Abstract
Cyclooxygenase 2 (COX-2) has an established role in postnatal kidney development. 15-Hydroxyprostaglandin dehydrogenase (15-PGDH) is recently identified as an endogenous inhibitor of COX-2, limiting the production of COX-2-derived prostanoids in several pathological conditions. The present study was undertaken to examine the regulation of renal 15-PGDH expression during postnatal kidney development in rats compared with COX-2. qRT-PCR and immunoblotting demonstrated that 15-PGDH mRNA and protein in the kidney were present in neonates, peaked in the second postnatal week, and then declined sharply to very low level in adulthood. Immunostaining demonstrated that at the second postnatal week, renal 15-PGDH protein was predominantly found in the proximal tubules stained positive for Na/H exchanger 3 and brush borders (periodic acid-Schiff), whereas COX-2 protein was restricted to macular densa and adjacent thick ascending limbs. Interestingly, in the fourth postnatal week, 15-PGDH protein was redistributed to thick ascending limbs stained positive for the Na-K-2Cl cotransporter. After 6 wk of age, 15-PGDH protein was found in the granules in subsets of the proximal tubules. Overall, these results support a possibility that 15-PGDH may regulate postnatal kidney development through interaction with COX-2.
Keywords: COX-2, postnatal kidney development, prostaglandin, proximal tubule, 15-PDGH
prostaglandins (pgs) represent a diverse family of autacoids generated by cyclooxygenase (COX)-mediated metabolism of arachidonic acid. Four PGs (PGD2, PGE2, PGF2α, and PGI2) and another COX metabolite, thromboxane A2, are the primary bioactive derivatives (4, 20, 29, 36). These primary bioactive prostanoids exert their divergent effects on target tissues in an autocrine or paracrine manner via their own selective receptors (20). In the kidney, PGE2 is a predominant arachidonic acid metabolite and plays an important role in renal functions, including regulation of vascular smooth muscle tonus, glomerular filtration, renin release, and tubular sodium and water transport (5, 8). In the renal medulla, PGE2 inhibits Na+ reabsorption at the medullary thick ascending limbs of Henle and antagonizes antidiuretic hormone action in collecting ducts (5).
COXs are the primarily important enzymes for PG synthesis, which convert arachidonic acid to PGG2 and PGH2. There are two isoforms of COXs, a constitutive isoform, COX-1, and an inducible isoform, COX-2 (28, 36). Although both enzymes are expressed in the kidney, tissue distributions of these isoforms are different (1). COX-1 is constitutively expressed in medullary collecting ducts and medullary interstitial cells whereas COX-2 is expressed in the macula densa of the juxtaglomerular apparatus and associated cortical thick ascending limb of Henle (cTAL) in the renal cortex and interstitial cells in the renal medulla (7). The expression of COX-2 is induced by various stimuli, such as low salt intake (40), loop diuretics (13), and water deprivation (39), and PGE2 synthesized by COX-2 regulates glomerular filtration, renin release in the renal cortex, and tubular absorption of sodium and/or water in the medulla (6), suggesting that immediate production of PGE2 by COX-2 might be important for the regulation of those physiological functions. Nephrogenesis in rodents continues after birth. Abundant evidence suggests an essential role of COX-2 in postnatal kidney development (12, 26). COX-2 null mice developed impairment in maturation of the nephrogenic zone during the postnatal period, leading to chronic renal failure and hypertension (2, 10, 17). Interestingly, this phenotype depends on genetic background. Pharmacological inhibition of COX-2 in rats results in postnatal kidney developmental abnormalities and chronic renal failure, similar to the phenotype in COX-2 null mice (14, 35). In support of the role of COX-2 in postnatal kidney development, COX-2 expression in the kidney is subjected to developmental regulation with a peak level in the second postnatal week (43).
The steady-state kidney levels of active prostaglandins depend on the relative rates of biosynthesis and inactivation. It has been proposed that two major steps are involved in prostaglandin inactivation: uptake from the plasma membrane by prostaglandin transporters and oxidation by NAD+-dependent 15-hydroxyprostaglandin dehydrogenase (15-PGDH) (21, 22, 34). Pichaud et al. (24) noted that 15-PGDH is the main enzyme of prostaglandin degradation. By catalyzing the conversion of the 15-hydroxyl group of prostaglandins into a keto group, this ubiquitous enzyme strongly reduces the biological activity of these molecules (24). Previous reports have shown that 15-PGDH was expressed in the proximal tubule, cortical, and outer medullary thick ascending limb, and collecting duct of the kidney but not in the macula densa or papilla (41). Kidney 15-PGDH activity has been reported to be increased in obstructive kidneys (10) but inhibited by estradiol (5). However, in contrast to the overwhelming information about renal function of COX-2, little is known about 15-PGDH. Therefore, the present study was designed to elucidate temporal, spatial, and cytological aspects of 15-PGDH expression during postnatal kidney development.
MATERIALS AND METHODS
Animals.
Sprague-Dawley (SD) rats of different ages were purchased from Harlan (Indianapolis, IN). Wistar-Kyoto (WKY) rats of different ages were purchased from Charles River (Wilmington, MA). Rats were maintained on normal rat chow. Twenty-four-hour urine was collected using metabolic cages. All protocols employing rats were conducted in accordance the principles and guidance of the University of Utah Institutional Animal Care and Committee.
Immunohistochemistry.
Under anesthesia, kidneys were removed and fixed with 4% paraformaldehyde. The tissues were subsequently embedded in paraffin, and 3-μm sections were cut. Immunohistochemical staining was performed using an EnVision TM FLEX Mini Kit (Dako, Carpinteria, CA) according to the manufacturer's instructions. In brief, after deparaffinization and rehydration through xylene and graded alcohol, the slides were treated with Target Retrieval Solution (50× Tris/EDTA buffer, pH 9) and sequentially incubated with peroxidase-blocking reagent, primary antibody, EnVision/horseradish peroxidase (HRP), and DAB plus chromogen. Then, the slides were counterstained with hematoxylin and visualized under the microscope. Anti-15-PGDH antibody (1:100 dilution) and anti-COX-2 antibody (1:200 dilution) were purchased from Cayman (Ann Arbor, MI); anti-Na/H exchanger 3 (NHE3) antibody (1:200 dilution) was purchased from Millipore (Billerica, MA); and anti-Na-K-2Cl cotransporter (NKCC2) antibody (1: 200 dilution) and anti-aquaporin-2 (AQP2) antibody (1:200 dilution) were purchased from Biosynthesis (Lewisville, TX).
Periodic acid-Schiff staining.
Under anesthesia, kidneys are removed and fixed with 4% paraformaldehyde. The tissues were subsequently embedded in paraffin, and 3-μm sections were cut. After deparaffinization and rehydration through xylene and graded alcohol, the slides were stained with periodic acid-Schiff (PAS) and counterstained by hematoxylin.
Quantitative RT-PCR.
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA), and first-strand cDNAs were synthesized from 2 μg of total RNAs in a 20-ml reaction using SuperScript (Invitrogen). The first-strand cDNAs served as the template for quantitative PCR (qPCR) performed in the Applied Biosystems 7900 Real Time PCR System using SYBR green PCR reagent (Applied Biosystems, Foster City, CA). The amplification was carried out for 40 cycles with conditions of 15-s denaturation at 95°C and 1-min annealing at 60°C. The sequence of oligonucleotides used for PCR is listed as follows: 15-PGDH sense: 5′-AGGACTCTGCTCACGAAGGA-3′ and antisense: 5′-TGACATGGATTGGAACAGCA-3′; and GAPDH sense: 5′-GTCTTCACTACCATGGAGAAGG-3′ and antisense: 5′-TCATGGATGACCTTGGCCAG-3′.
Immunoblotting.
The kidney lysates were stored at −80°C until assayed. Protein concentrations were determined using Coomassie reagent. An equal amount of the whole tissue protein was denatured at 100°C for 10 min, separated by SDS-PAGE, and transferred onto nitrocellulose membranes. The blots were blocked overnight with 5% nonfat dry milk in Tris-buffered saline (TBS), followed by incubation with primary antibody overnight. The blots were washed with TBS followed by incubation with horseradish peroxidase-conjugated secondary antibody. Immune complexes were detected using ECL methods. The immunoreactive bands were quantified using the Gel and Graph Digitizing System (Silk Scientific, Tustin, CA).
Immunoprecipitation.
The urine of rats was stored at −80°C until assayed. Immunoprecipitation was performed using a Pierce Protein A/G Magnetic IP/Co-IP Kit (Thermo Scientific, Rockford, IL) according to the manufacturer's instructions. Five hundred microliters urine was used for each reaction.
Data analysis.
Data are summarized as means ± SE. Statistical analysis was performed using one-way ANOVA with the Bonferroni correction or Student's t-test as appropriate. P < 0.05 was considered statistically significant.
RESULTS
15-PGDH mRNA and protein expression in developing rat kidneys.
qRT-PCR was used to investigate 15-PGDH mRNA expression in the kidney of SD rats at different ages. As indicated in Fig. 1, developmental regulation of renal 15-PGDH mRNA expression was apparent in rat pups compared with the constitutive glyceraldehyde-3-phosphate dehydrogenase mRNA. The 15-PGDH signal was detected at low levels in newborns, which gradually increased with age, reaching the peak level at postnatal day 14 (PND14), and decreased thereafter, returning to the baseline level at PND42.
Fig. 1.

15-Hydroxyprostaglandin dehydrogenase (15-PGDH) mRNA expression in the kidneys of Sprague-Dawley (SD) rats at different ages. Renal 15-PGDH mRNA expression was detected SD rats at various ages by quantitative (q) RT-PCR. Values are means ± SE.; n = 5 in each group.
In principle, the protein expression pattern of 15-PGDH as assessed by immunoblot analysis was similar to its mRNA albeit with more robust variations. In contrast to the constant β-actin expression, 15-PGDH protein was detected at low levels in the newborns, gradually increasing to the peak level at PND14, and decreasing sharply with age; 15-PGDH protein became nearly undetectable after PND28 (Fig. 2).
Fig. 2.

15-PGDH protein expression in the kidneys of SD rats during different ages. A: renal 15-PGDH protein expression was detected in SD rats at various ages by Western blotting. B: 15-PGDH densitometry. Values are means ± SE.; n = 5 in each group.
Cellular localization of 15-PGDH protein in the developing rat kidney.
We performed immunostaining to determine the cellular localization of 15-PGDH in the kidneys of SD and WKY rats at various ages. The results indicated distinct expression patterns during postnatal kidney development. In light of the peak expression of 15-PDGH at PND14, we chose this time point for detailed immunostaining analysis.
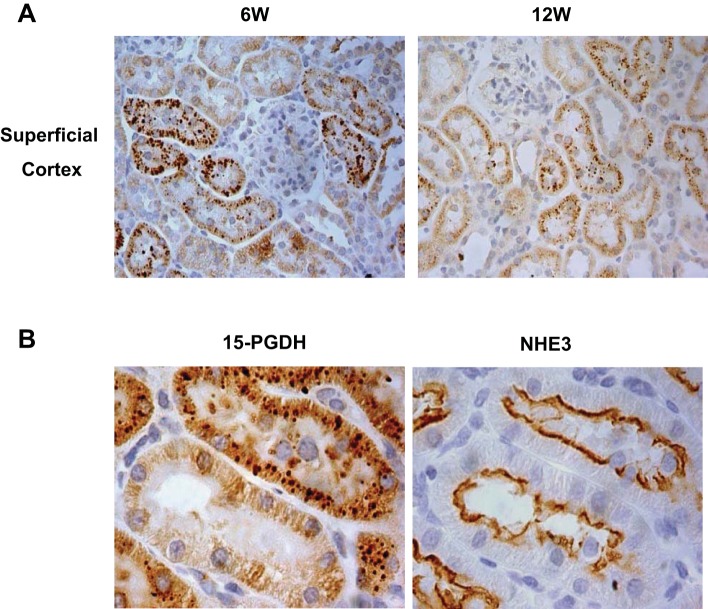
As shown in Fig. 3, at PND14 a 15-PGDH signal was predominantly detected in the deep cortex with a relatively weak signal in the superficial cortex and almost no signal in outer and inner medullas (Fig. 3, A and C). Rabbit IgG staining was used as a negative control (Fig. 3B). 15-PGDH blocking peptide was also used to confirm the specificity of the antibody against 15-PGDH (Fig. 3B). Within the deep cortex at PND14, immunostaining of consecutive sections demonstrated the colocalization of 15-PDGH with NHE3, which is known to be predominantly expressed in the proximal tubule, and to a lesser extent in the thin limb and thick ascending limb of Henle's loop (Fig. 4B). Brush borders are present in the proximal tubule but not in the thin limb and thick ascending limb of Henle's loop and therefore can be used to discriminate the proximal from the latter two nephron segments. Using PAS staining and immunohistochemistry of 15-PGDH of consecutive sections, we further confirmed that 15-PGDH immunoreactivity was detected in the renal tubules that contained brush borders, confirming the localization in the proximal tubule (Fig. 4A). Interestingly, at PND28, in contrast to the reduced total renal 15-PGDH protein abundance, immunostaining demonstrated redistribution of 15-PGDH protein to the outer medulla (Fig. 5A). At this location, the 15-PGDH-positive tubules were stained positive with anti-NKCC2 antibody, confirming the localization in the thick ascending limb (Fig. 5B). In addition, collecting ducts (AQP2 positive) had very weak 15-PGDH signal (Fig. 5C), indicating a substantially low 15-PGDH expression in the collecting ducts. Interestingly, in the superficial cortex 15-PGDH was present in granules in many tubular epithelial cells and the lumens at PND42 and PND84 (Fig. 6A). The granules were detected in some but not all NHE3-positive tubules (Fig. 6B).
Fig. 3.
Localization of 15-PGDH protein in the kidneys of 2-wk-old SD rats. A: immunostaining of kidney slides with anti-15-PGDH antibody in 2-wk-old SD rats (×50). B: 15-PGDH-blocking peptide was used to confirm the specificity of the antibody against 15-PGDH. Rabbit IgG staining was used as a negative control. C: immunostaining of kidney slides with anti-15-PGDH antibody in 2-wk-old SD rats (×400). Shown are representatives of 2–3 experiments.
Fig. 4.
Localization of 15-PGDH protein in renal proximal tubules of 2-wk-old SD rats. A: immunohistochemistry of 15-PGDH and periodic acid-Schiff (PAS) staining on consecutive kidney sections in 2-wk-old SD rats (×400). B: immunostaining with anti-15-PGDH and anti-Na/H exchanger 3 (NHE3) antibodies on consecutive kidney sections in 2-wk-old SD rats (×1,000). Shown are representatives of 2–3 experiments.
Fig. 5.
Localization of 15-PGDH protein in the kidneys of 4-wk-old SD rats. A: immunostaining of kidney slides with anti-15-PGDH antibody in 4-wk old SD rats (×400). B: immunostaining with anti-15-PGDH and anti-Na-K-2Cl cotransporter (NKCC2) antibodies on consecutive kidney sections in 2-wk-old SD rats (×1,000). C: immunostaining with anti-15-PGDH and anti-aquaporin-2 (AQP2) antibodies on consecutive kidney sections in 2-wk-old SD rats (×1,000). Shown are representatives of 2–3 experiments.
Fig. 6.
Localization of 15-PGDH protein in renal superficial cortex of 6- and 12-wk old SD rats. A: immunostaining of kidney slides with anti-15-PGDH antibody in 6- and 12-wk-old SD rats (×1,000). B: immunostaining with anti-15-PGDH and anti-NHE3 antibodies on consecutive kidney sections in 6-wk-old SD rats (×1,000). Shown are representatives of 2–3 experiments.
The localization of COX-2 in the kidney was also examined in the present study (Fig. 7). In the kidney cortex, prostaglandins derived from COX-2 play an important role in regulation of vascular tone, renin biosynthesis, and excretion (11). In normal adult rat cortex, only isolated macula densa cells are COX-2 positive (12). Using immunochemistry staining, we confirmed previous reports that the COX-2-positive cells were localized to macula densa and adjacent cortical thick ascending limbs (arrows). A 15-PGDH signal was absent in COX-2-positive macula densa and adjacent cortical thick ascending limb at both PND14 and PND28 (Fig. 7. arrows), again supporting the localization of 15-PGDH in the proximal tubule.
Fig. 7.
Localization of 15-PGDH protein and cyclooxygenase 2 (COX-2) protein in the kidneys of 2-, 4-, and 6-wk-old SD rats. Immunostaining was done with anti-15-PGDH and anti-COX-2 antibodies on consecutive kidney sections in 2-, 4-, and 6-wk-old SD rats (×1,000). Shown are representatives of 2–3 experiments.
In this study, 4- and 12-wk-old WKY rats were also used to detect the renal localization of 15-PGDH protein. The expression pattern of 15-PGDH in the kidney of WKY rats was the same as that of SD rats during postnatal development (data not shown).
Urinary 15-PGDH protein levels in SD and WKY rats.
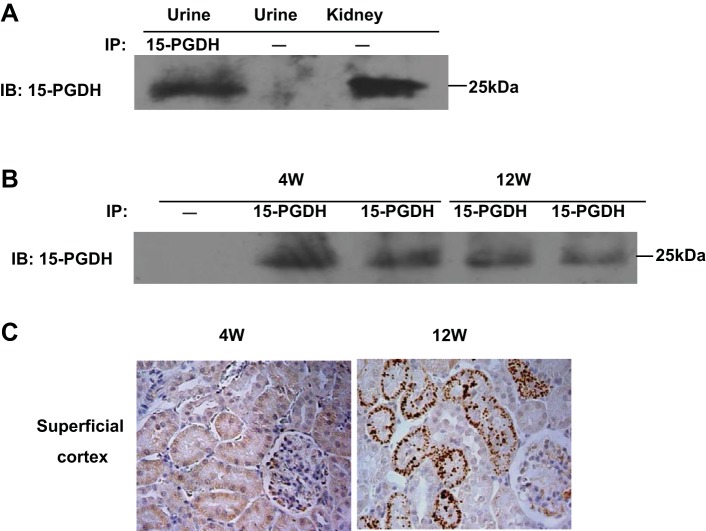
The granular appearance and also its presence in the lumens suggests a possibility that 15-PGDH might be secreted to the urine. Accordingly, we performed immunoprecipitation to detect the urinary 15-PGDH protein levels of SD and WKY rats. As shown in Fig. 8A, 15-PGDH protein was detectable in the urine of 12-wk-old SD rats after immunoprecipitation using anti-15-PGDH antibody. To test whether this phenomenon was dependent on the rat strain, we performed parallel studies in 4- and 12-wk-old WKY rats. As seen in SD rats, urinary 15-PGDH was detectable in 4- and 12-wk-old WKY rats (Fig. 8B). These results demonstrated urinary excretion of 15-PGDH protein in different rat strains. We subsequently investigated the relationship between granular appearance and urinary excretion of 15-PGDH.
Fig. 8.
Urinary excretion of 15-PGDH protein in rats at different ages. A: urinary 15-PGDH protein level was detected at 12-wk-old SD rats by immunoprecipitation. B: urinary 15-PGDH protein level was detected at 4- and 12-wk-old Wistar-Kyoto (WKY) rats by immunoprecipitation. C: immunostaining of kidney slides with anti-15-PGDH antibody in 4- and 12-wk-old WKY rats (×400). Shown are representatives of 2–3 experiments.
As shown in Fig. 8C, the granular appearance of 15-PGDH was detected in 12- but not 4-wk-old WKY rats, in contrast to the constant levels of urinary 15-PDGH, suggesting no association between the granular appearance and urinary excretion of 15-PGDH.
DISCUSSION
The present study examined the renal expression and localization of 15-PGDH, a key enzyme in prostaglandin degradation, during postnatal development. In this study, qRT-PCR and immunoblotting consistently demonstrated that 15-PGDH mRNA and protein are low at birth, rise to a peak level in the first 2 postnatal weeks, and gradually decline to very low levels in adulthood. Consistent with our results, Moel et al. (16) examined the renal PGE2 degradation in rats aged 20 (30.7 g), 31 (101 g), and 120 days (413 g), and their results showed that renal PGE2 degradation was highest in 20-day-old rats and decreased with age. It is known that the immature kidney is more sensitive to prostaglandins than adult kidneys because of increased affinity for the receptor (15). In immature kidneys, PGE2 acts through the EP3 receptor to suppress AVP-stimulated cAMP generation via activation of Gi. Resistance to AVP seems to be one of the major factors limiting urine concentration in immature kidneys. Thus the ability of immature kidneys to concentrate urine is lower than in adult kidneys. Excessive PGE2 activity may lead to severe water and electrolyte disorders, especially in premature babies. Clinically, infants with hyperprostaglandin E syndrome (HPS) are present with the typical pattern of impaired tubular reabsorption in the thick ascending limb of Henle's loop, including salt wasting, isosthenuric, or hyposthenuric polyuria, and hypercalciuria with subsequent nephrocalcinosis (9, 27, 37). Therefore, it is important to limit the content of PGE2 in immature kidneys, which is likely accomplished by the high activity of renal 15-PGDH during the postnatal period.
COX-1 and -2 are thought to be the rate-limiting enzymes for PGE2 synthesis. Ogawa et al. (23) showed that COX-1 expression was constant in the kidney; COX-1 mRNA expression did not change with age. Lipopolysaccharide treatment did not alter COX-1 mRNA expression in infant or adult rats. In contrast, renal COX-2 in rodents is subjected to postnatal regulation (23, 43). COX-2 expression was low at birth, increased gradually to reach a peak at PND14, and decreased gradually to very low levels in the adults, a pattern almost analogous to that of 15-PGDH. However, despite the same time frame of the induction of renal COX-2 and 15-PGDH, they have distinct cellular localization. At PND14, the induction of 15-PGDH occurred primarily in the proximal tubule, whereas the induction of COX-2 was mostly restricted to macular densa and adjacent cortical thick ascending limbs. These findings support the concept that 15-PGDH may function as an endogenous antagonist of COX-2 during the postnatal period, therefore preventing the overproduction of COX-2-derived products and also ensuring the precise location of action of these products. Consistent with this possibility, a large body of evidence demonstrates that 15-PGDH functions as an endogenous COX-2 antagonist in the states of carcinogenesis and inflammation (11, 18, 19, 30, 38). Downregulation of 15-PGDH was observed in various cancer tissues with concomitant induction of COX-2 (11, 18, 19, 30, 38). In gastric cancer specimens, 15-PGDH expression was found to be inversely correlated with the COX-2 level and lymph node metastasis (11, 38). IL-1β, TNF-α, and phorbol ester, which are well known inducers of COX-2 expression, significantly downregulated the expression of 15-PGDH in human lung adenocarcinoma cells (31, 33). Conversely, the anti-inflammatory cytokine IL-10 upregulated the expression of 15-PGDH by antagonizing the 15-PGDH downregulation induced by proinflammatory cytokines IL-1β and TNF-α, in villous and chorionic trophoblasts (25). When the level of 15-PGDH expression was increased by transfection with pcDNA3 or adenovirus harboring 15-PGDH, the level of COX-2 expression induced by IL-1β was decreased in a manner dependent on the level of 15-PGDH expression (31, 33). This was further supported by the finding that suppression of 15-PGDH expression by 15-PGDH small interfering (si) RNA led to a further increase in the IL-1β-induced expression of COX-2 (31, 33). In another experiment, siRNA knockdown of 15-PGDH resulted in a substantial increase in PGE2 production in gastric cancer (MKN-28) cells and enhanced the anchorage-independent growth of these cells (32). The expression of COX-2 protein is also responsible for attenuation of 15-PGDH expression. Interactions between COX-2 and 15-PGDH are of potential importance in kidney function. In the kidney cortex, COX-2-derived prostaglandins from the macula densa and/or vasculature have been implicated in vasodilation and stimulation of renin biosynthesis and release. In low salt-treated animals, COX-2 expression increased in the macula densa and adjacent thick ascending limbs, whereas 15-PGDH expression in these sites was minimal (7, 41). On the contrary, in high salt-treated animals, COX-2 expression in the macula densa/cortical thick ascending limbs decreased, whereas 15-PGDH expression in the macula densa increased (7, 41). Additionally, the renal inner medulla has the highest activity of COX-2 but no expression of 15-PGDH (3, 7, 42). Together, it seems reasonable to speculate that 15-PGDH may negatively regulate the renal COX-2 activity in the postnatal developmental kidney of rats.
In the present study, 15-PGDH was detected in both the cytoplasm and the lumens of proximal tubules as aggregated granules, raising a possibility that 15-PGDH may translocate from the cytoplasm to the lumen for excretion to the urine. In support of this notion, 15-PGDH protein was indeed present in the urine of both SD and WKY rats after 6 wk of age. However, 4-wk-old WKY rats had 15-PGDH protein present in the urine comparable to that of 12-wk-old WKY rats but exhibited no granular staining of 15-PGDH. This result does not favor a direct correlation between the granular appearance in the proximal tubules and urinary excretion of 15-PGDH. Therefore, the functional implication of the granular appearance of 15-PGDH protein in the proximal tubules of adult rats remains unclear and needs to be explored in future studies.
In summary, the present study investigated the regulation of renal 15-PGDH expression during postnatal rat kidney development. At the whole tissue level, 15-PGDH mRNA and protein in the kidney were gradually increased after birth, reaching the peak level at PND14, and then declining sharply to a very low level in adulthood, a pattern almost analogous to that of COX-2. At the cellular level, however, renal 15-PGDH protein during the postnatal period was predominantly found in the proximal tubule whereas COX-2 protein was restricted to the macular densa and adjacent cortical thick ascending limbs. Overall, the anatomic evidence supports the concept that 15-PGDH may function as an endogenous inhibitor of COX-2 during postnatal kidney development.
GRANTS
This work was supported by National Natural Science Foundation of China Grant No. 31330037, National Basic Research Program of China 973 Program 2012CB517600 (No. 2012CB517602), a Veterans Affairs Merit Review, and National Institutes of Health Grant DK094956. T. Yang is an Established Investigator of the American Heart Association and a Research Career Scientist in the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.L., Z.J., Y.S., L.Z., and M.D. performed experiments; Y.L., Z.J., Y.S., L.Z., M.D., and T.Y. analyzed data; Y.L. prepared figures; Y.L. drafted manuscript; R.C., A.Z., and T.Y. edited and revised manuscript; A.Z. and T.Y. interpreted results of experiments; T.Y. provided conception and design of research; T.Y. approved final version of manuscript.
REFERENCES
- 1.Campean V, Theilig F, Paliege A, Breyer M, Bachmann S. Key enzymes for renal prostaglandin synthesis: site-specific expression in rodent kidney (rat, mouse). Am J Physiol Renal Physiol 285: F19–F32, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378: 406–409, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Farman N, Pradelles P, Bonvalet JP. PGE2, PGF2α, 6-keto-PGF1α, and TxB2 synthesis along the rabbit nephron. Am J Physiol Renal Fluid Electrolyte Physiol 252: F53–F59, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Grose JH, Gbeassor FM, Lebel M. Differential effects of diuretics on eicosanoid biosynthesis. Prostaglandins Leukot Med 24: 103–109, 1986 [DOI] [PubMed] [Google Scholar]
- 5.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol 70: 357–377, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol 281: F1–F11, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94: 2504–2510, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imig JD. Eicosanoid regulation of the renal vasculature. Am J Physiol Renal Physiol 279: F965–F981, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Konrad M, Leonhardt A, Hensen P, Seyberth HW, Kockerling A. Prenatal and postnatal management of hyperprostaglandin E syndrome after genetic diagnosis from amniocytes. Pediatrics 103: 678–683, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev 13: 1561–1574, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Wang X, Lu Y, Du R, Luo G, Wang J, Zhai H, Zhang F, Wen Q, Wu K, Fan D. 15-Hydroxyprostaglandin dehydrogenase is a tumor suppressor of human gastric cancer. Cancer Biol Ther 10: 780–787, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Madsen K, Stubbe J, Yang T, Skott O, Bachmann S, Jensen BL. Low endogenous glucocorticoid allows induction of kidney cortical cyclooxygenase-2 during postnatal rat development. Am J Physiol Renal Physiol 286: F26–F37, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Mann B, Hartner A, Jensen BL, Kammerl M, Kramer BK, Kurtz A. Furosemide stimulates macula densa cyclooxygenase-2 expression in rats. Kidney Int 59: 62–68, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Matson JR, Stokes JB, Robillard JE. Effects of inhibition of prostaglandin synthesis on fetal renal function. Kidney Int 20: 621–627, 1981 [DOI] [PubMed] [Google Scholar]
- 15.Melendez E, Reyes JL, Escalante BA, Melendez MA. Development of the receptors to prostaglandin E2 in the rat kidney and neonatal renal functions. Dev Pharmacol Ther 14: 125–134, 1989 [PubMed] [Google Scholar]
- 16.Moel DI, Cohn RA, Penning J. Renal prostaglandin E2 synthesis and degradation in the developing rat. Biol Neonate 48: 292–298, 1985 [DOI] [PubMed] [Google Scholar]
- 17.Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 83: 473–482, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, Lawrence E, Dannenberg AJ, Lovgren AK, Luo G, Pretlow TP, Newman RA, Willis J, Dawson D, Markowitz SD. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci USA 103: 12098–12102, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Na HK, Park JM, Lee HG, Lee HN, Myung SJ, Surh YJ. 15-Hydroxyprostaglandin dehydrogenase as a novel molecular target for cancer chemoprevention and therapy. Biochem Pharmacol 82: 1352–1360, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, functions. Physiol Rev 79: 1193–1226, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Nomura T, Chang HY, Lu R, Hankin J, Murphy RC, Schuster VL. Prostaglandin signaling in the renal collecting duct: release, reuptake, and oxidation in the same cell. J Biol Chem 280: 28424–28429, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Nomura T, Lu R, Pucci ML, Schuster VL. The two-step model of prostaglandin signal termination: in vitro reconstitution with the prostaglandin transporter and prostaglandin 15 dehydrogenase. Mol Pharmacol 65: 973–978, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Ogawa T, Tomomasa T, Hikima A, Kobayashi Y, Nakano K, Fukabori Y, Morikawa A. Developmental changes in cyclooxygenase mRNA expression in the kidney of rats. Pediatr Nephrol 16: 618–622, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Pichaud F, Delage-Mourroux R, Pidoux E, Jullienne A, Rousseau-Merck MF. Chromosomal localization of the type-I 15-PGDH gene to 4q34-q35. Hum Genet 99: 279–281, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Pomini F, Caruso A, Challis JR. Interleukin-10 modifies the effects of interleukin-1beta and tumor necrosis factor-alpha on the activity and expression of prostaglandin H synthase-2 and the NAD+-dependent 15-hydroxyprostaglandin dehydrogenase in cultured term human villous trophoblast and chorion trophoblast cells. J Clin Endocrinol Metab 84: 4645–4651, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Reverte V, Tapia A, Moreno JM, Rodriguez L, Salazar F, Llinas MT, Salazar FJ. Renal effects of prolonged high protein intake and COX2 inhibition on hypertensive rats with altered renal development. Am J Physiol Renal Physiol 301: F327–F333, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Seyberth HW, Koniger SJ, Rascher W, Kuhl PG, Schweer H. Role of prostaglandins in hyperprostaglandin E syndrome and in selected renal tubular disorders. Pediatr Nephrol 1: 491–497, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol 62: 167–215, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Soma M, Manku MS, Jenkins DK, Horrobin DF. Prostaglandins and thromboxane outflow from the perfused mesenteric vascular bed in spontaneously hypertensive rats. Prostaglandins 29: 323–333, 1985 [DOI] [PubMed] [Google Scholar]
- 30.Tai HH. Prostaglandin catabolic enzymes as tumor suppressors. Cancer Metastasis Rev 30: 409–417, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Tai HH, Tong M, Ding Y. 15-Hydroxyprostaglandin dehydrogenase (15-PGDH) and lung cancer. Prostaglandins Other Lipid Mediat 83: 203–208, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiel A, Ganesan A, Mrena J, Junnila S, Nykanen A, Hemmes A, Tai HH, Monni O, Kokkola A, Haglund C, Petrova TV, Ristimaki A. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in gastric cancer. Clin Cancer Res 15: 4572–4580, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Tong M, Ding Y, Tai HH. Reciprocal regulation of cyclooxygenase-2 and 15-hydroxyprostaglandin dehydrogenase expression in A549 human lung adenocarcinoma cells. Carcinogenesis 27: 2170–2179, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Uchida S, Nonoguchi H, Endou H. Localization and properties of NAD+-dependent 15-hydroxyprostaglandin dehydrogenase activity in the rat kidney. Pflügers Arch 404: 278–284, 1985 [DOI] [PubMed] [Google Scholar]
- 35.van der Heijden BJ, Carlus C, Narcy F, Bavoux F, Delezoide AL, Gubler MC. Persistent anuria, neonatal death, and renal microcystic lesions after prenatal exposure to indomethacin. Am J Obstet Gynecol 171: 617–623, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38: 97–120, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Welch TR. The hyperprostaglandin E syndrome: a hypercalciuric variant of Bartter's syndrome. J Bone Miner Res 12: 1753–1754, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, Lawrence E, Lutterbaugh J, Lu S, Willson JK, Luo G, Hensold J, Tai HH, Wilson K, Markowitz SD. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci USA 101: 17468–17473, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang T, Schnermann JB, Briggs JP. Regulation of cyclooxygenase-2 expression in renal medulla by tonicity in vivo and in vitro. Am J Physiol Renal Physiol 277: F1–F9, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol Renal Physiol 274: F481–F489, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Yao B, Xu J, Harris RC, Zhang MZ. Renal localization and regulation of 15-hydroxyprostaglandin dehydrogenase. Am J Physiol Renal Physiol 294: F433–F439, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Zhang MZ, Hao CM, Breyer MD, Harris RC, McKanna JA. Mineralocorticoid regulation of cyclooxygenase-2 expression in rat renal medulla. Am J Physiol Renal Physiol 283: F509–F516, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Zhang MZ, Wang JL, Cheng HF, Harris RC, McKanna JA. Cyclooxygenase-2 in rat nephron development. Am J Physiol Renal Physiol 273: F994–F1002, 1997 [DOI] [PubMed] [Google Scholar]