Abstract
Iron is an essential trace mineral that plays a number of important physiological roles in humans, including oxygen transport, energy metabolism, and neurotransmitter synthesis. Iron absorption by the proximal small bowel is a critical checkpoint in the maintenance of whole-body iron levels since, unlike most other essential nutrients, no regulated excretory systems exist for iron in humans. Maintaining proper iron levels is critical to avoid the adverse physiological consequences of either low or high tissue iron concentrations, as commonly occurs in iron-deficiency anemia and hereditary hemochromatosis, respectively. Exquisite regulatory mechanisms have thus evolved to modulate how much iron is acquired from the diet. Systemic sensing of iron levels is accomplished by a network of molecules that regulate transcription of the HAMP gene in hepatocytes, thus modulating levels of the serum-borne, iron-regulatory hormone hepcidin. Hepcidin decreases intestinal iron absorption by binding to the iron exporter ferroportin 1 on the basolateral surface of duodenal enterocytes, causing its internalization and degradation. Mucosal regulation of iron transport also occurs during low-iron states, via transcriptional (by hypoxia-inducible factor 2α) and posttranscriptional (by the iron-sensing iron-regulatory protein/iron-responsive element system) mechanisms. Recent studies demonstrated that these regulatory loops function in tandem to control expression or activity of key modulators of iron homeostasis. In health, body iron levels are maintained at appropriate levels; however, in several inherited disorders and in other pathophysiological states, iron sensing is perturbed and intestinal iron absorption is dysregulated. The iron-related phenotypes of these diseases exemplify the necessity of precisely regulating iron absorption to meet body demands.
Keywords: duodenum, divalent metal-ion transporter 1, ferroportin 1, hephaestin, hepcidin
iron is abundant in the earth's crust, and most forms of life, including mammals, have evolved to utilize iron in many biological processes. For example, iron is required to support oxygen delivery to tissues, for the control of cellular growth and differentiation, and for energy metabolism. The role of iron in these physiological processes revolves around the ability of the metal to exist in two stable oxidation states [ferric (Fe3+) and ferrous (Fe2+)]. This chemical property of iron underlies its ability to participate in reduction-oxidation (electron transfer) reactions and also leads to its potential toxicity if not properly managed by cells and tissues. Free iron can participate in Fenton chemistry, whereby oxygen free-radicals are produced, and these in turn can damage numerous biological molecules (e.g., membrane lipids, proteins, DNA). Mammals have thus developed complex regulatory mechanisms that manage iron absorption, transport, and recycling.
Unlike most essential nutrients, no active excretory mechanisms exist for iron in humans, although small amounts are lost via exfoliation of skin and gastrointestinal cells, and in bile and urine. Body iron levels are thus principally controlled by modulation of iron absorption in the duodenum and proximal jejunum, which allows absorption to be precisely matched to unregulated losses. The mechanisms that regulate iron absorption also allow for appropriate increases or decreases according to physiological demand. Large variations in iron status and stores among individuals could, however, be misconstrued as indicative of imprecise regulation of intestinal iron absorption. This might be true if one assumed that iron status/stores were directly related to the capacity of the small intestine to absorb iron. This is not the case though since the amount of bioavailable iron is often limiting, which can partially explain variations in iron stores. Moreover, various pathologies can influence the relationship between the rate of iron absorption and body iron levels. Absorption of dietary iron by the proximal intestine is thus accurately regulated by cellular and systemic factors to ensure that overall body iron levels are maintained at adequate levels.
Iron balance is controlled in part by the liver-derived, serum-borne, peptide hormone hepcidin (HEPC), which functions to block intestinal iron absorption and inhibit iron release from stores. Molecules that regulate HEPC expression, such as transferrin receptor 2 (TFR2), HFE, hemojuvelin (HJV), and matriptase-2, “sense” body iron levels allowing appropriate modulation of iron absorption. HEPC exerts its influence on iron homeostasis by binding to the iron export protein ferroportin 1 (FPN1), causing its internalization and degradation (80). FPN1 is expressed in cells that absorb (enterocytes) and store [hepatocytes and reticuloendothelial (RE) macrophages] iron. HEPC expression is induced by high body iron stores, and by infection and inflammation. During iron deficiency and tissue hypoxia, when HEPC production is very low, additional regulatory mechanisms are invoked to upregulate intestinal iron absorption.
Perturbations in iron absorption can have significant physiological consequences. Many iron-related disorders in humans occur when dietary iron absorption is inappropriately regulated, resulting in excessive iron accumulation in various tissues and subsequent oxidative damage or, in some cases, serum iron depletion. The most common of these pathologies is a group of genetic, iron-overload disorders, collectively referred to as hereditary hemochromatosis (HH). In most forms of HH, HEPC expression is inappropriately low, leading to increased iron absorption. Diseases associated with reduced iron absorption are much less common, attesting to the essentiality of the metal. Anemias that do not respond to oral iron supplementation have also been described, with one form, iron-refractory iron-deficiency anemia (IRIDA), being recently linked to alterations in HEPC expression (27).
FORMS OF IRON IN THE DIET AND BIOAVAILABILITY
Iron in foods exists principally as heme and nonheme (or inorganic) iron. Heme iron is derived predominantly from hemoglobin and myoglobin in meats. Heme iron absorption is efficient and largely uninfluenced by other dietary constituents. A candidate intestinal heme transporter, heme carrier protein 1 (HCP1), was proposed (109), but recent evidence suggests that it is most likely a folate transporter (and not a physiologically relevant heme transporter) (94). This issue is still, however, not fully resolved (64). Conversely, nonheme (and largely ferric) iron, found in both meat and plant foods, is highly insoluble, and its bioavailability is influenced by many dietary components. Gastric acid and ascorbic acid promote reduction and solubilization of dietary ferric iron and thus improve absorption. Furthermore, dietary factors commonly found in plants, such as phytate, oxalate, polyphenols, and tannins, decrease the absorption of nonheme iron. The chronic use of proton pump inhibitors for gastric acid reflux, Helicobacter pylori infection, and inflammatory conditions (e.g., celiac disease) also decrease nonheme iron absorption (16).
NONHEME IRON TRANSPORT BY DUODENAL ENTEROCYTES
Although multiple dietary sources of iron exist, the transport of nonheme iron has been most intensively studied and will be the focus of this review. Many of the proteins that mediate nonheme iron absorption have been identified (Table 1 and Fig. 1).
Table 1.
Proteins involved in the absorption of nonheme iron
| Name | Protein | Function |
|---|---|---|
| Duodenal cytochrome b | DCYTB | Ferric iron reduction for absorption via DMT1 |
| Solute carrier family 11 (proton-coupled divalent metal-ion transporter), member 2 | DMT1 | BBM ferrous iron/proton cotransporter; also transports a range of other divalent metal ions, including Mn2+ and Cd2+; may transport Cu during iron deprivation |
| Solute carrier family 40 (iron-regulated transporter), member 1 | FPN1 | BLM ferrous iron exporter; HEPC target |
| Ferritin, light polypeptide/Ferritin, heavy polypeptide 1 | FTL/FTH1 | Intracellular iron storage |
| Hephaestin | HEPH | BLM ferroxidase; a soluble, cytosolic form may also exist |
| HEPC antimicrobial peptide | HEPC | Liver-derived, circulating peptide hormone; binds to FPN1 and mediates its internalization and degradation |
FPN1, ferroportin 1; BLM, basolateral membrane; HEPC, hepcidin; FOX, ferroxidase.
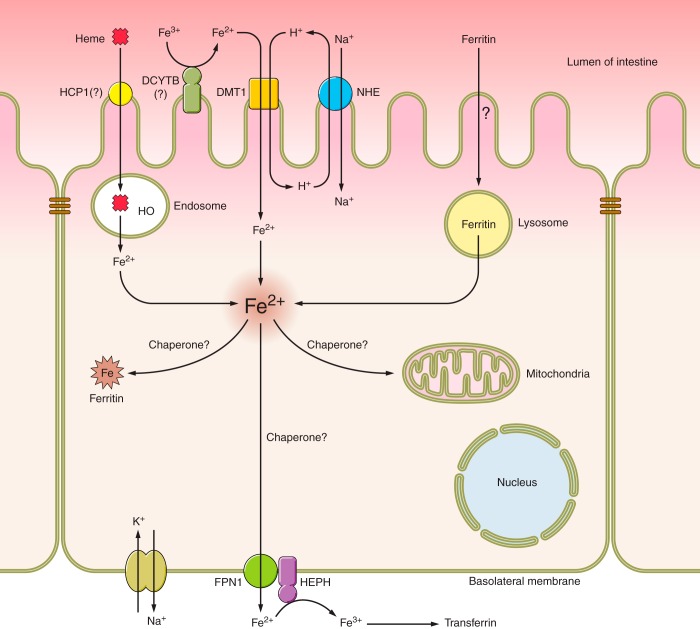
Fig. 1.
Mechanisms of iron absorption in the mammalian duodenum. A single enterocyte is depicted with the transport machinery responsible for assimilation of dietary iron. Iron may be derived from heme or ferritin or it may occur as free nonheme iron. Heme iron transport is probably mediated by endocytosis of heme followed by iron liberation from heme within endosomes by heme oxygenase (HO). Whether HCP1 and/or other proteins are involved in this process remains to be determined. Nonheme ferric iron must be reduced, possibly by duodenal cytochrome b (DCYTB) or other cell surface ferrireductases, and subsequently transported into cells via divalent metal-ion transporter 1 (DMT1). The proton gradient that fuels DMT1 activity is maintained by the combined actions of an apical sodium/hydrogen exchanger (NHE) and the basolateral Na+-K+-ATPase. Iron from ferritin is absorbed into enterocytes via an unknown mechanism and is likely then freed within lysosomes. Iron derived from all three dietary sources likely forms a single intracellular iron pool. Whether iron chaperones exist in enterocytes is unknown and thus how iron trafficks within cells after absorbance is not clear. Iron destined for export traverses the basolateral membrane (BLM) via ferroportin 1 (FPN1). The exit of ferrous iron is functionally coupled with iron oxidation via hephaestin (HEPH) and possibly other ferroxidases (not shown). Ultimately, ferric iron then binds to transferrin in the interstitial fluids or in the vasculature and is distributed throughout the body.
Ferric Iron Reduction
Dietary nonheme iron exists predominantly in the oxidized or ferric (Fe3+) form, yet ferrous iron (Fe2+) is likely the form that is transported into enterocytes. Ferric iron, therefore, must be reduced before transport. Recent investigations have identified a brush-border membrane (BBM) ferrireductase, duodenal cytochrome b (DCYTB) (72), that may mediate this process. Interestingly, DCYTB facilitates the reduction of ferric iron via electron transfer from intracellular ascorbate (66, 118), providing one potential mechanism by which vitamin C enhances iron absorption. DCYTB is strongly upregulated in duodenal enterocytes during iron deficiency (17, 62) and acute hypoxia (63). Moreover, DCYTB can account for the inducible ferrireductase activity in the mouse intestine during iron deficiency, pregnancy, and hypoxia (14). These data are suggestive of an important physiological role for DCYTB in intestinal iron transport. However, recent investigations in knockout (KO) mice showed that DCYTB is not required for efficient iron absorption (50). It is thus likely that, under basal conditions, other reductases (e.g., a STEAP protein) (84) are important, or that dietary components or gastrointestinal secretions provide the reducing power necessary to promote iron absorption. Enzymatic reduction may not, therefore, be rate limiting. It is also important to consider that these observations were made in laboratory rodents. It is a logical prediction that the physiology of nonheme iron transport could be different among mammalian species, especially since rats and mice can synthesize ascorbic acid and humans cannot.
Iron Uptake Across the BBM
Subsequent to reduction of dietary ferric iron, ferrous iron (Fe2+) is transported across the BBM of enterocytes via divalent metal-ion transporter 1 (DMT1; encoded by the SLC11A2 gene) (67). DMT1 is a multipass, transmembrane protein that mediates proton-coupled, ferrous iron uptake. Protons, which provide the driving force for iron transport, are likely provided by the action of a BBM sodium/hydrogen exchanger (probably NHE3) that acidifies the unstirred water layer. DMT1 is so named because it can transport other divalent cations (49), including manganese and cobalt (108). Some studies suggest DMT1 also transports copper (4, 116), but others refute this contention (68). Irrespective of its substrate profile in different model systems, physiological data implicate DMT1 as being an essential intestinal iron importer, as exemplified by the severe iron-deficiency anemia that results from deletion (48) or mutation (34, 35) of the gene in rodents. Humans with SLC11A2 mutations also suffer from severe systemic iron deficiency (6, 75), exemplifying the essentiality of DMT1 for efficient acquisition of dietary nonheme iron.
Iron Trafficking and Storage Within Enterocytes
After ferrous iron is transported across the BBM into enterocytes, it is likely chelated by small-molecular-weight organic acids (e.g., citrate), amino acids, or intracellular proteins. The poly-r(C)-binding proteins are iron-trafficking proteins (chaperones) that have been identified recently (65, 110), but whether they are expressed in mammalian enterocytes is unknown. How iron trafficks to different intracellular compartments (e.g., mitochondria) within enterocytes is thus unclear. Irrespective of specific trafficking mechanisms, intracellular iron is rapidly transferred across the basolateral membrane (BLM) by FPN1 when body iron demands are high. When demand is low, iron can be stored in ferritin, an intracellular iron storage protein complex consisting of heavy (H) and light (L) chain subunits that form a hollow sphere accepting up to 4,500 iron atoms. Most iron stored in ferritin is likely lost via subsequent exfoliation of intestinal epithelial cells. Interestingly, a recent study described a protective role for ferritin H in regulation of intestinal iron absorption during conditions of iron overload (117). These authors showed that intestine-specific ferritin H deletion led to a twofold increase in iron absorption in iron-loaded mice, suggesting that ferritin H works in conjunction with systemic signals (e.g., HEPC) to limit iron flux.
Iron Efflux Across the BLM
FPN1 (encoded by the SLC40A1 gene) is the only ferrous iron export protein identified to date in mammals (73). FPN1 is highly expressed in enterocytes, RE macrophages and hepatocytes, consistent with its established roles in iron absorption and recycling. The essentiality of FPN1 is exemplified by knockout of the gene in mice, which causes severe iron-deficiency anemia (25). SLC40A1 gene mutations have been described in humans, and, although rare, they collectively represent an important subset of iron-loading disorders (8, 24, 71). Affected individuals have varying phenotypes depending upon how the mutations alter FPN1 protein function. Importantly, these observations clearly exemplify the critical, nonredundant role of FPN1 in intestinal iron absorption.
Iron Oxidation and Transferrin Binding
Ferrous iron exits enterocytes via FPN1-mediated transport, but ferric iron is required for binding to transferrin in the interstitial fluids. Transferrin-bound iron is then distributed via the circulation throughout the body. Iron efflux must thus be coupled to oxidation. Earlier studies postulated that the oxidation step required an enzyme catalyst, since chemical oxidation is likely inadequate to supply the large amount of iron required by the bone marrow to maintain erythropoeisis (85, 86). In the intestine, iron oxidation is, in part, mediated by hephaestin (HEPH), which is a membrane-anchored, multicopper ferroxidase (FOX) (11). Mice harboring a mutation in the Heph gene [sex-linked anemia (sla) mice] exhibit moderate iron deficiency, particularly during the rapid growth period in early life (2). HEPH has homology in the FOX domain (∼50% amino acid identity) to the liver-derived, circulating, multicopper FOX ceruloplasmin (CP) (119). In vitro (51, 126) and in vivo (125) approaches have demonstrated FPN1 and HEPH colocalization on or near the BLM of duodenal enterocytes. Furthermore, recent investigations reported immunoreactive HEPH and FOX activity in the cytosolic (soluble) fraction of isolated rodent enterocytes (98). This protein-mediated FOX activity was only partially contributed by HEPH, since robust activity remained in enterocytes isolated from Heph KO mice (97). Although the nature of this cytosolic FOX is currently unknown, this redundant FOX activity likely complements HEPH, thus ensuring adequate iron absorption during times of increased demand.
CP may also influence intestinal iron transport, since it is undoubtedly present in the interstitial fluids within the lamina propria of intestinal villi. Cp KO mice, however, do not show a noticeable disruption in iron absorption (52). Moreover, CP added to the basal side of differentiated Caco-2 cells grown in bicameral cell culture inserts did not influence iron transport (127). Conversely, CP was necessary for increased iron absorption accompanying stimulated erythropoiesis (13), since iron absorption did not increase to the same extent in Cp KO mice following bleeding as it did in wild-type mice. Furthermore, copper contained within CP and HEPH is required for enzymatic activity (i.e., electron transfer), perhaps explaining why copper-deficient animals absorb less iron (20). Additional experimentation is required to determine the relative contributions of enterocyte (membrane-bound HEPH and cytosolic FOXs) and serum-derived (CP) FOXs to intestinal iron transport.
REGULATION OF INTESTINAL IRON ABSORPTION
Iron absorption is precisely regulated by a range of systemic and cellular mediators (Fig. 2). Liver-derived, circulating HEPC regulates duodenal iron absorption and also modulates iron release from stores in hepatocytes and RE macrophages (which recycle iron from senescent erythrocytes). Iron demand to support erythrocyte production in the bone marrow is the strongest stimulator of iron absorption, but overall physiological requirements for iron also influence this process. Iron absorption thus increases when body iron stores are low or when the erythropoietic rate is high, and decreases in the reverse situations. Absorption is also increased during chronic hypoxia, pregnancy, and in the suckling period (as discussed below).
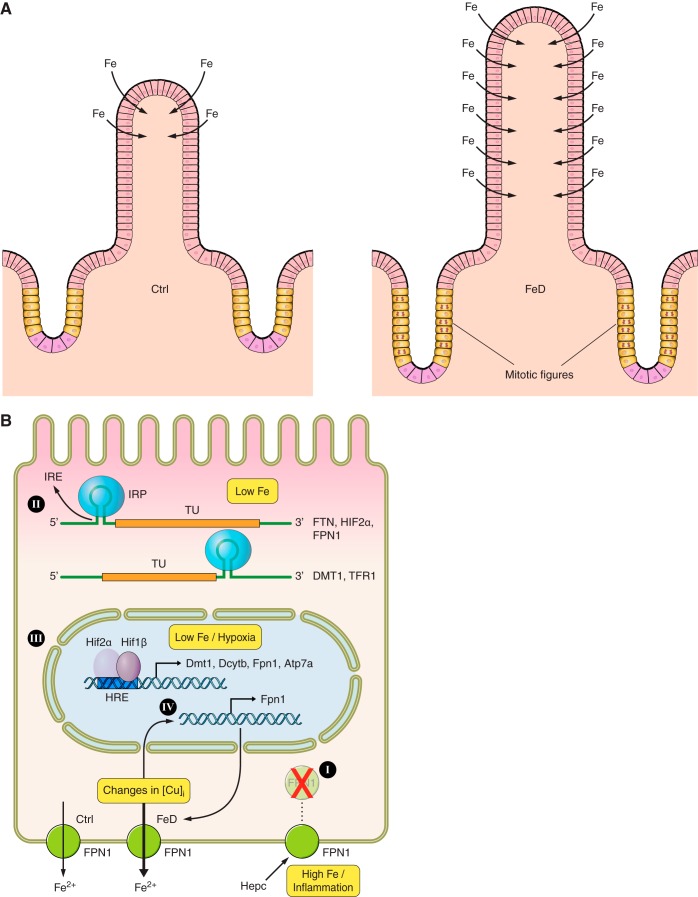
Fig. 2.
Systemic and mucosal regulation of iron absorption. During iron deficiency (FeD), morphological adaptations occur within the intestinal mucosa (A). These include increased mucosal thickness, increased villus length and width, enhanced mitosis of stem cells in the crypts (exemplified by more “mitotic figures”), and absorption of iron via enterocytes over a greater length of the villus. The net result is to maximize the capacity of the small intestine to extract iron from the diet. Additional adaptations occur within duodenal enterocytes in response to changes in intracellular metal-ion concentrations or to alterations in body iron status (B). When body iron stores are replete or during iron overload, HEPC binds to FPN1 on the BLM of enterocytes and causes its internalization and degradation, effectively blocking iron export (I). Iron absorption is also regulated locally by changes in intracellular iron levels, which alter interactions between stem-loop structures in mRNA transcripts [iron-responsive elements (IREs)] and cytosolic iron-sensing proteins [iron-regulatory proteins (IRPs)] (II). The result is that the translation of certain transcripts is blocked (e.g., ferritin [FTN], HIF2α, FPN1) and other transcripts are stabilized (e.g., DMT1, TFR1). Moreover, during iron deficiency and/or low oxygen conditions, a hypoxia-inducible trans-acting factor, hypoxia-inducible factor (HIF) 2α, is stabilized in enterocytes, promoting its dimerization with a HIF1β subunit and induction of gene transcription via interaction of the complex with a hypoxia-responsive cis-acting element (HRE) on target gene promoters (III). This results in increased iron transport via apical and BLM transport processes. Alterations in intracellular copper homeostasis, which occur during iron deficiency (FeD), have also been shown to affect iron transport in enterocytes (IV). Knockdown of a copper transporter (ATP7A), which presumably alters intracellular copper distribution and/or cuproenzyme synthesis, increases FPN1 gene transcription and enhances iron efflux. TFR1, transferrin receptor 1; Ctrl, control (normal iron status); FeD, iron-deficient; TU, transcriptional unit.
Early studies established that intestinal iron absorption is stimulated when body iron stores decrease, and the term “stores regulator” was coined to describe this phenomenon (33). Iron absorption is also enhanced when erythropoiesis is stimulated (e.g., by blood loss or acute hemolysis), since hemoglobin production in developing erythrocytes requires large amounts of iron (31). The term “erythroid regulator” has been used to describe this physiological adaptation (33). Iron absorption also increases in response to tissue hypoxia. While in part this may relate to changes in the erythropoietic rate, a component of the response relates specifically to oxygen levels (96). For example, iron absorption increases during hypoxia before an increase in erythrocyte production (53), demonstrating that hypoxia exerts a direct effect on the gut. Consistent with this observation, iron regulatory molecules in the liver and genes encoding iron transporters in the duodenum respond directly to hypoxia (90).
Iron absorption also increases during pregnancy (5). During gestation, iron requirements are high due to expansion of the maternal erythroid mass and the iron needed by the developing fetus. The underlying trigger probably relates to both a reduction in maternal iron stores and relative tissue hypoxia. Moreover, during the perinatal and neonatal periods, iron requirements of humans are high, and iron absorption from breast milk is very efficient. The high iron absorption of neonates appears to be predominantly due to active transport mechanisms, as occur in adults, but the relative “leakiness” of the neonatal epithelium, which allows passive absorption of solutes, likely makes some contribution. A recent study also suggested an anatomic adaptation at this developmental stage, whereby more iron is absorbed in the distal portions of the gastrointestinal tract in neonatal rats than in adult animals (39). Furthermore, recent work has also demonstrated that iron absorption in neonates is refractory to HEPC (21), despite the fact that HEPC signaling is intact at this developmental stage. Although the mechanisms are not fully understood, this leads to a high capacity to absorb iron from an iron-poor diet at a time of great physiological need.
Intestinal Iron Homeostasis During Iron Overload and Infection/Inflammation
The liver-derived peptide hormone HEPC has emerged as an important regulator of systemic iron homeostasis (81). HEPC decreases circulating iron levels by blocking intestinal iron absorption and inhibiting iron release from stores. Thus, mice overexpressing Hepc develop severe iron-deficiency anemia (82). HEPC expression is induced when body iron stores are elevated, and during infection and inflammation, with the net result being lower serum iron levels. An inverse relationship between hepatic HEPC secretion and expression and activity of duodenal iron transporters (e.g., DMT1, FPN1) has been established (40). Furthermore, the importance of HEPC in maintaining iron balance is exemplified by the phenotype of humans with mutations in the HAMP gene (104), who develop an early onset, severe iron-loading disorder (termed juvenile hemochromatosis) due to unregulated (and inappropriately increased) intestinal iron absorption.
The physiological effects of HEPC are mediated by interaction of the circulating peptide with FPN1 on the plasma membrane of target cells, resulting in internalization and eventual degradation of FPN1 (22), thus limiting iron efflux (80). Duodenal enterocytes represent an important HEPC target, probably explaining the effect of this peptide on intestinal iron transport (103). Interestingly, recent studies have also supported novel roles for HEPC in the intestine. For instance, using in vitro and ex vivo approaches, it was shown that HEPC promotes the proteosomal degradation of DMT1 expressed on the BBM of duodenal enterocytes (7). Conceivably, additional FPN1-independent aspects of how HEPC modulates intestinal iron flux may be discovered in the future.
In accordance with its role in regulating iron homeostasis, HEPC expression is altered according to body iron needs. As might be anticipated, HEPC production decreases during iron deficiency (83), hypoxia, and pregnancy (74), and when erythropoiesis is stimulated, allowing adequate iron absorption and efficient iron release from stores. Conversely, HEPC expression increases when body iron levels are adequate or elevated, effectively reducing intestinal absorption and allowing excess iron to be stored in RE macrophages and hepatocytes. Inflammation also induces HEPC expression, likely explaining the hypoferremia that accompanies chronic inflammatory conditions (83).
Much knowledge of how HEPC regulates systemic iron homeostasis has been attained from investigation of several inherited iron-loading disorders, collectively referred to as HH. In healthy individuals, iron absorption can be appropriately downregulated when iron stores are replete, but, in patients with HH, iron absorption is chronically increased, allowing accumulation of the metal in liver, pancreas, heart, and other tissues. In addition to HAMP (104), mutations in the HJV (also called HFE2) gene may also cause an early onset (juvenile) hemochromatosis (88). The most common form of HH, however, results from mutations in the HFE gene, which causes a less severe, adult-onset iron loading (32). HFE encodes a membrane-bound major histocompatibility complex class I-like protein. In northern Europeans, ∼1 in 200 individuals are homozygous for the most common HFE mutation that is linked to iron loading. Many individuals that carry HFE mutations, however, do not develop iron-related tissue damage, suggesting that genetic modifiers exist or that environmental or dietary factors are also important for disease pathogenesis. Less commonly, adult-onset HH may result from mutations in the TFR2 gene (9). The phenotype of these patients is essentially indistinguishable from that of individuals with HFE-associated iron loading.
Although the severity of symptoms varies in patients harboring these genetic mutations, the resulting phenotypes have several shared features: 1) iron absorption is inappropriately high; 2) iron accumulates with a periportal distribution in the liver (predominantly in hepatocytes); 3) macrophages have low iron content, particularly in early stages of the disease; and 4) the mutant genes are all highly expressed in hepatocytes. This latter observation suggests that the proteins encoded by these genes may form parts of a common regulatory network. Consistent with this prediction, it was subsequently shown that patients with these various subtypes of HH all display inappropriately low expression or absence of HEPC, even though body iron levels are elevated (36). Thus, HFE, TFR2, and HJV are all integral parts of the signaling pathway that regulates expression of the HAMP gene in hepatocytes. These studies, and others not mentioned here, have paved the way for a detailed analysis of how HEPC expression is modulated in response to changes in body iron status, changes in erythropoeitic demand for iron, and during infection and inflammation. Accordingly, specific mechanisms of HEPC regulation have recently emerged, whereby various signaling pathways (e.g., the BMP/SMAD pathway) influence transcription of the HAMP gene in hepatocytes. This topic has been reviewed recently (43, 60).
Regulation of Intestinal Iron Absorption During Iron Depletion and Hypoxia
Additional control of iron absorption occurs at the level of the enterocyte, which ultimately determines how much iron is acquired from the diet. Regulation of iron homeostasis in enterocytes goes beyond the FPN1-HEPC axis, involving specific physiological adaptations that have evolved to maximize iron extraction from the diet when the demand for iron increases. These adaptations involve direct effects on enterocyte gene transcription, posttranscriptional control of mRNA stability, and morphological remodeling of the epithelium, probably all in response to alterations in intracellular iron levels in enterocytes. Moreover, a role for copper in the control of duodenal iron absorption, with direct effects upon enterocytes, has recently emerged.
The iron-regulatory protein/iron-responsive element system.
Intestinal DMT1 expression is strongly upregulated by iron deprivation and consequent hypoxia (17, 38, 100), probably via transcriptional and posttranscriptional mechanisms. The DMT1 transcript contains an iron-responsive element (IRE) (a stem-loop structure) in its 3′-untranslated region, which interacts with cytosolic iron-sensing proteins [iron-regulatory proteins (IRP) 1 and/or IRP2]. When intracellular iron is low, IRPs bind to the DMT1 3′-IRE and stabilize the transcript, ultimately leading to increased protein production (1, 42, 59). Conversely, when iron is abundant, the IRPs do not bind to the IRE, and the transcript becomes less stable, resulting in lower DMT1 protein levels. Interestingly, two DMT1 3′-splice variants exist, with and without the IRE (55). The variant with the IRE (+IRE) is the form predominantly expressed in the duodenum (25, 115). Recent data, however, suggest that the DMT1 3′-IRE may be functional (i.e., bound by IRPs during iron deprivation) only in suckling mice and not in adults (41). Whether this is representative of other mammalian species as well is unknown.
DMT1 expression is also transcriptionally regulated by hypoxia-inducible factor 2α (HIF2α) (69, 107) (as detailed below). Interestingly, the HIF2α transcript contains a 5′-IRE (105). IRP binding in this case would be expected to repress translation. A recent study provides evidence that IRP1 specifically regulates HIF2α translation (3) in mouse duodenum and, in part, thus regulates iron flux. This and other recent studies provide emerging evidence that the IRP/IRE system and HIF-mediated regulation of gene transcription function in tandem in the duodenum to provide precise control of iron absorption (3, 41).
FPN1 regulation in the duodenum, like DMT1, occurs via transcriptional and posttranscriptional mechanisms. First, FPN1 protein levels are modulated via interaction with serum-borne HEPC, which triggers internalization and eventual degradation of FPN1. This effectively blocks iron efflux from duodenal enterocytes. Second, because one FPN1 transcript variant contains a 5′-IRE (128), FPN1 protein expression may be attenuated by a translational block via the IRPs. Although the FPN1 variant with the IRE and the one lacking the IRE are both expressed in the duodenum, recent evidence in mice suggests that the +IRE variant predominates, even under conditions of iron restriction (41). This counterintuitive observation is suggested by these authors to imply that IRP regulation of FPN1 protein translation is of less functional significance than HEPC-mediated control of FPN1 protein turnover, at least in the duodenum (of mice). However, the IRP/IRE system contributes at least in part to the regulation of FPN1 expression in the duodenum, since mice lacking both IRPs specifically in the intestine fail to fully repress FPN1 under conditions of iron excess, when HEPC expression and secretion is enhanced (41). Last, FPN1 is also regulated at the level of gene transcription, like DMT1, via transactivation by HIF2α (114). Importantly, FPN1 thus represents a unique molecular link connecting cell-specific control of iron homeostasis via the IRP/IRE system, and HIF2α-mediated transcriptional regulation with systemic regulation via HEPC.
Hypoxia and iron absorption.
The intestinal epithelium exists in a natural state of hypoxia, with enterocytes on the upper part of the villus that are furthest from the capillary bed in the lamina propria being most significantly affected (15). Epithelial hypoxia increases between meals when blood flow to the gut decreases. More significant intestinal hypoxia results from iron deprivation, which impairs hemoglobin synthesis (and thus oxygen transport). Early studies showed that iron transport increased in rats (87) and mice (95) that were deprived of oyxgen. Because the identity of intestinal iron transporters was unknown at this time, these investigations did not provide mechanistic insight into how hypoxia increased iron absorption. Not surprisingly then, hypoxia is an important driver of intestinal iron absorption.
The molecular response to hypoxia is mediated by HIFs. HIFs form heterodimers containing a hypoxia-responsive α-subunit (HIF1, 2, or 3α) and a constititively expressed β-subunit [called HIF1β (or ANRT)]. When oxygen levels are adequate (∼21% O2), the cytosolic α-subunits are hydroxylated, ubiquinated, and rapidly degraded in lysosomes. During hypoxic conditions, however, the HIFα subunits are stabilized (i.e., not hydroxylated), allowing them to translocate to the nucleus and interact with the β-subunit. The net result is DNA binding by the complex and modulation of gene transcription. Interestingly, the enzymes that hydroxylate the HIFα subunits (causing their subsequent degradation), the prolyl hydroxylases, are iron-dependent enzymes (30). Therefore, when iron is low, prolyl hydroxylase activity decreases, and the HIFα subunits are more stable. Activity of the HIFs is thus regulated by oxygen and iron levels.
Recent studies showed that HIF2α was upregulated in the intestinal epithelium of mice during iron deprivation, whereas HIF1α levels were unaltered (69, 107). Furthermore, Dcytb, Dmt1, and Fpn1 were shown to be direct HIF2α targets (69, 107, 114); their induction presumably mediates the increase in iron absorption noted during low-iron/low-oxygen conditions. HIF2α was also preferentially stabilized in iron-deprived Caco-2 cells (54). Moreover, a genome-wide gene expression screen, combined with bioinformatics analysis of the promoters, suggested that many transactivated genes in iron-deprived Caco-2 cells were direct HIF2α targets. These studies collectively emphasize the important role that HIF2α signaling plays in the intestinal epithelium during alterations in iron or oxygen levels. Furthermore, it is important to consider whether this regulatory mechanism also influences iron absorption during other pathological situations with concurrent hypoxia (e.g., various inflammatory conditions or cancer). One recent study indeed showed that HIF2α transactivated DMT1 expression in colonic tumors (which are hypoxic), supporting the concept that iron plays an important role in the pathogenesis of cancer (124).
Morphological adaptations of the intestinal mucosa to iron deprivation.
In addition to HIF-mediated changes in gene transcription, morphological adaptations occur in the intestinal epithelium as part of the compensatory response to iron deprivation. Under normal conditions, enterocytes in the upper half of the villus make the largest contribution to iron absorption; however, during iron deficiency, enterocytes from the lower part of the villus participate in iron absorption (111). In this study, increased villus width and length was also documented. Another investigation performed using iron-deficient rats demonstrated that villus height, mucosal thickness, and epithelial surface area increased in the jejunum (121). An additional, more recent investigation reconfirmed these previous observations and extended them to show increased cell proliferation in the crypts of iron-deficient rats, as indicated by an increase in the number of noted mitotic cell divisions (19). The latter study hypothesized that induction of a lipoxygenase (Alox15) altered eicosanoid biosynthesis in the gut during iron deficiency, perhaps providing a mechanistic explanation for these morphological adaptations. Whether similar compensatory, morphological adaptations occur in iron-deficient humans is unknown.
The influence of copper on intestinal iron absorption.
Recognition of physiologically relevant interactions between iron and copper stem from observations made during the 1800s in Europe. It was noted that young women working in copper factories did not suffer from a common affliction at that time, called chlorosis (i.e., probably iron-deficiency anemia) (37, 44). More recent investigations have shown that copper is redistributed to tissues vital for control of iron homeostasis during iron deprivation, including the intestinal mucosa (29), the liver (100), and blood (28, 112). Serum FOX activity is also enhanced in iron-deprived rodents, which corresponded with higher hepatic CP protein expression (99). Moreover, the expression/activity of HEPH in duodenal enterocytes is also affected by copper levels (10, 12, 101). These data suggest that copper, either directly or indirectly, modulates intestinal iron homeostasis.
Several recent lines of experimental pursuit provide evidence that copper influences iron absorption directly at the level of duodenal enterocytes. First, copper homeostasis-related genes [such as those encoding copper-transporting ATPase 1 (ATP7A) and an intracellular copper-binding protein (metallothionein)] are upregulated in parallel with iron transport-related genes (e.g., DMT1, DCYTB, FPN1) in the proximal small intestine of iron-deprived rats (17, 18). ATP7A was also induced in mice fed a low-iron diet (46) and in iron-deprived rat intestinal epithelial (IEC-6) cells. (123). Furthermore, the Atp7a gene is transactivated by HIF2α during low iron/hypoxia (122, 123), demonstrating coordinate regulation with DCYTB, DMT1, and FPN1 (69, 107, 114). Another copper transporter (CTR1) expressed in the intestine may also be regulated by HIF2α (92). These data have led to the postulate that ATP7A is a molecular link between iron and copper in the intestinal mucosa.
Altered ATP7A expression likely influences intracellular copper levels (or distribution), since it has dual functions in enterocytes: pumping copper into the trans-Golgi network (TGN) (to support cuproenzyme synthesis) and pumping copper across the BLM (to mediate copper efflux) (89). Because HEPH is a copper-containing protein that may be synthesized in the TGN, ATP7A may be required for HEPH expression/activity. This was directly tested by silencing ATP7A in rat IEC-6 cells (47). ATP7A knockdown caused a significant reduction in membrane FOX activity (presumably mediated by HEPH), but, surprisingly, transepithelial iron flux increased. So, although ATP7A may not be absolutely required to deliver copper for the biosynthesis of HEPH, at least in this model system, diminished HEPH activity did not negatively influence iron transport. Transcriptional induction of FPN1 expression was suggested to be the mechanism by which iron flux was enhanced in cells lacking ATP7A (47). Other studies also suggested that FPN1 expression was influenced by copper levels (58, 70). Collectively, these studies reveal molecular details of iron-copper interactions in enterocytes, and provide rationale for further investigation.
PERTURBATIONS IN INTESTINAL IRON TRANSPORT ASSOCIATED WITH VARIOUS PATHOLOGICAL STATES
Intestinal iron absorption is altered in several important clinical conditions. These pathologies may result from mutations in genes encoding iron transporters or regulatory molecules, or, alternatively, iron absorption may be secondarily perturbed by physiological changes associated with different diseases. Relevant human disorders that display altered intestinal iron absorption are summarized in Table 2.
Table 2.
Selected pathological states associated with perturbed iron absorption
| Hereditary Hemochromatosis | Classification | Gene# | Iron Absortion | HEPC Level | Resulting Phenotpye |
|---|---|---|---|---|---|
| Type 1 | Primary iron overload | HFE | Increased | L | Parenchymal iron overload, liver disease, arthropathy |
| Type 2A | Primary iron overload | HFE2 (HJV) | Increased | VL | Severe parenchymal iron overload, cardiac disease, liver cirrhosis, endocrine failure, diabetes, arthropathy |
| Type 2B | Primary iron overload | HAMP | Increased | VL-Ab | Same as type 2A |
| Type 3 | Primary iron overload | TFR2 | Increased | L | Parenchymal iron overload, liver disease |
| Type 4; “transport defective” | Primary iron overload | SLC40A1 | Reduced | N-H | Parenchymal and reticuloendothelial iron overload in the liver |
| Type 4; “HEPC resistance” | Primary iron overload | SLC40A1 | Increased | N-H | Parenchymal iron overload |
| Iron-Related Anemias | |||||
| Refractory iron-deficiency anemia | Iron-deficiency anemia | TMPRSS6 | Reduced | H | Hypochromic, microcytic anemia |
| Refractory iron-deficiency anemia | Iron-deficiency anemia | SLC11A2 | Reduced | L-N | Hypochromic, microcytic anemia |
| Hereditary atransferrinemia | Iron-loading anemia | TF | Increased | L | Hemosiderosis of the heart and liver |
| β-Thalassemia | Iron-loading anemia | HBB | Increased | L-N | Parenchymal and reticuloendothelial iron overload, anemia, reticulocytosis |
| Sickle cell anemia | Iron-loading anemia | HBB | Increased | L-N | Same as β-thalassemia |
| X-linked sideroblastic anemia | Iron-loading anemia | ALAS2 | Increased | L* | Same as β-thalassemia |
Mutated gene causing disorder.
L, low; VL, very low; Ab, absent; N, normal; H, high.
Predicted levels based on current knowledge of HEPC regulation.
Genetic Defects in Intestinal Iron Transporters
SLC11A2 and SLC40A1 mutations have been infrequently identified in humans. Because DMT1 and FPN1 are expressed in many cell types, these mutations have wide-ranging pathophysiological effects. Mutations in the SLC40A1 gene result in so-called Ferroportin Disease (or type 4 hemochromatosis), which is an autosomal dominant form of iron loading (91). The mutant FPN1 protein may have a reduced capacity to export iron due to transport or trafficking defects, or its interactions with HEPC may be perturbed (23, 26). In the first case, lack of FPN1 activity traps iron in enterocytes, causing an initial systemic iron deficiency in affected individuals. Ultimately, however, lack of FPN1-mediated iron export leads to iron loading in cells (and tissues) which store iron, including hepatocytes and RE macrophages. Eventually, the bone marrow is deprived of iron required for erythrocyte production, and anemia ensues. Moreover, a compensatory increase in FPN1 expression from the remaining functional allele in the intestine occurs, enhancing iron absorption, and exacerbating tissue iron accumulation (120). The second class of SLC40A1 mutations perturbs the interaction of the FPN1 protein with HEPC so endocytosis from the BLM is impaired; iron absorption thus remains inappropriately high, leading to body iron accumulation (26).
SLC11A2 mutations in humans are exceedingly rare (56), which attests to the nonredundant role of DMT1 in iron metabolism. Development of severe microcytic, hypochromic anemia typifies these patients, but, surprisingly, some of them load iron in the liver (75). This unexpected phenotype would be unlikely if DMT1 activity was abolished. In these patients, relative tissue hypoxia and downregulation of HEPC expression likely trigger increases in intestinal iron absorption, which must secondarily lead to hepatic iron accumulation. In this scenario, mutant DMT1 must retain residual activity (or an alternative iron transport pathway must be activated, which seems unlikely). The phenotype of another patient with an SLC11A2 mutation supports this possibility, since this individual suffers from severe iron-deficiency anemia but does not load iron in the liver. This SLC11A2 mutation leads to an amino acid substitution in a highly conserved residue in the first transmembrane domain (G75R), which is predicted to abolish iron transport activity (57).
Pathological Conditions That Alter the Regulation of Iron Absorption
As detailed above, most inherited disorders of iron homeostasis involve mutations in the HAMP gene, or in genes encoding proteins that regulate HAMP transcription. HAMP or HJV mutations lead to extremely low or absent HEPC expression, secondarily resulting in enhanced iron absorption and subsequent severe tissue iron loading (88, 104). In patients with HFE or TFR2 mutations, HEPC reduction is less profound, and, as a result, the development of the iron-overload phenotype is more gradual (36, 79). These autosomal-recessive disorders, with the exception of HFE-related hemochromatosis, are all quite rare.
HEPC deficiency leads to iron loading, so HEPC overexpression would be postulated to cause iron deficiency. This is indeed the case, as is exemplified by mutations in the TMPRSS6 gene, which causes many clinical cases of IRIDA in humans (45). The TMPRSS6 gene encodes matriptase-2, an enzyme that functions via the BMP/SMAD signaling pathway to negatively regulate HAMP transcription (77). Interestingly, single nucleotide polymorphisms in the TMPRSS6 gene have been linked to variations in iron homeostasis among different human populations (113), exemplifying the important role that matriptase-2 plays in determining body iron levels. Furthermore, a recent investigation suggests that TPMRSS6 could be a therapeutic target to treat iron-overload diseases (106).
Altered Iron Absorption Secondary to Primary Pathological States
Iron absorption may be secondarily perturbed in a number of pathological conditions. One common cause of inefficient iron absorption (and consequent anemia) relates to a reduction in the absorptive surface area of the gut, as commonly occurs in Celiac disease, inflammatory bowel diseases, and short bowel syndrome. Blood loss associated with various gastrointestinal disorders and diarrhea may also result in anemia. In other situations, morphological changes in the gut epithelium are not apparent, but additional physiological disturbances may alter HEPC expression and activity, as discussed below.
Iron-loading anemias.
Because the bone marrow is the predominant site of iron usage to support erythropoiesis, perturbations in erythrocyte production can result in changes in HEPC expression and consequent alterations in iron absorption. Moreover, it has been known for decades that ineffective erythropoiesis increases iron absorption (102). In disorders such as β-thalassemia, sideroblastic anemia, or acute hemolysis, the enhanced erythroid drive reduces HEPC expression, leading to excessive intestinal iron absorption.
Chronic liver disease.
Iron levels are altered in many chronic liver diseases, but the underlying physiological mechanism(s) are not fully understood. It is likely, however, that altered HEPC expression and subsequent changes in intestinal iron absorption are involved. For example, diminished HEPC synthesis and hepatic iron loading have been linked with excessive alcohol consumption (61) and hepatitis C virus infection (93). Furthermore, HEPC levels are increased in obesity, which is associated with reduced body iron levels (76). This likely reflects the inflammatory underpinnings of the metabolic syndrome.
Anemia of chronic disease.
It is widely accepted that acute or chronic inflammation, associated with infection, cancer, rheumatoid arthritis, and other inflammatory conditions, causes hypoferremia. Reduced plasma iron results from cytokine-mediated induction of HEPC expression (e.g., by IL-6), resulting in decreased iron absorption and iron release from RE macrophages (78). The development of HEPC antagonistic drugs would be potentially very useful in treating this common form of anemia.
CONCLUSIONS
Among essential dietary nutrients, the homeostasis of iron is unique given that free iron is highly reactive and no active excretory mechanisms have evolved in humans. The lack of a process for ridding the body of iron demonstrates the critical requirement for this nutrient and probably reflects the fact that early humans did not have consistent, readily available sources of highly bioavailable iron. As a result, humans and other mammals have developed complex regulatory mechanisms to control body iron content at the level of absorption in the proximal small intestine. In health, iron absorption is precisely matched to iron losses, but in numerous clinical conditions this balance is disrupted, resulting in the pathological consequences of iron overload and iron deficiency.
Iron absorption is regulated systemically by HEPC. Many of the complexities of HEPC regulation have been revealed, and this has shown that HEPC is an integral part of each of the signaling pathways formerly known as the “stores,” “erythropoetic” and “inflammatory” regulators of iron homeostasis. HEPC is induced when body iron stores are high, and during infection and inflammation, and is reduced when erythropoietic demand increases and during iron deficiency. Furthermore, discovery of HIF2α-mediated regulation of genes encoding iron transporters has provided a mechanistic explanation as to why hypoxia increases assimilation of dietary iron. HIF2α also alters copper homeostasis in duodenal enterocytes, supporting the possibility that copper is important in some respect for control of intestinal iron transport.
Despite these advances, many aspects of intestinal iron homeostasis remain to be elucidated, and numerous pertinent questions remain to be answered. A few of those will be delineated here.
1) Given the physiological importance of iron and the absorption process, do redundant backup systems exist? This is likely the case since intestine-specific inactivation of the SLC11A2 and SLC40A1 genes does not result in lethality (25, 48). Yet, mice without these intestinal transporters suffer from significant iron-deficiency anemia, demonstrating the near essentiality of these two iron transport systems.
2) Is DCYTB required for basal iron transport into enterocytes, or it is only required when demand increases? Its coregulation by HIF2α-mediated transcriptional activation (along with DMT1 and FPN1) and its robust induction during iron deficiency hint at an important role for this BBM ferrireductase. Additionally, is enzymatic iron reduction rate-limiting for iron absorption, or do dietary factors and/or gastrointestinal secretions supply the necessary reducing power?
3) How does iron traffick within enterocytes after it is absorbed, and how it is delivered to FPN1? Given its propensity to mediate production of reactive oxygen species, free iron is likely very low in cells. Do specific iron-binding proteins or chaperones thus exist in enterocytes? Are the poly-r(C)-binding proteins expressed in enterocytes?
4) FPN1 and HEPH have been detected within the cytosol of enterocytes. Where within the cells are they located, and what is their function there? Do they physically and/or functionally interact? How is export coupled to iron oxidation by HEPH?
5) What are the relative contributions of HEPH, CP, and cytosolic FOXs to intestinal iron transport? Is enzymatic oxidation of ferrous iron rate-limiting for iron absorption? Can CP compensate for the loss of HEPH activity? What is the nature of the cytosolic FOXs in enterocytes? Are they required to complement HEPH activity when iron absorption increases? How do intracellular copper concentrations or distribution influence iron transport? Is ATP7A required for the biosynthesis of HEPH?
6) Does HEPC have additional biologically relevant effects on enterocytes, independent of its action on FPN1? How might HEPC regulate DMT1 protein trafficking on the BBM of duodenal enterocytes?
These unresolved issues and important questions will undoubtedly occupy the efforts of many investigators in this area of scientific pursuit over the next several years.
GRANTS
The writing of this manuscript was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1 R01 DK-074867 (J. F. Collins). G. J. Anderson is the recipient of a Senior Research Fellowship from the National Health and Medical Research Council of Australia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.G., G.J.A., and J.F.C. prepared figures; S.G., G.J.A., and J.F.C. drafted manuscript; S.G., G.J.A., and J.F.C. edited and revised manuscript; J.F.C. approved final version of manuscript.
REFERENCES
- 1.Anderson CP, Shen M, Eisenstein RS, Leibold EA. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta 1823: 1468–1483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson GJ, Frazer DM, McKie AT, Vulpe CD. The ceruloplasmin homolog hephaestin and the control of intestinal iron absorption. Blood Cells Mol Dis 29: 367–375, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Anderson SA, Nizzi CP, Chang YI, Deck KM, Schmidt PJ, Galy B, Damnernsawad A, Broman AT, Kendziorski C, Hentze MW, Fleming MD, Zhang J, Eisenstein RS. The IRP1-HIF-2alpha axis coordinates iron and oxygen sensing with erythropoiesis and iron absorption. Cell Metab 17: 282–290, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arredondo M, Mendiburo MJ, Flores S, Singleton ST, Garrick MD. Mouse divalent metal transporter 1 is a copper transporter in HEK293 cells. Biometals 27: 115–123, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Batey RG, Gallagher ND. Role of the placenta in intestinal absorption of iron in pregnant rats. Gastroenterology 72: 255–259, 1977 [PubMed] [Google Scholar]
- 6.Blanco E, Kannengiesser C, Grandchamp B, Tasso M, Beaumont C. Not all DMT1 mutations lead to iron overload. Blood Cells Mol Dis 43: 199–201, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Brasse-Lagnel C, Karim Z, Letteron P, Bekri S, Bado A, Beaumont C. Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology 140: 1261–1271, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Callebaut I, Joubrel R, Pissard S, Kannengiesser C, Gerolami V, Ged C, Cadet E, Cartault F, Ka C, Gourlaouen I, Gourhant L, Oudin C, Goossens M, Grandchamp B, de Verneuil H, Rochette J, Ferec C, Le Gac G. Comprehensive functional annotation of 18 missense mutations found in suspected haemochromatosis type 4 patients. Hum Mol Genet [Epub ahead of print] 2014 Apr. 25 [DOI] [PubMed] [Google Scholar]
- 9.Camaschella C, Roetto A, Cali A, De Gobbi M, Garozzo G, Carella M, Majorano N, Totaro A, Gasparini P. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet 25: 14–15, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Attieh ZK, Dang T, Huang G, van der Hee RM, Vulpe C. Decreased hephaestin expression and activity leads to decreased iron efflux from differentiated Caco2 cells. J Cell Biochem 107: 803–808, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Attieh ZK, Su T, Syed BA, Gao H, Alaeddine RM, Fox TC, Usta J, Naylor CE, Evans RW, McKie AT, Anderson GJ, Vulpe CD. Hephaestin is a ferroxidase that maintains partial activity in sex-linked anemia mice. Blood 103: 3933–3939, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Huang G, Su T, Gao H, Attieh ZK, McKie AT, Anderson GJ, Vulpe CD. Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J Nutr 136: 1236–1241, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Cherukuri S, Potla R, Sarkar J, Nurko S, Harris ZL, Fox PL. Unexpected role of ceruloplasmin in intestinal iron absorption. Cell Metab 2: 309–319, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Choi J, Masaratana P, Latunde-Dada GO, Arno M, Simpson RJ, McKie AT. Duodenal reductase activity and spleen iron stores are reduced and erythropoiesis is abnormal in Dcytb knockout mice exposed to hypoxic conditions. J Nutr 142: 1929–1934, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7: 281–287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins JF, Anderson GJ. Molecular mechanisms of intestinal iron tranport. In: Physiology of the Gastrointestinal Tract (5th ed.) New York, NY: Elsevier, 2012 [Google Scholar]
- 17.Collins JF, Franck CA, Kowdley KV, Ghishan FK. Identification of differentially expressed genes in response to dietary iron deprivation in rat duodenum. Am J Physiol Gastrointest Liver Physiol 288: G964–G971, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Collins JF, Hu Z. Promoter analysis of intestinal genes induced during iron-deprivation reveals enrichment of conserved SP1-like binding sites (Abstract). BMC Genomics 8: 420, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins JF, Hu Z, Ranganathan PN, Feng D, Garrick LM, Garrick MD, Browne RW. Induction of arachidonate 12-lipoxygenase (Alox15) in intestine of iron-deficient rats correlates with the production of biologically active lipid mediators. Am J Physiol Gastrointest Liver Physiol 294: G948–G962, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Coppen DE, Davies NT. Studies on the roles of apotransferrin and caeruloplasmin (EC 1.1631) on iron absorption in copper-deficient rats using an isolated vascularly- and luminally-perfused intestinal preparation. Br J Nutr 60: 361–373, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Darshan D, Wilkins SJ, Frazer DM, Anderson GJ. Reduced expression of ferroportin-1 mediates hyporesponsiveness of suckling rats to stimuli that reduce iron absorption. Gastroenterology 141: 300–309, 2011 [DOI] [PubMed] [Google Scholar]
- 22.De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell 18: 2569–2578, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Domenico I, Ward DM, Nemeth E, Vaughn MB, Musci G, Ganz T, Kaplan J. The molecular basis of ferroportin-linked hemochromatosis. Proc Natl Acad Sci USA 102: 8955–8960, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del-Castillo-Rueda A, Moreno-Carralero MI, Cuadrado-Grande N, Alvarez-Sala-Walther LA, Enriquez-de-Salamanca R, Mendez M, Moran-Jimenez MJ. Mutations in the HFE, TFR2, and SLC40A1 genes in patients with hemochromatosis Gene 508: 15–20, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1: 191–200, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Drakesmith H, Schimanski LM, Ormerod E, Merryweather-Clarke AT, Viprakasit V, Edwards JP, Sweetland E, Bastin JM, Cowley D, Chinthammitr Y, Robson KJ, Townsend AR. Resistance to hepcidin is conferred by hemochromatosis-associated mutations of ferroportin. Blood 106: 1092–1097, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, Khovananth K, Mudd S, Mann N, Moresco EM, Beutler E, Beutler B. The serine protease TMPRSS6 is required to sense iron deficiency. Science 320: 1088–1092, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ece A, Uyanik BS, Iscan A, Ertan P, Yigitoglu MR. Increased serum copper and decreased serum zinc levels in children with iron deficiency anemia. Biol Trace Elem Res 59: 31–39, 1997 [DOI] [PubMed] [Google Scholar]
- 29.El-Shobaki FA, Rummel W. Binding of copper to mucosal transferrin and inhibition of intestinal iron absorption in rats. Res Exp Med 174: 187–195, 1979 [DOI] [PubMed] [Google Scholar]
- 30.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Erlandson ME, Walden B, Stern G, Hilgartner MW, Wehman J, Smith CH. Studies on congenital hemolytic syndromes, IV. Gastrointestinal absorption of iron. Blood 19: 359–378, 1962 [PubMed] [Google Scholar]
- 32.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R, Jr, Ellis MC, Fullan A, Hinton LM, Jones NL, Kimmel BE, Kronmal GS, Lauer P, Lee VK, Loeb DB, Mapa FA, McClelland E, Meyer NC, Mintier GA, Moeller N, Moore T, Morikang E, Prass CE, Quintana L, Starnes SM, Schatzman RC, Brunke KJ, Drayna DT, Risch NJ, Bacon BR, Wolff RK. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet 13: 399–408, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Finch C. Regulators of iron balance in humans. Blood 84: 1697–1702, 1994 [PubMed] [Google Scholar]
- 34.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA 95: 1148–1153, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleming MD, Trenor CC, 3rd, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet 16: 383–386, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Fleming RE, Britton RS, Waheed A, Sly WS, Bacon BR. Pathogenesis of hereditary hemochromatosis. Clin Liver Dis 8: 755–773, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Fox PL. The copper-iron chronicles: the story of an intimate relationship. Biometals 16: 9–40, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Frazer DM, Inglis HR, Wilkins SJ, Millard KN, Steele TM, McLaren GD, McKie AT, Vulpe CD, Anderson GJ. Delayed hepcidin response explains the lag period in iron absorption following a stimulus to increase erythropoiesis. Gut 53: 1509–1515, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frazer DM, Wilkins SJ, Anderson GJ. Elevated iron absorption in the neonatal rat reflects high expression of iron transport genes in the distal alimentary tract. Am J Physiol Gastrointest Liver Physiol 293: G525–G531, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Frazer DM, Wilkins SJ, Becker EM, Vulpe CD, McKie AT, Trinder D, Anderson GJ. Hepcidin expression inversely correlates with the expression of duodenal iron transporters and iron absorption in rats. Gastroenterology 123: 835–844, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Galy B, Ferring-Appel D, Becker C, Gretz N, Grone HJ, Schumann K, Hentze MW. Iron regulatory proteins control a mucosal block to intestinal iron absorption. Cell Rep 3: 844–857, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Galy B, Ferring-Appel D, Kaden S, Grone HJ, Hentze MW. Iron regulatory proteins are essential for intestinal function and control key iron absorption molecules in the duodenum. Cell Metab 7: 79–85, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Ganz T. Iron homeostasis: fitting the puzzle pieces together. Cell Metab 7: 288–290, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Guggenheim KY. Chlorosis: the rise and disappearance of a nutritional disease. J Nutr 125: 1822–1825, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Guillem F, Lawson S, Kannengiesser C, Westerman M, Beaumont C, Grandchamp B. Two nonsense mutations in the TMPRSS6 gene in a patient with microcytic anemia and iron deficiency. Blood 112: 2089–2091, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Gulec S, Collins JF. Investigation of iron metabolism in mice expressing a mutant Menke's copper transporting ATPase (Atp7a) protein with diminished activity (Brindled; Mo (Br) (/y)). PLoS One 8: e66010, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gulec S, Collins JF. Silencing the Menkes copper-transporting ATPase (Atp7a) gene in rat intestinal epithelial (IEC-6) cells increases iron flux via transcriptional induction of ferroportin 1 (Fpn1). J Nutr 144: 12–19, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest 115: 1258–1266, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388: 482–488, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Gunshin H, Starr CN, Direnzo C, Fleming MD, Jin J, Greer EL, Sellers VM, Galica SM, Andrews NC. Cybrd1 (duodenal cytochrome b) is not necessary for dietary iron absorption in mice. Blood 106: 2879–2883, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han O, Kim EY. Colocalization of ferroportin-1 with hephaestin on the basolateral membrane of human intestinal absorptive cells. J Cell Biochem 101: 1000–1010, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA 96: 10812–10817, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hathorn MK. The influence of hypoxia on iron absorption in the rat. Gastroenterology 60: 76–81, 1971 [PubMed] [Google Scholar]
- 54.Hu Z, Gulec S, Collins JF. Cross-species comparison of genomewide gene expression profiles reveals induction of hypoxia-inducible factor-responsive genes in iron-deprived intestinal epithelial cells. Am J Physiol Cell Physiol 299: C930–C938, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hubert N, Hentze MW. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proc Natl Acad Sci USA 99: 12345–12350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iolascon A, De Falco L. Mutations in the gene encoding DMT1: clinical presentation and treatment. Semin Hematol 46: 358–370, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Iolascon A, De Falco L, Beaumont C. Molecular basis of inherited microcytic anemia due to defects in iron acquisition or heme synthesis. Haematologica 94: 395–408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jenkitkasemwong S, Broderius M, Nam H, Prohaska JR, Knutson MD. Anemic copper-deficient rats, but not mice, display low hepcidin expression and high ferroportin levels. J Nutr 140: 723–730, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang L, Garrick MD, Garrick LM, Zhao L, Collins JF. Divalent metal transporter 1 (Dmt1) mediates copper transport in the duodenum of iron-deficient rats and when overexpressed in iron-deprived HEK-293 cells. J Nutr 143: 1987–1933, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knutson MD. Iron-sensing proteins that regulate hepcidin and enteric iron absorption. Annu Rev Nutr 30: 149–171, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Kohgo Y, Ohtake T, Ikuta K, Suzuki Y, Torimoto Y, Kato J. Dysregulation of systemic iron metabolism in alcoholic liver diseases. J Gastroenterol Hepatol 23, Suppl 1: S78–S81, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Latunde-Dada GO, Van der Westhuizen J, Vulpe CD, Anderson GJ, Simpson RJ, McKie AT. Molecular and functional roles of duodenal cytochrome B (Dcytb) in iron metabolism. Blood Cells Mol Dis 29: 356–360, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Latunde-Dada GO, Xiang L, Simpson RJ, McKie AT. Duodenal cytochrome b (Cybrd 1) and HIF-2alpha expression during acute hypoxic exposure in mice. Eur J Nutr 50: 699–704 [DOI] [PubMed] [Google Scholar]
- 64.Le Blanc S, Garrick MD, Arredondo M. Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism. Am J Physiol Cell Physiol 302: C1780–C1785, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Leidgens S, Bullough KZ, Shi H, Li F, Shakoury-Elizeh M, Yabe T, Subramanian P, Hsu E, Natarajan N, Nandal A, Stemmler TL, Philpott CC. Each member of the poly-r(C)-binding protein 1 (PCBP) family exhibits iron chaperone activity toward ferritin. J Biol Chem 288: 17791–17802, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo X, Hill M, Johnson A, Latunde-Dada GO. Modulation of Dcytb (Cybrd 1) expression and function by iron, dehydroascorbate and Hif-2alpha in cultured cells. Biochim Biophys Acta 1840: 106–112, 2014 [DOI] [PubMed] [Google Scholar]
- 67.Mackenzie B, Garrick MD. Iron Imports. II. Iron uptake at the apical membrane in the intestine. Am J Physiol Gastrointest Liver Physiol 289: G981–G986, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Mackenzie B, Takanaga H, Hubert N, Rolfs A, Hediger MA. Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1). Biochem J 403: 59–69, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest 119: 1159–1166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matak P, Zumerle S, Mastrogiannaki M, El Balkhi S, Delga S, Mathieu JR, Canonne-Hergaux F, Poupon J, Sharp PA, Vaulont S, Peyssonnaux C. Copper deficiency leads to anemia, duodenal hypoxia, upregulation of HIF-2alpha and altered expression of iron absorption genes in mice. PLoS One 8: e59538, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McDonald CJ, Wallace DF, Crawford DH, Subramaniam VN. Iron storage disease in Asia-Pacific populations: the importance of non-HFE mutations. J Gastroenterol Hepatol 28: 1087–1094, 2013 [DOI] [PubMed] [Google Scholar]
- 72.McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A, Peters TJ, Raja KB, Shirali S, Hediger MA, Farzaneh F, Simpson RJ. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 291: 1755–1759, 2001 [DOI] [PubMed] [Google Scholar]
- 73.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 5: 299–309, 2000 [DOI] [PubMed] [Google Scholar]
- 74.Millard KN, Frazer DM, Wilkins SJ, Anderson GJ. Changes in the expression of intestinal iron transport and hepatic regulatory molecules explain the enhanced iron absorption associated with pregnancy in the rat. Gut 53: 655–660, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mims MP, Guan Y, Pospisilova D, Priwitzerova M, Indrak K, Ponka P, Divoky V, Prchal JT. Identification of a human mutation of DMT1 in a patient with microcytic anemia and iron overload. Blood 105: 1337–1342, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Munoz M, Botella-Romero F, Gomez-Ramirez S, Campos A, Garcia-Erce JA. Iron deficiency and anaemia in bariatric surgical patients: causes, diagnosis and proper management. Nutr Hosp 24: 640–654, 2009 [PubMed] [Google Scholar]
- 77.Nai A, Pagani A, Mandelli G, Lidonnici MR, Silvestri L, Ferrari G, Camaschella C. Deletion of TMPRSS6 attenuates the phenotype in a mouse model of beta-thalassemia. Blood 119: 5021–5029, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol 122: 78–86, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood 105: 1803–1806, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA 98: 8780–8785, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA 99: 4596–4601, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 110: 1037–1044, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood 108: 1388–1394, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osaki S. Kinetic studies of ferrous ion oxidation with crystalline human ferroxidase (ceruloplasmin). J Biol Chem 241: 5053–5059, 1966 [PubMed] [Google Scholar]
- 86.Osaki S, Johnson DA, Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem 241: 2746–2751, 1966 [PubMed] [Google Scholar]
- 87.Osterloh KR, Simpson RJ, Snape S, Peters TJ. Intestinal iron absorption and mucosal transferrin in rats subjected to hypoxia. Blut 55: 421–431, 1987 [DOI] [PubMed] [Google Scholar]
- 88.Papanikolaou G, Samuels ME, Ludwig EH, MacDonald ML, Franchini PL, Dube MP, Andres L, MacFarlane J, Sakellaropoulos N, Politou M, Nemeth E, Thompson J, Risler JK, Zaborowska C, Babakaiff R, Radomski CC, Pape TD, Davidas O, Christakis J, Brissot P, Lockitch G, Ganz T, Hayden MR, Goldberg YP. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet 36: 77–82, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J 15: 6084–6095, 1996 [PMC free article] [PubMed] [Google Scholar]
- 90.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest 117: 1926–1932, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis 32: 131–138, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Pourvali K, Matak P, Latunde-Dada GO, Solomou S, Mastrogiannaki M, Peyssonnaux C, Sharp PA. Basal expression of copper transporter 1 in intestinal epithelial cells is regulated by hypoxia-inducible factor 2alpha. FEBS Lett 586: 2423–2427, 2012 [DOI] [PubMed] [Google Scholar]
- 93.Price L, Kowdley KV. The role of iron in the pathophysiology and treatment of chronic hepatitis C. Can J Gastroenterol 23: 822–828, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127: 917–928, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Raja KB, Pippard MJ, Simpson RJ, Peters TJ. Relationship between erythropoiesis and the enhanced intestinal uptake of ferric iron in hypoxia in the mouse. Br J Haematol 64: 587–593, 1986 [DOI] [PubMed] [Google Scholar]
- 96.Raja KB, Simpson RJ, Pippard MJ, Peters TJ. In vivo studies on the relationship between intestinal iron (Fe3+) absorption, hypoxia and erythropoiesis in the mouse. Br J Haematol 68: 373–378, 1988 [DOI] [PubMed] [Google Scholar]
- 97.Ranganathan PN, Lu Y, Fuqua BK, Collins JF. Discovery of a cytosolic/soluble ferroxidase in rodent enterocytes. Proc Natl Acad Sci USA 109: 3564–3569, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ranganathan PN, Lu Y, Fuqua BK, Collins JF. Immunoreactive hephaestin and ferroxidase activity are present in the cytosolic fraction of rat enterocytes. Biometals 25: 687–695, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ranganathan PN, Lu Y, Jiang L, Kim C, Collins JF. Serum ceruloplasmin protein expression and activity increases in iron-deficient rats and is further enhanced by higher dietary copper intake. Blood 118: 3146–3153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ravia JJ, Stephen RM, Ghishan FK, Collins JF. Menkes Copper ATPase (Atp7a) is a novel metal-responsive gene in rat duodenum, and immunoreactive protein is present on brush-border and basolateral membrane domains. J Biol Chem 280: 36221–36227, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reeves PG, Demars LC, Johnson WT, Lukaski HC. Dietary copper deficiency reduces iron absorption and duodenal enterocyte hephaestin protein in male and female rats. J Nutr 135: 92–98, 2005 [DOI] [PubMed] [Google Scholar]
- 102.Rivella S. Ineffective erythropoiesis and thalassemias. Curr Opin Hematol 16: 187–194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood 106: 2196–2199, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, Loukopoulos D, Camaschella C. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet 33: 21–22, 2003 [DOI] [PubMed] [Google Scholar]
- 105.Sanchez M, Galy B, Muckenthaler MU, Hentze MW. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat Struct Mol Biol 14: 420–426, 2007 [DOI] [PubMed] [Google Scholar]
- 106.Schmidt PJ, Toudjarska I, Sendamarai AK, Racie T, Milstein S, Bettencourt BR, Hettinger J, Bumcrot D, Fleming MD. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(−/−) mice and ameliorates anemia and iron overload in murine beta-thalassemia intermedia. Blood 121: 1200–1208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab 9: 152–164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shawki A, Knight PB, Maliken BD, Niespodzany EJ, Mackenzie B. H(+)-coupled divalent metal-ion transporter-1: functional properties, physiological roles and therapeutics. Curr Top Membr 70: 169–214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell 122: 789–801, 2005 [DOI] [PubMed] [Google Scholar]
- 110.Shi H, Bencze KZ, Stemmler TL, Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science 320: 1207–1210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith MW, Debnam ES, Dashwood MR, Srai SK. Structural and cellular adaptation of duodenal iron uptake in rats maintained on an iron-deficient diet. Pflugers Arch 439: 449–454, 2000 [DOI] [PubMed] [Google Scholar]
- 112.Sourkes TL, Lloyd K, Birnbaum H. Inverse relationship of heptic copper and iron concentrations in rats fed deficient diets. C J Biochem 46: 267–271, 1968 [DOI] [PubMed] [Google Scholar]
- 113.Tanaka T, Roy CN, Yao W, Matteini A, Semba RD, Arking D, Walston JD, Fried LP, Singleton A, Guralnik J, Abecasis GR, Bandinelli S, Longo DL, Ferrucci L. A genome-wide association analysis of serum iron concentrations. Blood 115: 94–96, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, Gonzalez FJ, Shah YM. Hypoxia-inducible factor-2alpha mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 140: 2044–2055, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tchernitchko D, Bourgeois M, Martin ME, Beaumont C. Expression of the two mRNA isoforms of the iron transporter Nramp2/DMTI in mice and function of the iron responsive element. Biochem J 363: 449–455, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tennant J, Stansfield M, Yamaji S, Srai SK, Sharp P. Effects of copper on the expression of metal transporters in human intestinal Caco-2 cells. FEBS Lett 527: 239–244, 2002 [DOI] [PubMed] [Google Scholar]
- 117.Vanoaica L, Darshan D, Richman L, Schumann K, Kuhn LC. Intestinal ferritin H is required for an accurate control of iron absorption. Cell Metab 12: 273–282, 2010 [DOI] [PubMed] [Google Scholar]
- 118.Vlachodimitropoulou E, Naftalin RJ, Sharp PA. Quercetin is a substrate for the transmembrane oxidoreductase Dcytb. Free Radic Biol Med 48: 1366–1369, 2010 [DOI] [PubMed] [Google Scholar]
- 119.Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 21: 195–199, 1999 [DOI] [PubMed] [Google Scholar]
- 120.Wallace DF, Subramaniam VN. Non-HFE haemochromatosis. World J Gastroenterol 13: 4690–4698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wayhs ML, Patricio FS, Amancio OM, Pedroso MZ, Neto UF, Morais MB. Morphological and functional alterations of the intestine of rats with iron-deficiency anemia. Braz J Med Biol Res 37: 1631–1635, 2004 [DOI] [PubMed] [Google Scholar]
- 122.Xie L, Collins JF. Transcription factors Sp1 and Hif2alpha mediate induction of the copper-transporting ATPase (Atp7a) gene in intestinal epithelial cells during hypoxia. J Biol Chem 288: 23943–23952, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xie L, Collins JF. Transcriptional regulation of the Menkes copper ATPase (Atp7a) gene by hypoxia-inducible factor (HIF2α) in intestinal epithelial cells. Am J Physiol Cell Physiol 300: C1298–C1305, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xue X, Taylor M, Anderson E, Hao C, Qu A, Greenson JK, Zimmermann EM, Gonzalez FJ, Shah YM. Hypoxia-inducible factor-2alpha activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res 72: 2285–2293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yeh KY, Yeh M, Glass J. Interactions between ferroportin and hephaestin in rat enterocytes are reduced after iron ingestion. Gastroenterology 141: 292–299, 2011 [DOI] [PubMed] [Google Scholar]
- 126.Yeh KY, Yeh M, Mims L, Glass J. Iron feeding induces ferroportin 1 and hephaestin migration and interaction in rat duodenal epithelium. Am J Physiol Gastrointest Liver Physiol 296: G55–G65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zerounian NR, Linder MC. Effects of copper and ceruloplasmin on iron transport in the Caco 2 cell intestinal model. J Nutr Biochem 13: 138–148, 2002 [DOI] [PubMed] [Google Scholar]
- 128.Zhang DL, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab 9: 461–473, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]