Abstract
The aim of this study was to determine the mechanism of initiation of transient upper esophageal sphincter relaxation (TUESR) caused by gastric air distension. Cats (n = 31) were decerebrated, EMG electrodes were placed on the cricopharyngeus, a gastric fistula was formed, and a strain gauge was sewn on the lower esophageal sphincter (n = 8). Injection of air (114 ± 13 ml) in the stomach caused TUESR (n = 18) and transient lower esophageal sphincter relaxation (TLESR, n = 6), and this effect was not significantly (P > 0.05) affected by thoracotomy. Free air or bagged air (n = 6) activated TLESR, but only free air activated TUESR. Closure of the gastroesophageal junction blocked TUESR (9/9), but not TLESR (4/4), caused by air inflation of the stomach. Venting air from distal esophagus during air inflation of the stomach prevented TUESR (n = 12) but did not prevent air escape from the stomach to the esophagus (n = 4). Rapid injection of air on the esophageal mucosa always caused TUESR (9/9) but did not always (7/9) cause an increase in esophageal pressure. The time delay between the TUESR and the rapid air pulse was significantly more variable (P < 0.05) than the time delay between the rapid air pulse and the rise in esophageal pressure. We concluded that the TUESR caused by gastric air distension is dependent on air escape from the stomach, which stimulates receptors in the esophagus, but is not dependent on distension of the stomach or esophagus, or the TLESR. Therefore, the TUESR caused by gastric air distension is initiated by stimulation of receptors in the esophageal mucosa.
Keywords: upper esophageal sphincter, lower esophageal sphincter, stomach, transient lower esophageal sphincter relaxation, transient upper esophageal sphincter relaxation
prior studies (11) have found that the transient relaxation of the lower esophageal sphincter (TLESR) caused by air distension of the stomach is often associated with transient upper esophageal sphincter relaxation (TUESR), which was termed a “microburp.” These investigators theorized (11, 12) that this TUESR is an anticipatory or volitional response linked to the TLESR reflex such that the same mechanisms underlying TLESR also underlay activation of the TUESR. On the other hand, others (2) have suggested that air influx from the stomach causing a rapid rate of esophageal pressure increase activates the TUESR. Therefore, the relationship between TLESR and TUESR is unclear.
Both proposed theories are problematic. The theory that gastric air escape causes the TUESR by causing a rapid increase in esophageal pressure (2) is contradicted by the finding that 16% of TUESR's were found to not be associated with a rise in esophageal pressure (11). Therefore, esophageal distension due to an increase in intraluminal pressure is not likely the primary stimulus for TUESR. The theory that TUESR is caused by a volitional or anticipatory response linked to the TLESR reflex (11) is contradicted by the finding that the TUESR is readily activated in decerebrate animals, which are devoid of volition or anticipation (5, 7, 8). Moreover, no positive data supporting the theory that the TUESR is an anticipatory or volitional response linked to the TLESR reflex have been provided. Therefore, neither previously proposed hypothesis accounts for the initiation of TUESR caused by air distension of the stomach.
The objective of this study, therefore, was to determine the mechanism of initiation of the TUESR associated with gastric air distension. We sought to determine the relationship between TLESR and TUESR, and whether the TUESR is initiated by reflexes activated by distension of the stomach or by the escape of air from the stomach stimulating the esophagus. If it is found that the TUESR is independent of the TLESR and dependent upon escape of air from the stomach, then an additional aim was to determine the nature of the stimulus that causes initiation of TUESR.
METHODS
Two sets of experiments were conducted to address the objectives of this study: 1) determination of whether TUESR is linked to gastric air escape or the TLESR reflex and 2) determination of the nature of the stimulus most related to activation of the TUESR.
Experimental Protocols
Determination of whether TUESR is linked to gastric air escape or the TLESR reflex.
We hypothesized that if we prevented gastric air escape or the effects of gastric air escape on the esophagus, but preserved all of the other aspects of the gastric distension reflex that causes TLESR, then the absence of TUESR would provide experimental evidence for the essential role of gastric air escape, but not the TLESR, in initiating the TUESR. To accomplish this goal we devised three experimental procedures, as described below, after first defining the control response of the reflex activation of TLESR and TUESR caused by gastric air distension in the decerebrate cat. In seven animals, two of the following three experimental studies were performed in the same animal, and in 15 animals only one of the following studies was performed in each animal.
PROTOCOL 1: CONTROL RESPONSE OF THE LOWER AND UPPER ESOPHAGEAL SPHINCTER RESPONSES TO GASTRIC AIR DISTENSION.
The stomach was inflated with free air (n = 22) until cricopharyngeus (CP) EMG inhibition, i.e., TUESR, occurred, and the volume of air needed to activate TUESR was recorded.
PROTOCOL 2: EFFECT OF DISTENDING STOMACH WITH A BAG OF AIR.
The nondistensible plastic bag was inserted in the stomach and was inflated up to two times the volume of air needed to activate TUESR (n = 8) in the control procedure of protocol 1, and the effects on the CP EMG and/or lower esophageal sphincter (LES) tone were recorded.
PROTOCOL 3: DISTENDING THE STOMACH WITH AIR BUT PREVENTING GAS ESCAPE.
The gastroesophageal junction (GEJ) was gently occluded, and air was injected in the stomach up to two times the volume of air needed to activate TUESR (n = 7) in the control procedure of protocol 1, and the effects on CP EMG and/or and LES tone were recorded.
PROTOCOL 4. EFFECT OF VENTING AIR FROM THE DISTAL ESOPHAGUS.
The effects of venting air from the distal esophagus on activation of TUESR by gastric air distension were tested (n = 12). Air was vented to prevent gastric air escape from having a mechanical effect on the esophagus. The sound of air movement through the esophageal vent was recorded to confirm gastric air escape.
Determination of the nature of the stimulus most related to activation of the TUESR.
We hypothesized that if initiation of the TUESR was temporally closely related to the rapid air pulse and not to the rise in esophageal pressure then activating the TUESR was not due to stimulation of tension receptors in the muscular wall of the esophagus.
PROTOCOL 5: TEMPORAL RELATIONSHIP BETWEEN INITIATION OF TUESR AND DIFFERENT ESOPHAGEAL MECHANICAL STIMULI.
TUESR was activated by rapid air injection on the esophageal mucosa while esophageal pressure was monitored (n = 8). The times between initiation of the TUESR and the air pulse or increase in esophageal pressure were calculated and compared.
Animal Preparation
All studies were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. Cats (n = 31) were fasted overnight and decerebrated. The animals were anesthetized using isoflurane (3%), the ventral neck region was exposed, the trachea was intubated, and the carotid arteries were ligated. The skull was exposed, and a hole over a parietal lobe was made using a trephine. The hole was enlarged using rongeurs, the central sinus was ligated and cut, and the brain was severed midcollicularly using a metal spatula. The forebrain was then suctioned out of the skull, and the blood vessels of the Circle of Willis were coagulated by suction through cotton balls soaked in warm saline. The boney sinuses were filled with bone wax, the exposed brain was covered with paraffin oil-soaked cotton balls, and the skin over the skull was sewn closed. The animals were then placed supine on a heating pad (Harvard Homeothermic monitor), and the body temperature was maintained between 38 and 40°C.
All animals received the following surgical preparation. An incision was made on the ventral surface of the neck, and the larynx and pharynx were exposed. The trachea was cannulated, and EMG electrodes were placed on the thyrohyoideus, CP, and cricothyroideus. The CP is the primary muscle (4) of the upper esophageal sphincter (UES), and its actions were considered responses of the UES; therefore, transient CP EMG inhibition was considered TUESR. The abdomen was opened along the ventral midline, and the gastric corpus was exposed. A fistula of the proximal stomach was formed using a 3-ml plastic syringe that exited the abdominal cavity. The femoral vein was cannulated for infusion of saline, and the femoral artery was cannulated to record blood pressure.
Some animals (n = 8) had a strain gauge sewn on the LES. In these animals the diaphragm at its ventral border was opened, and the animals were placed on a ventilator with tidal volume set at 15 ml/kg and 20 breaths/min. The diaphragm was sectioned along the midline down the ventral mediastinum through the central tendon toward the GEJ. The phrenic vein was ligated, the GEJ was exposed, the phrenic nerves were transected, and a thread was sewn on the GEJ to mark the location at which the GEJ intersected with the diaphragm. The LES was then fully exposed, and a strain gauge was sewn on the ventral side of the LES 1–2 cm from the GEJ. The strain gauge was sewn under tension in the direction to record from the circular muscle of the LES. Sewing the strain gauge under tension allowed the gauge to record both relaxation and contraction of the LES (5, 6, 8). Care was taken to avoid damage to the vagal innervation of the esophagus or stomach.
We chose to record LES tone using a strain gauge rather than manometry because manometry is much more prone to artifact than strain gauge recordings, that is, manometry will record pressure no matter where the source is while the strain gauge will only record from what it is sewn on. Placing the strain gauge required thoracotomy, which had positive and negative consequences. The negative consequence is that the placement of the strain gauge required additional surgery, open chest, and artificial ventilation that might have altered responses. To control for this, we conducted similar studies in animals without an LES strain gauge and thus without thoracotomy and artificial ventilation. The positive aspect of thoracotomy was that it removed possible confounding factors like the effects of the crural diaphragm or other nonesophageal or nongastric factors on the responses.
In some (n = 9) animals, a 1-ft-length of Teflon-insulated flexible wire (1 mm diameter) was wrapped around the GEJ, and both ends of the wire were fed through a 10-cm-long segment of rigid plastic tubing that was led out of the abdomen. The abdomen was then closed with sutures. The wire ligature remained loosely in place until the end of the experiment so that its exact position relative to the GEJ could be determined. When occlusion of the GEJ was required, the ends of the wire were pulled through the tubing and held in place by a small alligator clip. This occlusion was firm enough to close the GEJ but gentle enough to not damage the GEJ and its innervation.
In some (n = 12) animals, a 10-in. piece of thin-walled plastic tubing 4 mm in diameter was inserted through the mouth in the esophagus until the tip was 2–3 cm from the GEJ. This tubing allowed gas entering the distal esophagus to be expelled from the esophagus rather than to stimulate the esophagus. In four of these animals, the end of the tubing outside of the animals was fitted with a Y-shaped connector. A mini microphone with built in low-noise high-gain IC preamp (VideoSecu MICO1D) was sealed within in one branch of the Y, which allowed recording of the sound of air movement at the end of the tubing in the esophagus. This allowed for the recording of gas escape from the stomach. During the experiment, the effects of venting were tested by examining the difference in responses between clamping (not vented) and not clamping (vented) the end of the tube that was outside of the animal. We only conducted this study in animals that had not been subjected to a thoracotomy because we wanted this study to have as little surgical manipulation of the esophagus as possible.
Stimulation Techniques
Gastric air distension.
The stomach was distended with air in two ways: free air (n = 18) and bagged air (n = 7). Free air was injected in the stomach through a catheter attached to the gastric fistula. For bagged air distension, a nondistensible plastic bag holding 300–350 ml of air was inserted in the proximal stomach through the gastric fistula. In both cases, air was injected slowly by hand using a 60- or 100-ml syringe. The perfusion pressures of these air injections were recorded manometrically, which were used as stimulus markers. The primary stimulus for TLESR is volume of air in the stomach and not gastric pressure (1, 14); therefore, the volume of air injected was recorded and not gastric pressure.
Esophageal rapid air puff.
A narrow (1-mm-diameter) catheter with a side hole (created by a 25-gauge needle) 1 cm from its tip was inserted in the esophagus (n = 8) through the UES such that the tip was 10–12 cm from the CP. The other end of the catheter was connected to a Picospritzer that was set to deliver a mild air pressure pulse of 8–12 psi for 0.5 s. This pulse was delivered tangential to the surface of the esophagus and was a very mild and soft air puff. The side pressure of the injection catheter was recorded and was used as a stimulus marker.
Recording Techniques
Manometry.
The intraluminal pressure of the esophagus was recorded using a solid-state manometric catheter (Gaeltec Medical Instruments). The recording site was 7 cm from the CP. Gastric air perfusion pressure, esophageal rapid air injection pressure, and blood pressure were recorded using Statham pressure transducers. The output signals from these devices were attached to low-level DC preamplifiers (Grass P122) set at 3 Hz high-frequency cutoff filtration. The manometric signals were stored on computer.
Electromyography.
Bipolar Teflon-coated stainless steel wires (AS 636; Cooner Wire, Chatsworth, CA) bared for 2–3 mm were placed in each muscle, and the wires were fed in differential amplifiers (A-M Systems 1800). The electrical activity was filtered (bandpass of 0.1–3.0 KHz) and amplified 1,000–10,000 times before feeding into the computer.
Sound of gastric air escape.
A small microphone was inserted in one arm of the Y tube at the end of the gas escape vent to record the sound of air movement to verify gas escape from the stomach in response to gastric injection of air in those studies where there was no UES or LES response to verify gastric gas escape.
Data Reduction and Analysis
The mean and SE of the volume of air required to activate TUESR were calculated before and after conducting the procedures to determine the role of gastric air escape in the initiation of TUESR. The mean and SE of the time delays between the initiation of TUESR and the rapid air pulse and esophageal pressure rise were calculated and compared. Differences between means of two groups were tested using Student's t-test. A paired t-test was used when comparing different responses from the same animal, otherwise the nonpaired test was used. The coefficient of variation, SE divided by mean, was calculated to determine the variability of mean values. A P value of 0.05 or less was considered statistically significant.
RESULTS
Effect of Gastric Air Distension on LES and CP EMG
We found that injection of air (114 ± 13 ml) in the stomach of all animals tested (n = 18) caused a TUESR (Figs. 1–6) and a decrease (Figs. 2 and 4) in the tone of the LES (n = 6), i.e., TLESR. The volume of air needed to activate TUESR (Figs. 1–6) did not differ significantly (P > 0.05) whether a thoracotomy (103 ± 25 ml, n = 6) was performed or not (119 ± 15 ml, n = 12).
Fig. 1.
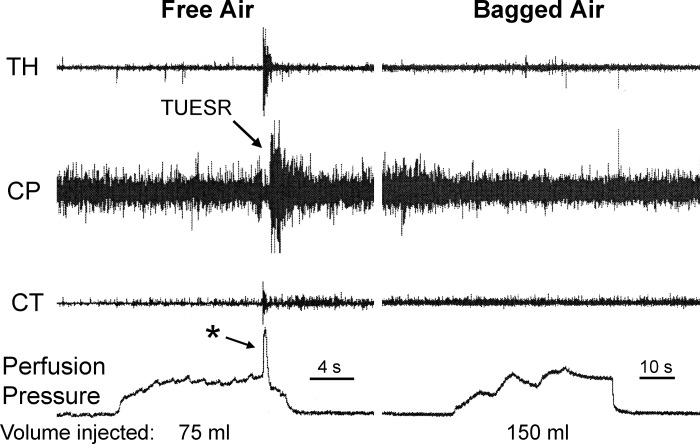
Comparison of the effects of free air and bagged air on the upper esophageal sphincter (UES) in unthoracotomized animal. We found that injection of air in the proximal stomach caused the transient upper esophageal sphincter relaxation (TUESR), but injection of two times that volume of bagged air had no effect on the UES. Therefore, the stimulus for activation of TUESR is gas escape from the stomach and not gastric distension. Note also the rapid increase in gastric perfusion pressure (*) associated with the TUESR. TH, thyrohyoideus; CP, cricopharyngeus; CT, cricothyroideus.
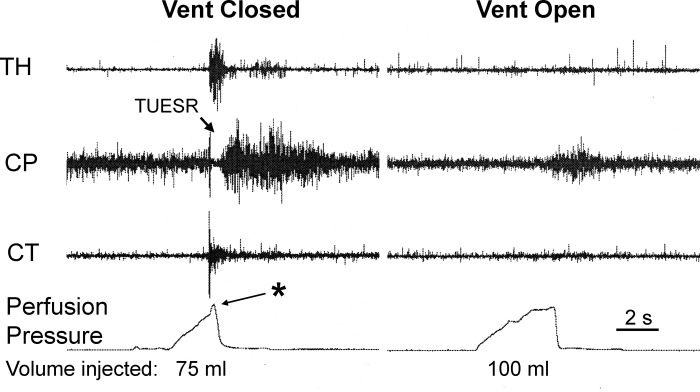
Fig. 6.
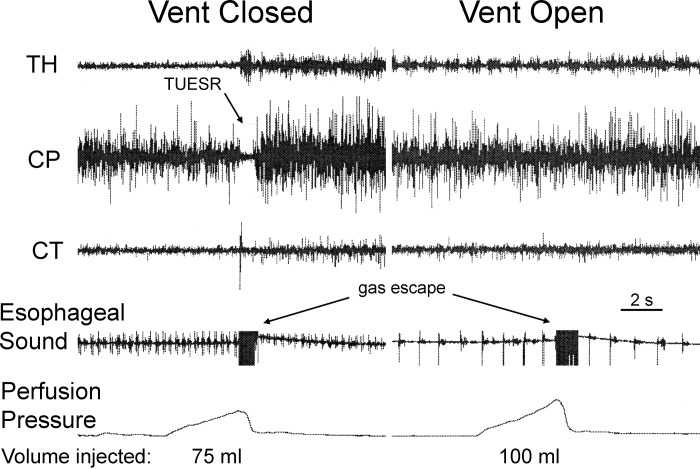
The effects of venting the distal esophagus during gastric air injection on TUESR and the sound of gastric air escape. We found that venting the distal esophagus prevented the activation of TUESR by gastric air injection but did not prevent the sound of air escaping the stomach. Therefore, allowing air escape from the esophagus prevented activation of the TUESR but not release of air from the stomach causing the TLESR. See Fig. 1 for definition of symbols.
Fig. 2.
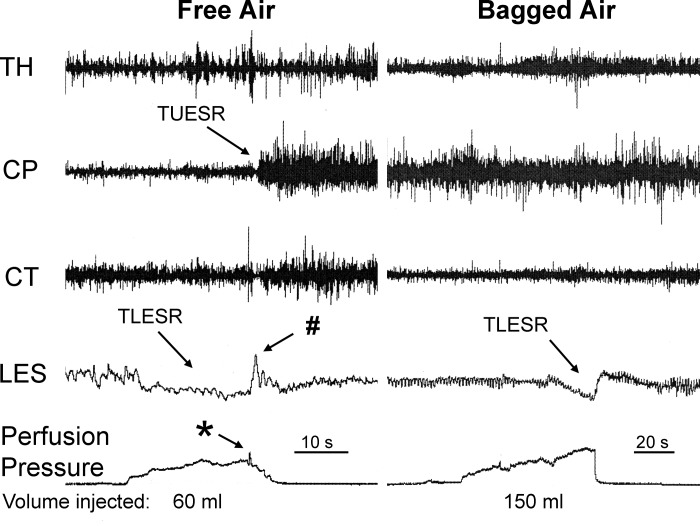
Comparison of the effects of free air and bagged air on the UES and lower esophageal sphincter (LES) in thoracotomized animal. We found that injection of air in the proximal stomach caused the TUESR and transient lower esophageal sphincter relaxation (TLESR), but injection of over two times that volume of bagged air activated TLESR but not TUESR. Therefore, the stimulus for activation of TUESR is gas escape from the stomach and not gastric distension, but the stimulus for activation of the TLESR is gastric distension. Note also the rapid increase in gastric perfusion pressure (*) and LES contraction during the TLESR (#) associated with TUESR. See Fig. 1 for definitions of other symbols.
Fig. 4.
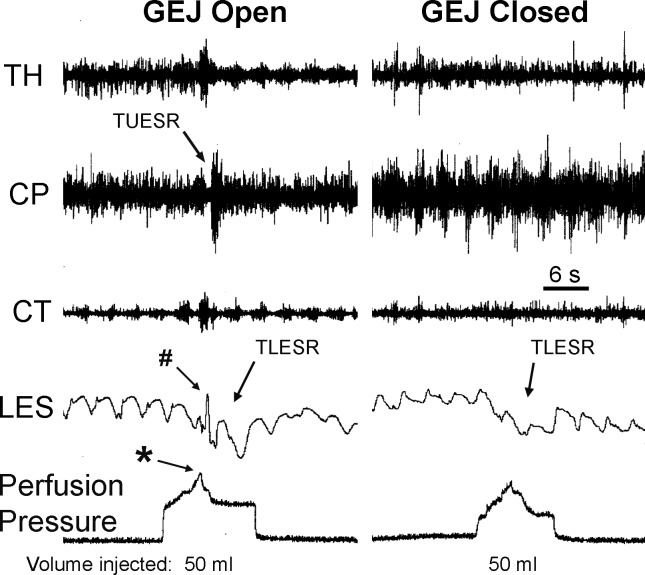
The effects of GEJ closure during gastric air injection on the UES and LES in thoractomized animals. We found that injection of 50 ml of air in the proximal stomach caused TUESR and TLESR when the GEJ was open, but injection of the same volume of air when the GEJ was closed caused only TLESR. Therefore, gastric gas escape activates the TUESR, and gastric distension activates the TLESR. Note the rapid increase in gastric perfusion pressure (*) and LES contraction during the TLESR (#) associated with TUESR. See Figs. 1 and 2 for definition of symbols.
Comparison of the Effects of Free Air and Bagged Air Inflation of the Stomach on TUESR
We compared the effects of free air and bagged air (n = 6) in the same animals with (n = 3) or without (n = 3) thoracotomy and found that, while both stimuli always (3 out of 3 animals) activated the TLESR, only free air caused (6 out of 6 animals) TUESR (Figs. 1 and 2). The volume of free air needed to activate TUESR in this study was 82 ± 9 ml (n = 6), whereas we failed to activate TUESR (P < 0.01) with as much as 175 ± 11 ml (n = 6) of bagged air.
Effect of Occlusion of GEJ on TUESR Caused by Gastric Air Distension
We found that restricting the movement of air out of the stomach prevented the TUESR (Figs. 3 and 4) in nine out of nine animals, but not the TLESR (Fig. 4) in four out of four animals. The volume of air needed to activate TUESR was 103 ± 13 ml (n = 9), but as much as 189 ± 27 ml (n = 9) of air in GEJ-occluded animals did not (P < 0.01) activate TUESR. In this group of animals, the volume of air needed to activate TUESR with free air in thoractomized (100 ± 20 ml, n = 4) and unthoracotomized animals (105 ± 20, n = 5) was not significantly different (P > 0.05).
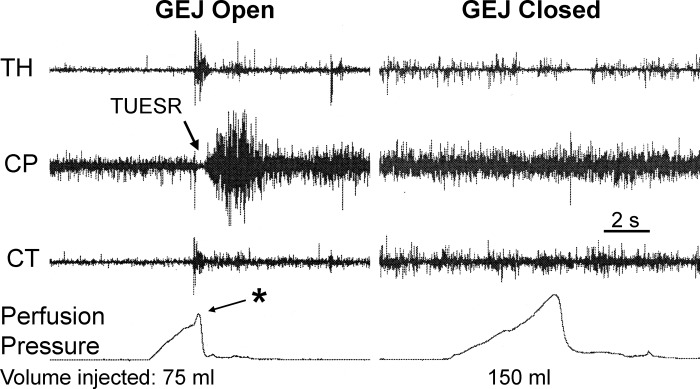
Fig. 3.
Effect of gastroesophageal junction (GEJ) closure on gastric air injection-induced TUESR. We found that injection of 75 ml of air in the proximal stomach caused TUESR when the GEJ was open, but injection of 150 ml of air when the GEJ was closed did not activate the TUESR. Therefore, gastric gas escape and not gastric distension activated TUESR. Note the rapid increase in gastric pressure associated with the TUESR (*). See Fig. 1 for definition of symbols.
Effect of Venting the Esophagus of Air
We found that venting air from the distal esophagus (n = 12) prevented TUESR (Fig. 6) caused by gastric air distension but did not prevent the sound of air movement (Fig. 6) that occurred just before the TUESR (n = 12). The volume of air needed to activate TUESR with the vent in place and clamped was 125 ± 14 ml (n = 12), whereas the volume of air needed to cause the sound of air movement out of the stomach with the vent open was 135 ± 16 ml (n = 12), which was not significantly different (P > 0.05). The sound of air movement was recorded in four of these animals, and the volume of air needed to cause the sound of air movement out of the stomach with the vent open was 112 ± 12 ml, which was not significantly different (P > 0.05) from the volume of air needed with the vent closed. The sound of air movement began 0.22 ± 0.01 s before TUESR began with a coefficient of variation of 8%.
Relationship of TUESR to Air Pulse and Increase in Esophageal Pressure
We found that the TUESR always occurred after the esophageal air pulse (Fig. 7) at a delay of 0.27 ± 0.2 s (n = 9). On the other hand, the esophageal pressure rise occurred (Fig. 7) at or before the TUESR (3 out of 9 animals), after the TUESR (4 out of 9 animals), or did not occur (2 out of 9 animals). In addition, the coefficient of variation of the time delay between air pulse and TUESR was significantly (P < 0.05) less than that between the rise in esophageal pressure and TUESR (Fig. 8). The delay and coefficient of variation of the time between air pulse and TUESR were not significantly different (P > 0.05) from that between the sound of air movement and TUESR.
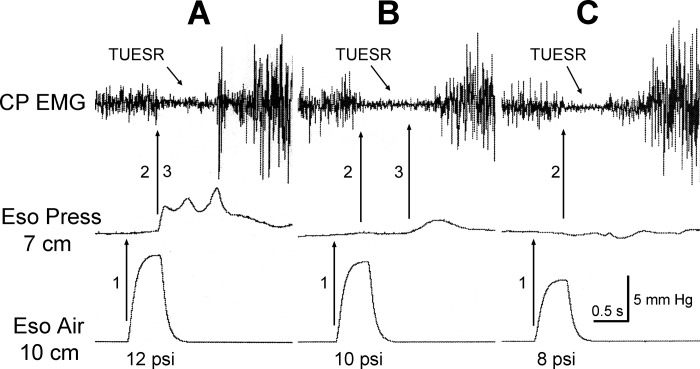
Fig. 7.
Temporal relationships among the esophageal air pulse, esophageal pressure increase, and TUESR. Note that esophageal air pulse always (9 out of 9) occurred (A, B, and C) before the TUESR. On the other hand, the rise in esophageal pressure occurred at or before (3 out of 9) the TUESR (A), after (4 out of 9) the TUESR (B), or did not (2 out of 9) occur (C). In addition, the time delays between the esophageal air pulse and TUESR were very consistent (A, B, and C), whereas the time delays between the rise in esophageal pressure and TUESR were highly variable (A and B). Therefore, the TUESR is temporally closely related to the esophageal air pulse but not the increase in esophageal pressure. The esophageal air pulse was applied 10 cm from the CP, and its timing was marked by recording the infusion pressure. This pressure was not a measure of the magnitude of the stimulus or response. Esophageal pressure was recorded at 7 cm from the CP using manometry. The pressure scale is for the esophageal pressure recording and not the air pulse recording.
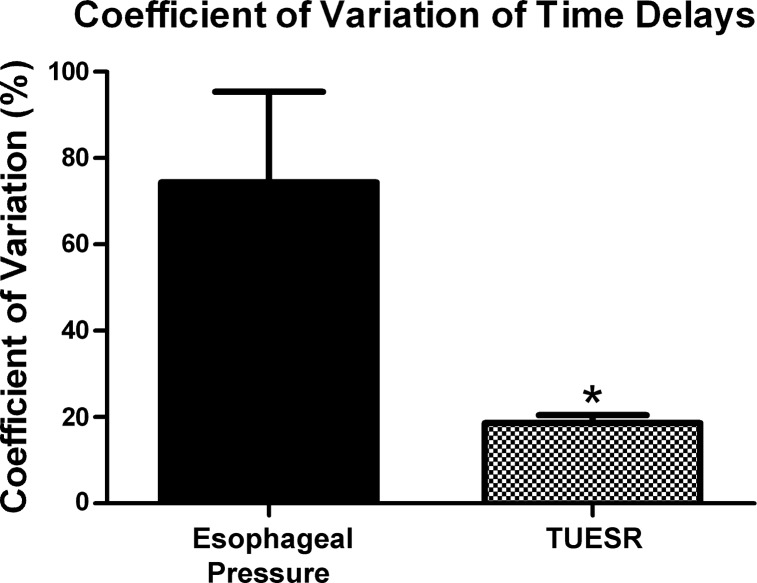
Fig. 8.
Coefficient of variation of time delays between the TUESR and esophageal pressure rise or esophageal air pulse. The time delay between esophageal air pulse and TUESR was significantly (*P < 0.05) less than that of the time delay between the rise of esophageal pressure and TUESR. Therefore, the esophageal air pulse is more closely related to the TUESR than the rise in esophageal pressure.
Other Responses Associated with TUESR
We found two other responses associated with TUESR. In 8 out of 18 animals we found a rapid short-lasting increase in gastric perfusion pressure (Figs. 1–5) that occurred 0.26 ± 0.06 s before the TUESR. This effect was never observed if the TUESR did not occur. In addition, in four out of eight animals we found a contractile response of the LES that occurred during the TLESR (Figs. 2 and 4) that occurred 0.35 ± 0.06 s after the beginning of TUESR. This response was never observed if the TUESR did not occur.
Fig. 5.
The effect of venting the distal esophagus during gastric air injection on TUESR. We found that venting the distal esophagus prevented the activation of TUESR by gastric air injection. However, venting did not prevent the sound of gas escape for the stomach that occurred at the initiation of the TUESR, but this sound was not recorded in this study. Therefore, allowing air escape from the esophagus prevented activation of the TUESR. Note the rapid increase in gastric perfusion pressure (*) associated with TUESR. See Fig. 1 for definition of symbols.
DISCUSSION
We found in three different types of studies that the TUESR associated with gastric air inflation required air stimulation of the esophagus. The inflation of the stomach with air freely able to leave the stomach activated TUESR, whereas air confined to the stomach did not, yet both caused TLESR. Blocking the exit of air from the stomach also prevented TUESR, but not TLESR. Finally, not allowing air that escapes the stomach from stimulating receptors in the esophagus, or venting the air outside of the animal, prevented TUESR. Therefore, the TUESR associated with gastric air distension is due to stimulation of receptors in the esophagus by air escaping the stomach, and it is not related to gastric distension itself or the reflexes caused by gastric distension, e.g., TLESR.
The concept that the TUESR associated with TLESR is mediated by a conscious process, i.e., a voluntary or an anticipatory response (11), is not supported by any positive data in this study or in the literature. To the contrary, our studies were performed in decerebrate animals, which readily exhibited TUESR caused by gastric air distension, yet decerebrate animals are incapable of volition or anticipation.
The theory that the TUESR is caused by an anticipatory or volitional response linked to the TLESR reflex was based in large part on the failure of investigators (11) to find a strong correlation between the rise in esophageal pressure and the TUESR after gas escape from the stomach. We attempted to model this effect by applying a very mild short-duration air puff locally directed to the esophageal mucosa and found that such a stimulus activated the TUESR and a rise in intraluminal pressure. However, as found in the human studies (11), the rise in esophageal pressure was not correlated with initiation of the TUESR. The timing of the pressure rise was highly variable, and sometimes no pressure rise was recorded at all, yet the TUESR always occurred. Therefore, we conclude, as did the authors of the human study (11), that the rise in esophageal intraluminal pressure is not the primary stimulus for TUESR.
Unlike in the human study (11), however, we were able to control the stimulus, and we found that, while the rise in esophageal pressure was not correlated with the initiation of the TUESR, the application of the pressure pulse itself was highly related to the initiation of the TUESR. Therefore, not only is the TUESR fully dependent on the gas escape from the stomach, but the timing of the application of the air pulse is highly correlated with the timing of the initiation of the TUESR. The conclusion that air stimulation of the esophagus activated TUESR was further supported by our observation that the time of occurrence of the sound of air abruptly leaving the stomach during gastric air injection was closely and very consistently related to the time of occurrence of the TUESR.
It is possible in our study, as well as in the human study (11), that a rise in esophageal pressure or an esophageal contraction occurred somewhere in the esophagus that was not recorded, but this does not change the conclusion. It was the local air pulse that was always highly correlated and closely timed, i.e., 0.27-s interval, with the TUESR, and any nonlocal esophageal distension or contraction would have had to occur at a delayed time with a much lower correlation with the TUESR. Therefore, if these responses occurred, they would have occurred too late to cause the TUESR. Moreover, we clearly demonstrated previously (7), as described below, that esophageal distension or contraction leads only to UES contraction and not relaxation. Therefore, even if these responses occurred, they could not have accounted for activation of the TUESR.
Gas escape in the esophagus causes the TUESR, but human (11) and animal data found that it is not a rise in esophageal pressure due to gastric gas escape that is associated with initiation of the TUESR. This implies that an air stimulus applied to the esophagus may be capable of activating reflexes without causing esophageal distension. The esophagus has two sets of mechanoreceptors: muscular (9, 10) and mucosal (3, 15). The muscle mechanoreceptor is a slowly adapting tension receptor that is activated by tension in the esophageal wall caused by distension, e.g., increase in intraluminal pressure, or contraction (9, 10). On the other hand, the mucosa contains rapidly adapting tension receptors that can be activated by very light touch of the mucosa as would occur by a localized puff of air (3, 15). In our prior studies (7) we found that removal of the esophageal mucosa or bathing the esophageal mucosa in lidocaine eliminated the inhibition of CP EMG caused by rapid injection of air in the esophagus but did not block secondary peristalsis or the esophago-UES contractile reflex (7). In addition, we found (7) that esophageal distension or contraction causes CP EMG excitation and not inhibition. Therefore, an air pulse applied to the esophagus, as occurs after injection of air in the stomach, can activate reflexes, e.g., TUESR, through mucosal receptors without causing esophageal distension or contraction, and the only esophageal receptors found to be associated with UES relaxation are those in the mucosa.
Prior studies have found a strong association between TLESR and belching (12, 13, 16). The TUESR associated with TLESR has been described as a microburp, that is, a brief period of UES inhibition associated with gas venting. This description is consistent with the recent characterization of the belch response in chronically prepared dogs (8). The belch has been defined as a three-phase process: gas escape, barrier elimination, and gas transport. These phases are independent physiological processes that are activated in sequence during the full belch but can occur independently. The gas escape phase includes not only TLESR but also longitudinal contraction of the esophagus as well as inhibition of the hiatal fibers of the diaphragm. Human studies have also found that the TLESR is associated with these responses (12). If gas escapes during the TLESR, the gas can activate esophageal receptors to activate the barrier elimination phase of eructation that is characterized by the TUESR as well as contraction of the thyrohyoideus, which helps open the UES. The magnitude of the barrier elimination phase depends on the magnitude of gastric gas escape (2); therefore, small escape of gas can cause a microburp. The gas transport phase includes reverse peristalsis of the esophagus and the movement of gas out of the esophagus. Therefore, the TLESR is strongly related to the TUESR in that TLESR is the initial phase of the belch response, but the primary response of the belch is the TUESR.
In this study we observed contraction of the LES during the TUESR, and a similar response was observed previously in chronic studies of belching in the canine (8). We found previously that this LES response was associated with reverse peristalsis of the esophagus caused by gas escape from the stomach. In the current study we also observed a rapid increase in gastric perfusion pressure that was associated with the TUESR. This response only occurred at or immediately before the TUESR and not during the TUESR. In our prior canine study of eructation (8) we had observed a rapid contraction of the diaphragm associated with TUESR, but this response occurred during and not before the TUESR. In addition, in the current study, the increase in gastric perfusion pressure occurred even in animals in which the phrenic nerves had been transected; therefore, this response was not likely to be due to contraction of the diaphragm. We hypothesize that this increase in gastric perfusion pressure associated with the TUESR may have been due to a rapid and brief contraction of the proximal stomach at the very beginning of the eructation process. More studies are needed to confirm this hypothesis.
Considering that the TUESR and thus the microburp are not volitional or anticipatory responses linked to the TLESR, efforts to control the TUESR should not be directed at control of the TLESR, volition, or anticipation.
In conclusion, the TUESR activated by gastric air distension is due to activation of TLESR, which lowers the barrier between stomach and esophagus, allowing gas to escape. The escaping gas stimulates receptors in the esophagus, which activates the belch response, resulting in TUESR. Activation of the TUESR is not correlated with changes in esophageal pressure that effect wall tension; therefore, the TUESR is probably initiated by activation of the mucosal tension receptors previously shown to mediate CP EMG inhibition caused by air pulse stimulation of the esophagus. The greater the gas stimulation of the esophagus the greater the TUESR; therefore, small gas escape associated with TLESR can cause a microburp.
GRANTS
This study was supported by in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-25731.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: I.M.L. and R.S. conception and design of research; I.M.L. and B.K.M. performed experiments; I.M.L. analyzed data; I.M.L. interpreted results of experiments; I.M.L. prepared figures; I.M.L. drafted manuscript; I.M.L., B.K.M., and R.S. edited and revised manuscript; I.M.L., B.K.M., and R.S. approved final version of manuscript.
REFERENCES
- 1.Allocca M, Peganini R. Effect of phasic contractions and tone of the proximal stomach on triggering of transient lower esophageal sphincter relaxation. Dig Dis Sci 49: 710–714, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Babaei A, Bhargaca V, Mittal RK. Upper esophageal sphincter during transient lower esophageal sphincter relaxation: Effects of reflux and posture. Am J Physiol Gastrointest Liver Physiol 298: G601–G607, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clerc N, Mei N. Vagal mechanoreceptors located in the lower esophageal sphincter. J Physiol Lond 336: 487–498, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang IM. Upper esophageal sphincter. In: Goyal and Shaker's GI Online, edited by Goyal RK, Shaker R. New York, NY: Nature Publishing Group, 2006 [Google Scholar]
- 5.Lang IM, Medda BK, Jadcherla S, Shaker R. The role of the superior laryngeal nerve in esophageal reflexes. Am J Physiol Gastrointest Liver Physiol 302: G1445–G1457, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang IM, Sarna SK, Dodds WJ. Pharyngeal, esophageal and proximal gastric responses associated with vomiting. Am J Physiol Gastrointest Liver Physiol 265: G963–G972, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distention. Am J Physiol Gastrointest Liver Physiol 281: G1246–G1263, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Lang IM, Medda BK, Shaker R. Digestive and respiratory tract motor responses associated with eructation. Am J Physiol Gastrointest Liver Physiol 304: G1044–G1053, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mei N. Mechanorecepteurs vagaux digestifs chez le chat. Exp Brain Res 11: 502–514, 1970 [PubMed] [Google Scholar]
- 10.Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol Lond 512: 907–916, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandolfino JE, Ghosh SK, Zhang Q, Han A, Kahrilas PJ. Upper sphincter function during transient lower oesophageal sphincter relaxation (tLOSR); it is mainly about microburps. Neurogastroenterol Motil 19: 203–210, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Pandolfino JE, Zhang Q, Ghosh SK, Han A, Boniquit C, Kahrilas PJ. Transient lower esophageal sphincter relaxations and reflux: Mechanistic analysis using concurrent fluoroscopy and high resolution manometry. Gastroenterology 1312: 1725–1733, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Patrikios J, Martin CJ, Dent J. Relationship of transient lower esophageal sphincter relaxation to postprandial gastroesophageal reflux and belching in dogs. Gastroenterology 90: 545–551, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Penagini R, Carmagnola S, Cantu P, Alloca M, Bianchi PA. Mechnoreceptors of the proximal stomach: role in triggering transient lower esophageal sphincter relaxation. Gastroenterology 126: 49–56, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Satchell PM. Canine oesophageal mechanoreceptors. J Physiol Lond 346: 287–300, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyman JB, Dent J, Heddle R, Dodds WJ, Toouli J, Downton J. Control of belching by the lower oesophageal sphincter. Gut 31: 639–646, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]