmunoResearch).
3-D image reconstruction.
Consecutive transverse or longitudinal sections of the pyloric region were collected on glass slides and stained for α-SMA and/or GATA3 expression by immunofluorescence (see above). Each section was photographed with an Apotome microscope (Carl Zeiss, Jena, Germany) and AxioCam MR camera (Carl Zeiss), and the stack of images was manually aligned and reconstructed in three dimensions using Imaris software (Bitplane, Zurich, Switzerland).
RESULTS
Development and cellular composition of the gastric ligaments.
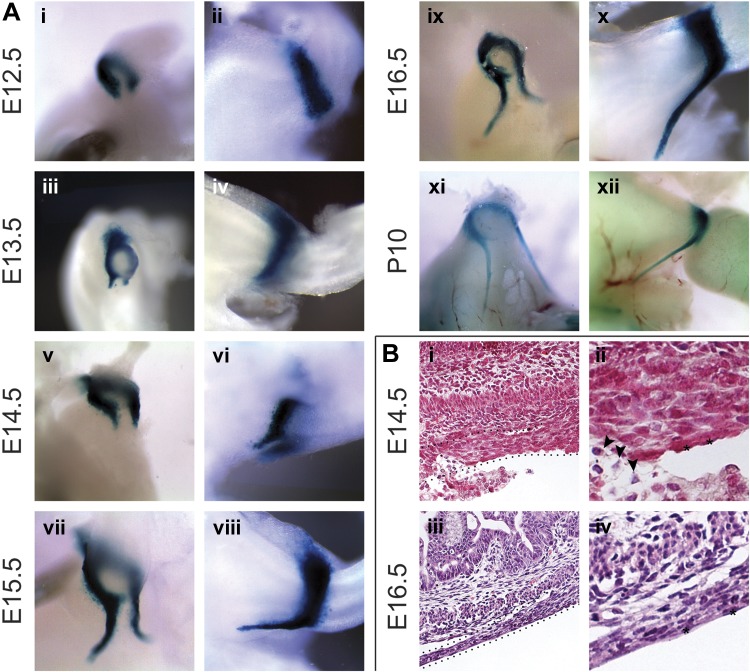
We previously demonstrated that Gata3 is expressed in the gastric ligaments at E14.5 and E18.5 (10). In the current study, we expand upon those findings by examining X-gal staining at the pyloric region in Gata3lacZ/+ embryos between E12.5 and postnatal day (P) 10. At E12.5, a thin, C-shaped band of X-gal staining is apparent at the pylorus, corresponding to nearly circumferential pyloric Gata3 expression (Fig. 1, Ai and Aii); a small gap in the X-gal staining (the open portion of the C) is detected on the ventral (lesser curvature) aspect of the pylorus (Fig. 1Ai). A day later, at E13.5, small cords of X-gal-positive cells, extending toward the esophagus, have formed at both edges of the ventral Gata3 expression gap (Fig. 1, Aiii and Aiv); these bilateral cellular cords correspond to the nascent gastric ligaments. Between E13.5 and E16.5, the gastric ligaments lengthen anteriorly, extending over the lesser curvature of the stomach to reach the connective tissue surrounding the distal esophagus (Fig. 1, Av–Ax). At P10, the gastric ligaments are still readily visible, and a contiguous Gata3 expression domain between the pyloric region and the gastric ligaments persists (Fig. 1, Axi and Axii). Thus, the aforementioned ventral gap in Gata3 expression is maintained throughout embryogenesis and the early postnatal period (Fig. 1, Ai, Aiii, Av, Avii, Aix, and Axi).
Fig. 1.
Morphogenesis and composition of the murine gastric ligaments. A: whole mount X-gal staining of Gata3lacZ/+ embryos. i and ii: The gastric ligaments have not yet developed at embryonic day (E) 12.5, although Gata3 is expressed in a C-shaped expression domain at the pylorus. The open portion of the “C” is ventral, which produces a Gata3 expression gap. iii and iv: At E13.5, bilateral Gata3-positive cellular cords (i.e., the nascent gastric ligaments) begin to extend anteriorly from either edge of the ventral Gata3 pyloric expression gap. v–x: From E14.5 to E16.5, the nascent gastric ligaments extend superficially and anteriorly over the ventral (lesser curvature) side of the stomach to reach the level of the esophagus. xi and xii: The gastric ligaments are still visible at postnatal day (P) 10. B: hematoxylin and eosin staining of wild-type (WT) embryos. i and ii: At E14.5, round-to-oval, mesenchymal-type cells compose the core of the ligaments; the ligament surface is lined by flattened, squamous-type serosal cells (asterisks) and anchored to the ventral stomach by loose connective tissue (arrowheads). iii and iv: By E16.5, the gastric ligaments have elongated and thinned but maintained their overall architecture. The cells of the ligament cores have adopted a spindle-shaped, smooth muscle-type morphology, with cellular and nuclear elongation parallel to the anteroposterior (long) axis of the gastrointestinal tract lumen. Orientation is as follows: stomach is left, and dorsal is up.
To assess the cellular morphology of the developing gastric ligaments, we examined the ventral side of the pyloric region in sectioned tissue from WT mice. At E14.5, the ligaments have begun to extend along the lesser curvature of the stomach, emerging from the pylorus and reaching toward the esophagus. The majority of cells within the ligament core have a mesenchymal-type cellular morphology, with round to oval nuclei and a moderate amount of homogenous eosinophilic cytoplasm (Fig. 1, Bi and Bii). On their surface, the ligaments are covered by a layer of flattened simple squamous-type epithelial cells with oval nuclei and scant cytoplasm, which likely correspond to peritoneal serosal cells. By E16.5, the ligaments have lengthened and thinned, extending anteriorly along the lesser curvature of the stomach, where they terminate in delicate connective tissue surrounding the base of the esophagus. The mesenchymal-type cells of the ligament core have adopted a more spindled, smooth muscle-type appearance with elongated nuclei, and the surface serosal cells have further flattened (Fig. 1, Biii and Biv).
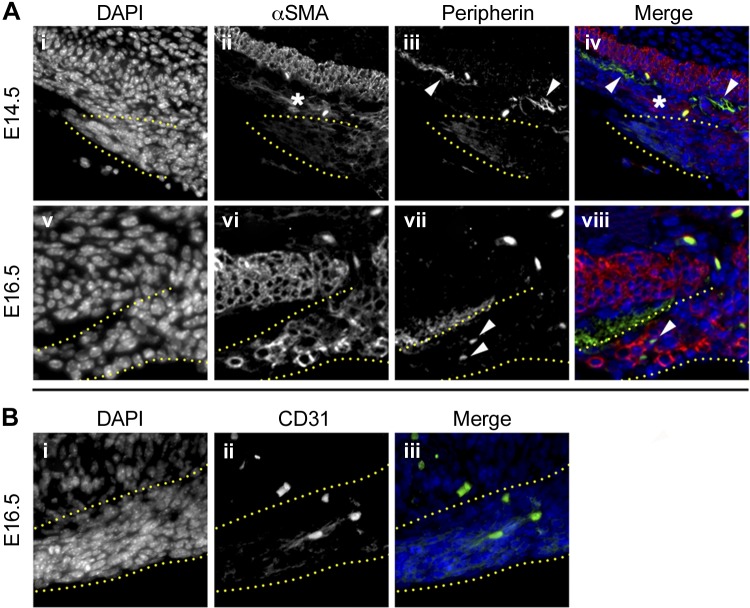
Next, to characterize the cell populations present within the gastric ligaments, we performed immunofluorescence on sectioned tissue for peripherin, α-SMA, and CD31 (to identify nervous, smooth muscle, and vascular tissue, respectively). At E14.5, none of these markers is expressed at significant levels in the gastric ligaments (Fig. 2, Aii and Aiii; data not shown). At this time point, however, the smooth muscle cells of the gastric ICM strongly express α-SMA (Fig. 2Aii), and robust peripherin expression is detected in ganglion cells of the gastric myenteric plexus (Fig. 2Aiii). By E16.5, differentiating smooth muscle cells within the gastric ligaments strongly upregulate expression of α-SMA (Fig. 2Avi), and peripherin and CD31 immunofluorescence reveals limited neurovascular tissue in these structures (Fig. 2, Avii and Bii). Thus, the gastric ligaments are primarily smooth muscle structures that mature between E14.5 and E16.5.
Fig. 2.
The gastric ligaments are predominantly smooth muscle structures. Immunofluorescence of WT pylorus at E14.5 (Ai–iv) and 16.5 (Av–viii, Bi–iii). Stains are listed above images. Ai–iv: at E14.5, α-smooth muscle actin (α-SMA) expression is low or absent within the developing gastric ligaments (outlined by yellow dots) and in the region of the gastric outer longitudinal muscle (OLM) (asterisk, Aii), since the smooth muscle has not yet matured. Comparatively, the mature gastric inner circular longitudinal muscle (ICM) strongly expresses α-SMA at this time point (bright staining above asterisk in Aii; red in Aiv). Peripherin expression is largely limited to the myenteric plexus adjacent to the gastric ICM (arrowheads in Aiii–iv). Av–viii and Bi–iii: at E16.5, the intensity of α-SMA staining in the gastric ligaments (outlined by yellow dotted lines) has increased, indicating robust smooth muscle differentiation (Avi and viii). However, there is limited expression of peripherin (arrowheads in Avii and Aviii) or CD31 (Bii and Biii), indicating that scant neurovascular tissue is present in the developing gastric ligaments. Orientation is as follows: stomach is left, and dorsal is up.
Loss of Gata3 or Nkx2–5 affects gene expression and smooth muscle maturation within the gastric ligaments.
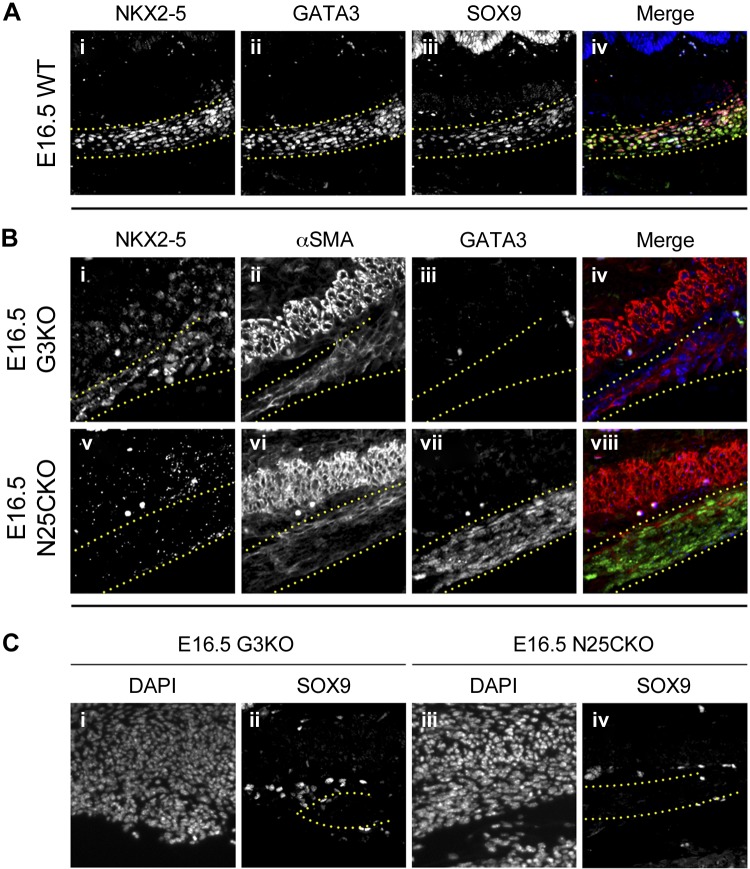
Previously, we demonstrated coexpression of Gata3, Nkx2–5, and Sox9 in smooth muscle cells of the dorsal pyloric OLM (5, 10). To investigate the expression of these transcription factors during ontogeny of the gastric ligaments, we performed immunofluorescence on sectioned tissue for GATA3, NKX2–5, and SOX9. At E16.5, NKX2–5 and GATA3 are strongly expressed in smooth muscle-type cells of the gastric ligaments (Fig. 3, Ai and Aii). SOX9 expression is detected in gastric epithelium and ganglion cells of the gastric myenteric plexus, both known sites of Sox9 expression (Fig. 3Aiii). In addition, a subset of GATA3/NKX2–5 double-positive smooth muscle-type cells within the gastric ligaments coexpress SOX9 (Fig. 3, Aiii and Aiv). No NKX2–5 or SOX9 expression is detected in surface serosal cells, although some of these cells express GATA3 (Fig. 3Aii).
Fig. 3.
Loss of Nkx2–5 or Gata3 leads to decreased smooth muscle maturation and abrogation of Sox9 expression within the gastric ligaments. Immunofluorescence of the pylorus in WT, Gata3lacZ/lacZ (G3KO), or CAGGCre-ERTM;Nkx2–5flox/flox (N25CKO) embryos at E16.5. Ai–iv: in WT embryos, GATA3 and NKX2–5 are coexpressed in the gastric ligaments. SOX9 is expressed in a subset of GATA3/NKX2–5 double-positive cells in the central portion of the ligament. Bi–viii and Ci–iv: expression of α-SMA, GATA3, NKX2–5, and SOX9 after germline loss of Gata3 (Bi–iv, Ci, and Cii) or conditional loss of NKX2–5 (Bv–viii, Ciii, and Civ). NKX2–5 expression persists in the absence of Gata3 (Bi), and GATA3 expression persists in the absence of Nkx2–5 (Bvii). α-SMA expression is reduced (Bii and Bvi) and SOX9 expression is lost (Cii and Civ) in the absence of Gata3 or Nkx2–5. The gastric ligaments are outlined with dotted yellow lines. Orientation is as follows: stomach is left, and dorsal is up.
We next examined the consequences of loss of Gata3 or Nkx2–5 on the morphology and gene expression of the nascent gastric ligaments. Germline Gata3 mutants (Gata3lacZ/lacZ or G3KO) die during early embryogenesis but can be pharmacologically rescued to birth by oral catecholamine administration to pregnant dams (4, 7, 10). In G3KO embryos at E16.5, GATA3 expression is not detected, confirming the germline absence of Gata3 (Fig. 3Biii). The gastric ligaments are present but attenuated (Fig. 3, Bi–iv), and, compared with the nearby gastric ICM cells, the residual mesenchymal cells of the gastric ligaments show weak α-SMA expression, suggesting impaired muscular maturation (Fig. 3Bii). Interestingly, NKX2–5 expression in the gastric ligaments is intact, indicating that Gata3 is not required for the initiation or maintenance of Nkx2–5 expression in these structures (Fig. 3Bi). In contrast, despite retained SOX9 expression in gastric epithelium and myenteric plexus ganglion cells (Fig. 3Cii; data not shown), no SOX9 expression can be detected in the gastric ligaments of G3KO embryos (Fig. 3Cii), suggesting that Gata3 is required for Sox9 expression in these maturing smooth muscle cells, independent of Nkx2–5.
Germline loss of Nkx2–5 is lethal during early embryogenesis because of its central role in heart development (8). Thus, to examine the role of this transcription factor in gastric ligament ontogeny, we combined a conditional Nkx2–5 allele (Nkx2–5flox/flox) with a tamoxifen-inducible Cre recombinase transgene driven from a ubiquitously expressed transgenic promoter (CAGGCre-ER) (10). Conditional mutant (CAGGCre-ER;Nkx2–5flox/flox or N25CKO) embryos were obtained at E16.5 after tamoxifen doses at E14.5 and E15.5, and the lack of NKX2–5 expression confirmed conditional loss of Nkx2–5 (Fig. 3Bv). In N25CKO embryos at E16.5, the gastric ligaments are present and similar in size and shape to WT embryos (Fig. 3, Bv–viii). However, similar to G3KO embryos, the mesenchymal cells of the gastric ligaments exhibit weak α-SMA expression relative to nearby gastric ICM cells, suggesting attenuated muscular maturation (Fig. 3Bvi). The residual mesenchymal cells within the gastric ligaments of N25CKO embryos continue to express GATA3, indicating that Nkx2–5 is not required for maintenance of Gata3 expression in these structures (Fig. 3Bvii). In addition, similar to G3KO embryos, SOX9 expression is completely lost in the gastric ligaments of N25CKO embryos (Fig. 3Civ). These results suggest that Nkx2–5 is continuously required for Sox9 expression in maturing smooth muscle cells of the gastric ligaments, independent of Gata3.
The gastric ligaments are physically contiguous with the pyloric OLM.
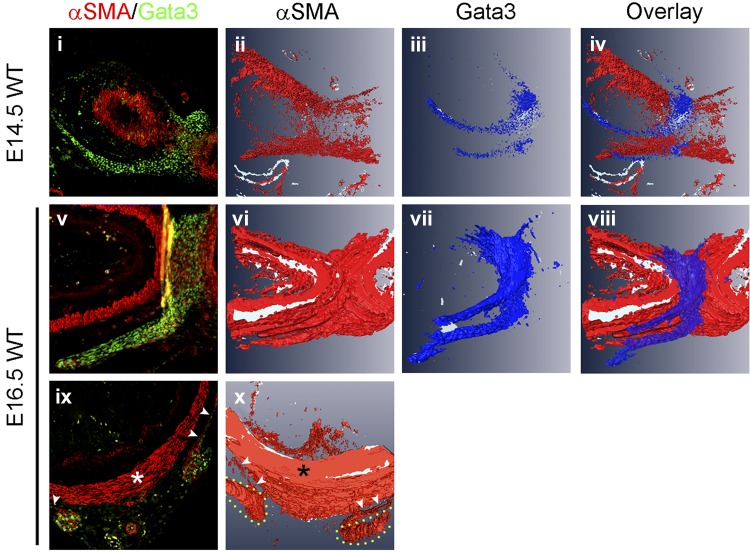
To better understand the anatomic relationship between the pyloric OLM and gastric ligaments, we analyzed serial sections of the pyloric region at E14.5 and E16.5. Immunofluorescence for α-SMA (which marks both ICM and OLM) and/or GATA3 (which marks only the pyloric OLM and not the ICM) (10) was performed on sectioned tissue, and the continuity of expression between the pyloric OLM and gastric ligaments was assessed in both individual sections and 3-D image reconstructions (Fig. 4).
Fig. 4.
The gastric ligaments are physically contiguous with the pyloric OLM. Individual sections (i, v, and ix) and 3-dimensional (3-D) image reconstruction (ii–iv, vi–viii, and x) of WT pylorus based on α-SMA and GATA3 immunofluorescence. i and iv: Superficial longitudinal sections at E14.5 (i) and E16.5 (iv) stained for α-SMA (red) and GATA3 (green). GATA3 expression at both time points highlights the physical contiguity between the gastric ligaments and pyloric OLM. ii–iv and vi–viii: At E14.5, 3-D image reconstruction emphasizes the absence of α-SMA expression in the gastric ligaments and shows that GATA3 is expressed in a saddle-shaped domain at the pylorus, the ends of which comprise the gastric ligaments. Two days later at E16.5, the smooth muscle of the OLM and gastric ligaments has matured. The GATA3 expression pattern has slightly expanded but is otherwise unchanged. At this time point, the α-SMA and GATA3 expression domains essentially completely overlap within the gastric ligaments. ix: Cross section at E16.5, stained for α-SMA (red) and GATA3 (green). The thick, α-SMA-positive pyloric ICM completely surrounds the pylorus (asterisk). The pyloric OLM (small white arrowheads) is a thin layer laterally and absent ventrally. GATA3 staining (green) is visible in the thin lateral OLM (small white arrowheads) and in the contiguous round structures that represent cross sections of the gastric ligaments. Physical continuity is visible between the pyloric OLM and the gastric ligaments (particularly on the right side of this section). x: 3-D reconstruction of α-SMA-stained regions emphasizes the continuity between the thin pyloric OLM (small white arrowheads) and the pyloric ligaments (outlined in yellow), with virtually absent ventral OLM. The pyloric ICM is a distinct muscular structure (asterisk).
Individual longitudinal sections through the pylorus, taken at a relatively superficial level, show a continuous domain of GATA3 staining between the dorsal pyloric OLM and ventral gastric ligaments at both E14.5 (Fig. 4i) and E16.5 (Fig. 4v). Continuity of the gastric ligaments with the pyloric OLM is further confirmed in individual cross sections through the middle of the pylorus (Fig. 4ix). The OLM is extremely thin (or missing altogether) on the ventral side of the pylorus (10), and, therefore, a gap in the GATA3 expression domain is apparent ventrally. Laterally, however, a clear continuity is visible between the thin OLM and the gastric ligaments (visualized as round structures that robustly express both GATA3 and α-SMA; Fig. 4ix). 3-D image reconstruction further emphasizes the single contiguous GATA3 expression domain (and, therefore, continuity between the pyloric OLM and gastric ligaments) at these time points (Fig. 4, ii–iv, vi–viii, and x).
Together, these data indicate that the contiguous pyloric OLM/gastric ligament structure is a saddle-shaped muscle surrounding the pyloric lumen and ICM, with the ligaments extending anteriorly, ventrally, and superficially toward the esophagus (Fig. 5); this muscular structure is therefore oriented in such a manner that contraction (or relaxation) of the contiguous muscle domain could alter the flexure of the pyloric region. Indeed, whole mount analysis of the pylorus from E18.5 G3KO embryos demonstrates altered morphology of the entire pyloric region, from antrum to duodenum (Fig. 6).
Fig. 5.
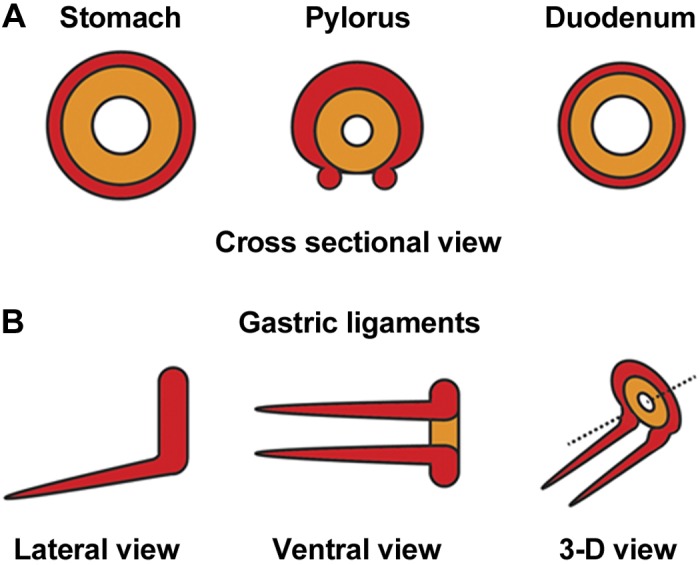
Proposed models of smooth muscle organization at and surrounding the pylorus. A: illustrations of ICM (orange) and OLM (red) arrangement in cross sections of stomach, pylorus, and duodenum. B: schematic depiction of the gastric ligaments, as viewed laterally, ventrally, and in 3-D. Together, the pyloric OLM and contiguous gastric ligaments form a saddle-shaped muscle that surrounds the ICM (except on the ventral side); the ligaments extend anteriorly and superficially over the ventral stomach, toward the esophagus. Orientation is as follows: stomach is left, and dorsal is up.
Fig. 6.
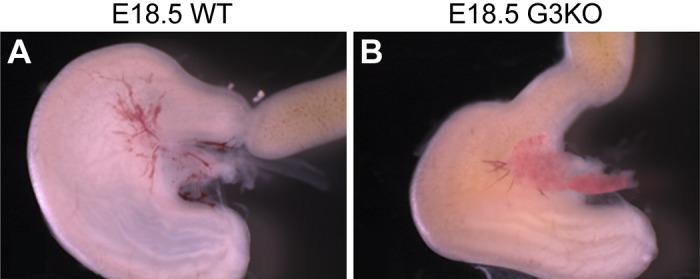
Loss of Gata3 alters the antral shape and the flexure of the pyloric region. Whole mount photomicrographs of pylorus from WT (A) and Gata3lacZ/lacZ (G3KO) (B) embryos at E18.5. Loss of Gata3 results in attenuation of the pyloric constriction (10), subtle but reproducible alteration of antral shape, and at the pylorus, the angle of flexure between the stomach and duodenum is altered. Orientation is as follows: stomach is left, and dorsal is up.
DISCUSSION
Here, we report the first molecular, cellular, and developmental characterization of the murine gastric ligaments. These structures are predominantly composed of smooth muscle cells that mature between E14.5 and E16.5. Gata3, Nkx2–5, and Sox9 are coexpressed in a subset of smooth muscle cells within the gastric ligaments, and loss of either Gata3 or Nkx2–5 impairs smooth muscle maturation and abrogates Sox9 expression. The gastric ligaments share these molecular characteristics with the pyloric OLM (10), and whole mount analysis of their ontogeny shows that, beginning at E13.5, the gastric ligaments emerge from a C-shaped domain of Gata3-expressing cells at the pylorus. In addition, 3-D image reconstruction of the pyloric region at two different time points demonstrates physical continuity between the gastric ligaments and pyloric OLM. Together, these results suggest that the gastric ligaments and pyloric OLM may function together as a smooth muscle unit at the pylorus. The antral and pyloric phenotypes of the Gata3 null animal are consistent with this hypothesis.
Our data indicate a similar epistatic relationship between Nkx2–5 and Gata3 in the gastric ligaments and pyloric OLM: Nkx2–5 expression is retained in the absence of Gata3 and vice versa. We also demonstrate that Sox9 is expressed in a subset of developing GATA3/NKX2–5 double-positive smooth muscle cells, and its expression is lost (concomitantly with strong α-SMA expression) in the absence of either Nkx2–5 or Gata3. This finding is similar to our previously published data regarding the genetic relationship between Sox9 and Nkx2–5 or Gata3 in pyloric OLM (10). Interestingly, the SOX9-expressing cells are centrally located within both the gastric ligaments and pyloric OLM; this pattern suggests that SOX9 may control expression of a soluble factor that regulates the maturation and size of both structures. Additionally, recent data suggest that the LIM homeodomain transcription factor Isl1 directly regulates Gata3 expression in pyloric OLM (6). While not specifically addressed, careful examination of the published data from that study suggests that Isl1 is expressed in the developing gastric ligaments at E14.5 and, therefore, might also directly regulate Gata3 expression in these structures. The mechanisms driving outgrowth of the gastric ligaments from the pyloric OLM and establishing Gata3, Nkx2–5, and Isl1 expression domains at the pylorus are currently unknown but will be an important area of future exploration.
As predominantly smooth muscle structures, the gastric ligaments differ from the other (peritoneal) ligaments of the stomach, which are critical for facilitating neurovascular connections between adjacent organs. Based on their contiguity with the pyloric OLM, as well as their position ventrally (essentially connecting the ventral pylorus to the distal esophagus), it is possible that these ligaments are necessary for determining the angle between the stomach and duodenum, in a manner similar to the proposed function of the ligament of Treitz at the duodenojejunal flexure (2). Given that the OLM of the duodenum is the presumed origin of the ligament of Treitz (2, 3, 11), our finding that the gastric ligaments appear to arise from the territory of the pyloric OLM suggests possible similar functions in regulating gastrointestinal motility. Indeed, a role for the gastric ligaments in pyloric flexure was suggested by Torgersen nearly 70 years ago, based on anatomic and radiological analysis of pyloric regions from multiple species (9). It will be important to further probe the contribution of the gastric ligaments to pyloric function and to analyze their character in the context of mouse models of pyloric dysfunction, including idiopathic hypertrophic pyloric stenosis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants F30-DK-082144 (A. M. Udager), F30-DK-089712 (A. Prakash), R01-DK-065850 (D. L. Gumucio), and P01-DK-062041 (D. L. Gumucio).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.P., A.M.U., and D.L.G. conception and design of research; A.P., A.M.U., and D.A.S. performed experiments; A.P., A.M.U., D.A.S., and D.L.G. analyzed data; A.P., A.M.U., D.A.S., and D.L.G. interpreted results of experiments; A.P., A.M.U., and D.L.G. prepared figures; A.P., A.M.U., and D.L.G. drafted manuscript; A.P., A.M.U., and D.L.G. edited and revised manuscript; A.P., A.M.U., D.A.S., and D.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Patrick Jay, James Douglas Engel, and Kim-Chew Lim for providing Nkx2–5flox/+ and Gata3lacZ/+ mice. We also appreciate excellent technical assistance from Brianna Sabol and Shawn Lopez, as well as the staff of the University of Michigan Microscopy and Image Analysis Laboratory.
REFERENCES
- 1.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244: 305–318, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Jit I. The development and the structure of the suspensory muscle of the duodenum. Anat Rec 113: 395–407, 1952 [DOI] [PubMed] [Google Scholar]
- 3.Jit I, Grewal SS. The suspensory muscle of the duodenum and its nerve supply. J Anat 123: 397–405, 1977 [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman CK, Zhou P, Pasolli HA, Rendl M, Bolotin D, Lim KC, Dai X, Alegre ML, Fuchs E. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev 17: 2108–2122, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Udager AM, Hu C, Qiao XT, Richards N, Gumucio DL. Dynamic patterning at the pylorus: formation of an epithelial intestine-stomach boundary in late fetal life. Dev Dyn 238: 3205–3217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Pan J, Wei C, Chen J, Liu Y, Liu J, Zhang X, Evans SM, Cui Y, Cui S. LIM homeodomain transcription factor Isl1 directs normal pyloric development by targeting Gata3 (Abstract). BMC Biol 12: 25, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet 25: 209–212, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2–5. Genes Dev 9: 1654–1666, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Torgersen J. The muscular build and movements of the stomach and duodenal bulb. Acta radiol Suppl 45: 1–191, 1942 [Google Scholar]
- 10.Udager AM, Prakash A, Saenz DA, Schinke M, Moriguchi T, Jay PY, Lim KC, Engel JD, Gumucio DL. Proper development of the outer longitudinal smooth muscle of the mouse pylorus requires Nkx2–5 and Gata3. Gastroenterology 146: 157–165 e110, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Zypen E, Revesz E. Investigation of development, structure and function of the phrenicocolic and duodenal suspensory ligaments. Acta Anat (Basel) 119: 142–148, 1984 [DOI] [PubMed] [Google Scholar]
- 12.van Doorninck JH, van Der Wees J, Karis A, Goedknegt E, Engel JD, Coesmans M, Rutteman M, Grosveld F, De Zeeuw CI. GATA-3 is involved in the development of serotonergic neurons in the caudal raphe nuclei. J Neurosci 19: RC12, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]