Abstract
There is evidence for developmental origins of vascular dysfunction yet little understanding of maturation of vascular smooth muscle (VSM) of regional circulations. We measured maturational changes in expression of myosin phosphatase (MP) and the broader VSM gene program in relation to mesenteric small resistance artery (SRA) function. We then tested the role of the sympathetic nervous system (SNS) in programming of SRAs and used genetically engineered mice to define the role of MP isoforms in the functional maturation of the mesenteric circulation. Maturation of rat mesenteric SRAs as measured by qPCR and immunoblotting begins after the second postnatal week and is not complete until maturity. It is characterized by induction of markers of VSM differentiation (smMHC, γ-, α-actin), CPI-17, an inhibitory subunit of MP and a key target of α-adrenergic vasoconstriction, α1-adrenergic, purinergic X1, and neuropeptide Y1 receptors of sympathetic signaling. Functional correlates include maturational increases in α-adrenergic-mediated force and calcium sensitization of force production (MP inhibition) measured in first-order mesenteric arteries ex vivo. The MP regulatory subunit Mypt1 E24+/LZ- isoform is specifically upregulated in SRAs during maturation. Conditional deletion of mouse Mypt1 E24 demonstrates that splicing of E24 causes the maturational reduction in sensitivity to cGMP-mediated vasorelaxation (MP activation). Neonatal chemical sympathectomy (6-hydroxydopamine) suppresses maturation of SRAs with minimal effect on a conduit artery. Mechanical denervation of the mature rat renal artery causes a reversion to the immature gene program. We conclude that the SNS captures control of the mesenteric circulation by programming maturation of the SRA smooth muscle.
Keywords: neural, mesenteric artery, myosin phosphatase, CPI-17, maturation
although there is increasing interest in the developmental origins of vascular disease (1, 39), there has been limited study of the functional maturation of the vascular system. In humans and other mammals, systemic blood pressure increases from birth through maturity. There is also evidence for maturational changes in the regulation of systemic circulations by constrictor and dilator signals dependent on the animal species and vascular bed (7, 31). Because of the steep inverse relationship between vessel diameter and resistance to flow, blood flow and pressure are predominately regulated in the small resistance arteries (SRAs). The SRAs express a unique combination of the slow and fast contractile gene programs underlying their mixed tonic and phasic contractile properties (15). There is little understanding of the developmental maturation of SRA smooth muscle, at least in part attributable to the small size, and its significance with regards to the regulation of blood flow in regional circulations. Myosin phosphatase (MP) is a key target of constrictor and dilator signals that regulate smooth muscle tone (21) and sets the sensitivity of the myofilaments to calcium/myosin light chain kinase (MLCK)-triggered force production. The regulated expression of its subunits is proposed to determine tissue-specific (phasic vs. tonic) and developmentally regulated smooth muscle function (reviewed in Ref. 11). Skipping of exon 24 (E24) of the MP regulatory subunit (Mypt1 E24-) in the mature tonic smooth muscle of the large vessels codes for a COOH-terminal leucine zipper (LZ) motif that is thought to be required for nitric oxide (NO)/cGMP-mediated activation of MP and desensitization of the myofilaments to calcium. In contrast, splicing of the 31-nt E24 in mature SRAs and phasic smooth muscle shifts the reading frame, coding for Mypt1 that lacks a COOH-terminal LZ motif and is not activated by cGMP (24, 25, 50) (reviewed in Ref. 11). The inhibitory subunit of MP, CPI-17, is more highly expressed in tonic vs. phasic smooth muscle (52) and is proposed to play more of a role in α-adrenergic-mediated inhibition of MP in SRAs vs. large arteries (27) (reviewed in Ref. 13). Other differences that characterize SRA or phasic vs. large artery or tonic smooth muscle include severalfold higher expression and activity of MP and MLCK, differences in myosin heavy and light chain splice variants, and increased γ- vs. α-actin (reviewed in Ref. 15).
In this study, we focused on MP and the broader smooth muscle gene program in the molecular and functional maturation of the smooth muscle of the rat mesenteric SRAs. To our surprise, this process does not begin until after the second postnatal week and is not complete until near sexual maturity. Given its late timing, we hypothesized that SRA maturation might be under an inductive influence and tested the role of sympathetic neural signaling for a number of reasons: 1) sympathetic nerves grow into the mesenteric circulation at postnatal day (PND)3–4 and form synapses by PND18–21 (22, 33, 34), approximating the timing of the initiation of SRA maturation; 2) sympathetic nerves may promote smooth muscle differentiation specifically in peripheral arteries that are highly innervated, such as rat tail and femoral arteries (9); 3) the well-established role of innervation in determining fast vs. slow gene programs in striated muscle (2, 45); 4) the sympathetic nervous system (SNS) is known to have trophic effects on the mature blood vessel wall (4, 16), and there has been a renewed interest in this field of research with the reemergence of denervation of the renal artery as a potentially effective treatment for hypertension (10). To test this, we examined the effect of chemical sympathetic denervation started at birth using an established method of 6-hydroxydopamine (6-OHDA) treatment (30). To determine whether continued SNS input is required for the maintenance of the peripheral artery gene program, the effect of mechanical denervation of the mature rat renal arteries was examined. In total, we provide evidence for a novel role for the SNS in the maturation and functional programming of the arterial smooth muscle of the mesenteric and renal circulations.
MATERIALS AND METHODS
Animals.
Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were bred, and offspring were weaned at PND21 (n = 42 offspring for developmental studies). Mypt1 E24 flox mice were generated using Zinc Finger Nuclease methodology in the inbred C57Bl/6J line via insertion of LoxP sequences ∼300 bp on either side of exon 24. Restriction enzyme sequences were designed next to each LoxP site to facilitate targeted integration genotyping. Mypt1 E24 flox mice were mated with smooth muscle-specific SMMHCCreERT2 mice (51) to obtain male mice of the genotype SMMHCCreERT2//Mypt1 F/+ (n = 4). Mice were subjected to five consecutive days of a single intraperitoneal injection of Tamoxifen (10 mg/kg; in sunflower oil) at 3 wk of age. The control group consisted of male mice that were treated with Tamoxifen as above and were positive for SMMHCCreERT2 and negative for the floxed alleles (n = 4). All mice were killed and blood vessels harvested at 8 wk of age. Mice were maintained in a C57BL/6J background. All animals were maintained on 12 h:12-h light/dark cycle and were provided food and water ad libitum. All animal protocols adhered to NIH guidelines and were approved by the Institutional Animal Care and Use Committee at the University of Maryland-Baltimore.
Neonatal chemical sympathectomy.
Neonatal rats (n = 20) were given 6-OHDA by intraperitoneal injection at a dose of 100 mg/kg on PND1 and 2 and 250 mg/kg on PND7, 14, 21, and 28 (30). Vehicle injection [0.87% sterile saline with 0.01% (wt/vol) ascorbic acid] followed the same protocol (n = 20). Pups treated with 6-OHDA or vehicle were each derived from four separate litters with five pups per group used per litter. Efficacy of sympathectomy was demonstrated by immunostaining for the neuronal marker TUJ (Class III neuronal β-tubulin) (Covance Laboratories, Evansville, IN) and the sympathetic marker tyrosine hydroxylase (Abcam, Cambridge, MA) (data not shown). There were no deaths up to PND35 in rats treated with 6-OHDA, and body weights were modestly but not significantly reduced [102.3 ± 7.8 g vs. 119 ± 4.7 g (P = 0.1; n = 6)]. A separate set of neonatal rats (n = 6) were given guanethidine sulfate (Santa Cruz Biotechnology, Santa Cruz, CA) by intraperitoneal injection at a dose of 50 mg/kg 5 days a week (9) from PND1–35. Vehicle injections (0.87% sterile saline) followed the same protocol (n = 6). Pups treated with guanethidine or vehicle were each derived from two separate litters with three pups per group used per litter. Separate litters were used for vehicle and experimental pups. Tissues [mesenteric arteries (MA) and aortas] were isolated for mRNA analysis at PND35. There was little variation between litters in the mRNAs measured by PCR.
Adult renal artery denervation.
Adult male Sprague Dawley rats (∼250 g; 10 wk old) were anesthetized with 2.5% isofluorane and the renal arteries exposed via a midline abdominal incision (n = 8–12). The adventitia and surrounding connective tissue was stripped away from the left renal artery followed by application of a 10% phenol in ethanol (90%) solution (3). The abdomen was then closed with 4–0 prolene. Sham animals were subjected to a midline incision without the denervation procedure (n = 8–12). Renal arteries were harvested at 4 or 14 days after surgery. Gene expression was similar at 4 and 14 days postsurgery; these groups were combined to reduce animal numbers.
RNA and protein analysis.
Total RNA was purified from tissue homogenates via RNEasy column purification (Qiagen, Valencia, CA) per the manufacturers' recommendations and reverse transcribed using random hexamers and Superscript III enzyme (1,000 U; Invitrogen, Carlsbad, CA). Ratios of Mypt1 alternative exon 24 splice variants were quantified using dye-labeled oligonucleotide primers flanking the alternative exon in PCR followed by gel separation and quantification of E24+ vs. E24- bands as previously described (43). mRNAs were measured by real-time PCR (StepOnePlus) using predesigned TaqMan probes and a Fast Advanced TaqMan Master Mix (Applied Biosystems, Foster City, CA) with cyclophilin A as an invariant internal control for normalization. Relative transcript abundance was calculated as fold change using the 2−ΔΔCt method. Protein lysates were prepared as previously described (43). In brief, tissues were homogenized in a lysis buffer containing 125 mM Tris·HCl (pH 6.8), 20% sucrose, 10% SDS, and 1% proteinase inhibitor cocktail (Sigma, St. Louis, MO). Proteins (10 μg) were loaded to Mini-PROTEAN TGX 4–15% Tris-glycine gels (Bio-Rad, Hercules, CA), separated at 100 V for 1.5 h, and then transferred to nitrocellulose membranes at 25 V for 2 h. LI-COR Odyssey blocking buffer (LI-COR, Lincoln, NE) was used to block membranes and dilute antibodies. The following primary antibodies were used: rabbit polyclonal total Mypt1 (1:3,000, Abcam), rabbit polyclonal specific for Mypt1 LZ+ and Mypt1 LZ- isoforms (1:3,000) (5), rabbit polyclonal CPI-17 (1:5,000, gift from Dr. Matsumi Eto), mouse monoclonal smooth muscle α-actin (1:3,000, Sigma), mouse monoclonal MLCK (1:3,000, Sigma), rabbit polyclonal Adra1a (1:1,000; Alomone Laboratories, Jerusalem, Israel), rabbit polyclonal P2rx1 (1:1,000, Alomone Laboratories), and rabbit polyclonal Npy1r (1:1,000, Alomone Laboratories). A rabbit monoclonal to cyclophilin A (1:3,000, Abcam) was used as a normalizer and was invariant. IRDye 800CW and 680LT goat anti-rabbit and goat anti-mouse IgG secondary antibodies (1:10,000) were used for detection (LI-COR). Bands were scanned in the Odyssey system and quantified in Image Studio 3.0 (LI-COR).
Vascular function.
First-order MA (MA1) 2 mm in length were dissected and placed in an ice-cold physiological saline solution (PSS) containing the following concentrations (mM): 119 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 25 NaHCO3, 1.2 NaH2PO4, 0.027 EDTA, and 5.5 glucose at a pH of 7.4 and 95% O2-5% CO2 bubbled into the solution. Arteries were mounted on a wire myograph (Model 610M; Danish Myo Technology, Aarhus, Denmark) for isometric force generation measurements. Starting tension was applied by stretching the vessel to IC90 as previously described (37). Vessels were then primed via addition of two separate doses of 10 μM phenylephrine to the bath and equilibrated in PSS for 20 min before the first experiment. Cumulative response curves to phenylephrine (1 nM-100 μM) were determined after the vessel reached a steady state of force production at each respective dose (∼3–4 min). Force was also activated by depolarization with 100 mM KCl. Force was continuously recorded and measured at steady state. Data are presented as active force generation (milliNewtons; mN).
A subset of vessels was permeabilized under pCa9 conditions with 1,000 U/ml α-toxin (Sigma). The pCa9 solution consisted of the following concentrations (mM): 60 potassium methane-sulfonate (KMS), 5 EGTA, 0.02 CaCl2, 9.3 MgCl2, 5.2 Na2ATP, 25 creatine phosphate, and 25 N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid with a final pH 7.1 with 1 N KOH. The free calcium solutions were designed via the MAXC computer program holding a constant ionic strength of 0.15. The maximal calcium solution (pCa4) was similar to pCa9 except for the following concentrations: 58.3 KMS, 5.2 CaCl2, 4.4 MgCl2. All solutions contained a cocktail of protease inhibitors consisting of leupeptin (1 μg/ml), pepstatin A (2.5 μg/ml), and phenylmethylsulfonyl fluoride (50 μM). Calcium sensitivity of the myofilaments was determined by activating the smooth muscle with pCa6 (1 μM calcium). Force was continuously recorded and measured at steady state. Once steady state was achieved, vessels were further activated via 10 μM phenylephrine (PE), and force was again continuously recorded and measured at steady state. Vasorelaxation responses were assessed in α-toxin permeabilized vessels with calcium clamped at pCa6 (1 μM). After steady-state force was achieved, vessels were exposed to cumulative concentrations of 8-Br-cGMP (1 nM- 100 μM) (Sigma). Vasorelaxation data is presented as a percentage of maximum force at each concentration of 8-Br-cGMP.
Statistics.
Statistical analysis and graph generation were completed with SigmaPlot software (SYSTAT, Chicago, IL). One-way ANOVA and a Bonferroni post hoc test were performed for vascular contractility assays comparing force generation at individual drug concentrations (PE, U-46619, 8-Br-cGMP) between PND21, PND35, and PND35 + 6-OHDA groups. Two-way ANOVAs were performed for the calcium sensitization experiments (Fig. 5, C–D) with postnatal day and constrictor activation (PE or KCl) used as factors comparing PND21, PND35, and PND35 + 6-OHDA groups. A Kruskal-Wallis ANOVA on ranks was used where applicable. One-way ANOVA and Student's t-test were used for Mypt1 splice variant analysis and real-time PCR comparisons. Protein expression analysis was performed using a Student's t-test comparing fold change to control. Statistical significance was accepted with P < 0.05.
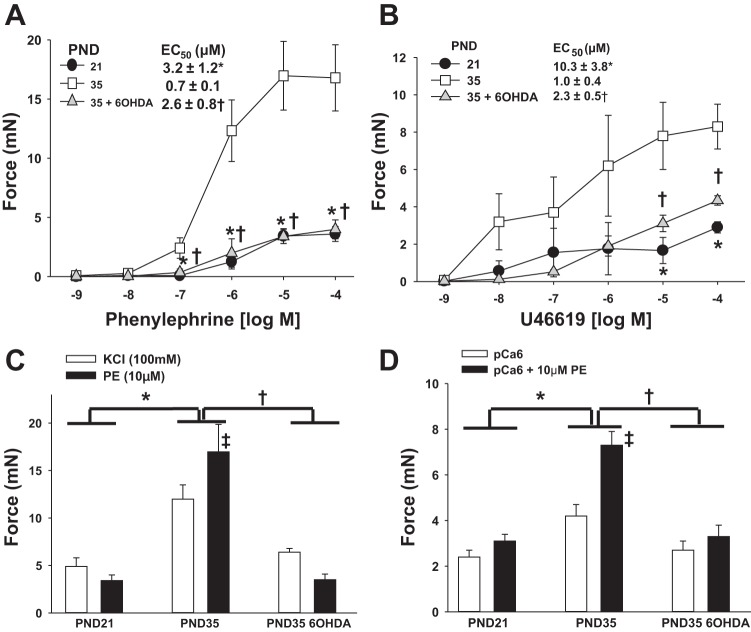
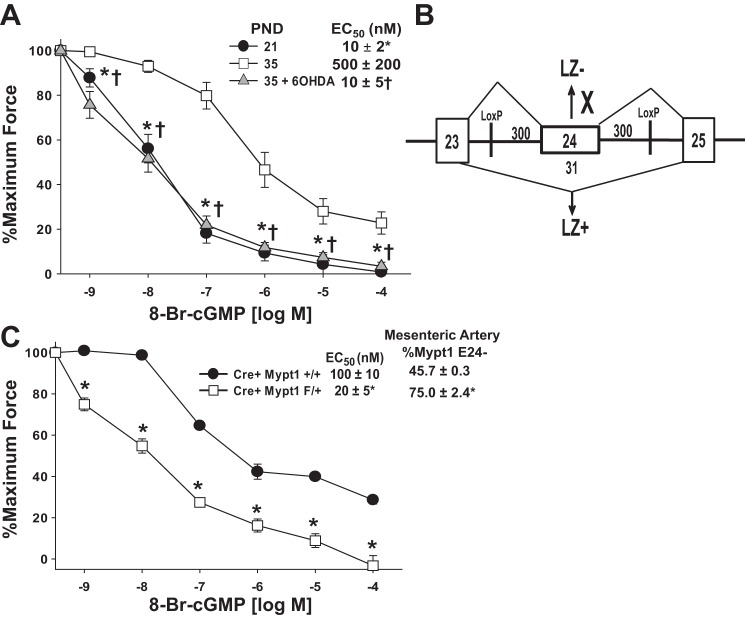
Fig. 5.
Maturation of MA constrictor function and its suppression by chemical sympathectomy. Newborn rats were treated with 6-OHDA to achieve chemical sympathectomy or vehicle and first-order MA (MA1) harvested at PND21 (vehicle) or PND35 (6-OHDA or vehicle). Force generation was measured in intact (A–C) or α-toxin- permeabilized (D) MA1s under isometric conditions in a wire myograph (see materials and methods). Data are plotted as developed force in milliNewtons (mN). A: dose response to the α-adrenergic agonist phenylephrine (PE). B: dose response to the thromboxane mimetic U-46619. C: force generated by KCl-induced depolarization (100 mM) vs. PE (10 μM) (2-way ANOVA). D: force generation in α-toxin-permeabilized MA1s activated with calcium alone (pCa6; 1 μM) or calcium and PE (10 μM) (2-way ANOVA). EC50 was calculated by standard curve analysis. All data are expressed as means ± SE. *P < 0.05, PND35 vehicle vs. PND21; †P < 0.05 PND35 6-OHDA vs. PND35 vehicle. C: ‡P < 0.05 within PND35 (KCl vs. PE activation). D: ‡P < 0.05 within PND35 (pCa6 vs. pCa6 + PE activation). n = 5–6/group.
RESULTS
Induction of contractile and neurovascular mRNAs and protein during postnatal MA maturation.
We began by examining the developmental expression of MP subunits and other contractile genes as indicators of the state of differentiation of the smooth muscle of small arteries within the rat MA arcade. There were no significant changes between PND3 and PND12 for Mypt1 splicing (data not shown). From PND12 to PND42, there was a nearly complete switch in the MP regulatory subunit Mypt1 from the slow (E24-) to the fast (E24+) splice variant mRNA (Fig. 1A; PND12 vs. PND42 P < 0.05). The inhibitory subunit of MP (CPI-17) mRNA was increased 60-fold (Fig. 1B; PND12 vs. PND35 P < 0.05), whereas there was a more modest fourfold induction of the regulatory subunit Mypt1 mRNA (Fig. 1C; PND12 vs. PND35 P < 0.05) and no change in a second inhibitory subunit family member (PHI-1) (Fig. 1B; PND12 vs. PND35 P = 0.7) or the catalytic subunit Ppp1cγ (Fig. 1C; PND12 vs. PND35 P = 0.1). The MLCK mRNA followed a pattern similar to that of Mypt1 except for a spike in its expression on PND35 (Fig. 1C; PND12 vs. PND35 P < 0.05). The induction of CPI-17 mRNA was part of a larger program of smooth muscle differentiation as evidenced by the induction of mRNAs for smooth muscle myosin heavy chain (PND12 vs. PND35 P < 0.05) and γ- and α-actin (Fig. 1B; PND12 vs. PND35 P < 0.05). Of note, the increased ratios of Mypt1 E24+/− and γ/α-actin distinguish mature mesenteric resistance vs. conduit (aortic) artery smooth muscle (14, 43) (reviewed in Ref. 15), the latter serving as a reference vessel in this study.
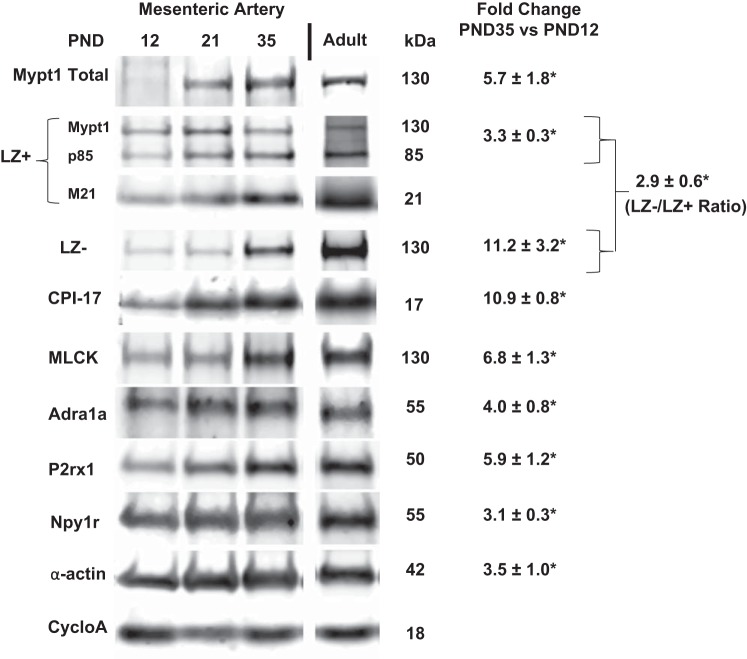
Fig. 1.
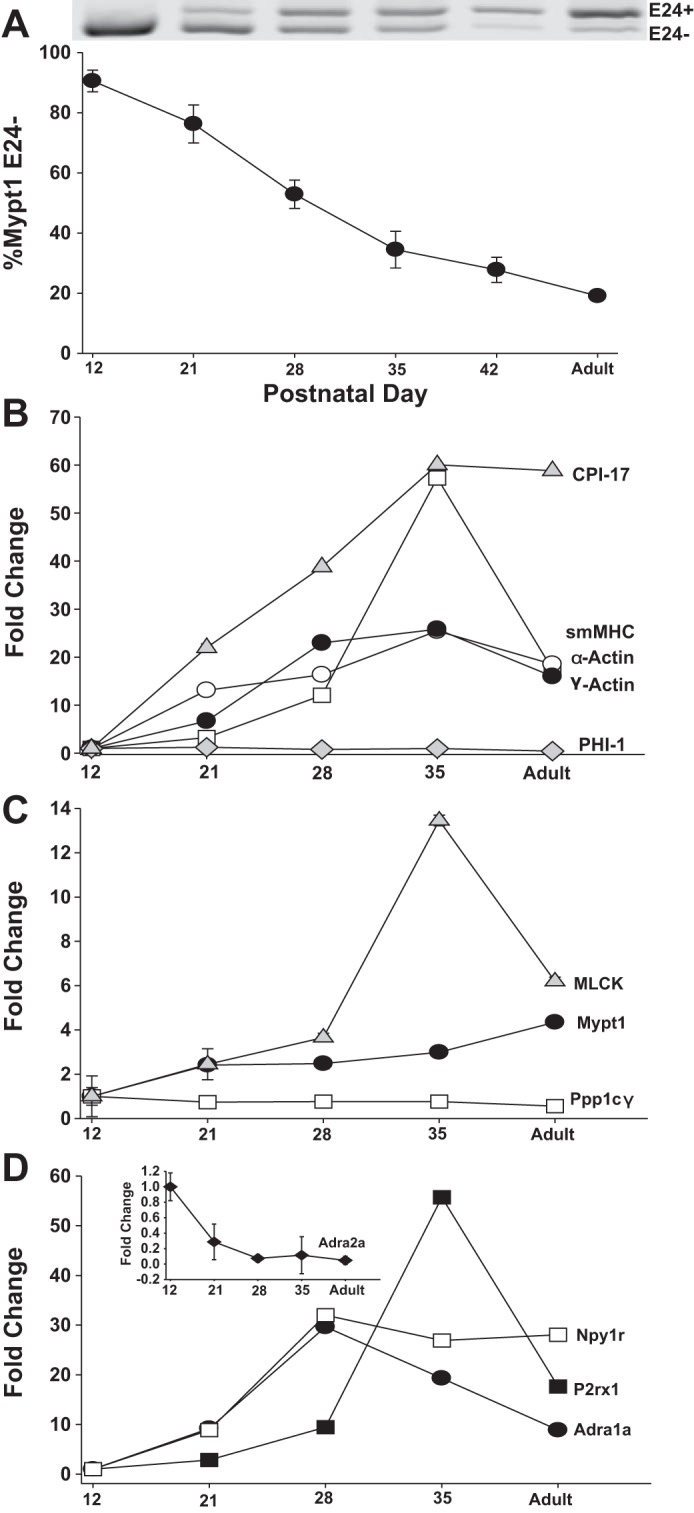
Postnatal maturation of rat mesenteric arterial (MA) smooth muscle gene expression. RNA was isolated from postnatal day (PND)12–42 and adult (10 wk) rat MA. A: representative gel of PCR products that contain (E24+) or lack (E24-) the 31-nt Mypt1 alternative exon 24 (E24) and graphed as %Mypt1 E24-. B–D: mRNAs were measured by qPCR using TaqMan probes normalized to cyclophilin A and reported as fold change vs. PND12 values. n = 3/PND. All data are expressed as means ± SE. MLCK, myosin light chain kinase.
As a first step in testing the relationship between SNS innervation and MA maturation, we examined the developmental time course of the expression of receptors for SNS signaling. The α1-adrenergic (Adra1a; PND12 vs. PND35 P < 0.05) and neuropeptide Y1 (Npy1r; PND12 vs. PND35 P < 0.05) receptor mRNAs were markedly induced during MA maturation, reaching a peak at PND28 (Fig. 1D), whereas the purinergic X1 (P2rx1) receptor mRNA was induced with a delayed time course with a spike at PND35 (PND12 vs. PND35 P < 0.05). In contrast, the α2-adrenergic receptor (Adra2a) mRNA was reduced by ∼90% (Fig. 1D, inset; PND12 vs. PND35 P < 0.05). There was modest or no change from PND12 to PND35 in mRNAs for receptors for endothelin (Ednra: 1.9-fold P < 0.05; Ednrb: 0.6-fold P = 0.3) and angiotensin (Agtr1a: 0.9-fold P = 0.8; Agtr1b: 0.8-fold P = 0.4; Agtr2: 0.1-fold P = 0.07).
Examination of MA lysates at PND12, 21, and 35 showed developmental induction of proteins that followed that of the corresponding mRNAs although, in some instances, the magnitude was less (Fig. 2). Of note, the developmental switch to the Mypt1 E24+ splice variant (Fig. 1A) translated into a significant increase in the Mypt1 LZ- isoform, which it encodes, and an increase in the ratio of the signals obtained with Mypt1 COOH-terminal LZ- vs. LZ+ (encoded by E24-) isoform-specific antibodies (P < 0.05). The ratio of MP inhibitory (CPI-17) to regulatory (Mypt1) protein subunits was increased approximately twofold between PND12 and PND35. The developmental trajectories in the expression of these contractile proteins were maintained into adulthood (10 wk of age; Fig. 2 and data not shown). The measurements of mRNA and protein were normalized to cyclophilin A, which was invariant.
Fig. 2.
Protein expression in MA during postnatal maturation. Representative images of proteins detected by Western blot using antibodies described in materials and methods comparing PND12, 21, 35, and adult (10 wk) MA. Data are expressed as fold change of PND35 compared with PND12. A rabbit polyclonal antibody raised against the Mypt1 leucine zipper (LZ) motif recognizes LZ motifs in Mypt family members Mypt1, p85, and M21. LZ-/LZ+ ratio reflects the ratio of signals obtained with Mypt1 (130 kDa) COOH-terminal LZ- vs LZ+ isoform-specific antibodies. Bands were imaged and directly quantified using the Licor Odyssey and normalized to values for cyclophilin A (cycloA). *P < 0.05, n = 3/PND. All data are expressed as means ± SE.
Chemical sympathectomy suppresses postnatal maturation of MA.
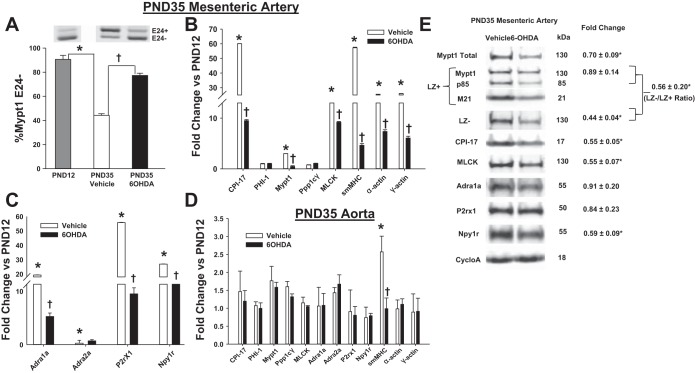
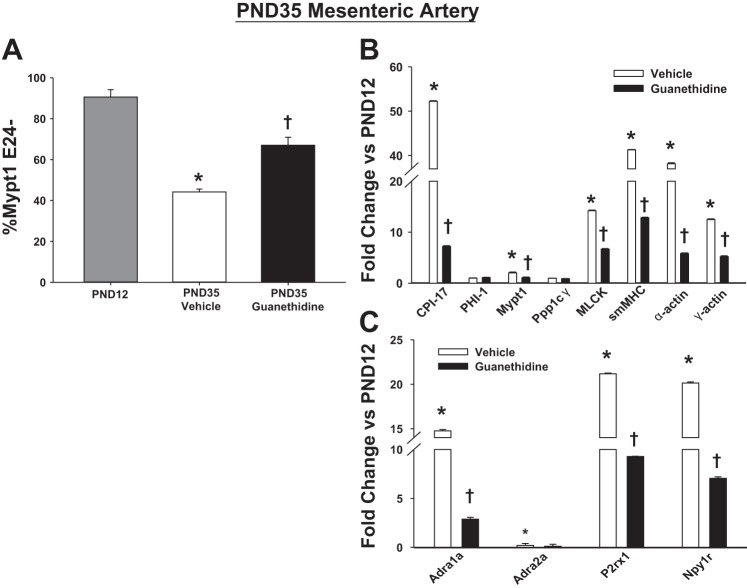
The timing of MA maturation beginning 2 wk postnatally, after sympathetic innervation (22, 33), suggested the hypothesis that sympathetic nerves may program its developmental maturation. To test this, rats were administered 6-OHDA 1 day after birth and regularly thereafter to achieve chemical sympathectomy as previously described (30). Chemical sympathectomy was confirmed by the absence of staining for markers of adrenergic nerves (tyrosine hydroxylase, class III neuronal β-tubulin) on the surface of PND35 MA (data not shown). Chemical sympathectomy markedly suppressed the developmental induction of splicing of Mypt1 E24 (Fig. 3A; P < 0.05) and contractile (Fig. 3B) and neurovascular (Fig. 3C) mRNAs as measured at PND35 and compared with vehicle-treated rats. Specificity of the effect of chemical sympathectomy is demonstrated by absence of an effect on mRNAs that were not developmentally induced in the MA (Fig. 3B: PHI-1, Ppp1cγ; Fig. 3C: Adra2a; and the normalizer cyclophilin A) and mRNAs, with the exception of smMHC (P < 0.05), in the thoracic aorta of the 6-OHDA-treated rats (Fig. 3D). A second method of neonatal chemical sympathectomy, treatment with guanethidine sulfate (9), similarly suppressed the developmental maturation of the MA measured at PND35 compared with PND12 rats for Mypt1 splicing (Fig. 4A) and vascular gene expression (Fig. 4, B and C).
Fig. 3.
Neonatal chemical sympathectomy specifically suppresses MA maturation. Newborn rats were treated with vehicle or 6-hydroxydopamine (6-OHDA) to achieve chemical sympathectomy as described in materials and methods. MA (A–C) and aorta (D) were assayed at PND35. A: representative gels of Mypt1 E24 splice variants (plotted as %Mypt1 E24-). mRNA levels from PND35 vehicle vs. PND35 6-OHDA (B and C) MA and aorta (D) normalized to untreated PND12 arteries. E: fold change in protein levels in PND35 6-OHDA (n = 3) MA vs. PND35 vehicle control (n = 3) normalized to cyclophilin A, which was invariant. LZ-/LZ+ is the ratio of the signal obtained with the Mypt1 LZ- rabbit polyclonal antibody divided by the signal obtained on different blots with the Mypt1 LZ+ rabbit polyclonal antibody. All data are expressed as means ± SE. *P < 0.05 PND12 vs. PND35 vehicle, †P < 0.05 PND35 6-OHDA vs. PND35 vehicle. n = 5–6/group.
Fig. 4.
Effect of neonatal chemical sympathectomy with guanethidine on mRNA in MA. Neonatal rats were treated with vehicle or guanethidine, and mRNAs from MA were assayed at PND35. A: Mypt1 splice variants in PND12 (untreated), PND35 vehicle, and PND35 guanethidine MA expressed as %Mypt1 E24-. B–C: mRNA levels measured via qPCR. Data are expressed as fold of change of PND35 guanethidine-treated rats and PND35 vehicle-treated rats normalized to PND12 MA. *P < 0.05 PND35 vehicle vs. PND12; †PND35 guanethidine vs. PND35 vehicle, n = 6/group.
The abundance of the contractile proteins in MA from PND35 rats treated with 6-OHDA was also significantly reduced compared with PND35 rats treated with vehicle (Fig. 3E). Of note, the ratio of CPI-17 to Mypt1 was reduced, and the ratio of the signals obtained with Mypt1 COOH-terminal LZ- vs. LZ+ (encoded by E24-) isoform-specific antibodies was decreased, each reversing the normal developmental trend. In contrast, for the neurovascular genes, the abundance of Npy1r protein was significantly reduced by chemical sympathectomy (P < 0.05; Fig. 3E), whereas there was no effect on the abundance of Adra1a and P2rx1, consistent with their regulation by posttranscriptional mechanisms.
Developmental maturation of MA contractile function and its suppression by sympathectomy.
The developmental increase in smooth muscle contractile proteins actin and myosin is predicted to increase maximum force, whereas the increase in MLCK is predicted to increase calcium-induced force. The developmental induction of the CPI-17 inhibitory subunit of MP is predicted to increase sensitivity to α-adrenergic-mediated inhibition of MP and thus calcium sensitization of force production. To test this, force production of intact or α-toxin-permeabilized and calcium-clamped MA1 was studied by wire myography under isometric conditions ex vivo. The dose response of intact PND21, PND35, and PND35 6-OHDA MA1s to the α-adrenergic agonist PE is shown in Fig. 5A. Maximum force to PE increased by 4.7-fold between PND21 and 35, and its efficacy was increased by a similar amount (Fig. 5A; P < 0.05). Neonatal chemical sympathectomy with 6-OHDA suppressed the maturational increase (Fig. 5A; P < 0.05), resulting in maximal force and EC50 that were not different from PND21 values. Similar results were obtained when the arteries were challenged with a different contractile agonist, the thromboxane mimetic U-46619 (Fig. 5B; P < 0.05). We next used two complementary assays to determine the extent to which differences in agonist-mediated force production may be due to inhibition of MP: 1) as an indirect indicator, the difference in maximum force produced with KCl depolarization vs. agonist in intact arteries (36), and 2) as a direct indicator, the ability of PE to augment force in permeabilized arteries with force activated at submaximal concentrations of calcium (8, 26). The PND35 MA generated 2.5-fold greater maximum force to KCl-induced depolarization compared with the PND21 MA (Fig. 5C; P < 0.05; two-way ANOVA) consistent with accretion of differentiated vascular smooth muscle. In response to maximum doses of PE (10 μM), the PND35 MA generated 1.4-fold greater force compared with KCl depolarization, whereas the PND21 MA generated less force (0.8-fold) (Fig. 5C; P < 0.05). In α-toxin-permeabilized arteries activated with submaximal concentrations of calcium (pCa6; 1 μM), PE induced an insignificant augmentation of force in PND21 MA (0.7 mN, P = 0.3) but a robust augmentation of force in PND35 MA (Fig. 5D; 3.1 mN; P < 0.05; two-way ANOVA). Chemical sympathectomy with 6-OHDA suppressed each of these maturational changes, such that contractile responses of PND35 6-OHDA MAs were not different from PND21 MAs (P = 0.8; two-way ANOVA). In total, these data demonstrate developmental maturation of α-adrenergic-mediated vasoconstriction that is in part mediated by increasing inhibition of MP (calcium sensitization) and suppressed by chemical sympathectomy.
Role of the developmental switch in Mypt1 isoforms in the maturation of MA responses to cGMP-mediated relaxation.
NO/cGMP activates MP through LZ-mediated heterodimerization of cGK1α with the regulatory subunit Mypt1 (25, 50). The developmental switch of Mypt1 to the E24+/LZ- isoform is predicted to decrease sensitivity of the smooth muscle to cGMP-mediated activation of MP and thus calcium desensitization of force production. To test this, MA were α-toxin permeabilized and force activated with submaximal concentrations of calcium (pCa6; 1 μM) followed by cumulative dose response to the nonhydrolyzable analog of cGMP, 8-Br-cGMP. The efficacy of 8-Br-cGMP for vasorelaxation was 50-fold less (P < 0.05) in PND35 vs. PND21 MA; maximal relaxation was also significantly decreased (P < 0.05; Fig. 6A). Chemical sympathectomy with 6-OHDA suppressed this maturational decrease in sensitivity to 8-Br-cGMP (P < 0.05 compared with PND35 arteries), such that contractile responses of 6-OHDA MAs were not different from PND21.
Fig. 6.
Maturation of MA dilator response to cGMP, suppression by chemical sympathectomy, and effect of deletion of mouse Mypt1 E24. A: newborn rats were treated with 6-OHDA to achieve chemical sympathectomy or vehicle and MA1s harvested at PND21 (vehicle) or PND35 (6-OHDA or vehicle). Force was activated in α-toxin-permeabilized MA1s with submaximal concentrations of calcium (pCa6; 1 μM). Plotted is a percentage of force remaining in response to cumulative doses of 8-Br-cGMP (n = 6). B: LoxP sequences were inserted in introns ∼300 nt up- and downstream of the mouse Mypt1 alternative exon 24 (not to scale). C: mice of the genotypes as shown (control: SMMHCCreERT2:Mypt1 E24 +/+ vs. experimental: SMMHCCreERT2:Mypt1 E24 f/+) were treated with tamoxifen at 3 wk of age to delete Mypt1 E24. At 8 wk of age, the percentage of transcripts in the MAs that were E24- (%Mypt1 E24-) had significantly increased. Force was activated in α-toxin-permeabilized MA1s with submaximal concentrations of calcium (pCa6; 1 μM). Plotted is a percentage of force remaining in response to cumulative doses of 8-Br-cGMP. EC50 was determined by standard curve analysis. All data are expressed as means ± SE. A: *P < 0.05 PND35 vehicle vs. PND21; †P < 0.05 PND35 6-OHDA vs. PND35 vehicle. C: *P < 0.05 Mypt1 E24 F/+ vs. Mypt1 E24 +/+ (n = 4).
To directly test the functional significance of the maturational switch of the MAs to the Mypt1 E24+/LZ- isoform, LoxP sites were inserted flanking this exon for the purpose of conditional deletion (Fig. 6B). Treatment of SMMHCCreERT2//f/+ mice with Tamoxifen from PND21–26 (cKO) shifted Mypt1 in the mature (8 wk old) mouse MA to ∼75% E24- (Fig. 5C; P < 0.05), similar to that of rat PND21 MA (Fig. 1A). MAs from control mature mice of the genotype SMMHCCreERT2//+/+ treated with Tamoxifen from PND21–26 were ∼46% E24-, similar to that of rat PND35 MA (Fig. 1A). Eight-week-old mouse MA1s were α-toxin permeabilized and activated with submaximal concentrations of calcium (pCa6; 1 μM) followed by cumulative dose response to 8-Br-cGMP. The shift of the mouse MA to the Mypt1 E24- isoform increased its sensitivity to 8-Br-cGMP under calcium clamp by fivefold and also increased maximal relaxation (P < 0.05; Fig. 6C). This shift in the dose response mirrors that in the rat developmental series (Fig. 6A), supporting the notion that the maturational induction of the Mypt1 E24+/LZ- isoform underlies the developmental functional deprogramming of MA to the NO second messenger cGMP.
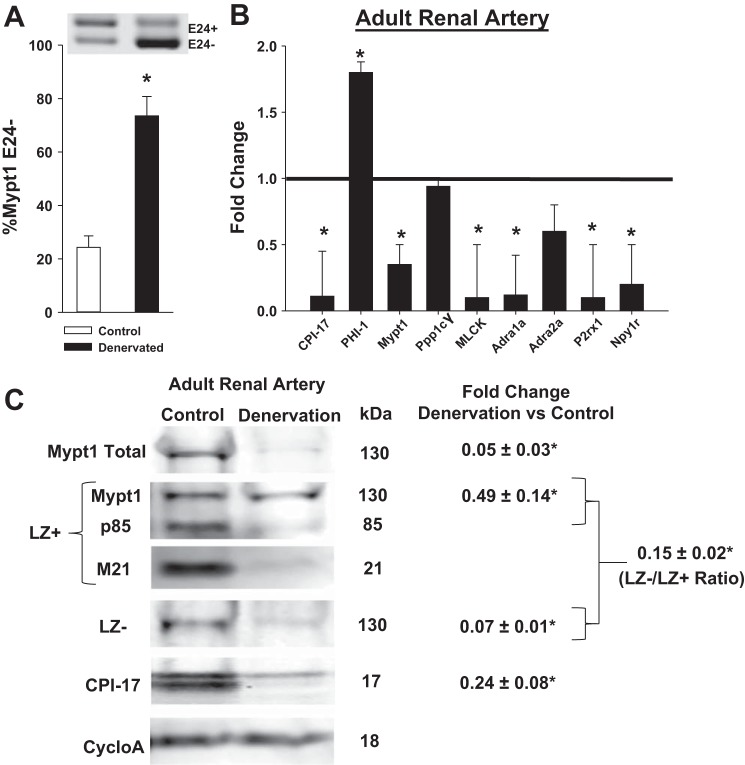
Denervation of the mature rat renal artery causes a reversion to the immature gene program.
To determine whether continued neural input is required to maintain the gene program of mature peripheral arterial smooth muscle, denervation was achieved by mechanical stripping of the adventitia of the mature rat renal artery (3 and materials and methods). mRNA and proteins were measured from renal arteries 4 and 14 days after denervation and compared with renal arteries from sham-operated rats. There were no differences in the effect at 4 vs. 14 days or in unilateral vs. bilateral denervation, so that these groups were combined for analysis. Denervation of the adult renal artery caused a change in the gene program similar to that of chemical sympathectomy in the neonate including reversion to the E24- isoform of Mypt1 (Fig. 7A; P < 0.05) and reductions in the mRNAs for MP inhibitory (CPI-17) and regulatory (Mypt1) subunits (P < 0.05) and no effect on the catalytic subunit (P = 0.4). The MP inhibitory subunit PHI-1 was induced 1.8-fold (P < 0.05; Fig. 7B). There was also reduction in mRNAs for MLCK and receptors of SNS signaling (Adra1a, P2rx1, Npy1r; P < 0.05; Fig. 6B) with no change in Adra2a (P = 0.07). The effect of renal artery denervation on the expression of the MP protein subunits followed the trends of the mRNAs (Fig. 7C). Mypt1 and CPI-17 protein were significantly reduced, as was the LZ- isoform of Mypt1 (P < 0.05); additionally there was a decrease in the Mypt1 LZ- signal normalized to the Mypt1 LZ+ signal (P < 0.05).
Fig. 7.
Mechanical denervation of the adult rat renal artery causes loss of the mature smooth muscle gene program. Adult male rat renal arteries were denervated by stripping of the adventitia followed by application of a solution of 10%phenol/90% ethanol (see materials and methods). Denervated renal arteries were assayed at postoperative days 4 and 14; these groups were not different and thus combined for analysis. A: representative gel of Mypt1 splice variants in sham (control) and denervated renal arteries expressed as %Mypt1 E24-. B: mRNA levels measured via qPCR as above. C: fold change in subunit expression in denervated (n = 3) renal arteries compared with sham surgery (n = 3) and normalized to cyclophilin A measured 4 days after surgery. LZ-/LZ+ is the ratio of the signal obtained with the Mypt1 LZ- rabbit polyclonal antibody divided by the signal obtained on different blots with the Mypt1 LZ+ rabbit polyclonal antibody. Data are expressed as fold change in renal arteries from denervated vs. sham surgery rats. *P < 0.05, n = 8–12/group.
DISCUSSION
In this study, we have demonstrated the molecular and functional maturation of rat MA under the inductive influence of the SNS. This process of maturation does not initiate until after the second postnatal week and extends throughout the juvenile period until sexual maturity, much different in timing than conduit arteries, such as the aorta (reviewed in Ref. 40). The smMHC transcript is the most specific indicator of vascular smooth muscle differentiation. Its greater than 50-fold increase is consistent with the accretion of differentiated smooth muscle in the vessel wall and concordant with the severalfold increase in MA maximal force production in the juvenile period. Of note, the developmental time course of induction of smooth muscle-enriched genes varies: 1) as in other contexts (40), the induction of smMHC is delayed relative to other indicators of differentiation such as actins and CPI-17; 2) there is a spike at PND35 in expression of procontractile transcripts smMHC, MLCK, and P2rx1. Whether this reflects a second inductive signal received at this specific stage requires further study. Over this same time period, there is also implementation of the fast smooth muscle gene program as indicated by the transition from Mypt1 E24- to E24+ splice variants and the increased ratio of γ- to α-actin. This slow-to-fast transition occurs in the maturation of prototypical phasic smooth muscle, such as intestines (18, 25) and portal vein (42) (reviewed in Ref. 15), as well as in other regional circulations (data not shown), suggesting a generalized phenomenon.
In contrast to striated muscle (2, 45), there is little information on inductive signals programming smooth muscle contractile diversity. The present study shows that this initiates after sympathetic innervation and is blocked by chemical sympathectomy. Consistent with a prior study (9), sympathectomy suppressed the differentiation of the highly innervated SRAs but had minimal effect on the lightly innervated conduit artery (aorta). This specificity supports the notion that there was not a generalized toxic effect, also supported by the good health and normal survival of the animals to 10 wk of age. Furthermore, a second method of blocking neonatal sympathetic nervous signals, treatment with guanethidine (9), similarly blocked MA maturation (Fig. 4). Which of the developmentally induced and highly expressed SNS receptors is required for MA maturation requires further study. Signaling pathways that have been implicated in the assembly of the vessel wall include TGF-β, platelet-derived growth factor-β, and Notch (19). However, these have not been studied in the process of the assembly and maturation of mesenteric or most regional circulations (46). Histological analysis of proximal rat jejunal (mesenteric) artery differentiation showed that at birth this 350-μm-diameter artery consists of a single layer of partially differentiated SMCs adjacent to the endothelium and surrounded by five cell layers of undifferentiated mesenchymal cells that begin to differentiate over the following 2 wk (53). Taken together, these data support a model in which the early differentiation of mesenteric SRA occurs adjacent to and under the inductive influence of endothelial cells, whereas the further differentiation and maturation of MA smooth muscle requires sympathetic neural signaling.
A major goal of this study was to determine the relationship between the molecular and functional maturation of the mesenteric circulation, which, because of resting sympathetic tone, contributes substantially to mature systemic vascular resistance and blood pressure. During normal development, increasing expression of α1-adrenergic receptors correlated with increasing sensitivity and maximum response to α-adrenergic stimulation. In this study, we compared PND21 with PND35; it is likely that greater differences would be evident at earlier ages, but the less mature arteries were too small for wire myography. This developmental maturation correlates well with the severalfold increase in rat systolic and diastolic blood pressure from PND1–60 and increasing α-agonist/antagonist pressor and depressor responses in vivo (35). In that study, ∼1/3 of the ∼85-mmHg maturational increase in resting systolic blood pressure was attributable to α-adrenergic tone. The massive upregulation of the inhibitory subunit of MP, CPI-17 (PPP1R14a), and increase in CPI-17:Mypt1 ratio suggested a postreceptor mechanism by which α-adrenergic responsiveness could be increased. This was demonstrated by the ability of PE to significantly augment force in permeabilized vessels under calcium clamp in PND35 but not PND21 MAs, indicating MP activation at PND35 but not PND21. It is likely that other targets of α-adrenergic signaling that control either calcium signaling or calcium sensitivity of the myofilaments are also developmentally regulated. Interestingly, a study of fetal vs. mature ovine middle cerebral arteries also demonstrated increased expression of CPI-17 protein and a proposed switch, based on chemical inhibitors, from Rho kinase to PKC-dependent enhancement of force production (20). Consistent with that study, we did not detect any change in the mRNAs of signaling intermediates, such as RhoA and Rock1 and 2 (data not shown), but a more comprehensive analysis of the developmentally regulated gene program is required. A recent study of smooth muscle-specific overexpression of CPI-17 demonstrated the positive relationship between CPI-17 levels and agonist-mediated contractile responses of adult mouse MA (49). Ultimately, defining the proportion of the maturational increase in MA α-adrenergic responsiveness that is specifically attributable to induction of CPI-17 will require gene loss-of-function mice, which are not currently available. In the present study, chemical sympathectomy markedly suppressed developmental induction of CPI-17 and agonist-mediated calcium sensitization of force production. Thus induction of CPI-17, as part of the SNS-inducible smooth muscle gene program, serves as a feed-forward mechanism by which SNS captures control of this regional circulation.
A second maturational change in MP is the near complete shift in the regulatory subunit Mypt1 from E24-/LZ+ to E24+/LZ-, as has been described in the maturation of other smooth muscle tissues implementing a fast gene program (18, 25, 42) (reviewed in Ref. 15). Biochemical and physiological experiments have suggested that the Mypt1 COOH-terminal LZ motif is required for cGK1α LZ-mediated heterodimerization and activation of MP (24, 25, 42, 50, 54) (reviewed in Ref. 11). Consistent with this model, the PND35 MA1 was significantly less sensitive than PND21 MA1 to the relaxing effects of 8-Br-cGMP under calcium clamp, the prototypical assay for measuring cGMP activation of MP (28, 25). An in vivo study also showed decreasing control by NO/cGMP in the postnatal maturation of a large animal mesenteric circulation (38); the arginine analog NG-monomethyl-l-arginine (l-NMMA) blocked flow-induced dilation of 3-day-old but not 34-day-old piglet mesenteric circulation, an effect that was ascribed to increased expression and activity of epithelial NO synthase (NOS) and guanylate cyclase. Additionally, there was no change in the sensitivity to the NO donor sodium nitroprusside. In contrast, in the rat, we did not observe developmental changes in levels of mRNAs for NOS1–3 or guanylate cyclase (data not shown) although it is still possible that there are developmental changes in the activity of these enzymes (reviewed in Ref. 6). In the present study, we directly tested the significance of the change in Mypt1 isoforms in isolation from the remainder of the gene program using a genetic approach. Cre-mediated excision of E24 shifted Mypt1 in the adult mouse MA from ∼40% E24- (LZ+) to ∼75% E24- (LZ+), modeling the change in isoforms in rat PND21 vs. PND35. This caused a similar fivefold shift in the sensitivity of permeabilized MA to cGMP-mediated calcium desensitization (activation of MP), establishing the fact that Mypt1 E24 splice variants determine sensitivity to cGMP in vivo. Whether the reduction in sensitivity to 8-Br-cGMP conferred by increased expression of the Mypt1 E24+/LZ- isoform represents the same activation of a smaller pool of MP enzymes or a different composition of this signaling pathway requires further study. Lastly, as is the case with α-adrenergic constriction discussed above, NO-mediated vasodilation has many targets, both cGMP dependent and independent (23, 29). Determining the relation between developmental changes in Mypt1 isoform expression and NO- and cGMP-dependent vasodilator function in vivo is now feasible through further study of these genetically engineered mice.
Mechanical denervation of the mature rat renal artery caused a reversion to the immature gene program, a general property of muscle phenotypic modulation (2). This suggests that continued sympathetic neural input is required to maintain the gene program of mature peripheral arterial smooth muscle. Because renal artery denervation in this well-established animal model (and in humans) involves tissue injury, it is possible that the injury or tissue inflammation plays a role in the observed changes. However, in a genetic mouse model of partial vascular endothelial growth factor deficiency, a defect in sympathetic innervation was associated with reductions in smooth muscle gene expression (smMHC, smoothelin) and contractile function specific to the mature resistance (saphenous) artery (48), similar to the effect of denervation reported in the present study. Surgical denervation of the human renal (41) and splanchnic (47) circulations predates medical therapies for human hypertension. Catheter-based radiofrequency ablation of the renal sympathetic nerves has reemerged as a potentially effective treatment for drug-resistant hypertension (12). A number of mechanisms have been proposed for the salutary effect in humans and animal models, including reductions in renal or splanchnic vascular resistance (17). The present study shows denervation-induced changes in renal vascular smooth muscle gene expression, providing a foundation for further studies of its effect on renovascular function and blood flow, particularly in models of hypertension.
In this study, we have shown that the SNS is specifically required for the maturation of resistance artery smooth muscle of the mesenteric circulation and the maintenance of the mature renal artery smooth muscle. The SNS developmentally captures control of the mesenteric circulation through the induction of receptor and postreceptor gene products needed for acute vasoconstriction in the regulation of blood flow (Fig. 8). There is also induction of splicing of E24 of the MP regulatory subunit, thereby turning down sensitivity to cGMP-mediated vasorelaxation, all consistent with its postnatal conversion from a low-to-high resistance state (reviewed in Ref. 44). How this may apply to larger animals including humans remains to be determined. Functional studies have suggested that regional circulations mature at different times dependent on the species and maturity at birth (7). Given how generalized the phenomenon of smooth muscle maturation is in the development of vascular and visceral organ systems, it is likely that the process is conserved in humans and other large animals although the timing may vary. There is increasing evidence for and interest in how developmental stressors in utero and postnatally, including sympathetic hyperactivity, may reprogram vascular (or other organ) systems, contributing to vascular dysfunction of maturity (1, 32, 39). The present study defines the developmental window of vascular smooth muscle plasticity and maturation under the control of the SNS in a rodent model, providing a framework for the study of how signals generated by SNS hyperactivity in critical developmental windows may adversely affect the maturation and function of the vascular system.
Fig. 8.
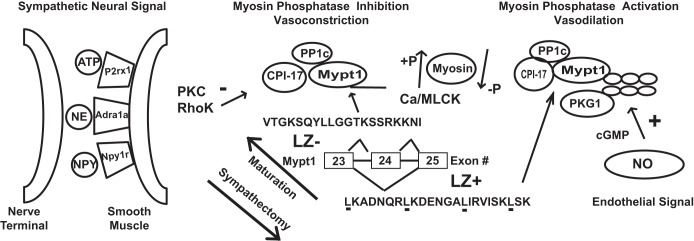
Model for developmental maturation of MA smooth muscle and signaling pathways regulating contractility. Maturation of MA under the inductive influence of the sympathetic nervous system (SNS) includes a switch to the E24+/LZ- isoform of Mypt1 and upregulation of smooth muscle contractile proteins (actin, myosin, MLCK, myosin light chain phosphatase), in particular massive induction of its signaling target the myosin phosphatase (MP) inhibitory subunit CPI-17. The receptors for SNS signaling including Adra1a, P2rx1, and Npy1r are also significantly developmentally upregulated. The E24- splice variant of Mypt1, coding for a COOH-terminal LZ, a motif required for nitric oxide (NO)/cGMP activation of MP (calcium desensitization of force production) is developmentally downregulated. The developmental maturation of MA smooth muscle is suppressed by chemical sympathectomy. Minus and plus signs denote inhibition and activation of MP, respectively. NPY, neuropeptide Y; NE, norepinephrine.
GRANTS
This work was supported by NIH grants RO1-HL066171 (S. Fisher), T32-HL072751 (J. Reho), and the NIA Short-Term Training Program on Aging T35AG036679 (J. Benjamin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J.R. and S.A.F. conceived idea, J.J.R. and S.A.F. designed and oversaw experiments, J.J.R., X.Z., J.E.B. performed experiments, collected and analyzed data. J.J.R. generated graphs and ran statistical analyses, J.J.R. X.Z., J.E.B, and S.A.F wrote the manuscript. S.A.F. supervised the project. All authors have approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Rachael Dippold and Alex Lloyd for technical assistance.
REFERENCES
- 1.Barker DJ, Bagby SP, Hanson MA. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2: 700–707, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 75: 19–37, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bello-Reuss E, Colindres RE, Pastoriza-Munoz E, Mueller RA, Gottschalk CW. Effects of acute unilateral renal denervation in the rat. J Clin Invest 56: 208–217, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevan RD. Trophic effects of peripheral adrenergic nerves on vascular structure. Hypertension 6: III19–III26, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Bhetwal BP, An CL, Fisher SA, Perrino BA. Regulation of basal LC20 phosphorylation by MYPT1 and CPI-17 in murine gastric antrum, gastric fundus, and proximal colon smooth muscles. Neurogastroenterol Motil 23: e425–e436, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boegehold MA. Endothelium-dependent control of vascular tone during early postnatal and juvenile growth. Microcirculation 17: 394–406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckley NM. Maturation of circulatory system in three mammalian models of human development. Comp Biochem Physiol A Comp Physiol 83: 1–7, 1986 [DOI] [PubMed] [Google Scholar]
- 8.Buus CL, Aalkjaer C, Nilsson H, Juul B, Moller JV, Mulvany MJ. Mechanisms of Ca2+ sensitization of force production by noradrenaline in rat mesenteric small arteries. J Physiol 510: 577–590, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damon DH. Sympathetic innervation promotes vascular smooth muscle differentiation. Am J Physiol Heart Circ Physiol 288: H2785–H2791, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Davis MI, Filion KB, Zhang D, Eisenberg MJ, Afilalo J, Schiffrin EL, Joyal D. Effectiveness of renal denervation therapy for resistant hypertension: a systematic review and meta-analysis. J Am Coll Cardiol 62: 231–241, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Dippold RP, Fisher SA. Myosin phosphatase isoforms as determinants of smooth muscle contractile function and calcium sensitivity of force production. Microcirculation 21: 239–248, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376: 1903–1909, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem 284: 35273–35277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatigati V, Murphy RA. Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J Biol Chem 259: 14383–14388, 1984 [PubMed] [Google Scholar]
- 15.Fisher SA. Vascular smooth muscle phenotypic diversity and function. Physiol Genomics 42A: 169–187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folkow B. Neurotrophic effects on the vascular bed. Acta Med Scand Suppl 672: 95–99, 1983 [DOI] [PubMed] [Google Scholar]
- 17.Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension 61: 806–811, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu K, Mende Y, Bhetwal BP, Baker S, Perrino BA, Wirth B, Fisher SA. Tra2beta protein is required for tissue-specific splicing of a smooth muscle myosin phosphatase targeting subunit alternative exon. J Biol Chem 287: 16575–16585, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29: 630–638, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Goyal R, Mittal A, Chu N, Shi L, Zhang L, Longo LD. Maturation and the role of PKC-mediated contractility in ovine cerebral arteries. Am J Physiol Heart Circ Physiol 297: H2242–H2252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartshorne DJ, Ito M, Erdodi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem 279: 37211–37214, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Hill CE. Selectivity in sympathetic innervation during development and regeneration in the rat. Experientia 41: 857–862, 1985 [DOI] [PubMed] [Google Scholar]
- 23.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 86: 1–23, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Huang QQ, Fisher SA, Brozovich FV. Unzipping the role of myosin light chain phosphatase in smooth muscle cell relaxation. J Biol Chem 279: 597–603, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Khatri JJ, Joyce KM, Brozovich FV, Fisher SA. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J Biol Chem 276: 37250–37257, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem 275: 9897–9900, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Kitazawa T, Kitazawa K. Size-dependent heterogeneity of contractile Ca2+ sensitization in rat arterial smooth muscle. J Physiol 590: 5401–5423, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem 272: 5063–5068, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res 106: 633–646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ljung B, Lundberg JM, Dahlstrom A, Kjellstedt A. Structural and functional ontogenetic development of the rat portal vein after neonatal 6-hydroxydopamine treatment. Acta Physiol Scand 106: 271–279, 1979 [DOI] [PubMed] [Google Scholar]
- 31.Longo LD, Goyal R. Cerebral artery signal transduction mechanisms: developmental changes in dynamics and Ca2+ sensitivity. Curr Vasc Pharmacol 11: 655–711, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loria AS, Brands MW, Pollock DM, Pollock JS. Early life stress sensitizes the renal and systemic sympathetic system in rats. Am J Physiol Renal Physiol 305: F390–F395, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luff SE. Development of neuromuscular junctions on small mesenteric arteries of the rat. J Neurocytol 28: 47–62, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Luff SE. Ultrastructure of sympathetic axons and their structural relationship with vascular smooth muscle. Anat Embryol (Berl) 193: 515–531, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Mills E, Smith PG. Mechanisms of adrenergic control of blood pressure in developing rats. Am J Physiol 250: R188–R192, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Morgan JP, Morgan KG. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol 351: 155–167, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41: 19–26, 1977 [DOI] [PubMed] [Google Scholar]
- 38.Nankervis CA, Nowicki PT. Role of nitric oxide in regulation of vascular resistance in postnatal intestine. Am J Physiol Gastrointest Liver Physiol 268: G949–G958, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Nuyt AM, Alexander BT. Developmental programming and hypertension. Curr Opin Nephrol Hypertens 18: 144–152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Page IH, Heuer GJ. The effect of renal denervation on the level of arterial blood pressure and renal function in essential hypertension. J Clin Invest 14: 27–30, 1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Payne MC, Zhang HY, Prosdocimo T, Joyce KM, Koga Y, Ikebe M, Fisher SA. Myosin phosphatase isoform switching in vascular smooth muscle development. J Mol Cell Cardiol 40: 274–282, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Payne MC, Zhang HY, Shirasawa Y, Koga Y, Ikebe M, Benoit JN, Fisher SA. Dynamic changes in expression of myosin phosphatase in a model of portal hypertension. Am J Physiol Heart Circ Physiol 286: H1801–H1810, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Reber KM, Nankervis CA, Nowicki PT. Newborn intestinal circulation. Physiology and pathophysiology. Clin Perinatol 29: 23–39, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 22: 269–278, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol 22: 2156–2165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc 152: 1501–1504, 1953 [DOI] [PubMed] [Google Scholar]
- 48.Storkebaum E, Ruiz de Almodovar C, Meens M, Zacchigna S, Mazzone M, Vanhoutte G, Vinckier S, Miskiewicz K, Poesen K, Lambrechts D, Janssen GM, Fazzi GE, Verstreken P, Haigh J, Schiffers PM, Rohrer H, Van der Linden A, De Mey JG, Carmeliet P. Impaired autonomic regulation of resistance arteries in mice with low vascular endothelial growth factor or upon vascular endothelial growth factor trap delivery. Circulation 122: 273–281, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Su W, Xie Z, Liu S, Calderon LE, Guo Z, Gong MC. Smooth muscle-selective CPI-17 expression increases vascular smooth muscle contraction and blood pressure. Am J Physiol Heart Circ Physiol 305: H104–H113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science 286: 1583–1587, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12–G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 14: 64–68, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol 535: 553–564, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolgar JR, Scott TM. The relationship between innervation and arterial structure in late prenatal and early postnatal development of the rat jejunal artery. J Anat 167: 57–70, 1989 [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Fisher SA. Conditioning effect of blood flow on resistance artery smooth muscle myosin phosphatase. Circ Res 100: 730–737, 2007 [DOI] [PubMed] [Google Scholar]