Abstract
Increasing Na delivery to epithelial Na channels (ENaC) in the connecting tubule (CNT) dilates the afferent arteriole (Af-Art), a process we call connecting tubule glomerular feedback (CTGF). We hypothesize that aldosterone sensitizes CTGF via a nongenomic mechanism that stimulates CNT ENaC via the aldosterone receptor GPR30. Rabbit Af-Arts and their adherent CNTs were microdissected and simultaneously perfused. Two consecutive CTGF curves were elicited by increasing luminal NaCl in the CNT. During the control period, the concentration of NaCl that elicited a half-maximal response (EC50) was 37.0 ± 2.0 mmol/l; addition of aldosterone 10−8 mol/l to the CNT lumen caused a left-shift (decrease) in EC50 to 19.3 ± 1.3 mmol/l (P = 0.001 vs. control; n = 6). Neither the transcription inhibitor actinomycin D nor the translation inhibitor cycloheximide prevented the effect of aldosterone (control EC50 = 34.7 ± 1.9 mmol/l; aldosterone+actinomycin D EC50 = 22.6 ± 1.6 mmol/l; P < 0.001 and control EC50 = 32.4 ± 4.3 mmol/l; aldosterone+cycloheximide EC50 = 17.4 ± 3.3 mmol/l; P < 0.001). The aldosterone antagonist eplerenone prevented the sensitization of CTGF by aldosterone (control EC50 = 33.2 ± 1.7 mmol/l; aldosterone+eplerenone EC50 = 33.5 ± 1.3 mmol/l; n = 7). The GPR30 receptor blocker G-36 blocked the sensitization of CTGF by aldosterone (aldosterone EC50 = 16.5 ± 1.9 mmol/l; aldosterone+G-36 EC50 = 29.0 ± 2.1 mmol/l; n = 7; P < 0.001). Finally, we found that the sensitization of CTGF by aldosterone was mediated, at least in part, by the sodium/hydrogen exchanger (NHE). We conclude that aldosterone in the CNT lumen sensitizes CTGF via a nongenomic effect involving GPR30 receptors and NHE. Sensitized CTGF induced by aldosterone may contribute to renal damage by increasing Af-Art dilation and glomerular capillary pressure (glomerular barotrauma).
Keywords: aldosterone, distal kidney tubules, arterioles, MR receptor, GPR30 receptor
increases in tubular NaCl concentration at the macula densa constrict the afferent arteriole (Af-Art) resulting in a decrease in the glomerular filtration rate (GFR). This phenomenon, called tubuloglomerular feedback (TGF), thus participates in the regulation of the GFR. In most nephrons, in addition to macula densa, another segment of the distal nephron, the connecting tubule (CNT), is in direct contact with the Af-Art of the same nephron (2, 8, 10, 39). Recently, we described a cross talk mechanism between the CNT and the Af-Art that we termed connecting tubule glomerular feedback (CTGF) (32, 34). CTGF is initiated by increases in Na concentration in the CNT lumen and results in dilation of the Af-Art. These physiological mechanisms regulate glomerular hemodynamics and electrolyte balance.
Aldosterone acts in the CNT and other nephron segments to regulate electrolyte homeostasis. There is now increasing evidence that in animal models with high aldosterone levels, as well as in patients with primary aldosteronism, aldosterone causes glomerular hypertension/hyperfiltration leading to glomerulosclerosis (9, 35). Although the mechanisms by which these detrimental effects of aldosterone occur remain unknown, it may be that aldosterone sensitizes CTGF, which dilates the Af-Art, thus leading to an elevation in glomerular capillary pressure and glomerular damage.
Historically it was thought that aldosterone acted solely via the classic genomic mechanism involving intracellular binding to the mineralocorticoid receptor (MR), thereby affecting transcription and protein synthesis; however, faster actions were also described that could not be explained by genomic mechanisms (3). Recently, a G protein-coupled receptor, GPR30, was shown to be involved in rapid actions of aldosterone in vascular smooth muscle cells (18), and in the nucleus ambiguous (4) that occur as fast as within 2 min.
In the present study, we hypothesize that aldosterone in the CNT lumen sensitizes CTGF acutely via a nongenomic mechanism by stimulating GPR30 receptors. We tested this hypothesis using a technique developed by us in which we simultaneously perfuse a microdissected rabbit Af-Art and its adherent CNT, thus avoiding the confounding influence of the multiple systems that regulate the renal microcirculation.
METHODS
New Zealand White male rabbits weighing 1.5–2 kg (Myrtle's Rabbitry) were given standard chow (Ralston Purina, St. Louis, MO) and tap water ad libitum and anesthetized with ketamine (50 mg/kg im), xylazine (10 mg/kg im), and pentobarbital sodium (25 mg/kg iv). All protocols were approved by Henry Ford Health System's Institutional Animal Care and Use Committee and adhered to APS's Guiding Principles in the Care and Use of Animals, including the provision to comply with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. We used rabbits because their CNTs are well demarcated and microdissection of the CNT and attached Af-Art is easier than in rats or mice. To isolate and microperfuse the Af-Art and CNT, we used methods similar to those described previously (30, 34). The kidneys were sliced along the corticomedullary axis and slices were placed in ice-cold minimum essential medium (MEM; GIBCO Laboratories, Grand Island, NY) containing 5% BSA (Sigma, St. Louis, MO). A single superficial Af-Art with its intact glomerulus was dissected together with the adherent CNT. The microdissected complex was transferred to a temperature-regulated perfusion chamber mounted on an inverted microscope with Hoffmann modulation. Both the Af-Art and CNT were cannulated with an array of concentric glass pipettes as described previously (5, 19). The Af-Art was perfused with MEM containing 5% BSA gassed with room air. Intraluminal pressure was measured by Landis' technique and maintained at 60 mmHg. The CNT perfusion solution contained (in mmol/l) 4 KHCO3, 10 HEPES, 0.5 Na acetate, 0.5 Na lactate, 0.5 K2HPO4, 1.2 MgSO4, 1 CaCO3, and 5.5 glucose, adding 1 mol/l NaCl to achieve the desired final NaCl concentration. Tubular perfusion was controlled by use of a syringe microperfusion pump (Harvard Apparatus) set to 20 nl/min (calibration checked to be ≈20 nl/min), which is within the range of physiological flow rates (15, 21). The bath was superfused with MEM containing 0.15% BSA at a rate of 1 ml/min.
Microdissection and cannulation of the Af-Art and CNT were completed within 90 min at 8°C, after which the temperature was gradually raised to 37°C. Once it was stable, a 30-min equilibration period was allowed before any measurements were taken. Since isolated arteries have little or no tone and our preliminary studies showed that increasing NaCl in the CNT perfusate caused only modest dilation of the Af-Art unless it was preconstricted, we performed all CTGF experiments with Af-Arts preconstricted with norepinephrine (NE; 2–5 × 10−7 mol/l). In each experiment, two consecutive concentration-response curves were generated by increasing luminal NaCl in the CNT from 0 to 5, 10, 20, 40, and 80 mmol/l (each NaCl concentration was maintained for 5 min). Af-Art diameter was measured in the region of maximal response to NE at three sites 3–5 μm apart and expressed as the average of these three measurements. Diameter was recorded at 5-s intervals with a video camera and measured with a computer equipped with Metavue image analysis software (MDS Analytical Technologies, Toronto, Ontario, Canada).
All drugs were added to the lumen of the CNT. The concentrations of the various reagents we used were based on previous publications: aldosterone (12), eplerenone (12), cycloheximide (23), actinomycin D (23), and G36 (7). Actinomycin D and cycloheximide were added to the nephron and we waited for 10 min before changing luminal NaCl concentration, based on previous literature showing inhibition of genomic effects (36, 37). G-36 was purchased from Azano Pharmaceuticals (Albuquerque, NM); all other chemicals were purchased from Sigma.
Statistics.
Values are expressed as means ± SE. Paired t-tests were used to compare CTGF (ΔAf-Art diameter) between the control and experimental periods. Hochberg's step-up procedure was used to adjust the P values for multiple comparisons. We found that the main effect of aldosterone was to sensitize CTGF, rather than to affect the maximal vasodilation that can be achieved in the Af-Art, thus we analyzed the data by comparing the concentration of NaCl in the CNT perfusate needed to achieve half of the maximal response (EC50). In other words, a decrease in EC50 indicates a left-shift in the CTGF curve. EC50 values were determined by SAS probit analysis (Statistical Analysis system, SAS Institute, Cary, NC). In experiments where one of the curves was flat, EC50 was not calculated.
RESULTS
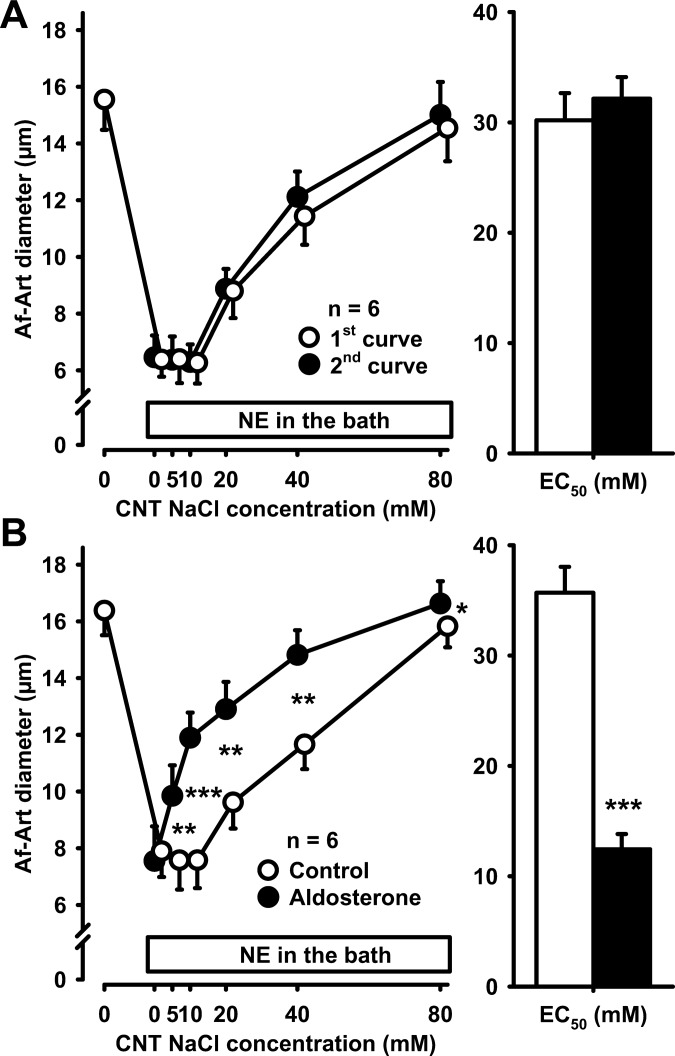
To show that the CTGF response remains stable over time, we performed two consecutive concentration-response curves to 0, 5, 10, 20, 40, and 80 mmol/l of NaCl in the CNT lumen. NE was administered to the Af-Art to cause an ∼50% decrease in its diameter when the attached CNT was perfused with 0 mmol/l NaCl. Increasing NaCl concentration in the CNT caused the Af-Art to relax in a concentration-dependent manner, restoring basal diameter at 80 mmol/l NaCl. We then returned CNT luminal NaCl concentration to zero and repeated the stepwise increase in NaCl concentration. The concentration of NaCl in the CNT perfusate needed to achieve the EC50 was 32.3 ± 1.4 mmol/l in the first curve and 30.9 ± 1.0 mmol/l in the second. Thus, the two curves were similar, confirming that the CTGF response is stable over time (Fig. 1A).
Fig. 1.
A: increasing NaCl concentration 2 consecutive times in the connecting tubule (CNT) dilated preconstricted afferent arterioles (Af-Arts) in a similar manner, indicating that connecting tubule glomerular feedback (CTGF) is stable and reproducible over time. B: adding 10−8 mol/l aldosterone to the CNT perfusate sensitized CTGF (*P < 0.05, **P < 0.01, ***P < 0.001; with vs. without aldosterone).
To study the effect of intratubular aldosterone on CTGF, we repeated the above described experiment, but added 10−8 mol/l aldosterone to the CNT perfusate during the second dose-response curve. When the CNT was perfused with 0 mmol/l NaCl, aldosterone did not affect Af-Art diameter. However, when CNT NaCl concentration was increased in the presence of aldosterone, CTGF-induced dilation of the Af-Art was sensitized, i.e., the curve was shifted to the left (control EC50 37.0 ± 2.0 mmol/l, aldosterone EC50 19.3 ± 1.3 mmol/l, P = 0.001, Fig. 1B). These data demonstrate that intratubular aldosterone sensitizes the CTGF response.
To study whether the effect of aldosterone on CTGF was genomic or nongenomic, we perfused the CNT during the second dose-response curve with both aldosterone and the transcription inhibitor actinomycin D 5 × 10−6 mol/l. Aldosterone sensitized CTGF in the presence of actinomycin D (control EC50 34.7 ± 1.9 mmol/l, aldosterone+actinomycin D EC50 22.6 ± 1.6 mmol/l, P < 0.001, Fig. 2A). In control experiments, we found that actinomycin D did not affect CTGF in the absence of exogenous aldosterone (control EC50 33.1 ± 1.9 mmol/l, actinomycin D EC50 32.6 ± 1.2 mmol/l, Fig. 2B).
Fig. 2.
A: adding 5 × 10−6 mol/l actinomycin D to the CNT perfusate did not prevent the sensitization of CTGF by aldosterone, suggesting that the effect of aldosterone on CTGF is not genomic. **P < 0.01; ***P < 0.001, control vs. aldosterone plus actinomycin D. B: actinomycin D alone did not affect CTGF in the absence of exogenous aldosterone.
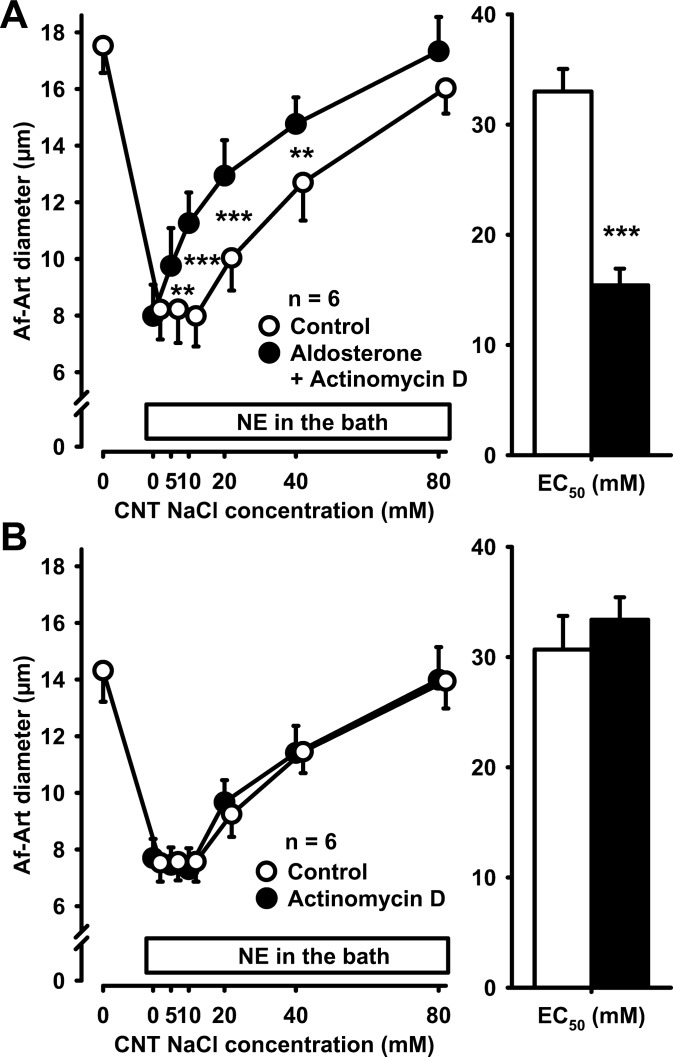
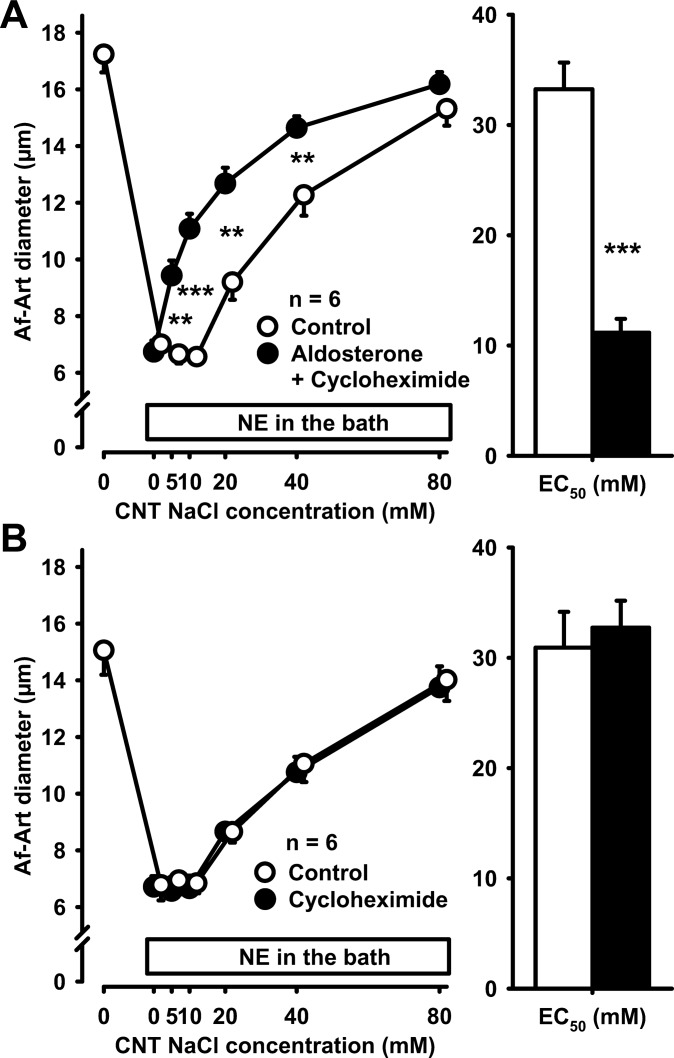
To confirm that the effect of aldosterone on CTGF was nongenomic, we repeated the previous experiment, but instead of actinomycin D we used the protein synthesis inhibitor cycloheximide (10−5 mol/l). Aldosterone also sensitized CTGF in the presence of cycloheximide (control EC50 32.4 ± 4.3 mmol/l, aldosterone + cycloheximide EC50 17.4 ± 3.3 mmol/l, P < 0.001, Fig. 3A). In control experiments, we found that cycloheximide does not affect CTGF in the absence of exogenous aldosterone (control EC50 33.9 ± 2.5 mmol/l, cycloheximide EC50 33.8 ± 2.0 mmol/l, Fig. 3B). Thus, neither actinomycin D nor cycloheximide prevented the sensitization of CTGF by aldosterone, indicating a direct nongenomic effect of aldosterone on CTGF.
Fig. 3.
A: adding 10−5 mol/l cycloheximide to the CNT perfusate did not prevent the sensitization of CTGF by aldosterone, suggesting that the effect of aldosterone on CTGF is not genomic. **P < 0.01; ***P < 0.001, control vs. aldosterone plus cycloheximide. B: cycloheximide alone did not affect CTGF in the absence of exogenous aldosterone.
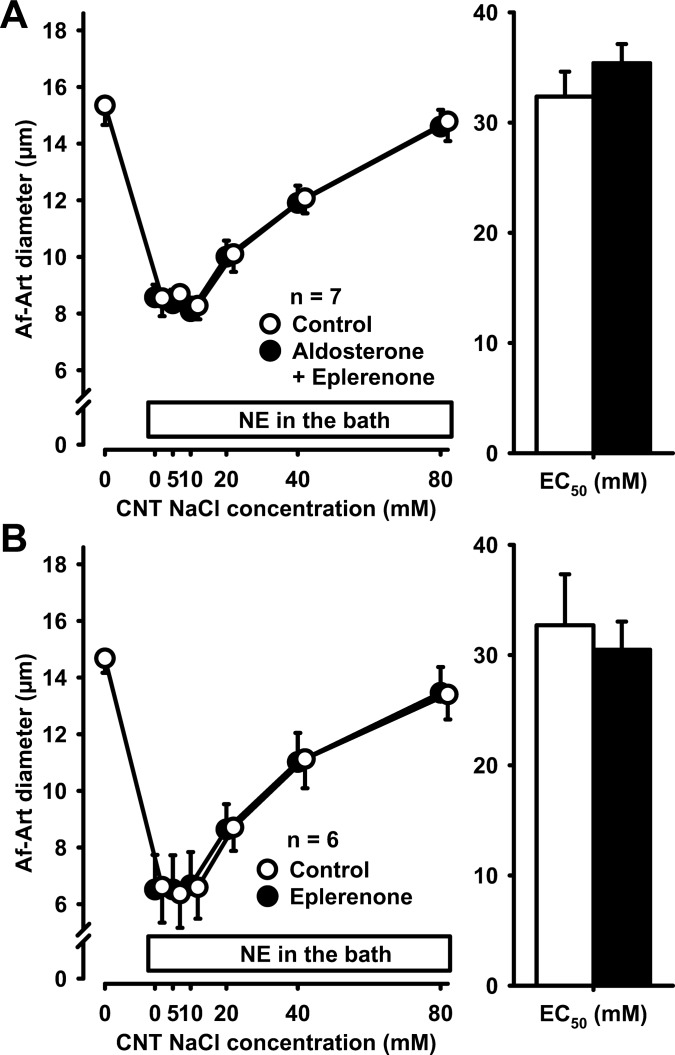
We then tested the effect of aldosterone on CTGF in the presence of the classical aldosterone antagonist eplerenone (previously thought to be selective for the intracellular MR, now recognized to also block the membrane-associated aldosterone receptor GPR30). Eplerenone (10−5 mmol/l) added to the CNT perfusate prevented the sensitization of CTGF by aldosterone and the CTGF curve remained unchanged (control EC50 33.2 ± 1.7 mmol/l, aldosterone+eplerenone EC50 33.5 ± 1.3 mmol/l, Fig. 4A). In control experiments, we found that eplerenone does not affect CTGF in the absence of exogenous aldosterone (control EC50 31.2 ± 2.2 mmol/l, eplerenone EC50 33.2 ± 2.2 mmol/l, Fig. 4B).
Fig. 4.
A: adding 10−5 mol/l eplerenone to the CNT perfusate prevented the sensitization of CTGF by aldosterone, suggesting that this effect is mediated by either the mineralocorticoid receptor (MR) or G protein-coupled receptor 30 (GPR30). B: eplerenone alone did not affect CTGF in the absence of exogenous aldosterone.
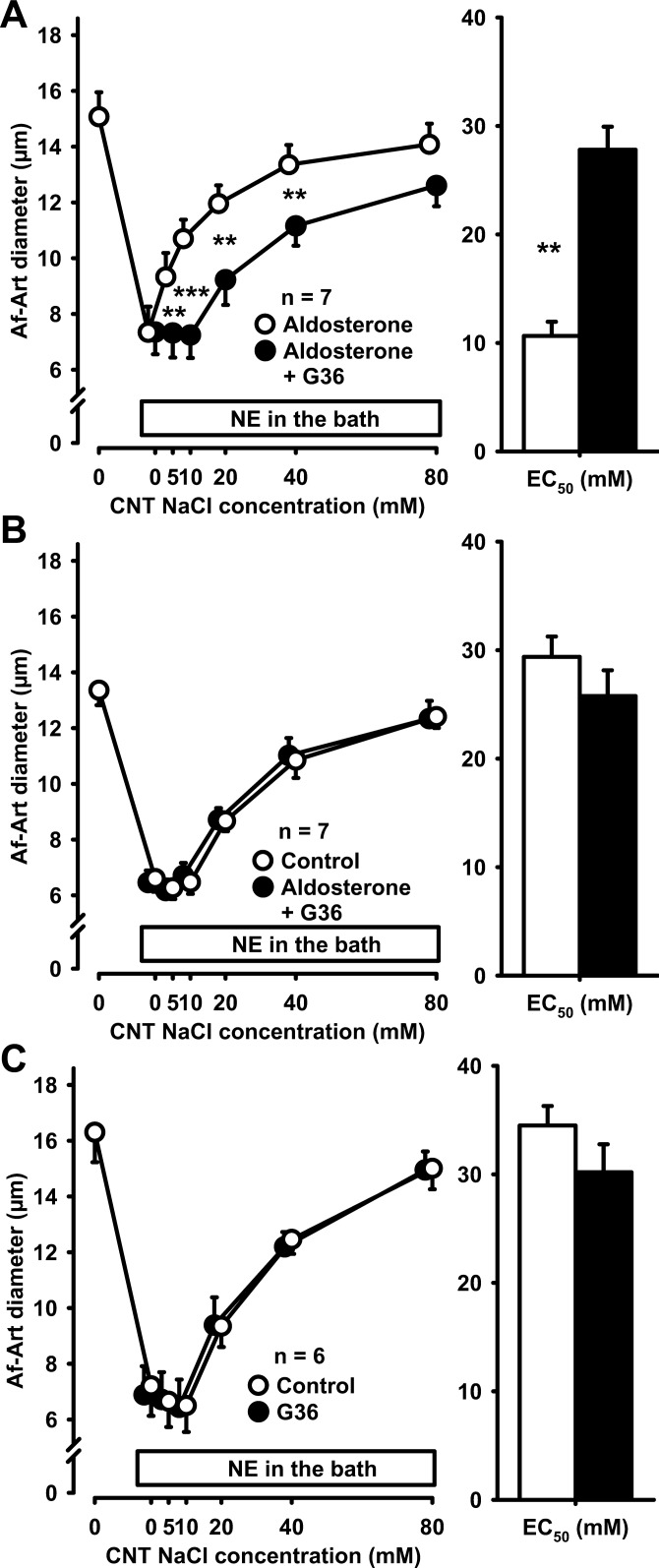
To test whether GPR30 played a role in the sensitization of CTGF by aldosterone, we used the specific GPR30 blocker G36 (10−5 mmol/l). In the presence of aldosterone, G36 attenuated CTGF (aldosterone alone EC50 16.5 ± 1.9 mmol/l, aldosterone + G36 EC50 29.0 ± 2.1 mmol/l, P < 0.05, Fig. 5A). These data indicate that GPR30 receptors mediate aldosterone-induced sensitization of CTGF. To further confirm these results, we performed a control CTGF response, followed by coadministration of aldosterone and G36 during the second period. We found that G36 completely prevented aldosterone-induced sensitization of CTGF (control EC50 30.0 ± 0.1 mmol/l, aldosterone+G36 EC50 30.5 ± 1.5 mmol/l, Fig. 5B). In control experiments, we found that G36 does not affect CTGF in the absence of exogenous aldosterone (control EC50 28.9 ± 1.2 mmol/l, G36 EC50 28.1 ± 1.7 mmol/l, Fig. 5C). These data indicate that aldosterone sensitizes CTGF via GPR30.
Fig. 5.
A: adding 10−5 mol/l G36 to the CNT perfusate attenuated the sensitization of CTGF by aldosterone, suggesting that this effect is mediated, at least in part, by GPR30 (**P < 0.01; ***P < 0.001, aldosterone vs. aldosterone plus G36). B: adding G36 to the CNT perfusate completely prevented the sensitization of CTGF by aldosterone, indicating that this effect is mainly mediated by GPR30. C: G36 alone did not affect CTGF in the absence of exogenous aldosterone.
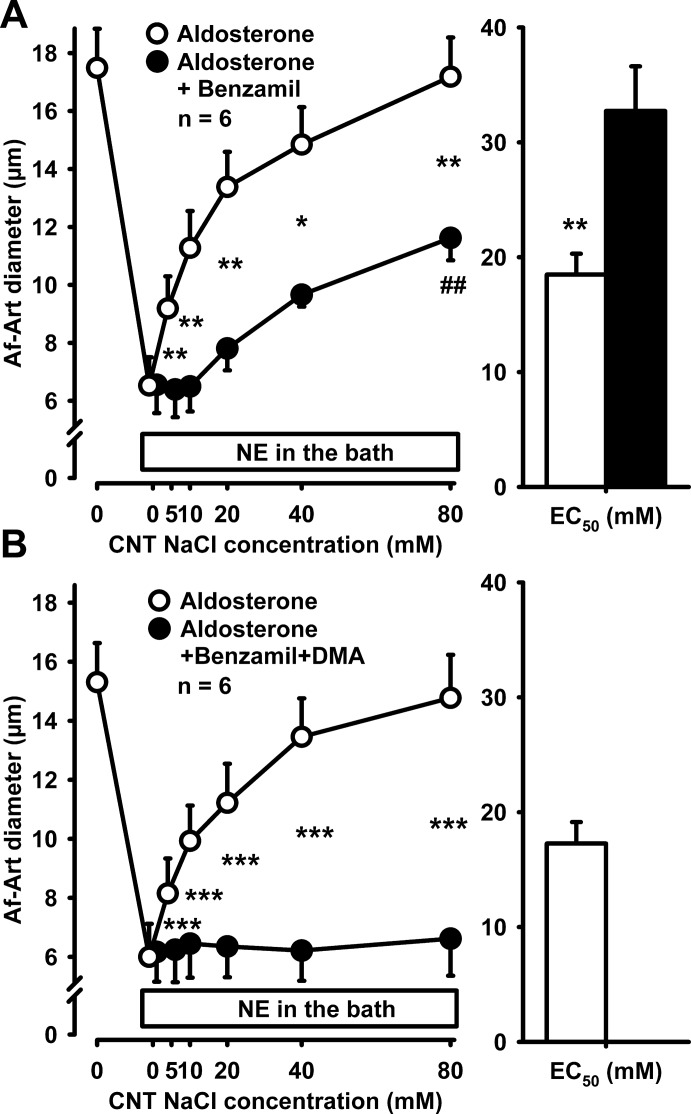
Since basal CTGF is mediated by ENaC, we tested whether aldosterone-induced sensitization was also mediated by ENaC, by using the ENaC inhibitor benzamil (10−6 mol/l). In the presence of aldosterone, benzamil added to the CNT perfusate only partially attenuated CTGF, indicating that the effect of aldosterone on CTGF is, at least in part, independent of ENaC (Fig. 6A). Since NHE mediates some of the effects of aldosterone in the distal nephron in a nongenomic manner, we next tested whether NHE mediated the effect of aldosterone on CTGF. Addition of the NHE inhibitor dimethylamiloride (DMA; 10−4 mol/l) together with benzamil to the CNT perfusate completely abolished both basal CTGF and its sensitization by aldosterone (Fig. 6B). These data suggest that the effect of aldosterone on CTGF is mediated, at least in part, by stimulation of sodium transport via NHE.
Fig. 6.
A: adding 10−6 mol/l benzamil to the CNT perfusate did not prevent aldosterone from inducing CTGF, suggesting that this nongenomic effect of aldosterone is, at least in part, independent from ENaC (*P < 0.05, **P < 0.01, aldosterone vs. aldosterone plus benzamil; ##P < 0.01 vs. 0 NaCl). B: adding 10−4 mol/l dimethylamiloride (DMA) and benzamil to the CNT perfusate completely abolished both basal CTGF and aldosterone-induced sensitization of CTGF (***P < 0.001, aldosterone vs. aldosterone plus DMA).
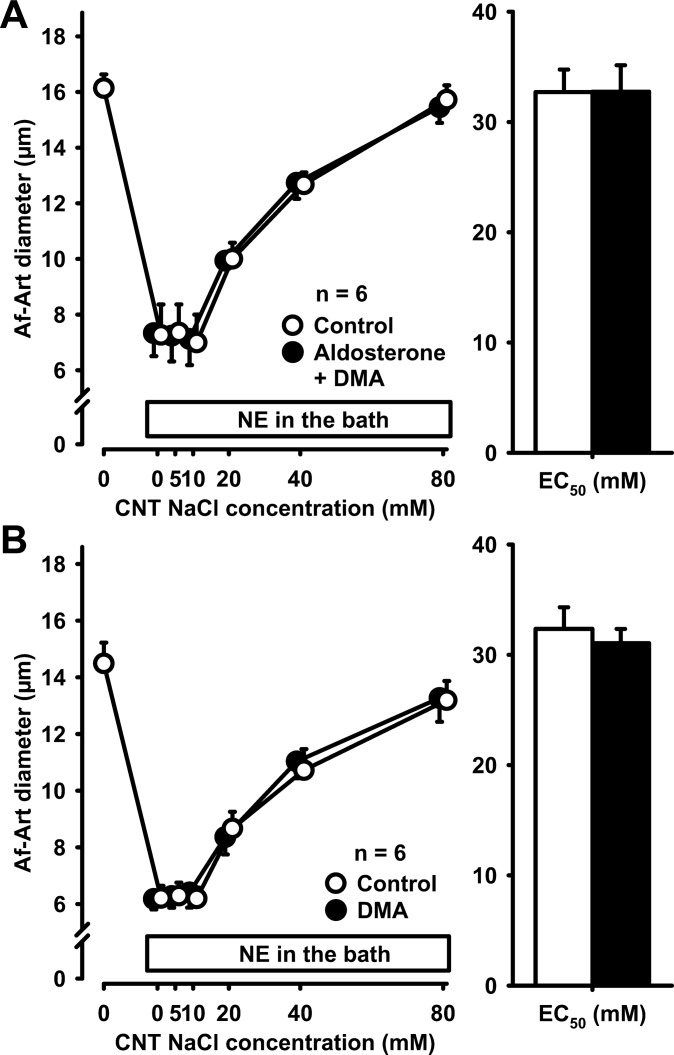
To elucidate whether aldosterone-induced sensitization was completely mediated by NHE, we combined aldosterone and DMA. Compared with control, aldosterone had no effect on CTGF when DMA was simultaneously perfused in the CNT, indicating that the effect of aldosterone on CTGF is completely dependent on NHE (Fig. 7A). Finally, to determine whether NHE participates to any degree in basal CTGF, we studied the effect of DMA alone. In the absence of exogenous aldosterone, DMA was devoid of effect (Fig. 7B), indicating that basal CTGF is not mediated by NHE.
Fig. 7.
A: adding 10−4 mol/l DMA to the CNT perfusate completely prevented the sensitization of CTGF by aldosterone, indicating that this effect is mediated by the sodium/hydrogen exchanger. B: DMA alone did not affect CTGF in the absence of exogenous aldosterone, indicating that the sodium/hydrogen exchanger does not participate in basal CTGF.
DISCUSSION
We investigated the regulation of CTGF by intratubular aldosterone and found that addition of aldosterone to the CNT lumen sensitized CTGF. These effects were mediated by the aldosterone GPR30 receptors and did not require transcription or protein synthesis, indicating that the effect of aldosterone on CTGF is nongenomic.
Aldosterone plays a key role in renal control of Na+, acid-base, and water balance by acting directly on the aldosterone-sensitive distal nephron which comprises the late distal convoluted tubule, the CNT, and the collecting duct (3, 12). Historically it was assumed that aldosterone could only act via the classic genomic mechanism involving binding to the intracellular receptor, MR, thereby affecting transcription and protein synthesis (3). In addition to its genomic effects, many experiments have shown that aldosterone can also elicit nongenomic responses by affecting signal transduction. These effects can be detected within seconds to few minutes and cannot be blocked by transcription and protein synthesis inhibitors, indicating the independence of these effects from the genomic effect of aldosterone (6, 40). It is well-known that aldosterone is the main hormonal regulator of ENaC-dependent Na+ transport in the distal nephron (25, 38). We reported that Na entry into the CNT via ENaC causes the attached Af-Art to dilate, a phenomenon we termed CTGF (34). Furthermore, we showed that ANG II, which is known to increase ENaC activity, sensitizes CTGF (31, 34). Here, we tested the possibility that aldosterone may sensitize CTGF. Indeed, we found that when aldosterone was added to the CNT, the dilation of the Af-Art in response to CNT NaCl was augmented; in other words, aldosterone sensitized CTGF. Our results also indicated that the effects of aldosterone to sensitize CTGF responses are not genomic, because they were not prevented by the transcription inhibitor actinomycin D, or the translation inhibitor cycloheximide. Furthermore, two additional findings support the nongenomic nature of the effect we observed; first, the effect is very quick (within 10 min), and second, the effect is completely blocked by the GPR30 receptor blocker.
The plasma concentration for aldosterone ranges widely and it depends on hydration status, potassium, and ANG II levels. Similarly to other studies, in our experiments we used aldosterone in the concentration of 10 mmol/l (1, 12), which is somewhat higher than the physiological plasma levels. This allowed us to study the mechanism of aldosterone action when its downstream effectors are maximally activated. Our findings indicated the aldosterone's action on CTGF was nongenomic in nature since they were not blocked by actonomycin D or cycloheximide, used at concentrations similar or up to five times higher than those used in previous studies reporting blockade of genomic effects (23). Also the fact that we used two drugs, one to block transcription and the other to block translation, makes our findings more reliable.
In the present study, we found that eplerenone completely blocked the effect of aldosterone on CTGF. Eplerenone, initially developed as a blocker of the intracellular MR, was recently found to also block the membrane-associated aldosterone receptor GPR30, thus these results indicate that the classical MR or the novel GPR30, or possibly both, is involved in aldosterone's action on CTGF. Nongenomic effects have been assumed to originate at the cell membrane, which is where most of the second messenger systems involved in rapid steroid actions are localized. Binding of aldosterone to membranes has been shown in porcine liver microsomes (27) as well as in human endothelial cells (41). Although the protein responsible for the binding could not be identified, it showed the characteristics of a GTP-binding protein (40). Despite all the efforts to identify the alternative receptor, no protein has been unequivocally validated. More recently, Gros et al. (17, 18) reported the involvement of GPR30 in the rapid effects of aldosterone in vascular smooth muscle cells and Brailoiu et al. (4) reported that GPR30 receptors mediated the effects of aldosterone in the nucleus ambiguous. GPR30 represents a heptahelical transmembrane protein acting as a G protein-coupled receptor (20). GPR30 receptors are expressed in the renal tubules, particularly in the CNT (16, 29).
Aldosterone can act rapidly via both GPR30 and classic MR; however, studies with MR antagonists such as spironolactone and eplerenone are confounded by the fact that these agents are also GPR30 antagonists (18). These MR antagonists can block GPR30-mediated effects of aldosterone as well as the effects of a GPR30 agonist on vascular smooth muscle cell (18). For this reason, we used a novel, specific antagonist of GPR30 receptors, namely G36. We found that G36 completely blocked the effect of aldosterone on CTGF; therefore, we concluded this rapid effect was due to GPR30. Our studies focused on the acute, nongenomic, effects of aldosterone, and we cannot rule out a genomic effect of classic MR in the long-term control of CTGF.
In addition to acting as a nongenomic receptor for aldosterone, GPR30 has been proposed to be an estrogen receptor (40). However, the concentrations of aldosterone needed for activating GPR30 are in the physiologic range for this hormone, while supraphysiologic concentrations of estrogens are required to have a similar effect (40). The fact that supraphysiologic concentrations of estrogens are needed to activate GPR30 also makes it unlikely that endogenous estrogens will participate in the regulation of CTGF. However, this possibility cannot be ruled out until further studies are conducted involving male and female animals.
We next posed the question of which sodium transporter in the CNT would be mediating the effect of aldosterone to sensitize CTGF. A natural candidate would be ENaC, since we repeatedly found that it mediates both basal CTGF and its sensitization by ANG II. However, although aldosterone is well-known to stimulate ENaC in a genomic manner, whether it can also do so in a nongenomic manner is not well-established. Aldosterone has been shown to stimulate subcellular translocation of ENaC in mouse cortical collecting duct cells in as little as 2 min (26), and also to activate sodium transport, as measured by patch-clamp, in a similar time frame (42). However, the physiological relevance of these findings has been debated (14). In our experiments, addition of the ENaC inhibitor was not able to completely block CTGF induced by aldosterone, indicating that the nongenomic effect of aldosterone to sensitize CTGF is, at least in part, independent of ENaC. The other sodium transporter that may play a role in the sensitization of CTGF by aldosterone is NHE, since it is present in the CNT and is known to respond to aldosterone in a nongenomic manner (13, 28). We found that inhibition of NHE with DMA, when added to the CNT together with benzamil, completely abolished both basal CTGF and its sensitization by aldosterone. We also found that DMA can completely prevent the effect of aldosterone on CTGF, but it does not affect basal CTGF. Taken together, these data indicate that aldosterone sensitizes CTGF by acting on NHE, but in the absence of aldosterone basal CTGF is mediated by ENaC and not NHE.
Fu et al. (12) recently reported that aldosterone inhibits TGF, a mechanism that constricts the Af-Art. Since CTGF causes Af-Art dilation antagonizing the physiological action of TGF, the coordinated action of aldosterone to inhibit TGF and sensitize CTGF could act to further dilate the Af-Art and increase glomerular filtration.
There is now increasing evidence that aldosterone plays an important role in the pathogenesis and progression of renal diseases (9, 22). We speculate that aldosterone may promote or aggravate the development of glomerulosclerosis by elevating glomerular capillary pressure through its dilator actions on Af-Art. Our results provided the first evidence that aldosterone can cause Af-Art dilation by sensitizing CTGF. This conclusion is in agreement with the clinical finding that glomerular capillary pressure was elevated in patients with primary aldosteronism (22).
In many respects, the actions of ANG II and aldosterone are synergistic. We reported that ANG II sensitizes CTGF, likely by increasing ENaC activity in the CNT (31, 33). Taken together, these findings suggest that CTGF is sensitized in pathophysiological conditions where the renin-angiotensin-aldosterone system is activated.
In summary, using an isolated perfused Af-Art with the CNT attached we found that intratubular aldosterone augmented CTGF response. The addition of actinomycin D and cycloheximide did not influence the effect of aldosterone on CTGF. Eplerenone and G36 completely blocked the effect of aldosterone. We conclude that aldosterone sensitized CTGF through a nongenomic action by acting on the aldosterone receptor GPR30.
GRANTS
This work was supported by National Institutes of Health Grant HL028982.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.R., M.A.D., J.L.G., and O.A.C. conception and design of research; Y.R. performed experiments; Y.R., M.A.D., J.L.G., and O.A.C. interpreted results of experiments; Y.R. and M.A.D. drafted manuscript; Y.R., M.A.D., J.L.G., P.L., K.K., H.W., E.L.P., and O.A.C. approved final version of manuscript; M.A.D. and E.L.P. analyzed data; M.A.D. prepared figures; M.A.D., J.L.G., P.L., K.K., H.W., E.L.P., and O.A.C. edited and revised manuscript.
REFERENCES
- 1.Arima S. Rapid nongenomic vasoconstrictor actions of aldosterone in the renal microcirculation. J Steroid Biochem Mol Biol 102: 170–174, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Barajas L, Powers K, Carretero OA, Scicli AG, Inagami T. Immunocytochemical localization of renin and kallikrein in the rat renal cortex. Kidney Int 29: 965–970, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Booth RE, Johnson JP, Stockand JD. Aldosterone. Adv Physiol Educ 26: 8–20, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Brailoiu GC, Benamar K, Arterburn JB, Gao E, Rabinowitz JE, Koch WJ, Brailoiu E. Aldosterone increases cardiac vagal tone via G protein-coupled oestrogen receptor activation. J Physiol 591: 4223–4235, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burg MB. Perfusion of isolated renal tubules. Yale J Biol Med 45: 321–326, 1972 [PMC free article] [PubMed] [Google Scholar]
- 6.Christ M, Meyer C, Sippel K, Wehling M. Rapid aldosterone signaling in vascular smooth muscle cells: involvement of phospholipase C, diacylglycerol and protein kinase C α. Biochem Biophys Res Commun 213: 123–129, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi S, Sklar LA, Hathaway HJ, Arterburn JB, Prossnitz ER. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol 127: 358–366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorup J, Morsing P, Rasch R. Tubule-tubule and tubule-arteriole contacts in rat kidney distal nephrons. A morphologic study based on computer-assisted three-dimensional reconstructions. Lab Invest 67: 761–769, 1992 [PubMed] [Google Scholar]
- 9.Epstein M. Aldosterone as a mediator of progressive renal disease: pathogenetic and clinical implications. Am J Kidney Dis 37: 677–688, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Faarup P. On the morphology of the juxtaglomerular apparatus. Acta Anat (Basel) 60: 20–38, 1965 [DOI] [PubMed] [Google Scholar]
- 11.Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 153: 2953–2962, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Y, Hall JE, Lu D, Lin L, Manning RD, Jr, Cheng L, Gomez-Sanchez CE, Juncos LA, Liu R. Aldosterone blunts tubuloglomerular feedback by activating macula densa mineralocorticoid receptors. Hypertension 59: 599–606, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gekle M, Golenhofen N, Oberleithner H, Silbernagl S. Rapid activation of Na+/H+ exchange by aldosterone in renal epithelial cells requires Ca2+ and stimulation of a plasma membrane proton conductance. Proc Natl Acad Sci USA 93: 10500–10504, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Good DW. Nongenomic actions of aldosterone on the renal tubule. Hypertension 49: 728–739, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Good DW, Wright FS. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol Renal Fluid Electrolyte Physiol 236: F192–F205, 1979 [DOI] [PubMed] [Google Scholar]
- 16.Grimont A, Bloch-Faure M, El AB, Crambert G. Mapping of sex hormone receptors and their modulators along the nephron of male and female mice. FEBS Lett 583: 1644–1648, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Gros R, Ding Q, Liu B, Chorazyczewski J, Feldman RD. Aldosterone mediates its rapid effects in vascular endothelial cells through GPER activation. Am J Physiol Cell Physiol 304: C532–C540, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Gros R, Ding Q, Sklar LA, Prossnitz EE, Arterburn JB, Chorazyczewski J, Feldman RD. GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension 57: 442–451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito S, Carretero OA. An in vitro approach to the study of macula densa-mediated glomerular hemodynamics. Kidney Int 38: 1206–1210, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Ji TH, Grossmann M, Ji I. G protein-coupled receptors. I. Diversity of receptor-ligand interactions. J Biol Chem 273: 17299–17302, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Khuri RN, Strieder WN, Giebisch G. Effects of flow rate and potassium intake on distal tubular potassium transfer. Am J Physiol 228: 1249–1261, 1975 [DOI] [PubMed] [Google Scholar]
- 22.Kimura G, Uzu T, Nakamura S, Inenaga T, Fujii T. High sodium sensitivity and glomerular hypertension/hyperfiltration in primary aldosteronism. J Hypertens 14: 1463–1468, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Le Moellic C, Ouvrard-Pascaud A, Capurro C, Cluzeaud F, Fay M, Jaisser F, Farman N, Blot-Chabaud M. Early nongenomic events in aldosterone action in renal collecting duct cells: PKCalpha activation, mineralocorticoid receptor phosphorylation, and cross-talk with the genomic response. J Am Soc Nephrol 15: 1145–1160, 2004 [PubMed] [Google Scholar]
- 24.Liu L, Duke BJ, Malik B, Yue Q, Eaton DC. Biphasic regulation of ENaC by TGF-α and EGF in renal epithelial cells. Am J Physiol Renal Physiol 296: F1417–F1427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loffing J, Korbmacher C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC). Pflügers Arch 458: 111–135, 2009 [DOI] [PubMed] [Google Scholar]
- 26.McEneaney V, Harvey BJ, Thomas W. Aldosterone regulates rapid trafficking of epithelial sodium channel subunits in renal cortical collecting duct cells via protein kinase D activation. Mol Endocrinol 22: 881–892, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer C, Christ M, Wehling M. Characterization and solubilization of novel aldosterone-binding proteins in porcine liver microsomes. Eur J Biochem 229: 736–740, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Oberleithner H, Weigt M, Westphale HJ, Wang W. Aldosterone activates Na+/H+ exchange and raises cytoplasmic pH in target cells of the amphibian kidney. Proc Natl Acad Sci USA 84: 1464–1468, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pradervand S, Zuber MA, Centeno G, Bonny O, Firsov D. A comprehensive analysis of gene expression profiles in distal parts of the mouse renal tubule. Pflügers Arch 460: 925–952, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Ren Y, Carretero OA, Garvin JL. Mechanism by which superoxide potentiates tubuloglomerular feedback. Hypertension 39: 624–628, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Ren Y, D'Ambrosio MA, Garvin JL, Carretero OA. Angiotensin II enhances connecting tubule glomerular feedback. Hypertension 56: 636–642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren Y, D'Ambrosio MA, Garvin JL, Wang H, Carretero OA. Possible mediators of connecting tubule glomerular feedback. Hypertension 53: 319–323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren Y, D'Ambrosio MA, Wang H, Peterson EL, Garvin JL, Carretero OA. Mechanisms of angiotensin II-enhanced connecting tubule glomerular feedback. Am J Physiol Renal Physiol 303: F259–F265, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int 71: 1116–1121, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Ribstein J, du CG, Fesler P, Mimran A. Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol 16: 1320–1325, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Ricci EP, Kucukural A, Cenik C, Mercier BC, Singh G, Heyer EE, Ashar-Patel A, Peng L, Moore MJ. Staufen1 senses overall transcript secondary structure to regulate translation. Nat Struct Mol Biol 21: 26–35, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawicki SG, Godman GC. On the differential cytotoxicity of actinomycin D. J Cell Biol 50: 746–761, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas W, McEneaney V, Harvey BJ. Aldosterone-induced signalling and cation transport in the distal nephron. Steroids 73: 979–984, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Vio CP, Figueroa CD, Caorsi I. Anatomical relationship between kallikrein-containing tubules and the juxtaglomerular apparatus in the human kidney. Am J Hypertens 1: 269–271, 1988 [DOI] [PubMed] [Google Scholar]
- 40.Wendler A, Albrecht C, Wehling M. Nongenomic actions of aldosterone and progesterone revisited. Steroids 77: 1002–1006, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Wildling L, Hinterdorfer P, Kusche-Vihrog K, Treffner Y, Oberleithner H. Aldosterone receptor sites on plasma membrane of human vascular endothelium detected by a mechanical nanosensor. Pflügers Arch 458: 223–230, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Zhou ZH, Bubien JK. Nongenomic regulation of ENaC by aldosterone. Am J Physiol Cell Physiol 281: C1118–C1130, 2001 [DOI] [PubMed] [Google Scholar]