Abstract
Dipeptidylpeptidase-4 (DPP-4) is a ubiquitously expressed transmembrane protein that removes NH2-terminal dipeptides from various substrate hormones, chemokines, neuropeptides, and growth factors. Two known substrates of DPP-4 include the incretin hormones glucagon-like peptide-1 (GLP-1) and gastric inhibitory peptide, which are secreted by enteroendocrine cells in response to postprandial hyperglycemia and account for 60–70% of postprandial insulin secretion. DPP-4 inhibitors (DPP-4i) block degradation of GLP-1 and gastric inhibitory peptide, extend their insulinotropic effect, and improve glycemia. Since 2006, several DPP-4i have become available for treatment of type 2 diabetes mellitus. Clinical trials confirm that DPP-4i raises GLP-1 levels in plasma and improves glycemia with very low risk for hypoglycemia and other side effects. Recent studies also suggest that DPP-4i confers cardiovascular and kidney protection, beyond glycemic control, which may reduce the risk for further development of the multiple comorbidities associated with obesity/type 2 diabetes mellitus, including hypertension and cardiovascular disease (CVD) and kidney disease. The notion that DPP-4i may improve CVD outcomes by mechanisms beyond glycemic control is due to both GLP-1-dependent and GLP-1-independent effects. The CVD protective effects by DPP-4i result from multiple factors including insulin resistance, oxidative stress, dyslipidemia, adipose tissue dysfunction, dysfunctional immunity, and antiapoptotic properties of these agents in the heart and vasculature. This review focuses on cellular and molecular mechanisms mediating the CVD protective effects of DPP-4i beyond favorable effects on glycemic control.
Keywords: vascular dysfunction, obesity, insulin resistance, diastolic dysfunction, incretin
epidemiological studies reveal that two out of three American adults are overweight or obese and at increased risk for progression to type 2 diabetes mellitus (T2DM) (58). Consumption of a Western-style diet, high in fat, high-fructose corn syrup, and salt, in concert with a sedentary lifestyle are major factors contributing to the pandemics of obesity and T2DM (96, 128). Among overweight/obese individuals there is a high incidence of insulin resistance that is a seminal risk factor for progression to cardiac, renal, and vascular maladaptive structural and functional abnormalities in individuals who are prediabetic, as well as those with T2DM (176). T2DM is classically recognized as a metabolic disorder yet develops as a collection of metabolic and immune derangements that predictably increase the risk for cardiovascular (CV) disease (CVD) and chronic kidney disease (CKD). The high incidence of CVD in T2DM in the setting of obesity can be explained, in part, by the interaction of several systemic maladaptations, such as, insulin resistance, chronic activation of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system with associated increases in inflammation, oxidative stress, and maladaptive immune responses (175). The vasculature is exquisitely sensitive to all of these abnormalities; consequently, vascular dysfunction is often the first derangement to occur in the progression to end organ/tissue damage in T2DM.
Although the conventional goal of diabetes therapies is to reduce plasma glucose and glucose toxicity (glycemic control), it has become increasingly apparent that development of novel safe and effective therapeutic strategies to improve long-term glycemic control should also have favorable direct and/or indirect effects on CVD and CKD outcomes. This more contemporary therapeutic approach is due, in part, to recent oberservations that some conventional diabetes therapies, although effective at controlling glycemia, may actually increase the risk of CVD events, increase hypoglycemic episodes and result in weight gain. As such, in 2008, the Food and Drug Administration issued more stringent testing requirements for drug companies to ensure that new diabetes drugs do not increase CVD risk (59). Indeed, it is known that the incidence of CVD events associated with T2DM can be reduced by therapeutic interventions targeting multiple CV and metabolic derangements inherent with T2DM, rather than interventions targeting a single risk factor, such as hyperglycemia (62). Therefore, there is a need for drugs that are effective not only for controlling glycemia, but also for improving CVD outcomes independently of glycemic control.
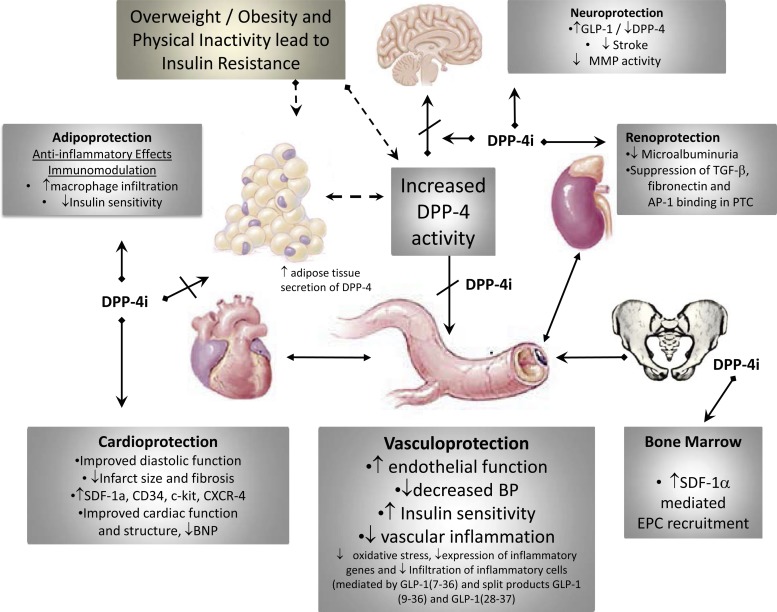
Accumulating evidence suggests that the modulation of incretin signaling by glucagon-like peptide-1 (GLP-1) agonists is beneficial in both glycemic control, as well as CV protection (182). In this regard, dipeptidylpeptidase-4 (DPP-4) inhibitors (DPP-4i), which have been developed specifically as incretin-based oral antihyperglycemia therapies, are now emerging as novel agents that have the potential to reduce the progression to CVD and CKD. DPP-4i exhibit multiple protective effects that collectively contribute to improvement in vascular function which can reduce the risk for development of vascular disease, heart failure (HF), and CKD (Fig. 1). The beneficial effects of these drugs, beyond glycemic control, occur by both GLP-1-dependent and -independent mechanisms (147, 150).
Fig. 1.
Pleiotropic effects of dipeptidylpeptidase-4 (DPP-4) inhibitors (DPP-4i) that benefit the vasculature. DPP-4i exhibit multiple protective effects that collectively contribute to improvement in vascular function which can reduce the risk for development of vascular disease, heart failure, and chronic kidney disease. In the setting of obesity/type 2 diabetes mellitus, circulating DPP-4 levels are elevated, due in part to elevated secretion of DPP-4 from inflamed visceral fat. Preclinical and clinical studies examining the efficacy of the DPP-4i have shown improvement in a number of cardiovascular disease (CVD) outcomes, as indicated in the gray boxes. SDF-1α, stromal cell-derived factor 1α; CXCR-4, C-X-C chemokine receptor type 4; BNP, brain natriuretic peptide; BP, blood pressure; GLP-1, glucagon-like peptide-1; MMP, matrix metalloproteinase; AP-1, activator protein-1; PTC, proximal tubule cell; EPC, endothelial progenitor cell.
Although most preclinical and clinical studies support a positive role for DPP-4i in the vasculature, a few studies indicate a negative impact on vascular function (11, 147, 167, 192). For instance, an increase in the rate of hospitalization has been reported for HF in patients treated with saxagliptin (3.5 vs. 2.8% by 2-yr Kaplan-Meier analysis), despite improvements in glycemic control (167). In another report, attenuation of flow-mediated dilation by sitagliptin and alogliptin has been reported (11). In contrast, vildagliptin improved endothelial function and alogliptin attenuated postprandial decrease in flow-mediated dilation in subjects without diabetes (142, 183). Further large-scale CV outcome studies will resolve the issue of excess HF risk. Importantly, the safety and beneficial effects of DPP-4i continue to be evaluated in several large CV outcomes studies, including the Trial to Evaluate Cardiovascular Outcomes after Treatment with Sitagliptin (TECOS) (69), Cardiovascular Outcomes Study of Alogliptin in Subjects With Type 2 Diabetes and Acute Coronary Syndrome (EXAMINE) (190, 191), Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus (SAVOR-TIMI) (167), and Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes (CAROLINA) (160). Although the use of GLP-1 receptor (GLP-1R) agonists and DPP-4i have been shown to be associated with development of pancreatitis and pancreatic and thyroid cancer in a few studies (26, 49), such adverse outcomes have not been demonstrated in many recent preclinical and clinical studies (46, 64, 111, 140, 167, 190).
DPP-4 Biology and Pharmacology
The incretin hormones GLP-1 and gastric inhibitory peptide (GIP) are secreted by enteroendocrine cells in response to postprandial hyperglycemia and account for 60–70% of postprandial insulin secretion (the incretin effect). Early studies demonstrated that the incretin effect was impaired in T2DM (139, 141). The recognition of the fundamental glucoregulatory role played by the intestinal-derived incretin hormones GLP-1 and GIP eventually led to the recent development of novel targeted therapies for treatment of T2DM. GLP-1 and GIP are key in regulating both postprandial and long-term glucose homeostasis by augmenting glucose-dependent pancreatic insulin secretion, suppressing glucagon release, slowing gastric emptying, enhancing satiety, and modulating the so-called “gut-brain axis” (24, 47). Once secreted, incretin hormones have a half-life of 1 to 2 min in plasma because of rapid degradation by the ubiquitous enzyme DPP-4. As such, direct administration of GLP-1 for lowering glucose levels may be limited because of enzymatic action of circulating DPP-4, which tends to be elevated especially in the setting of obesity (9, 100, 101, 107). One strategy to address this limitation has been the development of drugs to specifically inhibit DPP-4 to reduce catabolism of active GLP-1 and enhance its bioavailability. Several DPP-4i, including sitagliptin, saxagliptin, vildagliptin, linagliptin, and alogliptin, are now approved for use in the United Stated. Clinical trials have demonstrated that DPP-4i, alone or in combination with metformin or sulphonylureas, lower hemoglobin A1c (HbA1c) levels 0.6–0.9%, have low incidence of hypoglycemia, and are well tolerated because of a low incidence of side effects (90, 93, 133, 185).
The clinical pharmacology of DPP-4i inhibitors has been the subject of several recent comprehensive reviews and will not be discussed in detail here (12, 67, 163). Although DPP-4 inhibitors are structurally diverse, in general, they possess similar pharmacodynamic properties. As a monotherapy, or as an adjunct to conventional glucose lowering agents, DPP-4i are comparably effective at elevating plasma GLP-1 levels and lowering blood glucose and HbA1c, are generally weight neutral, and have similar safety and tolerability profiles (1, 3, 83, 134, 180). On the other hand, the pharmacokinetic profiles of the various DPP-4i compounds indicate differences in half-life, metabolism, distribution and bioavailability, plasma protein binding, and route of elimination that could have clinical relevance. Impaired kidney function, a common comorbidity in obesity and diabetes, is known to alter the elimination of alogliptin, sitagliptin, saxagliptin, and vildagliptin (67). As such, dose reduction is generally indicated in relation to the severity of renal dysfunction. In this regard, it is noteworthy that linagliptin is eliminated largely unmetabolized by a biliary/hepatic route, rather than by renal elimination like other DPP-4i. As such, linagliptin can be prescribed to patients with renal insufficiency without the need to decrease the dose (67).
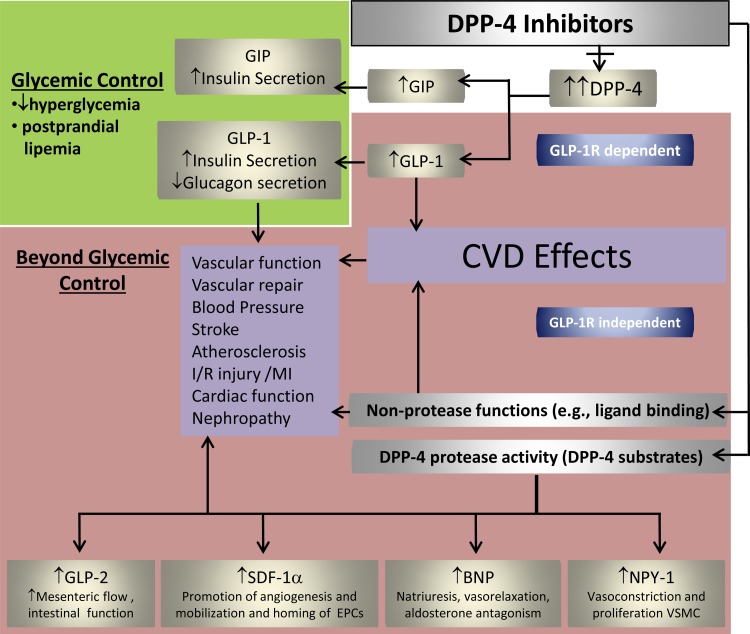
Most studies demonstrating vasculoprotection or cardioprotection by GLP-1 have used supraphysiological concentrations of native GLP-1 or GLP-1 analogs that elicit GLP-1 signaling beyond what is physiological. DPP-4i have been reported to induce a modest increase in circulating active GLP-1 levels above baseline (∼2- to 6-fold) in diabetic mice, rats, and humans (9, 40, 144, 155). Thus the extent of GLP-1-dependent responses to DPP-4i in the vasculature may be somewhat limited relative to those evoked in experimental studies by GLP-1 analogs. Moreover, DPP-4i may have the potential to exert either a broader range of beneficial effects or a different subset of benefits on overall CVD and CKD outcomes compared with cardioprotective and vasodilatory effects of GLP-1 analogs (15, 182). Accumulating evidence suggests that DPP-4i possess anti-inflammatory, antioxidant, and antiapoptotic effects in the vasculature and that these beneficial effects may also occur independent of GLP-1 (Fig. 2). This notion is based on the fact that DPP-4, in addition to its actions on incretin hormones, also acts on a number of other substrates, e.g., stromal cell-derived factor 1α (SDF-1α), as well as binds to specific proteins (e.g., fibronectin) that can contribute to the pathophysiology of CVD (202). Therefore, the GLP-1-independent effects of DPP-4i may include suppression of harmful effects of DPP-4. In this regard, administration of stable GLP-1 analogs does not affect DPP-4 activity that is often increased in plasma from patients with obesity, diabetes, or HF (107, 156). GLP-1 analogs are reported to result in small blood pressure (BP) reductions and modest weight loss (182). Some, but not all, studies have demonstrated small reductions in BP with DPP-4i (9, 114, 132, 145, 148, 152), and these effects may be independent of effects associated with weight loss, as DPP-4i tend to be body weight neutral. Given the advantage of oral administration of DPP-4i and their CV safety profile, these drugs are becoming second- and third-line therapies for glycemic reduction in diabetic patients. Importantly, emerging evidence suggests that DPP-4i may have neutral (167, 190) or positive effects on CVD outcomes (4, 89, 91, 182).
Fig. 2.
DPP-4 biology and pharmacology of DPP-4i. Incretin hormones, GLP-1, and gastric inhibitory peptide (GIP) regulate insulin secretion in a glucose-dependent manner. DPP-4i inhibit degradation of GLP-1 and GIP, thereby contributing to glycemic control. However, DPP-4i will have multiple effects beyond glycemic control that occur by GLP-1-independent mechanisms because of wide substrate specificity and direct effects of DPP-4 with other proteins. GLP-1R, GLP-1 receptor; I/R, ischemia-reperfusion; MI, myocardial infarction; NPY-1, neuropeptide Y-1; VSMC, vascular smooth muscle cell.
DPP-4 is a 766-amino-acid membrane protein (110 kDa) that was originally designated as lymphocyte cell surface protein, cluster of differentiation-26/antigen (CD26) for its role in T-cell activation. However, it is also expressed in other immune cells and nonimmune cells/tissues (5, 202). Tissues/organs expressing DPP-4 include blood vessels, adipose tissue, kidney, liver, pancreas, lymph nodes, spleen, bone, brain, lung, prostate, thymus, and uterus (109, 202). Various cytokines, such as interleukin (IL)-12 and interferon-γ, as well as transcription factors, such as hepatocyte nuclear factor-1α and nuclear factor-κB, regulate CD26/DPP-4 expression in various cells (16, 50). CD26/DPP-4 expression on CD4+ and CD8+ T cells has been reported to be higher in T2DM patients and reduced with active glucose control (109).
In addition to membrane-associated DPP-4 (mDPP-4), there is a soluble circulating form of DPP-4 (sDPP-4) that lacks the transmembrane and cytoplasmic domains of mDPP-4 and is largely responsible for degradation of the majority of newly synthesized GLP-1 and GIP (42, 73). Evidence suggests that proteolytic cleavage of mDPP-4 is the major source of circulating sDPP-4 (107). Although liver epithelium and lymphocytes are often cited as sources of sDPP-4 (37), the sources of sDPP-4 and factors that regulate circulating levels are largely unknown. In this regard, a recent study demonstrated that adipose tissue is a significant source of sDPP-4 and that insulin and tumor necrosis factor-α can induce shedding of sDPP-4 (107). Thus the elevated levels of sDPP-4 reported in obesity/T2DM may be related to the consequences of insulin resistance (e.g., elevated insulin and/or glucose), hyperglycemia, and adipose tissue inflammation (elevated tumor necrosis factor-α), which promote DPP-4 shedding from adipose tissue (107). Insulin resistance is associated with increased expression and release of DPP-4 (107, 202).
DPP-4 specifically cleaves dipeptides from incretin as well as a number of non-incretin peptide substrates containing a penultimate proline or alanine residue at the NH2-terminus (131). Importantly, some of these peptides, including stromal-cell derived factor-1α (SDF-1α), brain natriuretic peptide (BNP), neuropeptide Y (NPY), and peptide YY (PYY), have direct or indirect effects in the vasculature (Fig. 2). The effects of these substrates add considerable complexity to the potential mechanisms by which DPP-4i mediate effects on vascular/endothelial function. However, most of these peptides mediate a wide range of beneficial pleiotropic effects in the vasculature that are not imparted by GLP-1 agonists alone, although some DPP-4 substrates, such as NPY, exert vasoconstrictor effects in the setting of hypertension (182). Since DPP-4 activity is increased in obesity and T2DM, it is possible that inhibition of DPP-4 will not only increase GLP-1 but also enhance the activities of several beneficial substrates.
In addition to enzymatic actions, DPP-4 also has additional nonenzymatic functions involving binding to adjacent membrane proteins, including adenosine deaminase, caveolin, kidney, Na+/H+ exchanger, thromboxane A2 receptor, and C-X-C chemokine receptor type 4, as well as binding to extracellular matrix proteins such as fibronectin and collagens (202) (Fig. 2). For instance, the cysteine-rich region of DPP-4 contains binding sites for collagen and fibronectin that enable DPP-4 containing inflammatory T- helper cells to concentrate in areas of accumulating extracellular matrix proteins, such as those found in and around an affected blood vessel undergoing fibrosis and associated vascular stiffness (Fig. 3).
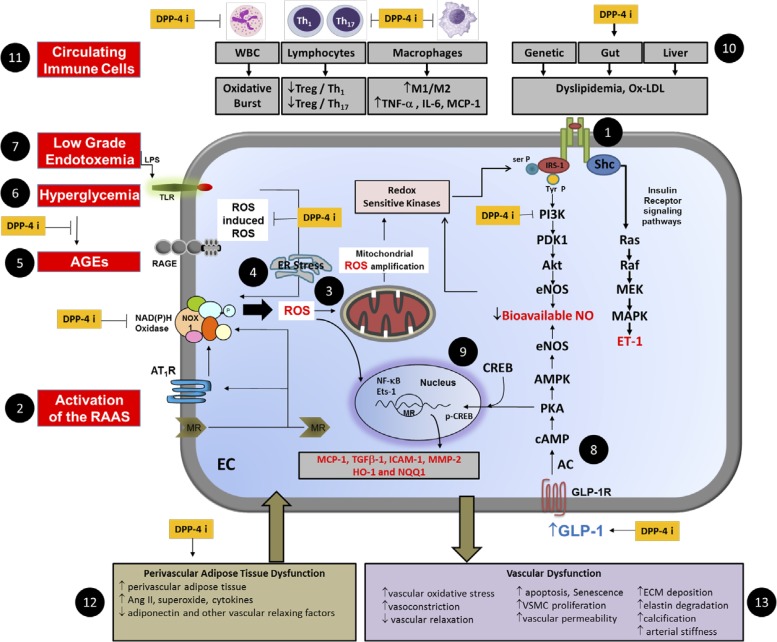
Fig. 3.
A schematic representation depicting deleterious effects of overnutrition and obesity in the vasculature and targets for DPP-4i. Numbered black circles 1 through 13 indicate key components of pathology. DPP-4i improve vascular dysfunction through GLP-1-dependent and -independent mechanisms through modulation of insulin (1) and renin-angiotensin-aldosterone system (RAAS) activation (2) leading to state of reactive oxygen species (ROS)-induced ROS and mitochondrial ROS amplification (3) and endoplasmic reticular (ER) stress (4). Advanced glycation end products (AGE) (5), hyperglycemia (6), and low-grade endotoxemia (7) collectively contribute to intracellular oxidative stress. The exacerbated state of oxidative stress induces activation of redox sensitive kinases that influence the phosphorylation state of insulin receptor substrate-1 (IRS-1) to reduce nitric oxide (NO) generation via the phosphoinositide 3-kinase (PI3K)/Akt/endothelial NO synthase (eNOS) signaling pathway while favoring signaling through the Shc Ras/MEK/MAPK to increase synthesis of endothelin-1 (ET-1). GLP-1 mediated activation of eNOS (8) and cAMP responsive element binding protein (CREB) (9) also contribute to vascular protection by DPP-4i. Dyslipidemia (10) and dysfunctional immunity (11) and dysfunctional visceral and perivascular adipose tissue (12) further contribute to vascular injury and inflammation. Thus the interaction of vascular cells, immune cells and adipose tissue causes abnormalities in vascular function and remodeling (13). DPP-4i (indicated by yellow boxes) modulate different components of pathways that could act to improve vascular function. RAGE, receptor for AGE; TLR, Toll-like receptor; Treg, regulatory T cells; Th, T-helper cells; WBC, white blood cell; MCP-1, monocyte chemoattractant protein-1; Ox-LDL, oxidized LDL; NOX, NADPH oxidase; AT1R, angiotensin type 1 receptor; MR, mineralocorticoid receptor; EC, endothelial cell; Ets-1, E26 transformation-specific-1; Ser P, serine phosphorylation; Tyr P, tyrosine phosphorylation; PDK1, 3-phosphoinositide-dependent protein kinase-1; AMPK, AMP-activated protein kinase; AC, adenylate cyclase; HO-1, hemoxygenase-1; NQQ1, quinine reductase.
DPP-4i and Vasculoprotection
Emerging evidence suggests that DPP-4i may have beneficial pleiotropic effects in the vasculature and heart that may reduce the risk of CVD, including hypertension, atherosclerosis, stroke, and heart, and CKD (Fig. 1).
Hypertension.
Approximately 70% of patients with diabetes have high BP, meaning they are twice as likely to have hypertension compared with individuals without diabetes (106). BP responses to DPP-4i therapy have been reported to be either neutral with saxagliptin and linagliptin (35, 185) or modestly reduced with sitagliptin (13, 105, 132, 133, 145). Most relevant clinical trials were of short and medium term; results of long-term trials are not yet available, so it cannot be concluded definitively that DPP-4i therapy is BP neutral in hypertensive diabetic humans.
In rodent models, sitagliptin and saxagliptin reduced BP in spontaneously hypertensive rats (SHRs) and young prehypertensive SHRs (114, 122, 148). Sitagliptin also reduced BP in Zucker diabetic fatty rats (56). We recently reported that linagliptin reduced radiotelemetric-derived measures of mean arterial pressure in mildly hypertensive, insulin-resistant Zucker obese (ZO) rats (9). The elevated BP in untreated ZO rats was associated with impaired endothelium-dependent vasodilation of small arterioles, and this endothelial defect was normalized by treatment with linagliptin.
In addition to reducing the enzymatic degradation of GLP-1 and GIP, DDP-4i decrease the degradation of several vasoactive peptides, such as BNP, substance P, PYY receptor (1–36), and NPY (22). Thus there is potential for DPP-4i therapy to either raise or lower BP based on these multiple actions and differing effects have been reported. To this point, DDP-4i therapy may be contraindicated in hypertensive patients with T2DM taking angiotensin-converting enzyme (ACE) inhibitors (ACEi) to lower BP. A recent study showed that sitagliptin increased BP in subjects with the metabolic syndrome when treated with enalapril (121). In this study, sitagliptin monotherapy slightly decreased BP and augmented the hypotensive effects of a submaximal dose of enalapril (5 mg/day). Interestingly, the combination of sitagliptin and a higher dose (10 mg/day) of enalapril that maximally inhibits ACE, neutralized the BP lowering effect of enalapril, and increased heart rate and circulating norepinephrine levels, suggesting that sitagliptin induces sympathetic nervous system activation under conditions of maximal ACEi. It has been suggested that the mild antihypertensive effect of DPP-4i in the absence of ACEi or in combination with a low dose of ACEi is mediated by attenuation of substance P metabolism. When ACE was inhibited maximally, DPP-4i decreased degradation of NPY (1–36), inactivated substance P, and increased sympathetic outflow to attenuate the antihypertensive effect of ACEi (86). These results were consistent with those from a study in SHRs showing that a combination of sitagliptin and ACEi increases BP (87). Taken together, these studies suggest that during combination DPP-4i and high-dose ACEi, activation of the sympathetic nervous system by substance P and decreased degradation of NPY(1–36) may offset decreased degradation of vasodilatory peptides. Clearly, further studies are needed to clarify the mechanism and roles of DDP-4i in BP regulation with regard to drug interactions. To address this concern, a recent study tested whether linagliptin affected BP when used in combination with the angiotensin receptor blocker (ARB) telmisartan in a model of renal hypertension. In contrast to the previous studies showing that DPP-4i therapy countered the BP-lowering effects of ACEi (86, 87, 121), the BP-lowering effects of telmisartan were not countered by linagliptin and tended to further improve BP in a rat model of renovascular hypertension (33). This study suggests the possibility that hypertension, renal function, and glycemic control may be more effectively managed in patients with T2DM treated with a combination of ARB/DPP-4i as opposed to ACEi/DPP-4i.
Atherosclerosis.
DDP-4i therapy may reduce the risk for further development of atherosclerosis via improved glucose regulation; however, there may be additional DPP-4i-mediated molecular mechanisms involved in blunting atherosclerosis or stabilizing plaque formation. In the setting of obesity/T2DM, a combination of hyperglycemia, elevated levels of circulating triglyceride (TG)-rich lipoproteins, and proinflammatory cytokines secreted from inflamed adipose tissue activates the endothelium, resulting in impaired insulin signaling, reduced nitric oxide (NO) synthesis and signaling, and recruitment of inflammatory cells to the vessel wall to incite conditions leading to atherosclerosis. The endothelium is the first barrier breached in the progression of atherosclerosis and damage and remodeling stem from a coordinated lipid and inflammatory cell infiltration (117). The beneficial effects of DPP-4i, independent of glycemic control, may occur through their effects on GLP-1 or GLP-1 split peptides. In this regard, a recent study demonstrates that GLP-1(7–37) and its metabolites, GLP-1(9–37) and GLP-1(28–37) reduce inflammation within atherosclerotic plaques in the aorta of apolipoprotein E (ApoE)−/− mice and improve plaque stability by decreasing the degradative actions of matrix metalloproteinase (MMP)-9 in the fibrous cap (25). Moreover, these split products also decreased plaque macrophage content, as well as expression of MMP-9. These anti-inflammatory effects were similar to GLP-1(7–37). Despite similar efficacy these compounds may work through different pathways to achieve plaque stabilization. Evidence suggests that in the absence of GLP-1R, GLP-1(9–37) and GLP-1(28–37) exert protective effects in the cardiovasculature (14, 15). Like GLP-1(7–37), GLP-1(9–37) does increase adenosine 3′5-cyclic monophosphate (cAMP) concentrations suggesting the possibility of similar signal transduction by an unidentified G protein-coupled receptor. Furthermore, the potential antiatherogenic properties of DPP-4i may arise from GLP-1 independent mechanisms through other DPP-4 substrates or pleiotropic effects of DPP-4i as discussed in section 4 below.
Stroke.
T2DM is major risk factor for degenerative neurological diseases, such as stroke (99) and Alzheimer's disease (158). GLP-1Rs are expressed in neurons from rodents and humans (66, 72), and GLP-1 and GLP-1 analogs readily cross the blood brain barrier (82), suggesting the possibility that incretin enhancer therapy could be neuroprotective (79). Experimental animal studies demonstrate that the GLP-1R agonist, exendin-4, reduces the severity of stroke in diabetic and nondiabetic rodent models (21, 39, 108). In rodent models of Alzheimer's disease GLP-1 analogs have been shown to ameliorate disease progression (130). Unlike GLP-1 or GLP-1 agonists, DPP-4i have not been reported to cross the blood brain barrier, and this raises the question whether DPP-4i exhibit comparable neuroprotection like that shown for GLP-1 analogs. A recent clinically relevant study tested the antistroke efficacy of linagliptin and a comparator glimepiride in middle-aged nondiabetic and diabetic mice (40). Stroke was induced by middle cerebral artery occlusion and T2DM was induced by high-fat diet feeding. Glimepiride was used because it lowers blood glucose but has no effect on incretin hormones. Linagliptin treatment reduced plasma DPP-4 activity and increased GLP-1 levels. This was associated with a significant increase in the number of surviving cortical neurons, despite no reduction in infarct size, in both nondiabetic and obese diabetic mice. Linagliptin reduced hyperglycemia in the diabetic model but did not affect glycemia in the nondiabetic model, suggesting that the efficacy of linagliptin in reducing stroke does not depend on glucose reduction. Like linagliptin, glimepiride was efficacious in reducing severity of stroke in nondiabetic mice but had little efficacy in the diabetes model. In a randomized, double-blind, noninferiority trial testing the 2-yr safety and efficacy of linagliptin versus glimepiride in patients with T2DM receiving metformin, but having inadequately controlled HbA1c levels, there were significantly fewer major CVD events (12 vs. 26% for linagliptin vs. glimepiride, respectively) (63). These findings were mostly attributed to a lower incidence of nonfatal stroke events in patients treated with linagliptin compared with glimepiride (P = 0.032). Although adjunctive linagliptin and glimepiride therapies were equally efficacious at improving glycemia, linagliptin was less likely to lead to hypoglycemic events or weight gain that was associated with glimepiride. The possible mechanisms for the observed antistroke efficacy of linagliptin are unknown at this point, but it is likely to be due, at least in part, to GLP-1-mediated effects in the brain as was indicated in a similar study using exendin-4 (39). In this regard, modulation of MMPs have been implicated in the pathogenesis of stroke (31, 92), and GLP-1 agonists suppress MMP-9 activity (143).
Heart disease.
A recent clinical study reported that diastolic, but not systolic, dysfunction is a highly prevalent (40%) comorbid condition in a large population of patients with early phase T2DM and no history of CVD (151). In the setting of overnutrition/obesity, diastolic dysfunction is often the earliest functional cardiac abnormality (161, 184, 199). Moreover, prediabetic insulin resistance and diastolic dysfunction may become more prevalent given the emerging pandemic in childhood/adolescent overweight/obesity (146). We recently tested the notion that the DPP-4i, linagliptin, could be useful in ameliorating pathophysiologic abnormalities in diastolic and vascular endothelial dysfunction in a clinically relevant rodent model of obesity associated with insulin resistance. We treated ZO rats with linagliptin for 8 wk (9), beginning at 8 wk of age when they exhibit both insulin resistance and diastolic dysfunction (203), CV manifestations that are seen in young obese humans with cardiorenal metabolic syndrome (176). Linagliptin markedly improved impaired diastolic function, and this was associated with improved vascular endothelial function and a reduction in BP.
DPP-4i could also be effective at improving cardiac function in more severe forms of heart disease associated with myocardial infarction (182, 200). A recent report demonstrated a correlation between circulating DPP-4 activity and cardiac dysfunction in patients with HF and in a rodent model of experimental HF (45). Earlier studies demonstrated activation of the cardioprotective signaling pathways by GLP-1, leading to improvement in coronary blood flow (172, 201), decreases in cardiomyoctye apoptosis (157), and reduction in infarct size following ischemia-reperfusion (I/R) injury (18, 143). In the setting of severe diastolic dysfunction, left atrial dilation, and abnormal myocardial perfusion, it has been shown that the foremost contributor to advanced left ventricular (LV) diastolic dysfunction was an ischemic myocardium (151). Thus the increased bioavailability of GLP-1 consistently observed with DPP-4i therapy could confer cardioprotection, especially in the ischemic myocardium. DPP-4i have been tested in experimental rodent models of myocardial infarction and ischemia and shown to have mostly positive effects (36, 76, 78, 162, 197, 198). In one study, linagliptin significantly reduced infarct size and area of fibrosis in male Wistar rats after I/R injury both in the short term (7 days post I/R) and long term (8 wk post-I/R) (78). Cardiac function was impaired in this model following I/R injury, yet diastolic function, as measured by a significant improvement in the maximum rate of LV pressure decline, was improved 8 wk following the I/R procedure. Linagliptin did not blunt the reduction in ejection fraction caused by I/R injury. Linagliptin treatment resulted in a 19-fold increase in plasma GLP-1 levels, and this likely contributed to the reduction in myocardial injury.
Recently, the beneficial effects of DPP-4i, independent of incretin hormone effects, have been shown in a model of uremic cardiomyopathy. Linagliptin treatment of rats with chronic uremic cardiomyopathy lowered the augmented BNP levels and heart tissue fibrosis markers, which likely led to improved cardiac function and structure (34). BNP is one of the off-target peptides implicated in the beneficial effects of DPP-4i in the cardiovasculature. BNP plays a critical role in regulating body fluid homeostasis, has vasodilator effects, and is a marker of HF. Degradation of BNP by DPP-4 lowers guanosine 3′5-cyclic monophosphate (cGMP) levels, resulting in reduced diuresis and natriuresis, as well as reduction in the contribution of BNP to vascular tone. The improvement of cardiac function and fibrosis after linagliptin treatment raises the possibility that elevated BNP in HF is initially the result of stress to the myocardium, and a subsequent decrease in BNP levels with DPP-4i therapy may be indicative of improvement in myocardial stress with concomitant lowering of BNP release. This paradoxical notion that DPP-4i therapy reduces circulating BNP levels was further validated in a recent study reporting elevated circulating DPP-4 activity in patients and rats with HF (45). In that study, the elevated level of BNP associated with experimental HF in rats was reduced in rats on DPP-4i therapy (sitagliptin) in concert with improvement in cardiac function. Taken together, DPP-4i appears to be efficacious in conditions of insulin resistance associated with early diastolic dysfunction in obesity, as well as more advanced cardiomyopathies.
Kidney disease.
Obesity and T2DM are associated with progressive loss of renal function. T2DM accounts for 30–40% of CDK and as much as 45% of end-stage renal disease (188). GLP-1Rs are expressed in the kidney, notably in podocytes (77) and the brush border microvilli of proximal tubule cells (38, 165), and DPP-4 is abundantly expressed in capillary endothelial cells (ECs) (170), as well as in the brush border membrane of proximal tubule cells where it assembles with the Na+/H+ exchanger-3 (65, 97). Moreover, renal activity of DPP-4 is elevated in conditions associated with development of diabetic nephropathy, such as in overnutrition/obesity and inflammatory states (195). Recent studies demonstrate that stable GLP-1 analogs and DPP-4i attenuate renal injury in rodent models of type 1 diabetes (102, 115). However, most DPP-4i are excreted through a renal route, thereby raising concern for their use in the presence of renal impairment. In this regard, linagliptin is an exception since it is eliminated mainly through a hepatobiliary route. The renal clearance of linagliptin is very low (<1%), thereby obviating the need for dose adjustment in patients with renal impairment (43, 60, 63, 68, 154). In addition, linagliptin has also been shown to reduce proteinuria in diabetic patients with overt diabetic nephropathy (70, 77). In support of this, we recently observed that linagliptin led to reductions in glomerular injury, proteinuria, and oxidative stress in ZO rats (unpublished data). In another study, linagliptin suppressed activation of transforming growth factor-β, reduced fibronectin expression, and decreased activator protein-1 binding in human kidney proximal tubule cells induced by high glucose (149). These studies highlight the pleiotropic renal effects of DPP-4i beyond glycemic control. Since CKD is associated with CVD, the renal effects of DPP-4i may contribute to improvement in CVD outcomes. In this regard, DPP-4i has been shown to improve cardiac fibrosis, cardiac function in a rodent model of uremic cardiomyopathy (34).
Cellular and Molecular Mechanisms of Action of DPP-4i in the Vasculature
Vascular dysfunction is the critical factor in progression to CVD and CKD and the associated vasculopathies that constitute early derangements mediating progression to end organ damage (5, 8, 137, 138). Vascular dysfunction is independently associated with CVD complications, including stroke, atherosclerosis, and heart and kidney disease (8, 135). In this regard, the development of endothelial dysfunction and vascular stiffness are the early events that contribute to the progression of CVD (6, 32, 186). In the setting of obesity/T2DM, endothelial dysfunction and arterial stiffness may result from multiple factors including insulin resistance, oxidative stress, dyslipidemia, adipose tissue dysfunction (e.g., visceral and perivascular inflammation), and dysfunctional immunity (Fig. 3) (5, 27, 189). Endothelial dysfunction, altered vascular tone, extracellular matrix remodeling, and adventitial dysfunction contribute to arterial stiffness (6, 110); however, arterial stiffness also contributes to endothelial dysfunction (6). These components of the pathophysiology of obesity are modulated by DPP-4i and thus may contribute to CVD protection.
Administration of native GLP-1 and GLP-1 analogs elicit both GLP-1R-mediated responses, as well as effects that are independent of the GLP-1R (15). Some GLP-1R-independent effects in the vasculature have been shown to be mediated by GLP-1(9–36), a stable DPP-4-generated metabolite of active GLP-1(7–36) (15). Specifically, it was shown that GLP-1(9–36) causes vasodilation and increased blood flow in the coronary vasculature, and these effects are mediated by NO-dependent cGMP release, effects that cannot be elicited by the DPP-4-resistant GLP-1 analog exendin-4. The stimulation of NO production occurs through the PKA/liver kinase B1/AMP-activated protein kinase-α/endothelial NO synthase (eNOS) axis (114). Hypothetically, use of GLP-1 analogs or DPP-4i could limit putative GLP-1R-independent, NO-dependent vasodilation since both therapies may have the effect of limiting generation of the metabolite GLP-1(9–36) (25). However, this potential loss of benefit may be offset by pleitropic NO-mediated direct effect of DPP-4i. In addition to the effects DPP-4i have on their substrates, they may also exert direct effects on vascular tone. In a recent study, linagliptin exerted the most potent vasodilatory effects in ex vivo preparations of aortic rings, followed by alogliptin and vildagliptin (103). The vasorelaxant effects of alogliptin and linagliptin are mediated by the NO/cGMP pathway (103, 169). Alogliptin-mediated increased eNOS phosphorylation and NO production in human umbilical vein ECs (HUVECs) were not inhibited by GLP-1R antagonists exendin 9–39, suggesting a GLP-1R-independent mechanism (169). Moreover, alogliptin caused vascular relaxation in preconstricted aortic segments yet had no effect on insulin-mediated activation of eNOS/Akt. Vasodilation by alogliptin occurred through Src kinase-mediated effects on eNOS/Akt (169). In this regard, we recently reported that an observed defect in endothelium-dependent vasodilation of skeletal muscle 1A arterioles from insulin-resistant ZO rats was abolished by treatment with linagliptin (9). This improvement occurred despite reduced vascular smooth muscle sensitivity to NO as assessed by vasodilatory responses to the NO donor sodium nitroprusside. The observed improvement in arteriolar dilation in linagliptin-treated ZO rats occurred in concert with a lowering of mean arterial pressure and improvement in diastolic function. Moreover, the specific improvement in EC function was associated with increased eNOS phosphorylation, suggesting that improvement in endothelium-dependent vasodilation with linagliptin may be the result of increased NO bioavailability, to overcome reduced NO sensitivity. Similarly, DPP-4i treatment (des-fluoro-sitagliptin) was shown to improve endothelium-dependent vasodilation to acetycholine in aortic rings but not vascular smooth muscle cell dilation to sodium nitroprusside in high-fat fed ApoE null mice (125). These findings do not exclude the possibility that DPP-4i may also lead to upregulation of other endothelium-dependent vasodilator species (i.e., prostacyclin and endothelium-derived hyperpolarizing factors).
Insulin resistance, oxidative stress, and RAAS activation.
Vascular dysfunction, including endothelial dysfunction, is mainly linked to insulin resistance (136). Insulin actions in vascular cells are mediated through the phosphoinositide 3-kinase (PI3K)/insulin receptor substrate (IRS)-1/Akt pathway on the one hand, which stimulates the production of vascular dilator NO, and the mitogenic Shc/Ras/MEK pathway on the other hand, leading to the generation of the vasoconstrictor edothelin-1 and other downstream growth pathways (136) (Fig. 3). The NO pathway is positively regulated by phosphorylation of eNOS at the serine-1177 residue through the PI3K/Akt pathway. Impairment of this pathway occurs by serine phosphorylation of IRS (17, 104). Vascular dysfunction in obesity/T2DM is associated with oxidative stress and the activation of redox sensitive kinases resulting in increased serine phosphorylation of IRS, which acts to impair NO production and vascular relaxation. However, edothelin-1 production is unaffected, thereby resulting in an imbalance in vasomediators favoring vascular contraction (Fig. 3).
Increased oxidative stress is largely caused by inappropriate activation of RAAS and accumulation of advanced glycation end products (AGE) (Fig. 3). Oxidative stress in vascular cells can be caused by either cellular reactive oxygen species (ROS) accumulation by NADPH oxidase activation or mitochondrial ROS production (6, 41). However, abnormal activation of NADPH oxidase resulting in increased intracellular ROS levels induces mitochondrial ROS generation, which in turn further activates NADPH oxidase, thereby causing a feed-forward accumulation of ROS. This phenomenon is often termed as “ROS-induced-ROS release” (204, 205). The accumulation of ROS also contributes to endoplasmic reticular stress, leading to insulin resistance and vascular dysfunction (7). Chronic hyperglycemia in diabetes leads to activation of receptor for AGE (RAGE) that transduces the effects of numerous ligands, including posttranslationally glycosylated proteins known as AGEs that are prooxidative, proinflammatory, prothrombotic, and profibrotic (23, 193). RAGE are present in cardiomyocytes, ECs, and vascular smooth muscle cells, as well as in cells recruited to these tissues during stress, such as monocytes, macrophages, and T cells (194).
AGE/RAGE-induced ROS formation induces release of DPP-4 from ECs, inciting a positive feedback loop whereby DPP-4 induces increased expression of RAGE to further exacerbate AGE effects in ECs (84). Furthermore, DPP-4i treatment significantly inhibits the AGE-induced ROS generation in concert with suppression of RAGE, ICAM-1, and plasminogen activator inhibitor-1 gene expression in ECs, thereby providing novel pathways by which DPP-4i suppresses DPP-4 release from the ECs (84). Thus the activation of the AGE/RAGE axis and its interaction with DPP-4 may, in part, explain why ECs and the vasculature are inordinately remodeled and damaged and prone to atherosclerosis and stroke in the setting of diabetes. Accumulating evidence suggests that GLP-1 and DPP-4i may ameliorate the deleterious effects of AGE/RAGE in ECs and cardiac tissue. GLP-1, via the GLP-1R and downstream activation of cAMP, is reported to reduce the expression of RAGE, generation of ROS, and inflammatory markers in HUVECs (85). The DPP-4i vildagliptin reduces RAGE gene and protein expression and suppresses oxidative stress, as well as thrombotic and inflammatory markers in the aorta of type 2 diabetic rats (126). Linagliptin blocks ROS formation in HUVECs generated by the positive feedback loop between AGE/RAGE and DPP-4 (84). Thus DPP-4i could act to protect the endothelium and vasculature in diabetes largely by reducing expression of RAGE and the associated oxidative stress and inflammation.
Microcirculation and vascular recruitment.
Recent evidence supports that GLP-1 plays a role in postprandial muscle uptake and use of glucose and insulin (29). In vivo, GLP-1 infusion increases microvascular recruitment that is coupled to muscle insulin uptake and glucose use. These effects are independent of insulin but are mediated by protein kinase A (PKA)-dependent NO release (Fig. 3). Thus it is likely that the effects of DPP-4i on endothelial function may be mediated by GLP-1 and its receptor or direct modulation of DPP-4 activity and pleitropic actions. Experimental and clinical studies support the notion that GLP-1 is directly beneficial to the vasculature [reviewed in Balakumar and Dhanaraj (13), Fadini and Avogaro (52), and Ussher and Drucker (182)].
GLP-2, mesenteric flow, and intestinal function.
GLP-2 is a 33-amino-acid peptide substrate of DPP-4 and produced by enteroendocrine cells (48, 74). The plasma levels of GLP-2 are low in the fasting state and rapidly rise after food intake. GLP-2 increases mesenteric blood flow (Fig. 2), facilitates nutrient absorption in the gut, enhances gut permeability, and exerts small bowel cytoprotection (48). The improvement in mesenteric blood flow is secondary to metabolic responses mediated through GLP-2 receptors (19, 20, 48, 74). These findings suggest potential benefits of DPP-4i on mesenteric vascular function.
Scenescence and apoptosis.
DPP-4i may also serve to improve vascular and EC function by ameliorating EC senescence common in the vasculature of animals and humans with obesity/T2DM. Recently, it was shown that treatment of HUVECs with uncleaved or active GLP-1 (7–36) reduced ROS-induced senescence and DNA damage (144). These antisenescent effects were mediated by GLP-1 binding to the GLP-1R and subsequent downstream signaling through the cAMP/PKA pathway rather than by PI3K/Akt. Additionally, GLP-1 induced a PKA-dependent phosphorylation of the transcription factor cAMP responsive element binding protein that promotes cell survival (Fig. 3). In the same study, these results were corroborated in Zucker diabetic fatty rats treated with vildagliptin. Treated rats had elevated GLP-1 levels, and this was associated with a reduction in the augmented levels of vascular cell senescence despite little improvement in glycemia. Whether the observed reduction in EC senescence with vildagliptin is common to all DPP-4i requires further studies. Interestingly, Ban et al. (15) reported that GLP-1 (9–36), the so-called “inactive form” of GLP-1, resulting from enzymatic cleavage of active GLP-1 (GLP-1 [7–36]) by DPP-4, exhibited antiapoptotic effects in EC and cardiomyocytes and that this effect was mediated independently of the GLP-1R. These GLP-1R-independent effects of GLP-1 (9–36) are likely to be minimal with DPP-4i therapy that drastically reduces degradation of GLP-1 (7–36).
Metaloproteinases.
MMPs have been implicated in vascular remodeling and disease, including stroke (31, 92, 153). It is possible that MMPs, proteins that play a central role in remodeling extracellular matrixes, mediate the antistroke efficacy of linagliptin and exendin-4. At least two studies suggest that GLP-1 may regulate MMP-9 expression and activity. Specifically, GLP-1R agonism is associated with decreased MMP-9 expression and activity in CV tissue (25, 143). We recently observed an increase in active MMP-9 in brain tissue from untreated ZO rats; linagliptin treatment normalized the level of active MMP-9 in the ZO brain (unpublished data). This preliminary observation raises the possibility that the antistroke efficacy of linagliptin could indirectly involve modulation of MMP-9 and that efficacy is likely due to GLP-1 or other DPP-4 substrates present in areas of the brain affected by stroke.
Dyslipidemia.
Animal studies demonstrate that DPP-4i (sitagliptin and linagliptin) or GLP-1R agonism (exendin-4) significantly reduce intestinal secretion of TG, cholesterol, and apolipoprotein B-48, suggesting that GLP-1 might directly regulate lipoprotein assembly and secretion in enterocytes (80, 123). A recent study showed that the DPP-4i alogliptin ameliorates postprandial elevation of TG-rich lipoproteins (lipemia) and endothelial dysfunction in nondiabetic humans following an oral fat load (142), suggesting a potential antiatherogenic role for DPP-4i in humans. Recent clinical studies have reported that DPP-4i such as vildagliptin (124) and sitagliptin (181) improve postprandial atherogenic TG-rich lipoprotein levels in patients with T2DM. Previous studies have shown that DPP-4i such as vildagliptin and sitagliptin decrease postprandial TG, remnant lipoprotein cholesterol and apolipoprotein B-48 levels after a fat-loading test in patients with T2DM (124, 181) and nonobese subjects without diabetes (142). However, the effects of other DPP-4i on postprandial lipid elevation-induced endothelial dysfunction have not been fully evaluated. Although linagliptin exhibited CVD benefits versus a comparator, as reported in a recent meta-analysis of patients with T2DM, it did not reveal a TG lowering effect (90), suggesting other pleitropic effects of linagliptin in the cardiovasculature.
Immune and inflammatory response.
Accumulating evidence suggests that a low-grade chronic inflammatory state accompanies obesity, insulin resistance, hypertension, atherosclerosis, and diabetes (164, 202). In these settings, there exists a suite of maladaptive immune responses involving effector and regulatory T cells (Tregs) and polarized macrophages that constitute an inflammatory phenotype, i.e., increased T-helper-17 cells (Th17)/M1 macrophages and reduced Tregs/M2. Tregs are a unique subset of T cells that play an important role in the preservation of self-tolerance and suppression of potentially inflammatory T cells (113). Tregs protect against insulin resistance, in part, by secreting anti-inflammatory cytokines, such as IL-10 and transforming growth factor-β (57, 113, 129, 159). Treg-derived IL-10 suppresses local oxidative stress by inhibiting NADPH oxidase to reduce levels of superoxide (95). On the other hand, Th17 cells secrete IL-17, and these cells can be either cytotoxic or protective depending on the type and site of inflammation (8). In obese mice and in patients with T2DM, Th17 cells are elevated (88).
DPP-4 is expressed widely in CD4 and CD8+ immune cells (37, 109), thus DPP-4i therapy may be beneficial in modulating abnormalities in innate and adaptive immunity (196). Indeed, DPP-4i treatment decreases the accumulation of M1 macrophages and increases levels of M2 macrophages in adipose tissue and in atherosclerotic lesions (51, 168, 171, 178). DDP-4i blunts proliferation and migration of vascular smooth muscle cells from the tunica media into the intima in response to cytokines secreted by damaged ECs to attenuate atherosclerosis. Alogliptin reduced aortic plaques and plaque macrophages in LDL receptor knockout mice fed a high-fat diet (168). Alogliptin also reduced inflammation in this model by inhibiting monocyte activation and chemotaxis. Notably, adipose tissue inflammatory macrophage content (CD11b+, CD11c+, Ly6Chi) was reduced concomitantly with upregulation of CD163 positive anti-inflammatory macrophages. Alogliptin showed similar antiatherogenic properties in diabetic ApoE-deficient mice by decreasing the augmented IL-6 and IL-1β expression in atherosclerotic plaques (178). The DPP-4i anagliptin reduced plaque formation and inhibited vascular smooth muscle cell migration in ApoE-deficient mice (51). Moreover, a recent study showed that linagliptin improved insulin sensitivity in a mouse model of diet-induced obesity, and this was accompanied by suppression of adipose tissue macrophage infiltration, suggesting an anti-inflammatory/antiatherogenic role for linagliptin (98), rather than through effects on circulating lipids (90). In addition, linagliptin also attenuated inflammation and improved wound healing in diabetic mice (166). These data suggest that the effect of DDP-4i on ECs and vascular smooth muscle cells may potentially contribute to the inhibition of atherosclerosis. GLP-1 agonists have also been shown to increase Tregs (71), and Tregs promote M2 polarization (113). Thus there is potential to enhance Treg function with DPP-4i therapy. Furthermore, the increase in circulating DPP-4 levels reported in obese patients and animals support the novel use of DPP-4i for suppression of low-grade inflammation and associated tissue insulin resistance as an additional therapeutic benefit to patients with obesity, the metabolic syndrome and T2DM.
Dysfunctional immunity can be mediated, in part, by activation of the RAAS, which is known to modulate macrophage polarization and Th17/Treg balance (75, 94, 120, 164). In this regard, telmisartan, a third generation ARB, improves insulin sensitivity and promotes macrophage polarization to an anti-inflammatory state in visceral adipose tissue (61). Thus coadministration of specific ARBs with DPP-4i could act to potentiate the incretin enhancing and anti-inflammatory effects of DPP-4i to further improve CVD outcomes. Indeed, recent evidence supports the notion that combination DPP-4i/ARB therapy may be emerging as a novel strategy to blunt metabolic and CVD complications of obesity (2, 173, 174).
The role for perivascular adipose tissue (PVAT) dysfunction in promoting vascular inflammation and function is increasingly recognized. Expansion of PVAT and immune cell infiltration that occurs in obesity and T2DM is associated with secretion of inflammatory cytokines and vasoactive hormones (Fig. 3) (177). Moreover, adiponectin from PVAT causes vasorelaxation and its levels are decreased in obesity (30, 118). Therefore, inhibition of DPP-4 in adipose tissue may contribute to vasculoprotection.
Endothelial repair and angiogenesis: the effects of DPP-4i in modulation of bone-derived endothelial progenitor cells and their mobilization and homing.
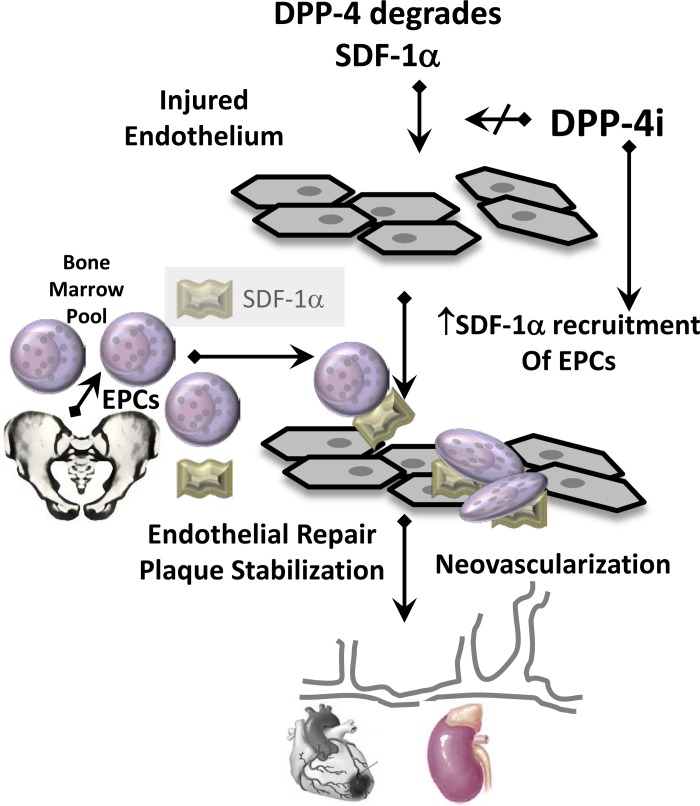
The vascular endothelium consists of terminally differentiated ECs that have little capacity to proliferate; as such, there is limited intrinsic reparative capacity. Repair of the injured endothelium is accomplished in large part by recruitment of circulating endothelial progenitor cells (EPCs). The role of endothelial repair is being increasingly recognized as a potential therapeutic target to improve endothelial dysfunction, myocardial ischemic injury, kidney injury, and bone marrow microangiopathy (10, 44, 112, 119). The endothelium of individuals who are obese and diabetic is prone to endothelial injury that can lead to endothelial dysfunction and microvascular and macrovascular complications. Patients with diabetes are reported to be deficient in numbers of circulating EPCs (55), whereas an enriched EPC pool predicts low CV mortality (187). Moreover, EPCs in patients with diabetes exhibit functional impairments because of reduced capacity to proliferate, as well as adhere, migrate, and incorporate into tubular structures (116, 179). In this regard, the chemokine SDF-1α, a known substrate of DPP-4, is emerging as an important regulator of vasculoprotective EPCs, important for endothelial repair and neoangiogenesis. Treatment with DPP-4i blunts the DPP-4-mediated degradation of SDF-1α, thereby enhancing recruitment of EPCs to vascular beds in need of repair or expansion (Fig. 4). Several DPP-4i have been shown to have effects on EPC expansion and recruitment. For example, linagliptin treatment ameliorated cardiac injury and reduced myocardial infarction size and fibrosis in a model of cardiac I/R injury in rats (78). This was associated with increased expression of CD34, c-kit, C-X-C chemokine receptor type 4, and SDF-1α. Since SDF-α is a substrate for DPP-4, these results imply decreased degradation of SDF-1α and increased recruitment of circulating EPCs that promote vascular repair and angiogenesis (170). In support of this, DPP-4 activity in the coronary sinus partly correlated with LV diastolic dysfunction and DPP-4i with vildagliptin reversed LV diastolic dysfunction through an SDF-1α-mediated effect on angiogenesis (170). Moreover, sitagliptin increases SDF-1α levels and circulating EPCs in patients with T2DM, and these effects were considered independent of GLP-1 based on unaltered nitrate/nitrite or plasma glucose levels (54). Sitagliptin also increases SDF-1α and circulating EPCs in a model of hindlimb ischemia (81). Since impaired angiogenesis and rarefaction is associated with obesity/T2DM and contributes to impaired diastolic dysfunction and renal injury, the efficacy of various DPP-4i in promoting SDF-1α-mediated effects may significantly contribute to improved CV outcomes. Incretin signaling has been shown to modulate bone remodeling (28). Therefore, DPP-4i may also improve bone repair by their effects on EPCs and SDF-1α, in addition to their actions on bone cells (28). Furthermore, DPP-4i may act to increase EPC numbers by potentiating the bone marrow mobilizing effects of granulocyte-colony stimulating factor-1 (53, 54). Moreover, ARBs have been shown to increase EPCs and improve vascular repair (127). These findings suggest that the combination of ARBs and DPP-4i have potential synergistic benefits on bone health and EPCs. Thus DPP-4i acts to improve CV outcomes by affecting improvements in endothelial function, ameliorating EC senescence and apoptosis, and promoting blood vessel repair (Fig. 4).
Fig. 4.
DPP-4i facilitates endothelial repair, plaque stabilization, and neovascularization by preventing the degradation of its substrate, SDF-1α, which recruits bone marrow derived EPCs to injured areas of the endothelium.
Conclusions
DPP-4i are potent, highly selective inhibitors of DPP-4 approved for treatment of T2DM as either monotherapy or in combination with conventional diabetes therapies. To date, most DPP-4i have good safety and tolerability profiles with low incidence of adverse events. The abnormal increase in circulating DPP-4 levels in individuals who are obese and diabeteic underscores the significance of targeting DPP-4 as a therapeutic strategy. The advantage of DPP-4i stems from its multiple beneficial actions that improve endothelial function to protect the vasculature. Preclinical evidence suggests that DPP-4i confers vasculoprotection and the potential vasculoprotective properties could reduce the risk for further development of the multiple comorbidities associated with obesity/T2DM, including hypertension and heart and kidney disease. The efficacy of DPP-4i is due, in part, to effects unrelated to improved glycemia. Potential benefits in the vasculature include increased NO generation, reductions in oxidative stress and inflammatory cell infiltration, and increased recruitment of EPCs, effects that collectively act to repair the injured endothelium and improve endothelial function (Fig. 1). Nonetheless, pending results from ongoing CV outcomes trials will be critical to further evaluate whether specific or collective benefits of DPP-4i therapy in the vasculature reduce CVD risk or mortality in T2DM, as well as elucidate potential risks not yet discovered. Further study is also needed to determine whether DPP-4i induce favorable alterations in immune and inflammatory responses that play a causative role in development and progression of vascular dysfunction and the associated CVD and CKD. Coadministration of DPP-4i and ARBs, but not ACEi, may be an attractive and safe therapeutic combination to treat patients with T2DM, especially those with hypertension.
GRANTS
This work was supported by an investigator initiated grant from Boehringer Ingelheim Pharmaceuticals (to V. G. DeMarco); National Heart, Lung, and Blood Institute Grants R01-HL-73101-01A1 and R01-HL-107910-01 (to J. R. Sowers); and Veterans Affairs Merit System Grant 0018 (to J. R. Sowers).
DISCLOSURES
V. G. DeMarco has received support from Boehringer Ingelheim Pharmaceuticals.
AUTHOR CONTRIBUTIONS
A.R.A., G.J., and V.G.D. performed experiments; A.R.A., J.R.S., G.J., and V.G.D. analyzed data; A.R.A., G.J., and V.G.D. interpreted results of experiments; A.R.A., J.R.S., G.J., and V.G.D. edited and revised manuscript; A.R.A., J.R.S., G.J., and V.G.D. approved final version of manuscript; G.J. and V.G.D. drafted manuscript; V.G.D. conception and design of research; V.G.D. prepared figures.
ACKNOWLEDGMENTS
We gratefully acknowledge Brenda Hunter for editorial assistance.
REFERENCES
- 1.Ahren B. Clinical results of treating type 2 diabetic patients with sitagliptin, vildagliptin or saxagliptin–diabetes control and potential adverse events. Best Pract Res Clin Endocrinol Metab 23: 487–498, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Alter ML, Ott IM, von Websky K, Tsuprykov O, Sharkovska Y, Krause-Relle K, Raila J, Henze A, Klein T, Hocher B. DPP-4 inhibition on top of angiotensin receptor blockade offers a new therapeutic approach for diabetic nephropathy. Kidney Blood Press Res 36: 119–130, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 298: 194–206, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Anagnostis P, Athyros VG, Adamidou F, Panagiotou A, Kita M, Karagiannis A, Mikhailidis DP. Glucagon-like peptide-1-based therapies and cardiovascular disease: looking beyond glycaemic control. Diabetes Obes Metab 13: 302–312, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Aroor A, McKarns S, Nistala R, Demarco V, Gardner M, Garcia-Touza M, Whaley-Connell A, Sowers JR. DPP-4 inhibitors as therapeutic modulators of immune cell function and associated cardiovascular and renal insulin resistance in obesity and diabetes. Cardiorenal Med 3: 48–56, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aroor AA, DeMarco VG, Jia G, Sun Z, Nistala R, Meininger GA, Sowers JR. The role of tissue renin-angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front Endocrinol (Lausanne) 4: 161, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aroor AR, Mandavia C, Ren J, Sowers JR, Pulakat L. Mitochondria and oxidative stress in the cardiorenal metabolic syndrome. Cardiorenal Med 2: 87–109, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism 62: 1543–1552, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aroor AR, Sowers JR, Bender SB, Nistala R, Garro M, Mugerfeld I, Hayden MR, Johnson MS, Salam M, Whaley-Connell A, Demarco VG. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin resistant male zucker obese rats. Endocrinology 154: 2501–2513, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care 34, Suppl 2: S285–S290, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayaori M, Iwakami N, Uto-Kondo H, Sato H, Sasaki M, Komatsu T, Iizuka M, Takiguchi S, Yakushiji E, Nakaya K, Yogo M, Ogura M, Takase B, Murakami T, Ikewaki K. Dipeptidyl peptidase-4 inhibitors attenuate endothelial function as evaluated by flow-mediated vasodilatation in type 2 diabetic patients. J Am Heart Assoc 2: e003277, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baetta R, Corsini A. Pharmacology of dipeptidyl peptidase-4 inhibitors: similarities and differences. Drugs 71: 1441–1467, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Balakumar P, Dhanaraj SA. Cardiovascular pleiotropic actions of DPP-4 inhibitors: a step at the cutting edge in understanding their additional therapeutic potentials. Cell Signal 25: 1799–1803, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Ban K, Kim KH, Cho CK, Sauve M, Diamandis EP, Backx PH, Drucker DJ, Husain M. Glucagon-like peptide (GLP)-1(9–36)amide-mediated cytoprotection is blocked by exendin(9–39) yet does not require the known GLP-1 receptor. Endocrinology 151: 1520–1531, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117: 2340–2350, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Bauvois B, Djavaheri-Mergny M, Rouillard D, Dumont J, Wietzerbin J. Regulation of CD26/DPPIV gene expression by interferons and retinoic acid in tumor B cells. Oncogene 19: 265–272, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes 62: 313–319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bose AK, Mocanu MM, Carr RD, Yellon DM. Myocardial ischaemia-reperfusion injury is attenuated by intact glucagon like peptide-1 (GLP-1) in the in vitro rat heart and may involve the p70s6K pathway. Cardiovasc Drugs Ther 21: 253–256, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Bremholm L, Hornum M, Andersen UB, Hartmann B, Holst JJ, Jeppesen PB. The effect of glucagon-like peptide-2 on mesenteric blood flow and cardiac parameters in end-jejunostomy short bowel patients. Regul Pept 168: 32–38, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Bremholm L, Hornum M, Andersen UB, Holst JJ. The effect of glucagon-like peptide-2 on arterial blood flow and cardiac parameters. Regul Pept 159: 67–71, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Briyal S, Gulati K, Gulati A. Repeated administration of exendin-4 reduces focal cerebral ischemia-induced infarction in rats. Brain Res 1427: 23–34, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Brown NJ. Cardiovascular effects of antidiabetic agents: focus on blood pressure effects of incretin-based therapies. J Am Soc Hypertens 6: 163–168, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 318: 1315–1321, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Burcelin R, Gourdy P, Dalle S. GLP-1-based strategies: a physiological analysis of differential mode of action. Physiology (Bethesda) 29: 108–121, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Burgmaier M, Liberman A, Möllmann J, Kahles F, Reith S, Lebherz C, Marx N, Lehrke M. Glucagon-like peptide-1 (GLP-1) and its split products GLP-1(9–37) and GLP-1(28–37) stabilize atherosclerotic lesions in apoe−/− mice. Atherosclerosis 231: 427–435, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 36: 2118–2125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl 111: S4–S9, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Ceccarelli E, Guarino EG, Merlotti D, Patti A, Gennari L, Nuti R, Dotta F. Beyond glycemic control in diabetes mellitus: effects of incretin-based therapies on bone metabolism. Front Endocrinol (Lausanne) 4: 73, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, Liu Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes 61: 888–896, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res 104: 541–549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaturvedi M, Kaczmarek L. MMP-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol 49: 563–573, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaudhary K, Kunal M, Sowers J, Aroor A. Uric acid–key ingredient in the recipe for cardiorenal metabolic syndrome. Cardiorenal Med 3: 208–220, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaykovska L, Alter ML, von Websky K, Hohmann M, Tsuprykov O, Reichetzeder C, Kutil B, Kraft R, Klein T, Hocher B. Effects of telmisartan and linagliptin when used in combination on blood pressure and oxidative stress in rats with 2-kidney-1-clip hypertension. J Hypertens 31: 2290–2299, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Chaykovska L, von Websky K, Rahnenfuhrer J, Alter M, Heiden S, Fuchs H, Runge F, Klein T, Hocher B. Effects of DPP-4 inhibitors on the heart in a rat model of uremic cardiomyopathy. PLoS One 6: e27861, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cobble ME, Frederich R. Saxagliptin for the treatment of type 2 diabetes mellitus: assessing cardiovascular data. Cardiovasc Diabetol 11: 6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connelly KA, Zhang Y, Advani A, Advani SL, Thai K, Yuen DA, Gilbert RE. DPP-4 inhibition attenuates cardiac dysfunction and adverse remodeling following myocardial infarction in rats with experimental diabetes. Cardiovasc Ther 31: 259–267, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Cordero OJ, Salgado FJ, Nogueira M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immunother 58: 1723–1747, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, Girardi AC. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol 301: F355–F363, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Darsalia V, Mansouri S, Ortsater H, Olverling A, Nozadze N, Kappe C, Iverfeldt K, Tracy LM, Grankvist N, Sjoholm A, Patrone C. Glucagon-like peptide-1 receptor activation reduces ischaemic brain damage following stroke in Type 2 diabetic rats. Clin Sci (Lond) 122: 473–483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darsalia V, Ortsater H, Olverling A, Darlof E, Wolbert P, Nystrom T, Klein T, Sjoholm A, Patrone C. The DPP-4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: a comparison with glimepiride. Diabetes 62: 1289–1296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 56: 325–330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 80: 952–957, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 13: 258–267, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Desouza CV. Does drug therapy reverse endothelial progenitor cell dysfunction in diabetes? J Diabetes Complications 27: 519–525, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Dos Santos L, Salles TA, Arruda-Junior DF, Campos LC, Pereira AC, Barreto AL, Antonio EL, Mansur AJ, Tucci PJ, Krieger JE, Girardi AC. Circulating dipeptidyl peptidase iv activity correlates with cardiac dysfunction in human and experimental heart failure. Circ Heart Fail 6: 1029–1038, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Drucker DJ. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes 62: 3316–3323, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest 117: 24–32, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol 76: 561–583, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 141: 150–156, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erickson RH, Gum JR, Lotterman CD, Hicks JW, Lai RS, Kim YS. Regulation of the gene for human dipeptidyl peptidase IV by hepatocyte nuclear factor 1 alpha. Biochem J 338: 91–97, 1999 [PMC free article] [PubMed] [Google Scholar]
- 51.Ervinna N, Mita T, Yasunari E, Azuma K, Tanaka R, Fujimura S, Sukmawati D, Nomiyama T, Kanazawa A, Kawamori R, Fujitani Y, Watada H. Anagliptin, a DPP-4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo E-deficient mice. Endocrinology 154: 1260–1270, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol 55: 10–16, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Fadini GP, Avogaro A. Potential manipulation of endothelial progenitor cells in diabetes and its complications. Diabetes Obes Metab 12: 570–583, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, Agostini C, Tiengo A, Avogaro A. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care 33: 1607–1609, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fadini GP, Sartore S, Agostini C, Avogaro A. Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care 30: 1305–1313, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Ferreira L, Teixeira-de-Lemos E, Pinto F, Parada B, Mega C, Vala H, Pinto R, Garrido P, Sereno J, Fernandes R, Santos P, Velada I, Melo A, Nunes S, Teixeira F, Reis F. Effects of sitagliptin treatment on dysmetabolism, inflammation, and oxidative stress in an animal model of type 2 diabetes (ZDF rat). Mediators Inflamm 2010: 592760, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15: 930–939, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307: 491–497, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Freemantle N. Commentary: What can we learn from the continuing regulatory focus on the thiazolidinediones? BMJ 341: c4812, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Friedrich C, Emser A, Woerle HJ, Graefe-Mody U. Renal impairment has no clinically relevant effect on the long-term exposure of linagliptin in patients with type 2 diabetes. Am J Ther 20: 618–621, 2013 [DOI] [PubMed] [Google Scholar]
- 61.Fujisaka S, Usui I, Kanatani Y, Ikutani M, Takasaki I, Tsuneyama K, Tabuchi Y, Bukhari A, Yamazaki Y, Suzuki H, Senda S, Aminuddin A, Nagai Y, Takatsu K, Kobayashi M, Tobe K. Telmisartan improves insulin resistance and modulates adipose tissue macrophage polarization in high-fat-fed mice. Endocrinology 152: 1789–1799, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580–591, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Gallwitz B, Rosenstock J, Rauch T, Bhattacharya S, Patel S, von Eynatten M, Dugi KA, Woerle HJ. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet 380: 475–483, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Giorda CB, Nada E, Tartaglino B, Marafetti L, Gnavi R. A systematic review of acute pancreatitis as an adverse event of type 2 diabetes drugs: from hard facts to a balanced position. Diabetes Obes Metab 2014 [DOI] [PubMed] [Google Scholar]
- 65.Girardi AC, Degray BC, Nagy T, Biemesderfer D, Aronson PS. Association of Na+-H+ exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule. J Biol Chem 276: 46671–46677, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7: 2294–2300, 1995 [DOI] [PubMed] [Google Scholar]
- 67.Golightly LK, Drayna CC, McDermott MT. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin Pharmacokinet 51: 501–514, 2012 [DOI] [PubMed] [Google Scholar]
- 68.Graefe-Mody U, Friedrich C, Port A, Ring A, Retlich S, Heise T, Halabi A, Woerle HJ. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin(*). Diabetes Obes Metab 13: 939–946, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Green JB, Bethel MA, Paul SK, Ring A, Kaufman KD, Shapiro DR, Califf RM, Holman RR. Rationale, design, and organization of a randomized, controlled Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) in patients with type 2 diabetes and established cardiovascular disease. Am Heart J 166: 983e7–989e7, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care 36: 3460–3468, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia 53: 730–740, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Hamilton A, Holscher C. Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport 20: 1161–1166, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140: 5356–5363, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Hansen LB. GLP-2 and mesenteric blood flow. Dan Med J 60: B4634, 2013 [PubMed] [Google Scholar]
- 75.Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Front Physiol 3: 128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hausenloy DJ, Whittington HJ, Wynne AM, Begum SS, Theodorou L, Riksen N, Mocanu MM, Yellon DM. Dipeptidyl peptidase-4 inhibitors and GLP-1 reduce myocardial infarct size in a glucose-dependent manner. Cardiovasc Diabetol 12: 154, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hocher B, Reichetzeder C, Alter ML. Renal and cardiac effects of DPP-4 Inhibitors–from preclinical development to clinical research. Kidney Blood Press Res 36: 65–84, 2012 [DOI] [PubMed] [Google Scholar]
- 78.Hocher B, Sharkovska Y, Mark M, Klein T, Pfab T. The novel DPP-4 inhibitors linagliptin and BI 14361 reduce infarct size after myocardial ischemia/reperfusion in rats. Int J Cardiol 167: 87–93, 2013 [DOI] [PubMed] [Google Scholar]
- 79.Holst JJ, Burcelin R, Nathanson E. Neuroprotective properties of GLP-1: theoretical and practical applications. Curr Med Res Opin 27: 547–558, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Hsieh J, Longuet C, Baker CL, Qin B, Federico LM, Drucker DJ, Adeli K. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 53: 552–561, 2010 [DOI] [PubMed] [Google Scholar]
- 81.Huang CY, Shih CM, Tsao NW, Lin YW, Huang PH, Wu SC, Lee AW, Kao YT, Chang NC, Nakagami H, Morishita R, Ou KL, Hou WC, Lin CY, Shyu KG, Lin FY. Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br J Pharmacol 167: 1506–1519, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hunter K, Holscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 13: 33, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huttner S, Graefe-Mody EU, Withopf B, Ring A, Dugi KA. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single oral doses of BI 1356, an inhibitor of dipeptidyl peptidase 4, in healthy male volunteers. J Clin Pharmacol 48: 1171–1178, 2008 [DOI] [PubMed] [Google Scholar]
- 84.Ishibashi Y, Matsui T, Maeda S, Higashimoto Y, Yamagishi S. Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cardiovasc Diabetol 12: 125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem Biophys Res Commun 391: 1405–1408, 2010 [DOI] [PubMed] [Google Scholar]
- 86.Jackson EK. Dipeptidyl peptidase IV inhibition alters the hemodynamic response to angiotensin-converting enzyme inhibition in humans with the metabolic syndrome. Hypertension 56: 581–583, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Jackson EK, Mi Z. Sitagliptin augments sympathetic enhancement of the renovascular effects of angiotensin II in genetic hypertension. Hypertension 51: 1637–1642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jagannathan-Bogdan M, McDonnell ME, Shin H, Rehman Q, Hasturk H, Apovian CM, Nikolajczyk BS. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol 186: 1162–1172, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jax T. Treatment of patients with diabetes with GLP-1 analogues or DPP-4-inhibitors: a hot topic for cardiologists? Clin Res Cardiol 98: 75–79, 2009 [DOI] [PubMed] [Google Scholar]
- 90.Johansen OE, Neubacher D, von Eynatten M, Patel S, Woerle HJ. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: a pre-specified, prospective, and adjudicated meta-analysis of a phase 3 programme. Cardiovasc Diabetol 11: 3, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jose T, Inzucchi SE. Cardiovascular effects of the DPP-4 inhibitors. Diab Vasc Dis Res 9: 109–116, 2012 [DOI] [PubMed] [Google Scholar]
- 92.Kaczmarek L. Mmp-9 inhibitors in the brain: can old bullets shoot new targets? Curr Pharm Des 19: 1085–1089, 2013 [DOI] [PubMed] [Google Scholar]
- 93.Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 344: e1369, 2012 [DOI] [PubMed] [Google Scholar]
- 94.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 59: 324–330, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol 31: 2534–2542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, Ruden D, Pietrobelli A, Barger JL, Fontaine KR, Wang C, Aronne LJ, Wright SM, Baskin M, Dhurandhar NV, Lijoi MC, Grilo CM, DeLuca M, Westfall AO, Allison DB. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond) 30: 1585–1594, 2006 [DOI] [PubMed] [Google Scholar]
- 97.Kenny AJ, Booth AG, George SG, Ingram J, Kershaw D, Wood EJ, Young AR. Dipeptidyl peptidase IV, a kidney brush-border serine peptidase. Biochem J 157: 169–182, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kern M, Kloting N, Niessen HG, Thomas L, Stiller D, Mark M, Klein T, Bluher M. Linagliptin improves insulin sensitivity and hepatic steatosis in diet-induced obesity. PLoS One 7: e38744, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khoury JC, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, Broderick JP, Kissela BM. Diabetes mellitus: a risk factor for ischemic stroke in a large biracial population. Stroke 44: 1500–1504, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kirino Y, Kamimoto T, Sato Y, Kawazoe K, Minakuchi K, Nakahori Y. Increased plasma dipeptidyl peptidase IV (DPP IV) activity and decreased DPP IV activity of visceral but not subcutaneous adipose tissue in impaired glucose tolerance rats induced by high-fat or high-sucrose diet. Biol Pharm Bull 32: 463–467, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Kirino Y, Sato Y, Kamimoto T, Kawazoe K, Minakuchi K. Altered dipeptidyl peptidase-4 activity during the progression of hyperinsulinemic obesity and islet atrophy in spontaneously late-stage type 2 diabetic rats. Am J Physiol Endocrinol Metab 300: E372–E379, 2011 [DOI] [PubMed] [Google Scholar]
- 102.Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota D, Sato C, Ogawa D, Makino H. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 54: 965–978, 2011 [DOI] [PubMed] [Google Scholar]
- 103.Kroller-Schon S, Knorr M, Hausding M, Oelze M, Schuff A, Schell R, Sudowe S, Scholz A, Daub S, Karbach S, Kossmann S, Gori T, Wenzel P, Schulz E, Grabbe S, Klein T, Munzel T, Daiber A. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc Res 96: 140–149, 2012 [DOI] [PubMed] [Google Scholar]
- 104.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K, Kadowaki T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 13: 294–307, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Kubota Y, Miyamoto M, Takagi G, Ikeda T, Kirinoki-Ichikawa S, Tanaka K, Mizuno K. The dipeptidyl peptidase-4 inhibitor sitagliptin improves vascular endothelial function in type 2 diabetes. J Korean Med Sci 27: 1364–1370, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lago RM, Singh PP, Nesto RW. Diabetes and hypertension. Nat Clin Pract Endocrinol Metab 3: 667, 2007 [DOI] [PubMed] [Google Scholar]
- 107.Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, Eckardt K, Kaufman JM, Ryden M, Muller S, Hanisch FG, Ruige J, Arner P, Sell H, Eckel J. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes 60: 1917–1925, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee CH, Yan B, Yoo KY, Choi JH, Kwon SH, Her S, Sohn Y, Hwang IK, Cho JH, Kim YM, Won MH. Ischemia-induced changes in glucagon-like peptide-1 receptor and neuroprotective effect of its agonist, exendin-4, in experimental transient cerebral ischemia. J Neurosci Res 89: 1103–1113, 2011 [DOI] [PubMed] [Google Scholar]
- 109.Lee SA, Kim YR, Yang EJ, Kwon EJ, Kim SH, Kang SH, Park DB, Oh BC, Kim J, Heo ST, Koh G, Lee DH. CD26/DPP4 levels in peripheral blood and t cells in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 98: 2553–2561, 2013 [DOI] [PubMed] [Google Scholar]
- 110.Leopold JA. Cellular and molecular mechanisms of arterial stiffness associated with obesity. Hypertension 62: 1003–1004, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li L, Shen J, Bala MM, Busse JW, Ebrahim S, Vandvik PO, Rios LP, Malaga G, Wong E, Sohani Z, Guyatt GH, Sun X. Incretin treatment and risk of pancreatitis in patients with type 2 diabetes mellitus: systematic review and meta-analysis of randomised and non-randomised studies. BMJ 348: g2366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin CP, Lin FY, Huang PH, Chen YL, Chen WC, Chen HY, Huang YC, Liao WL, Huang HC, Liu PL, Chen YH. Endothelial progenitor cell dysfunction in cardiovascular diseases: role of reactive oxygen species and inflammation. Biomed Res Int 2013: 845037, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, Zhao Y. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol 89: 130–142, 2011 [DOI] [PubMed] [Google Scholar]
- 114.Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, Pu Y, Zhu Z, Xu A, Lam KS, Chen ZY, Ng CF, Yao X, Huang Y. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension 60: 833–841, 2012 [DOI] [PubMed] [Google Scholar]
- 115.Liu WJ, Xie SH, Liu YN, Kim W, Jin HY, Park SK, Shao YM, Park TS. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther 340: 248–255, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 53: 195–199, 2004 [DOI] [PubMed] [Google Scholar]
- 117.Lusis AJ. Atherosclerosis. Nature 407: 233–241, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH, Heagerty AM. Perivascular adipose tissue-derived adiponectin activates BKCa channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol 304: H786–H795, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma XL, Sun XL, Wan CY, Ma JX, Tian P. Significance of circulating endothelial progenitor cells in patients with fracture healing process. J Orthop Res 30: 1860–1866, 2012 [DOI] [PubMed] [Google Scholar]
- 120.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension 56: 728–733, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mason RP, Jacob RF, Kubant R, Ciszewski A, Corbalan JJ, Malinski T. Dipeptidyl peptidase-4 inhibition with saxagliptin enhanced nitric oxide release and reduced blood pressure and sICAM-1 levels in hypertensive rats. J Cardiovasc Pharmacol 60: 467–473, 2012 [DOI] [PubMed] [Google Scholar]
- 123.Masuda D, Koybayashi T, Okada T, Nakaoka H, Kawase R, Nakatani K, Ohama T, Nishida M, Matsuyama A, Komuro I, Yamashita S. Treatment with linagliptin can attenuate postprandial hyperlipidemia (abstract). In: Proceedings of the 73rd Annual Scientific Sessions of the American Diabetes Association. Chicago, IL: 2013, p. A77 [Google Scholar]
- 124.Matikainen N, Manttari S, Schweizer A, Ulvestad A, Mills D, Dunning BE, Foley JE, Taskinen MR. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia 49: 2049–2057, 2006 [DOI] [PubMed] [Google Scholar]
- 125.Matsubara J, Sugiyama S, Sugamura K, Nakamura T, Fujiwara Y, Akiyama E, Kurokawa H, Nozaki T, Ohba K, Konishi M, Maeda H, Izumiya Y, Kaikita K, Sumida H, Jinnouchi H, Matsui K, Kim-Mitsuyama S, Takeya M, Ogawa H. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol 59: 265–276, 2012 [DOI] [PubMed] [Google Scholar]
- 126.Matsui T, Nishino Y, Takeuchi M, Yamagishi S. Vildagliptin blocks vascular injury in thoracic aorta of diabetic rats by suppressing advanced glycation end product-receptor axis. Pharmacol Res 63: 383–388, 2011 [DOI] [PubMed] [Google Scholar]
- 127.Matsuura K, Hagiwara N. The pleiotropic effects of ARB in vascular endothelial progenitor cells. Curr Vasc Pharmacol 9: 153–157, 2011 [DOI] [PubMed] [Google Scholar]
- 128.McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M, Benca RM, Biggio J, Boggiano MM, Eisenmann JC, Elobeid M, Fontaine KR, Gluckman P, Hanlon EC, Katzmarzyk P, Pietrobelli A, Redden DT, Ruden DM, Wang C, Waterland RA, Wright SM, Allison DB. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr 49: 868–913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McCaughan GW, Gorrell MD, Bishop GA, Abbott CA, Shackel NA, McGuinness PH, Levy MT, Sharland AF, Bowen DG, Yu D, Slaitini L, Church WB, Napoli J. Molecular pathogenesis of liver disease: an approach to hepatic inflammation, cirrhosis and liver transplant tolerance. Immunol Rev 174: 172–191, 2000 [DOI] [PubMed] [Google Scholar]
- 130.McClean PL, Parthsarathy V, Faivre E, Holscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci 31: 6587–6594, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 214: 829–835, 1993 [DOI] [PubMed] [Google Scholar]
- 132.Mistry GC, Maes AL, Lasseter KC, Davies MJ, Gottesdiener KM, Wagner JA, Herman GA. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol 48: 592–598, 2008 [DOI] [PubMed] [Google Scholar]
- 133.Monami M, Ahren B, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 15: 112–120, 2013 [DOI] [PubMed] [Google Scholar]
- 134.Monami M, Iacomelli I, Marchionni N, Mannucci E. Dipeptydil peptidase-4 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 20: 224–235, 2010 [DOI] [PubMed] [Google Scholar]
- 135.Moody WE, Edwards NC, Madhani M, Chue CD, Steeds RP, Ferro CJ, Townend JN. Endothelial dysfunction and cardiovascular disease in early-stage chronic kidney disease: cause or association? Atherosclerosis 223: 86–94, 2012 [DOI] [PubMed] [Google Scholar]
- 136.Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord 14: 5–12, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nakagawa T. Is endothelial dysfunction more deleterious than podocyte injury in diabetic nephropathy? Kidney Int 83: 1202–1203, 2013 [DOI] [PubMed] [Google Scholar]
- 138.Nakagawa T, Tanabe K, Croker BP, Johnson RJ, Grant MB, Kosugi T, Li Q. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat Rev Nephrol 7: 36–44, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 29: 46–52, 1986 [DOI] [PubMed] [Google Scholar]
- 140.Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care 36: 2126–2132, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91: 301–307, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Noda Y, Miyoshi T, Oe H, Ohno Y, Nakamura K, Toh N, Kohno K, Morita H, Kusano K, Ito H. Alogliptin ameliorates postprandial lipemia and postprandial endothelial dysfunction in non-diabetic subjects: a preliminary report. Cardiovasc Diabetol 12: 8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M, Drucker DJ. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 58: 975–983, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Sillje HH. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol 30: 1407–1414, 2010 [DOI] [PubMed] [Google Scholar]
- 145.Ogawa S, Ishiki M, Nako K, Okamura M, Senda M, Mori T, Ito S. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, decreases systolic blood pressure in Japanese hypertensive patients with type 2 diabetes. Tohoku J Exp Med 223: 133–135, 2011 [DOI] [PubMed] [Google Scholar]
- 146.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 307: 483–490, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oyama J, Higashi Y, Node K. Do incretins improve endothelial function? Cardiovasc Diabetol 13: 21, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pacheco BP, Crajoinas RO, Couto GK, Davel AP, Lessa LM, Rossoni LV, Girardi AC. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J Hypertens 29: 520–528, 2011 [DOI] [PubMed] [Google Scholar]
- 149.Panchapakesan UGS, Komala MG, Pegg K, Pollock CA. DPP4 inhibition in human kidney proximal tubular cells-renoprotection in diabetic nephropathy? J Diab Metab S9-007, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Panchapakesan U, Mather A, Pollock C. Role of GLP-1 and DPP-4 in diabetic nephropathy and cardiovascular disease. Clin Sci (Lond) 124: 17–26, 2013 [DOI] [PubMed] [Google Scholar]
- 151.Poulsen MK, Henriksen JE, Dahl J, Johansen A, Gerke O, Vach W, Haghfelt T, Hoilund-Carlsen PF, Beck-Nielsen H, Moller JE. Left ventricular diastolic function in type 2 diabetes mellitus: prevalence and association with myocardial and vascular disease. Circ Cardiovasc Imaging 3: 24–31, 2010 [DOI] [PubMed] [Google Scholar]
- 152.Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, Thomsen AB, Sondergaard RE, Davies M, Group LDS. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 375: 1447–1456, 2010 [DOI] [PubMed] [Google Scholar]
- 153.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 75: 346–359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ramirez G, Morrison AD, Bittle PA. Clinical practice considerations and review of the literature for the use of DPP-4 inhibitors in patients with type 2 diabetes and chronic kidney disease. Endocr Prac 19: 1025–1034, 2013 [DOI] [PubMed] [Google Scholar]
- 155.Rauch T, Graefe-Mody U, Deacon CF, Ring A, Holst JJ, Woerle HJ, Dugi KA, Heise T. Linagliptin increases incretin levels, lowers glucagon, and improves glycemic control in type 2 diabetes mellitus. Diabetes Ther 3: 10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ravassa S, Barba J, Coma-Canella I, Huerta A, Lopez B, Gonzalez A, Diez J. The activity of circulating dipeptidyl peptidase-4 is associated with subclinical left ventricular dysfunction in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 12: 143, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ravassa S, Zudaire A, Carr RD, Diez J. Antiapoptotic effects of Glp-1 in murine Hl-1 cardiomyocytes. Am J Physiol Heart Circ Physiol 300: H1361–H1372, 2011 [DOI] [PubMed] [Google Scholar]
- 158.Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med (Berl) 82: 510–529, 2004 [DOI] [PubMed] [Google Scholar]
- 159.Rocha VZ, Folco EJ. Inflammatory concepts of obesity. Int J Inflam 2011: 529061, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rosenstock J, Marx N, Kahn SE, Zinman B, Kastelein JJ, Lachin JM, Bluhmki E, Patel S, Johansen OE, Woerle HJ. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res 10: 289–301, 2013 [DOI] [PubMed] [Google Scholar]
- 161.Santos JL, Salemi VM, Picard MH, Mady C, Coelho OR. Subclinical regional left ventricular dysfunction in obese patients with and without hypertension or hypertrophy. Obesity (Silver Spring) 19: 1296–1303, 2011 [DOI] [PubMed] [Google Scholar]
- 162.Sauve M, Ban K, Momen MA, Zhou YQ, Henkelman RM, Husain M, Drucker DJ. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes 59: 1063–1073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Scheen AJ. Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes Obes Metab 12: 648–658, 2010 [DOI] [PubMed] [Google Scholar]
- 164.Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 126: 267–274, 2014 [DOI] [PubMed] [Google Scholar]
- 165.Schlatter P, Beglinger C, Drewe J, Gutmann H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul Pept 141: 120–128, 2007 [DOI] [PubMed] [Google Scholar]
- 166.Schurmann C, Linke A, Engelmann-Pilger K, Steinmetz C, Mark M, Pfeilschifter J, Klein T, Frank S. The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. J Pharmacol Exp Ther 342: 71–80, 2012 [DOI] [PubMed] [Google Scholar]
- 167.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mozenson O, McGuire DK, Ray KK, Leiter LA, Raz I; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 369: 1317–1326, 2013 [DOI] [PubMed] [Google Scholar]
- 168.Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, Xu X, Lu B, Moffatt-Bruce S, Durairaj R, Sun Q, Mihai G, Maiseyeu A, Rajagopalan S. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 124: 2338–2349, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shah Z, Pineda C, Kampfrath T, Maiseyeu A, Ying Z, Racoma I, Deiuliis J, Xu X, Sun Q, Moffatt-Bruce S, Villamena F, Rajagopalan S. Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vasc Pharmacol 55: 2–9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shigeta T, Aoyama M, Bando YK, Monji A, Mitsui T, Takatsu M, Cheng XW, Okumura T, Hirashiki A, Nagata K, Murohara T. Dipeptidyl peptidase-4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis-dependent and -independent actions. Circulation 126: 1838–1851, 2012 [DOI] [PubMed] [Google Scholar]
- 171.Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y, Amo K, Aoki K, Morimoto C, Takeda E, Terauchi Y. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes 60: 1246–1257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail 12: 694–699, 2006 [DOI] [PubMed] [Google Scholar]
- 173.Souza-Mello V, Gregorio BM, Cardoso-de-Lemos FS, de Carvalho L, Aguila MB, Mandarim-de-Lacerda CA. Comparative effects of telmisartan, sitagliptin and metformin alone or in combination on obesity, insulin resistance, and liver and pancreas remodelling in C57BL/6 mice fed on a very high-fat diet. Clin Sci (Lond) 119: 239–250, 2010 [DOI] [PubMed] [Google Scholar]
- 174.Souza-Mello V, Gregorio BM, Relvas-Lucas B, da Silva Faria T, Aguila MB, Mandarim-de-Lacerda CA. Pancreatic ultrastructural enhancement due to telmisartan plus sitagliptin treatment in diet-induced obese C57BL/6 mice. Pancreas 40: 715–722, 2011 [DOI] [PubMed] [Google Scholar]
- 175.Sowers JR. Diabetes mellitus and vascular disease. Hypertension 61: 943–947, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sowers JR, Whaley-Connel AT, Hayden MR. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med 1: 5–12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Szasz T, Bomfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag 9: 105–116, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ta NN, Schuyler CA, Li Y, Lopes-Virella MF, Huang Y. DPP-4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E-deficient mice. J Cardiovasc Pharmacol 58: 157–166, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 106: 2781–2786, 2002 [DOI] [PubMed] [Google Scholar]
- 180.Thomas L, Eckhardt M, Langkopf E, Tadayyon M, Himmelsbach F, Mark M. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazoli n-2-ylmethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J Pharmacol Exp Ther 325: 175–182, 2008 [DOI] [PubMed] [Google Scholar]
- 181.Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab 13: 366–373, 2011 [DOI] [PubMed] [Google Scholar]
- 182.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev 33: 187–215, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.van Poppel PC, Netea MG, Smits P, Tack CJ. Vildagliptin improves endothelium-dependent vasodilatation in type 2 diabetes. Diabetes Care 34: 2072–2077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Van Putte-Katier N, Rooman RP, Haas L, Verhulst SL, Desager KN, Ramet J, Suys BE. Early cardiac abnormalities in obese children: importance of obesity per se versus associated cardiovascular risk factors. Pediatr Res 64: 205–209, 2008 [DOI] [PubMed] [Google Scholar]
- 185.von Eynatten M, Gong Y, Emser A, Woerle HJ. Efficacy and safety of linagliptin in type 2 diabetes subjects at high risk for renal and cardiovascular disease: a pooled analysis of six phase III clinical trials. Cardiovasc Diabetol 12: 60, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension 62: 1105–1110, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353: 999–1007, 2005 [DOI] [PubMed] [Google Scholar]
- 188.Whaley-Connell A, Bomback AS, McFarlane SI, Li S, Roberts T, Chen SC, Collins AJ, Norris K, Bakris GL, Sowers JR, McCullough PA; on behalf of the Kidney Early Evaluation Program Investigators. Diabetic Cardiovascular Disease Predicts Chronic Kidney Disease Awareness in the Kidney Early Evaluation Program. Cardiorenal Med 1: 45–52, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Whaley-Connell A, Johnson MS, Sowers JR. Aldosterone: role in the cardiometabolic syndrome and resistant hypertension. Prog Cardiovasc Dis 52: 401–409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 369: 1327–1335, 2013 [DOI] [PubMed] [Google Scholar]
- 191.White WB, Pratley R, Fleck P, Munsaka M, Hisada M, Wilson C, Menon V. Cardiovascular safety of the dipetidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab 15: 668–673, 2013 [DOI] [PubMed] [Google Scholar]
- 192.Wu S, Hopper I, Skiba M, Krum H. Dipeptidyl peptidase-4 inhibitors and cardiovascular outcomes: Meta-analysis of randomized clinical trials with 55,141 participants. Cardiovasc Ther. 2014. April 21. 10.1111/1755-5922.12075 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 193.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res 106: 842–853, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yan SF, Ramasamy R, Schmidt AM. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev Mol Med 11: e9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang J, Campitelli J, Hu G, Lin Y, Luo J, Xue C. Increase in DPP-IV in the intestine, liver and kidney of the rat treated with high fat diet and streptozotocin. Life Sci 81: 272–279, 2007 [DOI] [PubMed] [Google Scholar]
- 196.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci 30: 600–607, 2009 [DOI] [PubMed] [Google Scholar]
- 197.Ye Y, Keyes KT, Zhang C, Perez-Polo JR, Lin Y, Birnbaum Y. The myocardial infarct size-limiting effect of sitagliptin is PKA-dependent, whereas the protective effect of pioglitazone is partially dependent on PKA. Am J Physiol Heart Circ Physiol 298: H1454–H1465, 2010 [DOI] [PubMed] [Google Scholar]
- 198.Yin M, Sillje HH, Meissner M, van Gilst WH, de Boer RA. Early and late effects of the DPP-4 inhibitor vildagliptin in a rat model of post-myocardial infarction heart failure. Cardiovasc Diabetol 10: 85, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, Kirchmair R, Bahlman F, Walter D, Curry C, Hanley A, Isner JM, Losordo DW. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation 111: 2073–2085, 2005 [DOI] [PubMed] [Google Scholar]
- 200.Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, Fischer R, Krieg L, Hirsch E, Huber B, Nathan P, Israel L, Imhof A, Herbach N, Assmann G, Wanke R, Mueller-Hoecker J, Steinbeck G, Franz WM. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell 4: 313–323, 2009 [DOI] [PubMed] [Google Scholar]
- 201.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther 317: 1106–1113, 2006 [DOI] [PubMed] [Google Scholar]
- 202.Zhong J, Rao X, Rajagopalan S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis 226: 305–314, 2013 [DOI] [PubMed] [Google Scholar]
- 203.Zhou X, Ma L, Habibi J, Whaley-Connel AT, Hayden MR, Tilmon RD, Brown AN, DeMarco VG, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial tissue remodeling through reductions in oxidative stress in the Zucker Obese rat. Hypertension 55: 880–888, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol 301: H647–H653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 1757: 509–517, 2006 [DOI] [PubMed] [Google Scholar]