Abstract
Early hyperglycemia after trauma increases morbidity and mortality. Insulin is widely used to control posttrauma glucose, but this treatment increases the risk of hypoglycemia. We tested a novel method for early posttrauma hyperglycemia control by suppressing hepatic glycogenolysis via β2-adrenoreceptor blockade [ICI-118551 (ICI)]. We have shown that, after severe trauma, obese Zucker (OZ) rats, similar to obese patients, exhibit increased acute lung injury compared with lean Zucker (LZ) rats. We hypothesized that OZ rats exhibit a greater increase in early posttrauma glucose compared with LZ rats, with the increased posttrauma hyperglycemia suppressed by ICI treatment. Orthopedic trauma was applied to both hindlimbs in LZ and OZ rats. Fasting plasma glucose was then monitored for 6 h with or without ICI (0.2 mg·kg−1·h−1 iv.) treatment. One day after trauma, plasma IL-6 levels, lung neutrophil numbers, myeloperoxidase (MPO) activity, and wet-to-dry weight ratios were measured. Trauma induced rapid hepatic glycogenolysis, as evidenced by decreased liver glycogen levels, and this was inhibited by ICI treatment. Compared with LZ rats, OZ rats exhibited higher posttrauma glucose, IL-6, lung neutrophil infiltration, and MPO activity. Lung wet-to-dry weight ratios were increased in OZ rats but not in LZ rats. ICI treatment reduced the early hyperglycemia, lung neutrophil retention, MPO activity, and wet-to-dry weight ratio in OZ rats to levels comparable with those seen in LZ rats, with no effect on blood pressure or heart rate. These results demonstrate that β2-adrenoreceptor blockade effectively reduces the early posttrauma hyperglycemia, which is associated with decreased lung injury in OZ rats.
Keywords: obesity, trauma, glucose, lung injury, β2-adrenoreceptor
unintentional injuries are the leading cause of mortality in individuals under 50 yr of age in the United States, with staggering medical expenses attributable to traumatic injury (10a, 43). Obese patients with severe orthopedic trauma are at a higher risk than lean patients for increased inflammation, multiorgan failure, prolonged hospital stay, and increased mortality (5, 8, 10, 12). Although the mechanisms responsible for the increased posttrauma complications in obese patients are unclear, intensive and specific treatments have been emphasized for these patients.
Posttrauma hyperglycemia has been recognized as a risk factor that exacerbates complications, organ dysfunction, and mortality (29, 41, 44). Several clinical studies have suggested that, in critically ill obese patients, impaired glucose homeostasis appears to be a better predictor of increased complications and mortality than body mass index (38, 41, 45). In addition, there is evidence that the early hyperglycemia within the first day after trauma is a more reliable predictor of poor outcomes and mortality compared with later increases in plasma glucose (7, 16). A study (45) monitoring glucose levels for 4 days after trauma showed that nonsurvivors only exhibited a significant increase in glucose (>150 mg/dl) within the first half day after trauma, with peak levels observed at admission. Together, these findings suggest that early (stress-induced) hyperglycemia plays an important role in contributing to the adverse outcomes after trauma. Therefore, the present study focused on glucose responses within 6 h after severe trauma in lean Zucker (LZ) and obese Zucker (OZ) rats and examined systemic inflammation and lung complications on the following day.
Glucose control with insulin is widely used in clinical practice after trauma. However, a number of studies have reported that insulin treatment also increases the incidence of hypoglycemic episodes, which can adversely affect outcomes after trauma (13, 30). Moreover, treatment of early hyperglycemia can be more challenging in obese patients, as the dosages of insulin and responses of glucose after insulin administration are difficult to predict and control due to insulin resistance. The present study was designed to answer an important question: is it possible to successfully control the early increase in glucose with minimal risk of hypoglycemia, especially in obese subjects? We tested a novel method for early glucose control in the context of obesity via the suppression of hepatic glycogenolysis, a major pathway for stress-induced hyperglycemia (31). Stress-induced hepatic glycogenolysis is partially mediated by β2-adrenoreceptor activation in the liver (3). In addition, pancreatic β2-adrenoreceptor activation can increase glucagon release and thus further increase hepatic glycogenolysis. Therefore, we tested the effect of a β2-adrenoreceptor antagonist [ICI-118551 (ICI)] on early glucose levels after severe trauma in an animal model of obesity.
OZ rats have been widely used as a model of obesity. Similar to obese subjects, OZ rats exhibit central obesity, insulin resistance, hyperlipidemia, and cardiovascular dysfunction (9, 46). We have previously shown that, compared with LZ rats, OZ exhibit exacerbated systemic inflammation and acute lung injury (ALI) after severe trauma (48). Given that early posttrauma hyperglycemia is a predictor of adverse outcomes (7, 16, 45), we hypothesized that OZ rats exhibit a greater increase in early posttrauma glucose compared with LZ rats, with the increased posttrauma hyperglycemia suppressed by ICI treatment.
METHODS
Animals
Male LZ and OZ rats (∼12 wk) were purchased from Harlan Laboratories (Indianapolis, IN). The experimental protocols for this study were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center and were carried out according to both the National Institutes of Health Guide for the Care and Use of Laboratory Animals and guidelines of the Animal Welfare Act.
Severe Orthopedic Trauma Protocols
Severe trauma was applied to both hindlimbs in LZ and OZ rats under anesthesia (5% isoflurane inhalation), as previously described (36a, 48). In brief, soft tissue injury was induced by crushing the muscle groups adjacent to both the femur and fibula followed by the injection of bone components (1.5 ml/leg) near the femur. During the insertion of the needle, the fibula was fractured in each leg. The femur and tibia bones used to make the bone component suspension to inject into LZ and OZ rats were harvested from previously euthanized LZ and OZ rats, respectively. Directly before the trauma, rats were given a subcutaneous injection of buprenorphine (0.01 mg/kg) to minimize discomfort, and every 8–12 h after trauma, rats were given an additional dosage (0.05 mg/kg).
Clinical classification defines “severe trauma” as being an injury severity score of >16. Evaluating our model using the clinical classification (44a), the estimated total injury severity score for the bilateral injury was >18 (48). Our aim was to mimic severe trauma from a large long bone (femur) fracture, in which soft tissue injury and the release of bone components are present at the same time. We induced fibula fracture to simulate the stress of long bone fracture, but unlike a femur fracture, this manipulation does not necessitate fixation surgery that could exacerbate outcomes (37, 39). Because the fibula is very small, we injected additional bone components to the injured area as described in previous studies (35, 36a, 48). These manipulations cannot completely mimic the exact circumstance of a trauma involving a long bone fracture in humans, as this is virtually impossible due to the highly heterogeneous nature of traumatic injury. Instead, this trauma protocol focused more on mimicking posttrauma outcomes rather than the exact injury components.
Experimental protocol 1: measurements within 6 h after severe trauma.
PROTOCOL 1.1: HYPERGLYCEMIC AND HEMODYNAMIC RESPONSES TO SEVERE TRAUMA.
Catheters were implanted in the carotid artery and jugular vein in LZ and OZ rats, as previously described (47). Animals were equilibrated and fasted for 5–6 h before the start of experiments. All measurements were performed in conscious rats with free access to only water. Blood pressure and heart rate were recorded via carotid catheters using a PowerLab system (model ML 118). Glucose levels were measured with a glucometer using blood sampled from tail tips pretreated with Sensorcaine (On Call Plus, ACON Laboratories). Blood pressure, heart rate, and glucose levels were recorded before (B0) and 20 min after (B1) the start of the infusion of ICI (0.2 mg·kg−1·h−1 iv), a β2-adrenoreceptor antagonist, or vehicle (0.9% saline) via the jugular catheter. After a 20-min infusion, severe trauma was induced, and blood pressure, heart rate, and glucose levels were then measured at 10, 15, 30, 60, 120, 180, 240, 300, and 360 min after the initiation of the trauma. Trauma was performed as described above, and animals were recovered from isoflurane anesthesia within 5 min. LZ and OZ rats received a total volume of ∼200 μl/100 g body wt throughout the 6 h of the intravenous infusion.
PROTOCOL 1.2: PLASMA CORTISOL, INSULIN, GLUCAGON, AND LIVER GLYCOGEN LEVELS.
In an additional set of experiments, LZ and OZ rats were fasted for 5–6 h, and half of the animals were then treated with severe trauma. Tail vein blood (<500 μl) was collected before and 1 h after trauma to measure cortisol levels by radioimmunoassay. Six hours after trauma, control and traumatized animals were decapitated, and blood samples were collected to measure insulin and glucagon by ELISA (R&D Systems, Minneapolis, MN). The medial lobe of the liver was collected to measure glycogen levels using a glycogen assay kit (AB65620, Abcam, Cambridge, MA). To confirm the inhibitory effect of ICI on hepatic glycogenolysis, liver glycogen contents were measured 6 h after trauma in an additional group of ICI-treated LZ and OZ rats.
Experimental protocol 2: measurements 1 day after severe trauma.
After the 6-h measurements, animals from protocols 1.1 and 1.2 were returned to the animal room (at 22°C, 12:12-h light-dark cycle) with food and water ad libitum. One day after trauma, rats were decapitated, and blood was collected to measure IL-6 levels by ELISA (R&D Systems). Circulating IL-6 levels have been previously demonstrated to be an early indicator of systemic inflammation after trauma (19, 20) and are exacerbated in obese patients (21). Lung lobes were collected to measure lung edema, neutrophil counts, and myeloperoxidase (MPO) activity as we have previously described (48). We collected lung lobes from decapitated rats to minimize the blood volume trapped in the lung circulation.
In brief, the lower left lung lobe was isolated and stored at room temperature for 3–4 wk until a stable weight was achieved. The wet-to-dry weight ratio was used as an index of pulmonary edema. The other lung lobes were fixed in formalin or liquid nitrogen immediately after isolation. After 1 day in formalin, tissues were transferred to 70% alcohol for histological analysis of neutrophil numbers using a Vectastain ABC Kit (Vector Laboratories, Marion, IA). HistoMark Black (KPL 54-75-00) was used to visualize positive staining, with eosin as a counterstain. An antibody specific for neutrophil elastase (Anti-Neutrophil Elastase antibody, ab21595, Abcam, 1:200) was used to identify neutrophils. Neutrophil quantification was calculated as the number of positive cells per ×40 high-magnification field. Six random scans per section were analyzed and averaged. Liquid nitrogen-fixed tissues were stored at −80°C for later measurements of MPO activity. Lung tissue was homogenized, and the activity of MPO was determined using an Invitrogen EnzChek Assay Kit (Life Technologies, Grand Island, NY). Enzyme activity was normalized by protein concentration.
Quantitative and Statistical Analyses
Data were compared using two-way ANOVA or repeated-measures ANOVA. Where significant effects occurred, individual groups were compared using the Holm-Sidak method. All data are presented as means ± SE. P values of <0.05 were accepted as statistically significant for all comparisons.
RESULTS
Measurements Within 6 h After Severe Trauma in LZ and OZ Rats
Posttrauma glucose and liver glycogen levels in LZ and OZ rats.
Basal glucose levels were not significantly different between LZ and OZ rats (Fig. 1A). ICI or saline infusion did not affect basal glucose levels in LZ or OZ rats. In Fig. 1A, B0 and B1 are the basal levels before and 20 min after ICI/saline infusion, respectively. Severe trauma immediately increased glucose levels in LZ and OZ rats, with OZ rats exhibiting significantly higher hyperglycemia compared with LZ rats. Glucose levels in LZ rats returned to baseline 240 min after trauma, whereas OZ rats remained hyperglycemic during the 6 h of measurements. ICI treatment completely blocked the initial increase in glucose levels in both LZ and OZ rats. Compared with B1, posttrauma glucose was only increased from 180 to 300 min in ICI-treated LZ rats. In ICI-treated OZ rats, glucose did not increase until 30 min after trauma. A significant suppression in glucose levels was observed from 10 to 30 min in ICI-treated LZ rats and from 10 to 240 min in ICI-treated OZ rats compared with their respective groups that had trauma but received no treatment.
Fig. 1.
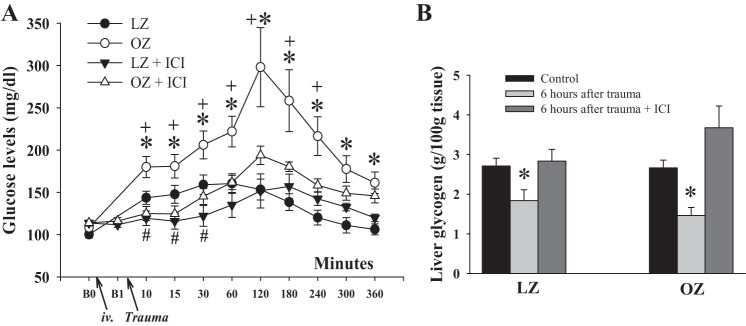
A: glucose curve in lean Zucker (LZ) and obese Zucker (OZ) rats with or without [ICI-118551 (ICI)] treatment within 6 h after trauma. B0 is the basal level before ICI or saline infusion, and B1 is the basal level before trauma. n ≥ 6 for each group. *P < 0.05, LZ vs. OZ rats; #P < 0.05, LZ vs. LZ + ICI rats; +P < 0.05, OZ vs. OZ + ICI rats. B: liver glycogen levels 6 h after trauma in LZ and OZ rats with or without ICI treatment. n = 5 for each group. *P < 0.05, trauma vs. control or ICI.
Hepatic glycogen concentrations were similar between control LZ and OZ rats (Fig. 1B). At 6 h after trauma, hepatic glycogen levels decreased similarly in LZ and OZ rats but were not significantly changed in ICI-treated rats compared with control levels.
Posttrauma blood pressure and heart rate in LZ and OZ rats.
Basal mean blood pressure and heart rate were similar between LZ and OZ rats before (B0) or after 20 min of ICI or saline infusion (B1; Fig. 2, A and B). ICI treatment had no effect on basal blood pressure and heart rate in LZ or OZ rats. Heart rate was increased immediately after trauma in both LZ and OZ rats, with LZ rats exhibiting higher heart rates within the first 15 min compared with OZ rats. Blood pressure was not significantly changed after trauma in LZ or OZ rats. ICI treatment had no effect on posttrauma blood pressure and heart rate in LZ or OZ rats.
Fig. 2.
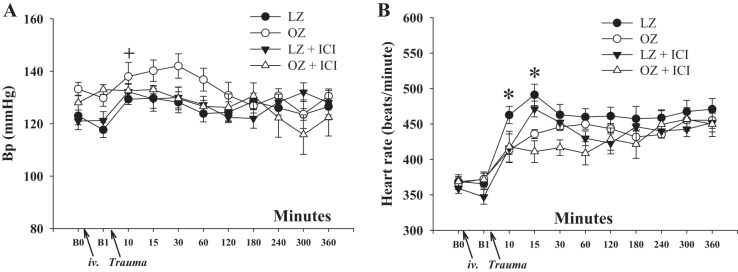
A and B: blood pressure (Bp; A) and heart rate (B) in LZ and OZ rats within 6 h after trauma with or without ICI treatment. B0 is the basal level before ICI or saline infusion, and B1 is the basal level before trauma. n ≥ 5 for each group. *P < 0.05, LZ vs. OZ rats; +P < 0.05 vs. B1 in all groups.
Plasma cortisol, insulin, and glucagon levels in LZ and OZ rats.
Basal cortisol levels were not different between LZ and OZ rats (Fig. 3A). One hour after trauma, cortisol levels were similarly increased in LZ and OZ rats. Insulin levels were significantly higher in OZ rats compared with LZ rats, whereas insulin levels 6 h after trauma were similar to baseline levels in LZ and OZ rats (Fig. 3B). OZ rats exhibited higher basal plasma glucagon levels compared with LZ rats, whereas 6 h after trauma, plasma glucagon levels decreased in OZ rats but remained unchanged in LZ rats (Fig. 3C).
Fig. 3.
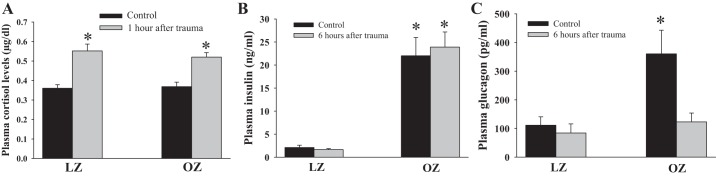
A: cortisol levels before and 1 h after trauma in LZ and OZ rats. n = 6 for each group. *P < 0.05, trauma vs. basal. B: plasma insulin levels in 6 h after trauma in LZ and OZ rats. n = 6 for each group. *P < 0.01, LZ vs. OZ rats. C: glucagon levels 6 h after trauma in LZ and OZ rats. n = 6 for each group. *P < 0.05 vs. LZ or OZ rats with 6 h after trauma.
Measurements 1 Day After Severe Trauma in LZ and OZ Rats
Plasma IL-6 levels in LZ and OZ rats with or without ICI treatment.
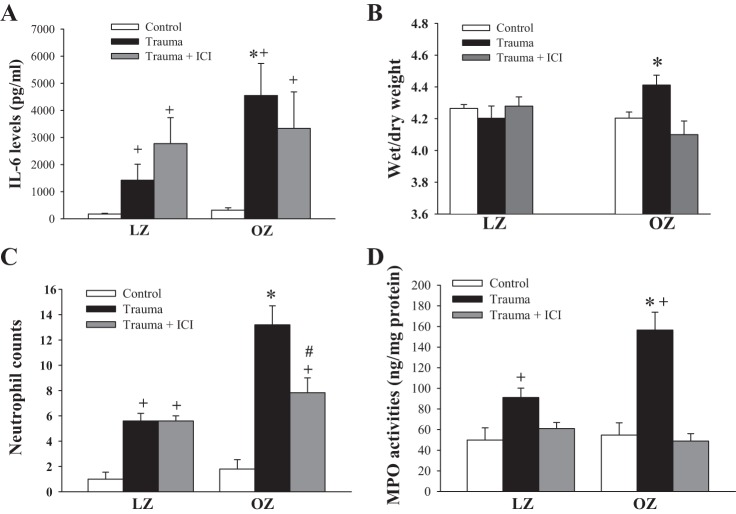
IL-6 levels were increased in all animal groups 1 day after severe trauma, with OZ rats exhibiting much greater increases in IL-6 compared with LZ rats (Fig. 4A). ICI treatment had no significant effect on plasma IL-6 levels in LZ and OZ rats.
Fig. 4.
A: IL-6 levels 1 day after trauma. n ≥ 6 for each group. *P < 0.05 vs. LZ rats with trauma; +P < 0.01 vs. control. B: wet-to-dry weight ratio 1 day after trauma. n ≥ 6 for each group. *P < 0.05 vs. control. C: lung neutrophil numbers 1 day after trauma. n ≥ 5 for each group. *P < 0.01 vs. LZ rats with trauma; +P < 0.01 vs. control; #P < 0.01, trauma vs. ICI within OZ rats. D: lung myeloperoxidase (MPO) activities 1 day after trauma. n ≥ 5 for each group. *P < 0.05 vs. LZ rats with trauma; +P < 0.05 vs. control.
Lung wet-to-dry weight ratio in LZ and OZ rats with or without ICI treatment.
The basal wet-to-dry weight ratio was similar between nontrauma LZ and OZ rats (Fig. 4B). One day after trauma, the wet-to-dry weight ratio was unchanged in LZ rats but was significantly increased in OZ rats. There was no increase in the wet-to-dry ratio in ICI-treated OZ rats.
Lung neutrophil infiltration and MPO activity.
Basal neutrophil numbers and MPO activities were similar between control LZ and OZ rats (Fig. 4, C and D). One day after trauma, neutrophil numbers and MPO activities were increased in all animal groups. OZ rats exhibited larger increases in neutrophil accumulation and MPO activity compared with LZ rats. ICI treatment significantly decreased lung neutrophil numbers in OZ rats but had no effect in LZ rats. MPO activity was normalized by ICI treatment in LZ and OZ rats.
DISCUSSION
The major findings of the present study were 1) trauma induced a rapid increase in plasma glucose levels due to β2-adrenoreceptor-mediated hepatic glycogenolysis; 2) compared with LZ rats, OZ rats exhibited increased and prolonged early posttrauma hyperglycemia, but there was no difference in hepatic glycogenolysis or the response to cortisol; 3) 6 h after trauma, glucose levels were still above baseline in OZ rats, but insulin levels were not significantly increased; and 4) ICI treatment decreased early posttrauma hyperglycemia and prevented the later development of ALI in OZ rats.
Activation of Hepatic β2-Adrenoreceptors Is Important for Early Posttrauma Hyperglycemia
Stress-induced hyperglycemia is thought to occur due to synergistic regulation by the hypothalamic-pituitary axis, inflammatory cytokines, and noradrenergic system (13). Traumatic stress can rapidly increase sympathetic nerve activity (SNA) and/or circulating catecholamines, as evidenced by the tachycardia and β2-adrenoreceptor-mediated increase in glucose immediately after trauma in the present study. Activation of β2-adrenoreceptors in the liver, directly, or in the pancreas, indirectly through glucagon release, can rapidly increase plasma glucose levels via hepatic glycogenolysis. We found that ICI treatment decreased hepatic glycogenolysis and posttrauma glucose, suggesting that hepatic glycogenolysis due to β2-adrenoreceptor activation plays an important role in mediating the early posttrauma hyperglycemia. In addition, ICI did not significantly affect blood pressure or heart rate, suggesting that the early activation of β2-adrenoreceptors after trauma did not occur in peripheral vessels, and thus the posttrauma glucose response was unlikely to be due to hemodynamic changes.
Impaired Glucose Uptake Is Involved in the Increased Early Posttrauma Hyperglycemia in OZ Rats
Liver glycogen levels were similar between LZ and OZ rats before and 6 h after trauma. Thus, the greater increase in posttrauma glucose in OZ rats does not appear to be due to an increased glycogenolytic rate. Cortisol can be rapidly released in response to a traumatic stress and participates in glucose homeostasis by increasing gluconeogenesis and inhibiting peripheral utilization of glucose. As a steroid hormone, cortisol cannot explain the rapid increase in glucose after trauma in LZ and OZ rats but might contribute to the relatively late (>1 h) increase in glucose. We compared cortisol levels between LZ and OZ rats before and 1 h after trauma because the difference in glucose levels between ICI-treated LZ and OZ rats appears to be most significant from 1 to 2 h after trauma. However, we found that cortisol levels were similarly increased in LZ and OZ rats, suggesting a minor role of cortisol in contributing to the exacerbated hyperglycemia in OZ rats.
Basal glucagon levels have been shown to be strongly correlated with body mass index (2). This is supported by our present study, which showed that OZ rats exhibited significantly higher basal levels of glucagon compared with LZ rats. Additionally, human and animal studies have demonstrated that obesity is associated with “glucagon resistance” and decreased glucagon receptors (6, 11, 36), which may explain why OZ rats exhibited higher basal glucagon levels but similar liver glycogen content compared with LZ rats. Six hours after trauma, glucagon levels were unchanged in LZ rats and were decreased in OZ rats. The peak increases in plasma glucagon may have been missed due to its short half-life (∼5 min). The decreased levels of glucagon 6 h after trauma in OZ rats were accompanied by hyperglycemia (>150 mg/dl; Fig. 1), suggesting that glucagon could be suppressed by the high glucose levels and did not play a role in exaggerating the hyperglycemia or hepatic glycogenolysis in OZ rats.
Six hours after trauma, insulin levels were not elevated above baseline levels despite the elevated glucose, suggesting a suppression of insulin release (Fig. 3B). A possible explanation for the absence of increased insulin levels is that trauma may also activate pancreatic α2-adrenoreceptors, which can inhibit insulin synthesis and secretion (17). For example, during exercise, insulin release can be suppressed due to increased SNA and β-cell α-adrenergic receptor activation (34). The inhibition in insulin secretion after trauma may also occur in LZ rats, but the impact appears to be minimal as glucose returned to baseline within 4 h after trauma in these animals. Therefore, the increased posttrauma hyperglycemia in OZ rats could be due, at least in part, to the combined effects of insulin resistance and suppressed insulin release. In addition, severe trauma may also decrease insulin sensitivity (26, 33, 50), so the glucose homeostasis in response to traumatic stress is complicated and warrants future studies to clearly elucidate these mechanisms.
Significance and Clinical Relevance
Clinical studies have shown that increased complications after trauma have a stronger correlation with plasma glucose levels than with body mass index or dyslipidemia (38, 41, 45). In addition, early posttrauma hyperglycemia has been found to be a more reliable predictor of poor outcomes and mortality compared with later increases in plasma glucose (7, 16). Hyperglycemia has been shown to increase ROS and affect neutrophil responses, including neutrophil retention (27) and exacerbated free radical release (28), which are important processes in innate immune responses (22, 49) and ALI. Indeed, our previous study (48) showed that early antioxidant treatment after severe trauma targeting the enzyme responsible for the neutrophil respiratory burst decreases systemic inflammation and prevents the development of ALI in OZ rats. The underlying mechanisms whereby early posttrauma hyperglycemia increases later adverse outcomes are unclear and beyond the scope of the present study.
We found increased ALI in OZ rats 1 day after trauma, as indicated by neutrophil retention, elevated lung MPO activity, increased capillary permeability (48), and lung edema. Inhibition of the increased early hyperglycemia by ICI treatment prevented the development of ALI in OZ rats. These results may provide novel insights into the mechanisms responsible for exacerbated morbidity and mortality and the treatment for the exaggerated early posttrauma hyperglycemia in obese patients. We did not use exogenous insulin to decrease early posttrauma hyperglycemia in OZ rats or to clamp posttrauma glucose at higher levels in LZ rats to confirm the impacts of early hyperglycemia. This is because the innate immune responses after trauma may be differentially affected by preexisting pathophysiological factors in obesity and diabetes (4, 25, 32, 41). Moreover, in addition to the risk of hypoglycemia, insulin treatment has been shown to elicit significant anti-inflammatory effects, which may confound the interpretation of the results (1, 14, 15, 24). Indeed, a number of studies have shown that diabetic patients with regular insulin treatment appear to be protected against sepsis-induced lung injury (18, 23).
Conclusions
OZ rats exhibited increased early posttrauma hyperglycemia compared with LZ rats, which was associated with exacerbated inflammation and the development of ALI. Treatment with a β2-adrenoreceptor antagonist effectively reduced the early posttrauma hyperglycemia and prevented ALI in OZ rats. This study uncovers important differences in glucose homeostasis in the context of obesity after trauma and provides novel insights into the possible use of β2-adrenoreceptor antagonists as a treatment for the exaggerated early posttrauma hyperglycemia seen in the obese population.
GRANTS
This work was supported by American Heart Association Grants AHA-12SDG12050525 and AHA-12POST12060126, National Institutes of Health Grants P20-GM-104357, HL-51971, and HL-89581, and a Basic Science Research Grant from the Orthopaedic Trauma Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.X. conception and design of research; L.X., S.L., P.M., J.C., and G.W.H. performed experiments; L.X., S.L., and P.M. analyzed data; L.X. and J.C. interpreted results of experiments; L.X. and S.L. prepared figures; L.X. drafted manuscript; L.X., S.L., P.M., J.C., and R.L.H. edited and revised manuscript; L.X., S.L., J.C., G.W.H., and R.L.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Merry Lindsey's laboratory and Haiyan Zhang for the technical help.
REFERENCES
- 1.Alba-Loureiro TC, Martins EF, Landgraf RG, Jancar S, Curi R, Sannomiya P. Role of insulin on PGE2 generation during LPS-induced lung inflammation in rats. Life Sci 78: 578–585, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Alford FP, Zimmet P, Chisholm DJ, Whitehouse S. Influence of obesity on basal glucagon levels in non-diabetic and diabetic Nauruans. Clin Endocrinol (Oxf) 19: 721–725, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Arinze IJ, Kawai Y. Adrenergic regulation of glycogenolysis in isolated guinea-pig hepatocytes: evidence that β2-receptors mediate catecholamine stimulation of glycogenolysis. Arch Biochem Biophys 225: 196–202, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Bellmeyer A, Martino JM, Chandel NS, Scott Budinger GR, Dean DA, Mutlu GM. Leptin resistance protects mice from hyperoxia- induced acute lung injury. Am J Respir Crit Care Med 175: 587–594, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belzberg H, Wo CC, Demetriades D, Shoemaker WC. Effects of age and obesity on hemodynamics, tissue oxygenation, and outcome after trauma. J Trauma 62: 1192–1200, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Bhathena SJ, Aparicio P, Revett K, Voyles N, Recant L. Effect of dietary carbohydrates on glucagon and insulin receptors in genetically obese female Zucker rats. J Nutr 117: 1291–1297, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Bochicchio GV, Bochicchio KM, Joshi M, Ilahi O, Scalea TM. Acute glucose elevation is highly predictive of infection and outcome in critically injured trauma patients. Ann Surg 252: 597–602, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Brown CV, Neville AL, Rhee P, Salim A, Velmahos GC, Demetriades D. The impact of obesity on the outcomes of 1,153 critically injured blunt trauma patients. J Trauma 59: 1048–1051, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Butcher JT, Goodwill AG, Stanley SC, Frisbee JC. Blunted temporal activity of microvascular perfusion heterogeneity in metabolic syndrome: a new attractor for peripheral vascular disease? Am J Physiol Heart Circ Physiol 304: H547–H558, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrnes MC, McDaniel MD, Moore MB, Helmer SD, Smith RS. The effect of obesity on outcomes among injured patients. J Trauma 58: 232–237, 2005 [DOI] [PubMed] [Google Scholar]
- 10a.Center for Disease Control and Prevention, National Center for Health Statistics. Accidents or Unintentional Injuries (online). http://www.cdc.gov/nchs/fastats/accidental-injury.htm [19 June 2014]. [Google Scholar]
- 11.Charbonneau A, Unson CG, Lavoie JM. High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. J Physiol 579: 255–267, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. Obesity increases risk of organ failure after severe trauma. J Am Coll Surg 203: 539–545, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Collier B, Dossett LA, May AK, Diaz JJ. Glucose control and the inflammatory response. Nutr Clin Pract 23: 3–15, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Dandona P, Chaudhuri A, Mohanty P, Ghanim H. Anti-inflammatory effects of insulin. Curr Opin Clin Nutr Metab Care 10: 511–517, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Donnelly M, Condron C, Murray P, Bouchier-Hayes D. Modulation of the glycemic response using insulin attenuates the pulmonary response in an animal trauma model. J Trauma 63: 351–357, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Duane TM, Ivatury RR, Dechert T, Brown H, Wolfe LG, Malhotra AK, Aboutanos MB. Blood glucose levels at 24 hours after trauma fails to predict outcomes. J Trauma 64: 1184–1187, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Fitzpatrick D, Purves D, Augustine G. Table 20:2. In: Neuroscience (3rd ed.). Sunderland, MA: Sinauer, 2004, p. 489 [Google Scholar]
- 18.Frank JA, Nuckton TJ, Matthay MA. Diabetes mellitus: a negative predictor for the development of acute respiratory distress syndrome from septic shock. Crit Care Med 28: 2645–2646, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Frink M, van Griensven M, Kobbe P, Brin T, Zeckey C, Vaske B, Krettek C, Hildebrand F. IL-6 predicts organ dysfunction and mortality in patients with multiple injuries. Scand J Trauma Resusc Emerg Med 17: 49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannoudis PV, Harwood PJ, Loughenbury P, Van Griensven M, Krettek C, Pape HC. Correlation between IL-6 levels and the systemic inflammatory response score: can an IL-6 cutoff predict a SIRS state? J Trauma 65: 646–652, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Gletsu N, Lin E, Zhu JL, Khaitan L, Ramshaw BJ, Farmer PK, Ziegler TR, Papanicolaou DA, Smith CD. Increased plasma interleukin 6 concentrations and exaggerated adipose tissue interleukin 6 content in severely obese patients after operative trauma. Surgery 140: 50–57, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Guo RF, Ward PA. Role of oxidants in lung injury during sepsis. Antioxid Redox Signal 9: 1991–2002, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Honiden S, Gong MN. Diabetes, insulin, and development of acute lung injury. Crit Care Med 37: 2455–2464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyun E, Ramachandran R, Hollenberg MD, Vergnolle N. Mechanisms behind the anti-inflammatory actions of insulin. Crit Rev Immunol 31: 307–340, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Hyun E, Ramachandran R, Cenac N, Houle S, Rousset P, Saxena A, Liblau RS, Hollenberg MD, Vergnolle N. Insulin modulates protease-activated receptor 2 signaling: implications for the innate immune response. J Immunol 184: 2702–2709, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Wu GH, Zhang B, Han YS, Zhuang QL. Acute insulin resistance following surgical trauma in rats. Exp Clin Endocrinol Diabetes 120: 315–22, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita K, Kraydieh S, Alonso O, Hayashi N, Dietrich WD. Effect of posttraumatic hyperglycemia on contusion volume and neutrophil accumulation after moderate fluid-percussion brain injury in rats. J Neurotrauma 19: 681–692, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Kummer U, Zobeley J, Brasen JC, Fahmy R, Kindzelskii AL, Petty AR, Clark AJ, Petty HR. Elevated glucose concentrations promote receptor-independent activation of adherent human neutrophils: an experimental and computational approach. Biophys J 92: 2597–2607, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laird AM, Miller PR, Kilgo PD, Meredith JW, Chang MC. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma 56: 1058–1062, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Li L, Messina JL. Acute insulin resistance following injury. Trends Endocrinol Metab 20: 429–435, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Losser MR, Damoisel C, Payen D. Bench-to-bedside review: glucose and stress conditions in the intensive care unit. Crit Care 14: 231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lumeng CN. Innate immune activation in obesity. Mol Aspects Med 34: 12–29, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma Y, Wang P, Kuebler JF, Chaudry IH, Messina JL. Hemorrhage induces the rapid development of hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol 284: G107–G115, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Marliss EB, Vranic M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes 51, Suppl 1: S271–S283, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Menzel CL, Pfeifer R, Darwiche SS, Kobbe P, Gill R, Shapiro RA, Loughran P, Vodovotz Y, Scott MJ, Zenati MS, Billiar TR, Pape HC. Models of lower extremity damage in mice: time course of organ damage and immune response. J Surg Res 166: e149–e156, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mighiu PI, Yue JT, Filippi BM, Abraham MA, Chari M, Lam CK, Yang CS, Christian NR, Charron MJ, Lam TK. Hypothalamic glucagon signaling inhibits hepatic glucose production. Nat Med 19: 766–772, 2013 [DOI] [PubMed] [Google Scholar]
- 36a.Mittwede PN, Xiang L, Lu S, Clemmer JS, Hester RL. A novel experimental model of orthopedic trauma with acute kidney injury in obese Zucker rats. Physiol Rep 1: e00097, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morley JR, Smith RM, Pape HC, MacDonald DA, Trejdosiewitz LK, Giannoudis PV. Stimulation of the local femoral inflammatory response to fracture and intramedullary reaming: a preliminary study of the source of the second hit phenomenon. J Bone Joint Surg Br 90: 393–399, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Mowery NT, May AK, Collier BC, Dossett LA, Gunter OL, Dortch MJ, Diaz JJ., Jr Glucose metabolism, not obesity, predicts mortality in critically ill surgical patients. Am Surg 76: 1377–1383, 2010 [PubMed] [Google Scholar]
- 39.Pape HC, Griensven MV, Hildebrand FF, Tzioupis CT, Sommer KL, Krettek CC, Giannoudis PV; Epoff Study Group. Systemic inflammatory response after extremity or truncal fracture operations. J Trauma 65: 1379–1384, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Pieracci F, Hydo L, Eachempati S, Pomp A, Shou J, Barie PS. Higher body mass index predicts need for insulin but not hyperglycemia, nosocomial infection, or death in critically ill surgical patients. Surg Infect (Larchmt) 9: 121–130, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soni A. Medical Expenditure Panel Survey. Top 10 Most Costly Conditions Among Men and Women, 2008: Estimates for the U.S. Civilian Noninstitutionalized Adult Population, Age 18 and Older (online).http://meps.ahrq.gov/data_stats/Pub_ProdResults_Details.jsp?pt=Statistical+Brief&opt=2&id=1006 [19 June 2014]. [Google Scholar]
- 44.Sperry JL, Frankel HL, Vanek SL, Nathens AB, Moore EE, Maier RV, Minei JP. Early hyperglycemia predicts multiple organ failure and mortality but not infection. J Trauma 63: 487–494, 2007 [DOI] [PubMed] [Google Scholar]
- 44a.SurgicalCriticalCare.net/AcuteCareSurgery.net. Injury Severity Score (online). http://www.surgicalcriticalcare.net/Resources/injury_severity_scoring.pdf [19 June 2014]. [Google Scholar]
- 45.Vogelzang M, Nijboer JM, van der Horst IC, Zijlstra F, ten Duis HJ, Nijsten MW. Hyperglycemia has a stronger relation with outcome in trauma patients than in other critically ill patients. J Trauma 60: 873–879, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol 294: H1658–H1666, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Xiang L, Clemmer JS, Lu S, Mittwede PN. Impaired blood pressure compensation following hemorrhage in conscious obese Zucker rats. Life Sci 93: 214–219, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiang L, Lu S, Mittwede PN, Clemmer JS, Hester RL. Inhibition of NADPH oxidase prevents acute lung injury in obese rats following severe trauma. Am J Physiol Heart Circ Physiol 306: H684–H689, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol 78: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Zhai L, Messina JL. Age and tissue specific differences in the development of acute insulin resistance following injury. J Endocrinol 203: 365–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]