Abstract
Fibrinogen (Fg) has been implicated in the pathogenesis of several fibrotic disorders by acting as a profibrotic ligand for a variety of cellular surface receptors and by modulating the provisional fibrin matrix formed after injury. We demonstrated increased renal Fg expression after unilateral ureteral obstruction and folic acid (FA) nephropathy in mice, respectively. Urinary Fg excretion was also increased in FA nephropathy. Using in vitro and in vivo approaches, our results suggested that IL-6 mediates STAT3 activation in kidney fibrosis and that phosphorylated (p)STAT3 binds to Fgα, Fgβ, and Fgγ promoters in the kidney to regulate their transcription. Genetically modified Fg heterozygous mice (∼75% of normal plasma Fg levels) exhibited only 3% kidney interstitial fibrosis and tubular atrophy after FA nephropathy compared with 24% for wild-type mice. Fibrinogenolysis through Ancrod administration after FA reduced interstitial fibrosis more than threefold compared with vehicle-treated control mice. Mechanistically, we show that Fg acts synergistically with transforming growth factor (TGF)-β1 to induce fibroblast proliferation and activates TGF-β1/pSMAD2 signaling. This study offers increased understanding of Fg expression and molecular interactions with TGF-β1 in the progression to kidney fibrosis and, importantly, indicates that fibrinogenolytics like Ancrod present a treatment opportunity for a yet intractable disease.
Keywords: kidney fibrosis, chronic kidney disease, fibrinogen, Ancrod, transforming growth factor-β1
chronic kidney disease (CKD) can be triggered by many pathological conditions that injure renal structures (glomerular, tubulointerstitial, or renovascular) and develops silently. Regardless of the initial cause, common CKD renal histological findings are generalized fibrosis and glomerulosclerosis (42). Fibrosis is characterized by aberrantly excessive deposition of extracellular matrix (ECM) materials like collagen and fibronectin by activated myofibroblasts (23). This leads to scar tissue formation, gradually replacing normal parenchymal tissue, and can progress to a loss of function and end-stage renal disease (47, 63).
A prominent coagulation factor, fibrinogen (Fg) is cleaved by thrombin to fibrin, forming the basic framework of the provisional matrix in wound healing and acts as center for inflammatory cell, endothelial cell, and fibroblast infiltration (15, 18). The quantity, timing, and location of provisional matrix deposition can have varied pathological effects. The persistence of fibrin matrices correlates with increased collagen accumulation and skin fibrosis in mice with inactive fibrin-degrading tissue plasminogen (16). In bleomycin-induced pulmonary fibrosis, enhanced fibrin matrix proteolysis reduced fibrosis, although complete abrogation of fibrin in Fg-deficient mice did not confer sufficient protection (32). Alternatively, fibrin deposition in a cholestatic model of liver fibrosis prevented toxic bile acid leakage and injury progression, whereas Fg depletion increased periportal necrosis and elevated liver injury biochemical markers (43).
Apart from fibrin matrix formation, Fg binds, through its heparin-binding domain to several growth factors, including transforming growth factor (TGF)-β family members (45). Fg has been shown to have profibrotic tendencies by transporting latent TGF-β1 into the damaged central nervous system (53). Fg accumulation in skeletal muscle augments TGF-β1 production by resident macrophages, stimulating fibroblasts to deposit ECM, producing fibrosis in Duchenne muscular dystrophy (61). An association with ICAM-1 promotes mucous hypersecretion in lung epithelial cells, as displayed in chronic airway diseases (37).
Fg deposition was observed in a number of kidney fibrosis experimental models (19, 24, 29, 44, 62). In unilateral ureteral obstruction (UUO), Fg knockout (KO) mice had reduced interstitial fibroblast proliferation and collagen deposition, resulting in reduced tubulointerstitial fibrosis (55).
In the present study, we aimed to better characterize the Fg contribution to kidney fibrosis and test if fibrinogenolysis can provide a therapeutic opportunity. Our results confirmed increased Fg expression by fibrotic kidneys in two distinct mouse models, UUO and folic acid (FA) nephropathy, as well as increased urinary Fg excretion in the initial stages of FA nephropathy. Using in vivo and in vitro approaches, we established that the IL-6/STAT3 signaling pathway could be an important upstream regulator of Fg transcription and translation in kidneys. Genetic and pharmacological Fg depletion in mice reduced kidney fibrosis after FA nephropathy, indicating a causal role of Fg in fibrosis development. Mechanistically, we showed that Fg could participate in the development of fibrosis by triggering TGF-β1 expression, the central mediator in this pathological process, and also by contributing to fibroblast proliferation.
MATERIALS AND METHODS
Animals.
Male C57BL/6 mice were used for the UUO procedure, and male BALB/c mice (body weight: 25–29 g) were used for experiments involving FA injection. Breeding pairs of mice with a mutated Fgα chain were kindly provided by Dr. Jay L. Degen (Children's Hospital Research Foundation, Cincinnati, OH). Wild-type (WT; Fg+/+), heterozygous (Fg+/−), and KO (Fg−/−) mice were generated through breeding in our animal facility, and age- and weight-matched male littermates were used in our experiments. Additional WT C57BL/6 and BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA). Animals were maintained in the central animal facility in transparent plastic cages containing wood chips free of any known chemical contaminants under conditions of 21 ± 1°C and 50–80% relative humidity at all times in an alternating 12:12-h light-dark cycle. Commercial rodent chow and water were available to animals ad libitum. All animals were acclimated to this environment for at least 1 wk before use in experiments. Animals were housed at 4–5 mice/cage, and each mouse was considered separately for data analysis. All animal maintenance and treatment protocols were in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Harvard Medical School Animal Care and Use Committees (Institutional Animal Care and Use Committees).
Animal procedures.
UUO in mice was performed under general anesthesia (50 mg/kg ip pentobarbital sodium) by ligation of the left ureter with two separate silk ties, and mice were euthanized after 3, 7, and 14 days. Control mice underwent sham surgery and were euthanized on day 7. Immediately after surgery, mice were injected with 1 ml normal saline subcutaneously (37°C) to replace the fluid lost and help the recovery of body temperature. Pain medication was provided for the first 2 days after surgery [buprenorphine (0.05 mg/kg sc) every 12 h; the first dose was given with the saline injection immediately after surgery and the other three doses were given in 50 μl normal saline]. Nephropathy was also induced chemically with a single intraperitoneal injection of 250 mg/kg FA in 0.3 M sodium bicarbonate. Normal mice, with no surgical or pharmacological interventions, were also included in the experiments. Ancrod (National Institute for Biological Standards and Control, Hertfordshire, UK) was delivered using 14-day miniosmotic pumps (Durect, Cupertino, CA). Pumps were loaded with a solution of Ancrod dissolved in normal saline such that they delivered 3 IU/day. Pumps were implanted under general anesthesia through a surgical incision subcutaneously on the back of the animals. After the incision was closed with sutures, a continuous infusion was allowed for 3 days before the FA injection was administered. Control mice received only normal saline vehicle. Mice received two doses of buprenorphine after surgery, with the first delivered immediately in 1 ml normal saline subcutaneously (37°C) and the second delivered 12 h afterward in 50 μl normal saline. The loading of the pumps was enough to provide continuous treatment for the first 11 days after FA injection, and animals were euthanized on day 14. Euthanasia was performed under isoflurane anesthesia. Blood and kidney samples were collected, and the thoracic cavity was opened to make sure that the animal was deceased.
Histopathology and histology scoring.
Kidneys were fixed in 10% neutral buffered formalin and embedded into paraffin. Periodic acid-Schiff or Masson's trichrome stainings were performed on 6-μm sections, and slides were evaluated under a light microscope by a renal pathologist (V. Bijol) in a blinded manner. Acute tubular injury was scored as nonexistent (grade 0), minimal (grade 0.5), mild (grade 1), moderate (grade 2), or severe (grade 3). Chronic changes were estimated as percentages of tubulointerstitial scarring, whereas global and segmental glomerulosclerosis were expressed as percentages of the total number of glomeruli per section.
RNA extraction and quantitative real-time RT-PCR.
Total RNA was isolated from flash-frozen tissue using TRIzol (Invitrogen, Grand Island, NY) according to the manufacturer's protocol. RNA concentration was measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). RNA (1 μg) was reverse transcribed into cDNA using a QuantiTect Reverse Transcription Kit from Qiagen (Valencia, CA). Quantitative RT-PCR was performed using a QuantiFast SYBR Green PCR Kit (Qiagen) on a CFX96 RT-PCR instrument (Bio-Rad Laboratories, Hercules, CA) with the following temperature profile: 3 min of enzyme activation at 95°C followed by 40 cycles of 95°C for 10 s and 55°C for 30 s. The primer pairs used are shown in Table 1. Gapdh was used as a reference gene for normalization.
Table 1.
List of primers used for quantitative RT-PCR and the chromatin immunoprecipitation assay
| Gene/Promoter | Sequence |
|---|---|
| Gene-specific primer pairs for quantitative RT-PCR | |
| Fgα | |
| Forward | 5′-TGTGGAGAGACATCAGAGTCAATG-3′ |
| Reverse | 5′-CGTCAATCAACCCTTTCATCC-3′ |
| Fgβ | |
| Forward | 5′-CTATGGCTGCTGCTGCTATTG-3′ |
| Reverse | 5′-GGCTCTTCCTTTCTCCTGTCAAC-3′ |
| Fgγ | |
| Forward | 5′-TGTGGCTACCAGAGATAACTGTTG-3′ |
| Reverse | 5′-ATGTCTTCCAGCGTTCGGAG-3′ |
| Kim-1 | |
| Forward | 5′-GGAAGTAAAGGGGGTAGTGGG-3′ |
| Reverse | 5′-AAGCAGAAGATGGGCATTGC-3′ |
| α-SMA | |
| Forward | 5′-GTCCCAGACATCAGGGAGTAA-3′ |
| Reverse | 5′-TCGGATACTTCAGCGTCAGGA-3′ |
| Fibronectin | |
| Forward | 5′-ATGTGGACCCCTCCTGATAGT-3′ |
| Reverse | 5′-GCCCAGTGATTTCAGCAAAGG-3′ |
| Collagen type I-α1 | |
| Forward | 5′-TGACTGGAAGAGCGGAGAGT-3′ |
| Reverse | 5′-GTTCGGGCTGATGTACCAGT-3′ |
| Promoter-specific primers for the chromatin immunoprecipitation assay | |
| Fgα | |
| Forward | 5′-TCTGGCTGGAGGAAAACAACC-3′ |
| Reverse | 5′-ACATTGGACTGTGTGTGGGATTC-3′ |
| Fgβ | |
| Forward | 5′-CACTGGAACAGAAAGCAGCACTGAA-3′ |
| Reverse | 5′-CGCAGTAGAGGCGTGCGGTT-3′ |
| Fgγ | |
| Forward | 5′-GTGGCAGATGGGAGGGAACCTGTC-3′ |
| Reverse | 5′-GACAGAGGCCGCGTGTTGCAA-3′ |
Fg, fibrinogen.
Immunoblot analysis and immunoprecipitation.
Immunblot analysis was performed as previously described (3). Protein estimation was performed by the Bradford method, and an equal amount of protein was run on a 12% or 15% polyacrylamide gel (PAGE). For nondenaturing gels, samples were loaded without heating and sample buffer without DTT or β-mercaptoethanol was used. The sources of primary antibodies were as follows: rabbit polyclonal Fg (Dako, Carpinteria, CA); goat polyclonal Fg (Nordic Immunological Labs, Eindhoven, The Netherlands); mouse monoclonal STAT3, rabbit monoclonal phosphorylated (p)STAT3, and polyclonal pERK1/2, ERK1/2, TGF-β1, and pSMAD2 (Cell Signaling Technology, Danvers, MA); mouse monoclonal α-tubulin, GAPDH, and α-smooth muscle actin (α-SMA; Sigma, St. Louis, MO); monoclonal rat IL-6, kidney injury molecule (Kim)-1, and mouse albumin (R&D Systems, Minneapolis, MN); rabbit polyclonal collagen type I-α1 (collagen 1A1; Novus Biologicals, Littleton, CO); and rabbit polyclonal fibronectin (Abcam, Cambridge, MA). Anti-GAPDH or anti-α-tubulin antibody was used as a loading control.
Immunoprecipation was performed as previously described (3, 4). Protein (500 μg) was used for IP, and 25 μg was kept as input, the volume was adjusted to 1 ml for immunoprecipation samples, and 5 μg rabbit polyclonal Fg or normal rabbit IgG antibody was added and incubated overnight at 4°C. Western blot analysis was performed with anti-Fg goat polyclonal and anti-TGF-β1 antibodies. α-Tubulin and rabbit IgG chain served as loading controls for input and IP, respectively.
Chromatin immunoprecipation assay.
STAT3 chromatin immunoprecipation in snap-frozen kidney tissue was performed as previously described (3). The Fg promoter-specific primers used for RT-PCR are shown in Table 1.
Cell lines and cell culture.
The rat kidney fibroblast cell line NRK-49F and liver carcinoma cell line Hep G2 were obtained from American Type Culture Collection (Manassas, VA) and maintained in DMEM (Cellgro, Manassas, VA) supplemented with 10% FBS (Invitrogen) at 37°C in a 5% CO2 incubator. Cells were treated with 100 ng/ml IL-6 (R&D Systems) and 200 nM dexamethasone (Sigma) and/or 10 μM Stattic (Calbiochem, Philadelphia, PA) and incubated for 24 h. Other cells were transfected with pRC/CMV (EV) or pRC/CMVSTAT3 (kind gift from Dr. David A. Frank, Department of Medical Oncology, Dana Farber Cancer Institute, Boston, MA) as previously described (3). Six hours posttransfection, cells were washed and further incubated for 18 h. Cells were then harvested and processed for RNA and protein isolation.
MTT assay.
A total of 7,500 NRK-49F cells were plated in a 96-well plate for 24 h in DMEM supplemented with 10% FBS. Cells were then serum starved for 24 h and pretreated for 1 h with 0.004 IU/ml Ancrod, 1 μM SB-431542, or a combination of both and then treated with 10 ng/ml TGF-β1 (Peprotech, Rocky Hill, NJ) and/or 1 mg/ml Fg (Sigma) in DMEM without phenol red. After 48 h of incubation, 1 mg/ml MTT (USB, Cleveland, OH) was added and incubated for another 2 h. The medium was aspirated, 100 μl isopropanol was added, and absorbance was measured at 595 nm taking 630 nm as a reference using SpectraMax Paradigm (Sunnyvale, CA). Absorbance obtained from untreated cells was taken as 100%. Experiments were performed twice with six replicates each time.
Immunofluorescence.
Cells were treated as described above in MTT assay and fixed with 3.7% paraformaldehyde. For F-actin staining, cells were incubated in 10 μM phalloidin (Life Technologies, Grand Island, NY) for 30 min at room temperature. For collagen staining, cells were blocked and incubated with collagen 1A1 antibody and FITC-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Cells were mounted in 4′,6-diamidino-2-phenylindole-containing mounting medium (Vector Laboratories, Burlingame, CA). Images were captured on a Carl Zeiss AxioImager.M2 using AxioVision SE64 software with a ×20 objective. Fluorescence intensity was quantified using ImageJ software.
Kidneys were fixed for 2 h in 4% paraformaldehyde followed by an overnight incubation in 30% sucrose at 4°C and embedded into OCT compound. Sections (6 μm) were blocked for 1 h in 5% donkey serum in PBS and incubated with a mixture of anti-Fg and Cy3-labeled anti-α-SMA (Sigma-Aldrich) in 5% donkey serum and PBS overnight at 4°C. Donkey anti-rabbit FITC-labeled secondary antibody (Jackson ImmunoResearch Laboratories) was used to detect the anti-Fg primary antibody. 4′,6-Diamidino-2-phenylindole-containing mounting medium (Sigma-Aldrich) was used for nuclear staining. Images were captured by a Nikon DS-QiMc camera attached to a Nikon Eclipse 90i fluorescence microscope using a ×60 oil-immersion objective (1.4 numerical aperture) and Nikon NIS Elements AR (version 3.2) software.
Luciferase assay.
NRK49-F cells were serum starved for 24 h and cotransfected with pGL3-Basic-FGG (Fgγ) and pRL-GAPDH plasmid constructs as previously described (3). Six hours posttransfection, cells were washed, treated with 100 ng/ml IL-6 and 200 nM dexamethasone, and further incubated for 24 h. For Stat3 transfection, cells were either transfected with pRC/CMV or pRC/CMVSTAT3. Six hours posttransfection, cells were washed and further incubated for 18 h. Cells were cotransfected with pGL3-Basic-FGG and pRL-Gapdh plasmid constructs and harvested after 24 h of incubation. For knockdown experiments, HepG2 cells were either transfected with control small interfering (si)RNA or Stat3 siRNA using Dharmafect 2, incubated for 6 h, washed, and further incubated for 48 h. Cells were then cotransfected with pGL3-Basic-FGG and pRL-Gapdh plasmids and harvested after 24 h of incubation. Luminescence was measured using the Dual-Glo Luciferase Assay System (Promega, Madison, WI) on a Veritas luminometer (Turner Biosystems, Sunnyvale, CA). Fgγ luciferase activity was normalized to the respective Gapdh luciferase activity.
Plasma biochemistry analysis.
For FA nephropathy progression experiments (Fig. 2), blood levels of urea nitrogen (BUN) and creatinine were measured on a VetScan VS2 machine using Critical Care Plus rotors (Abaxis, Union City, CA). For experiments testing genetic and pharmacological fibrinogenolysis effects on FA nephropathy (Figs. 5 and 6), BUN was measured using an InfinityUrea kit (Thermo Fisher Scientific) and serum creatinine was measured using Creatinine Analyzer II (Beckman Coulter).
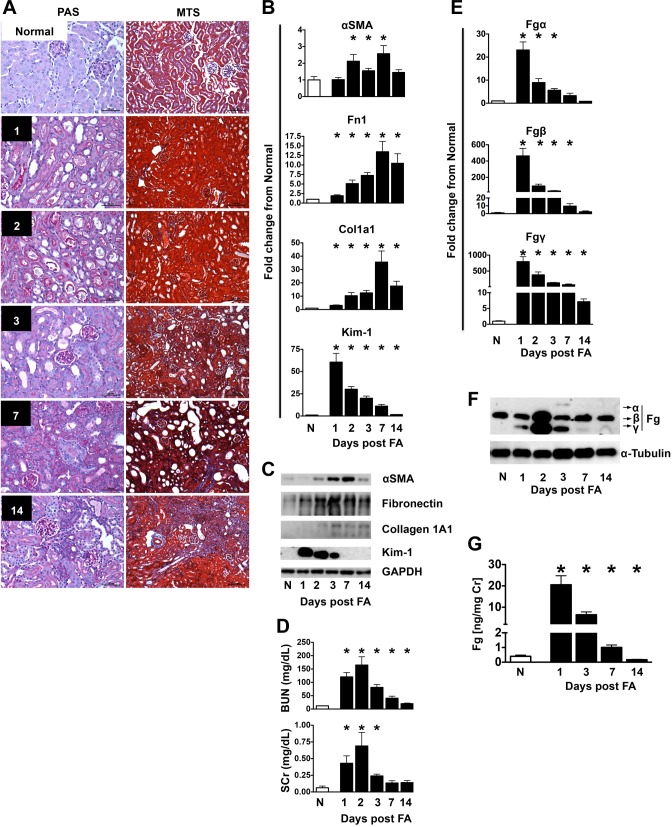
Fig. 2.
Evaluation of kidney fibrosis after folic acid (FA) injection. A: representative photomicrographs of Periodic acid-Schiff (PAS)- and MTS-stained slides indicating the progression from mild tubular injury on days 1–3 after FA injection to mild interstitial fibrosis on day 14. In this model of injury, tubulointerstitial damage is most prominent in the superficial renal cortex. Acute tubular injury is characterized by a low epithelial lining, distension of the tubular lumens, degenerative changes of epithelial cells (including vacuolization and single cell necrosis), and the presence of necrotic debris in tubular lumens. The initial injury at 1 day post-FA injection is manifested by mild tubular distension and a low epithelial lining in some tubules; some tubules reveal mild vacuolization of their cytoplasm. At 2 days, the injury is more obvious, and some tubules also reveal cell death and accumulation of cellular debris in tubular lumens. At 3 days, there is more prominent acute epithelial cell injury, with single cell necrosis, focal sloughing of the epithelium, and necrotic debris filling the lumens of some tubules. Regenerative changes can be seen in some of the tubules, including nuclear enlargement and increased mitotic activity. The cytoplasm is scant in some tubules, and they appear simplified; many proximal tubules do not contain brush borders. At 7 days, this simplification becomes more obvious; many tubules are still dilated and contain cellular debris but are lined with a low epithelial lining. Other tubules are small and simplified, with small cells and scant cytoplasm; the interstitium shows early collagen deposition in a few places. At 14 days, residual mild acute tubular injury is seen in some places, but mild parenchymal scarring is noted in several instances, with increased interstitial fibrosis, associated with tubular atrophy, mild nonspecific inflammation, and focal global and segmental glomerulosclerosis (affecting 2.5% of the glomeruli on average). Scale bars = 50 μm for PAS-stained images and 100 μm for MTS-stained images. B and C: determination of mRNA (B) and protein (C) expression of αSMA, Fn1, Col1A1, and Kim-1 in kidneys from FA-injected mice. Quantitative PCR data were normalized to GAPDH. Data are presented as means ± SE of fold changes from normal (N) mice; n = 8–10 mice/group. *P < 0.05 compared with normal mice. D: blood urea nitrogen (BUN) and serum creatinine (SCr) levels were increased early after FA injection but returned toward normal as animals progressed to kidney fibrosis. Data are presented as means ± SE of the group; n = 8–10 mice/group. *P < 0.05 compared with normal mice. E and F: determination of kidney mRNA (E) and protein (F) expression levels of Fg α-, β-, and γ-chains after FA injection in mice. Data were normalized to GAPDH. Data are presented as means ± SE of fold changes from normal mice; n = 8–10 mice/group. *P < 0.05 compared with normal mice. G: urinary excretion of Fg was significantly increased in FA-induced nephropathy in mice. n = 5 mice/group. *P < 0.05 compared with normal mice.
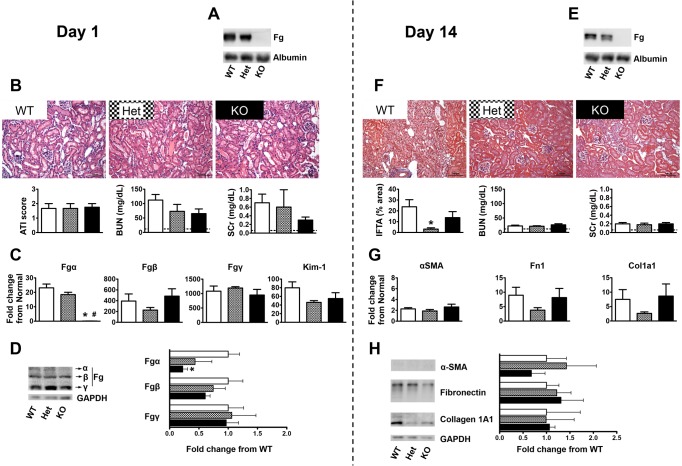
Fig. 5.
Genetic Fg depletion protects mice from FA-induced kidney fibrosis. The vertical dashed line separates results on day 1 after FA injection (acute kidney injury phase) from those on day 14 (kidney fibrosis phase). A: nondenaturing gel Western blot analysis showing reduced plasma Fg levels in mice with genetic disruption of the Fgα gene [wild-type (WT) have no alleles affected, heterozygous (Het) mice have one allele affected, and knockout (KO) mice have both alleles affected] on day 1 after FA injection. Plasma albumin was used as a loading control. B: investigation of acute injury pathology 1 day after FA injection revealed no differences between Fg WT, Het, and KO groups, as indicated by representative pictures of hematoxylin and eosin (H&E)-stained slides and quantification of the acute tubular injury (ATI) score. Biochemical analysis (BUN and SCr) confirmed the similarity of kidney injury for the three groups. Data are presented as means ± SE; n = 3–4 mice/group. The dashed line represents the mean of values in normal mice. C: kidney mRNA expression of Fgα confirmed the genetic depletion. No significant differences on day 1 were recorded for the three genetic groups in Fgβ, Fgγ, or Kim-1. Quantitative PCR data were normalized to GAPDH. Data are presented as means ± SE of fold changes from normal mice; n = 3–4 mice/group. *P < 0.05 compared with WT mice. D: representative immunoblots and quantification of kidney protein expression of Fg α-, β-, and γ-chains on day 1 after FA injection indicating no significant changes other than a reduction in the α-chain in KO mice. Data were normalized to GAPDH and are shown as means ± SE of fold changes from WT mice; n = 3–4 mice/group. *P < 0.05 compared with WT mice. E: mice with genetic disruption of the Fgα gene had reduced plasma Fg levels on day 14 after FA injection as shown by nondenaturing gel Western blot analysis. Plasma albumin was used as a loading control. F: representative pictures of MTS-stained slides and estimation of the tissue area presenting interstitial fibrosis and tubular atrophy (IFTA) indicating significant protection in Fg Het mice 14 days after FA injection. Data are presented as means ± SE; n = 4 mice/group. *P < 0.05 compared with WT mice. Plasma biochemical parameters (BUN and SCr) had reduced levels at this time point regardless of genotype. n = 5–6 mice/group. The dashed line represents the mean of values in normal mice. G: kidney mRNA expression of fibrosis markers α-SMA, Fn1, Col1A1 showing no significant differences on day 14 for the three Fg genetic groups. Quantitative PCR data were normalized to GAPDH. Data are presented as means ± SE of fold changes from normal mice; n = 5–6 mice/group. H: representative immunoblots and quantification of the protein expression of fibrosis markers in the kidney indicating no significant changes between Fg genotypes on day 14 after FA injection. Data were normalized to GAPDH and are shown as means ± SE of fold changes from WT mice; n = 4 mice/group.
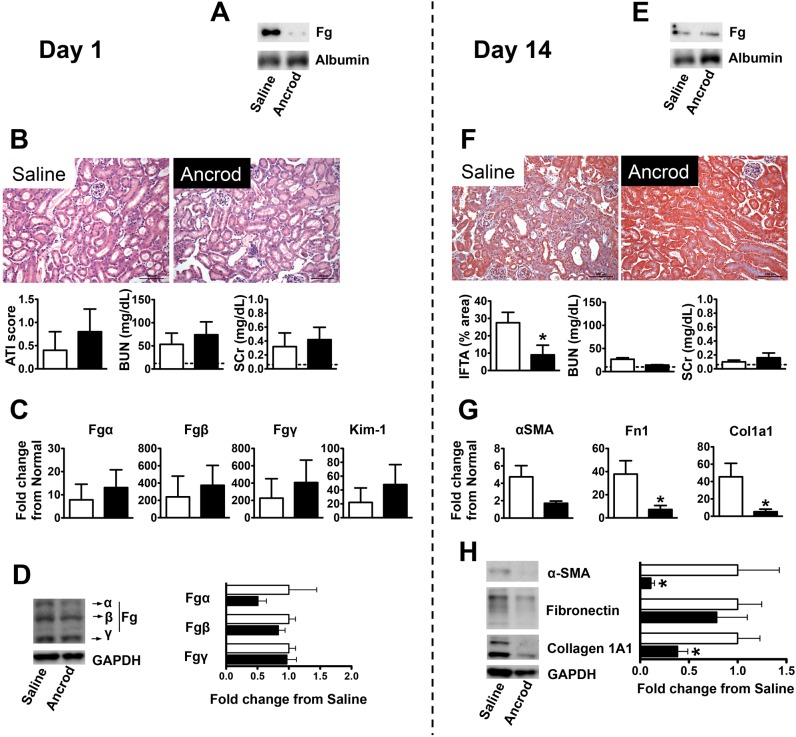
Fig. 6.
Pharmacological Fg depletion protects mice from FA-induced kidney fibrosis. The vertical dashed line separates results on day 1 after FA injection (acute kidney injury phase) from those on day 14 (kidney fibrosis phase). A: nondenaturing gel Western blot analysis showing reduced plasma Fg levels on day 1 after FA injection in mice that received a continuous peritoneal infusion of fibrinogenolytic Ancrod delivered by Alzet pumps (at a 3 U/day rate started 3 days before FA injection) compared with those that received saline vehicle. Plasma albumin was used as a loading control. B: histological (representative images of H&E-stained slides and ATI score) and biochemical (BUN and SCr) evaluation of acute injury revealing that kidneys were similarly affected 1 day after FA injection in mice that received fibrinogenolytic treatment (Ancrod) or vehicle (saline). Data are presented as means ± SE; n = 5 mice/group. The dashed line represents the mean of values in normal mice. C and D: kidney mRNA (C) and protein (D) expression (selected immunoblots and quantification) of Fgα, Fgβ, Fgγ, or Kim-1 were not significantly affected by the treatment. Quantitative PCR data were normalized to GAPDH. Data are presented as means ± SE of fold changes from normal mice. Western blot data were normalized to GAPDH and are shown as means ± SE of fold change from saline treatment; n = 5 mice/group. E: by day 14 after FA injection, plasma Fg levels were low and similar for mice that received a continuous peritoneal infusion of Ancrod or saline vehicle, as shown by nondenaturing gel Western blot analysis. The Alzet pumps ensured delivery of the drug up to day 11 after FA injection. Plasma albumin was used as a loading control. F: Ancrod treatment conferred significant protection from FA-induced kidney fibrosis after 14 days, as indicated by MTS (representative images shown and overall estimation of tissue area presenting interstitial fibrosis and tubular atrophy graphed). Plasma biochemical parameters (BUN and SCr) had reduced levels at this time point regardless of treatment. Data are presented as means ± SE; n = 5–6 mice/group. *P < 0.05 compared with saline treatment. The dashed line represents the mean of values in normal mice. G and H: Ancrod treatment resulted in reductions in kidney mRNA (G) and protein expression (H) of the fibrosis markers α-SMA, Fn1, and Col1A1. Quantitative PCR data were normalized to GAPDH. Data are presented as means ± SE of fold changes from normal mice. Western blot data were normalized to GAPDH and are shown as means ± SE of fold changes from saline treatment; n = 5–6 mice/group. *P < 0.05 compared with saline treatment.
Urine analysis.
Levels of urinary Fg were measured using species-specific Luminex assay kits from Millipore (Billerica, MA). These assays do not distinguish between intact Fg molecules, Fg chains, fibrin, or fibrin degradation products. Urinary creatinine levels, measured using the Creatinine Assay Kit (Cayman, Ann Arbor, MI) according to the manufacturers' protocols, were used for normalization.
Statistical analyses.
Results are reported as means ± SE for each group. Statistical analyses (Student's t-test for comparison of two groups or one-way ANOVA for comparison of three or more groups) were performed using GraphPad Prism5 (GraphPad Software, La Jolla, CA). Results were considered statistically significant if P < 0.05.
RESULTS
Fg expression markedly increases in kidney fibrosis mouse models.
After murine UUO, a well-established kidney fibrosis model (10), interstitial collagen deposition progressively increased on days 3, 7, and 14 (blue Masson's trichrome staining of kidney sections; Fig. 1A). Histologically, >75% of the section area showed fibrosis in UUO kidneys on day 14 with contralateral kidneys unaffected. Kidney mRNA and protein expression of established fibrosis markers (α-SMA, fibronectin, and collagen 1A1) were greatly increased in UUO compared with sham kidneys (Fig. 1, B and C). Contralateral kidneys showed much smaller increases only for fibronectin. As previously reported (1), gene and protein expression of Kim-1, a marker commonly associated with acute kidney injury (AKI), were also increased after UUO. A strong induction of Fg mRNA (Fig. 1D) and protein expression (Fig. 1E) of all three chains could be detected in UUO kidneys. Fgβ and Fgγ mRNA levels increased up to ∼100-fold over sham in UUO kidneys with a modest increase in contralateral kidneys (5-fold) as early as day 3 (Fig. 1D). To investigate if in fibrotic kidneys Fg is expressed in myofibroblasts, the interstitial cells most commonly associated with fibrosis development (23), we performed coimmunostaining of Fg with the myofibroblast marker α-SMA (Fig. 1F). There was increased Fg staining in UUO kidneys compared with contralateral kidneys 7 days after surgery. In UUO kidneys, the interstitial areas that showed more intense α-SMA staining, indicative of the presence of myofibroblasts, also stained for Fg, indicating a close spatial relation of the two and a possible mechanistic connection between Fg and fibroblasts that we investigated further in vitro. The interstitial presence of Fg in the kidney confirms previous results from our group (34, 38) and other groups (55).
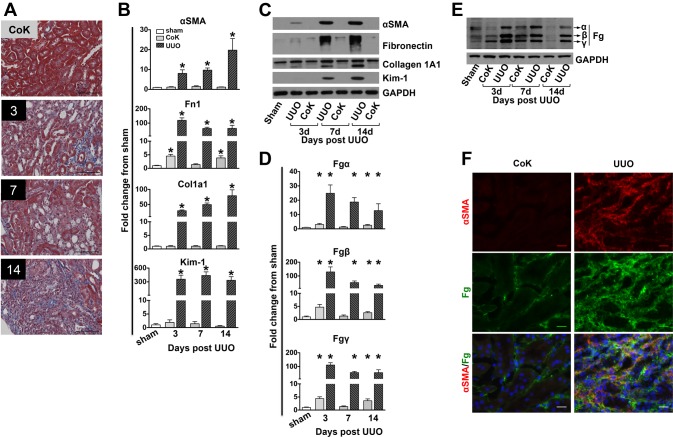
Fig. 1.
Kidney fibrosis evaluation after unilateral ureteral obstruction (UUO). A: representative images of formalin-fixed, Masson's trichrome stained (MTS) kidney histological sections of a 14-day contralateral kidney (CoK) versus UUO kidneys after 3, 7, and 14 days. Scale bar = 50 μm. B and C: determination of mRNA (B) and protein (C) expression of α-smooth muscle actin (α-SMA), fibronectin (Fn1), collagen type I-α1 (Col1A1), and kidney injury molecule (Kim)-1 in UUO and contralateral kidneys. Quantitative PCR data were normalized to GAPDH. Data are presented as means ± SE of fold changes from sham kidneys; n = 4–8 mice/group. *P < 0.05 compared with sham kidneys. D and E: determination of kidney mRNA (D) and protein (E) levels of fibrinogen (Fg) α-, β-, and γ-chains after UUO in mice. Data were normalized to GAPDH. Data are presented as means ± SE of fold changes from sham kidneys; n = 4–8 mice/group. *P < 0.05 compared with sham kidneys. F: Fg and α-SMA coimmunostaining indicating that myofibroblasts and Fg colocalize in the interstitial regions of fibrotic kidneys 7 days after UUO.
We have previously reported that urinary Fg excretion is increased in AKI animal models (34, 38). Since urine produced by the fibrotic kidney cannot be collected after UUO, urinary Fg excretion could not be tested. For this, we used a second kidney fibrosis mouse model induced by FA, which injures both kidneys but still allows them to produce urine (20). After a single FA intraperitoneal injection (250 mg/kg), mouse kidney damage progresses from AKI to fibrosis in 2 wk (64). We performed a detailed progression time course with samples collected from normal mice and samples collected on days 1, 2, 3, 7, and 14 after FA injection. Histological analysis (Fig. 2A) confirmed AKI on days 1–3 and tubulointerstitial fibrosis on day 14 (affecting 30% of the section area on average on day 14). These two stages were also reflected in levels of established markers. mRNA and protein expression of α-SMA, fibronectin, and collagen 1A1 increased toward days 7 and 14 as kidneys become fibrotic (Fig. 2, B and C). Contrary to the UUO model, Kim-1 expression was high in the AKI stage after FA but continuously decreased toward normal by day 14, when kidneys were fibrotic. Plasma levels of classic kidney function markers (BUN and creatinine) were also increased acutely after FA administration (Fig. 2D) but decreased toward normal on days 7 and 14, indicating sufficient remaining functional capacity. Kidney Fg gene expression was significantly elevated over normal, particularly in the AKI phase of the FA model (Fig. 2E). As for UUO, Fgβ and Fgγ chains registered the biggest increase, with peaks close to 500- and 800-fold on day 1, respectively. The increased protein matched the mRNA, with peak levels for all chains reached in the first 3 days and only Fgγ residual increased day 7 expression (Fig. 2F). Urinary Fg excretion in FA nephropathy increased 20-fold on day 1 but returned toward normal by day 14 (Fig. 2G).
STAT3 binds to Fg promoters in vitro and in vivo to regulate transcription.
The Fg genes are clustered within a 65-kb region on human chromosome 4 (4q23-4q32) (27, 36). Fgα and Fgγ chains reside on opposite DNA strands from Fgβ and are transcribed in the reverse direction. All three chains promoters contain known IL-6-responsive elements (CAAT-enhancing binding element and CTGGGAA motifs) (9). Therefore, we hypothesized that, as for liver Fg expression, IL-6 mediates STAT3 activation followed by translocation to the nucleus and binding to Fg promoters, leading to transcription (Fig. 3A). IL-6 protein expression was upregulated in 7- and 14-day UUO kidneys compared with contralateral kidneys (Fig. 3B). Increased STAT3 phosphorylation was also recorded for UUO kidneys. After FA, IL-6 and pSTAT3 were also increased up to day 7 postinjection (Fig. 3C). For both models, increased expression of these proteins was generally detected at time points when increased kidney Fg expression was also seen, as shown in Figs. 1 and 2.
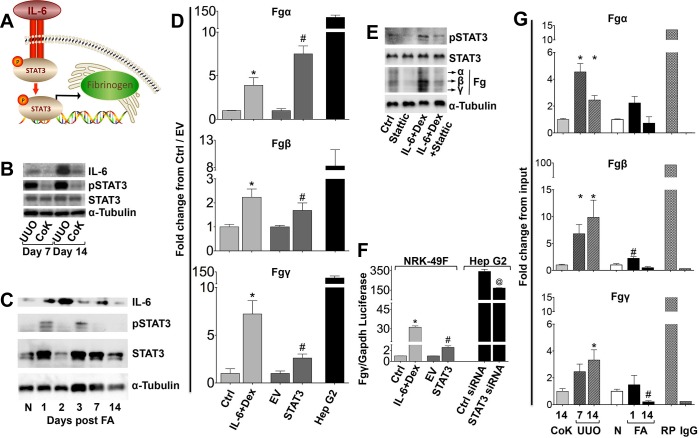
Fig. 3.
Renal Fg expression is regulated by IL-6/STAT3 signaling in mouse kidney fibrosis models, and the results were confirmed in vitro in fibroblasts. A: schematic of Fg synthesis by activation of STAT3 via IL-6. B: Western blots for IL-6, phosphorylated (p)STAT3, STAT3, and α-tubulin in mouse kidneys at 7 and 14 days after UUO. C: Western blots for IL-6, pSTAT3, STAT3, and α-tubulin in mouse kidneys progressing from normal to fibrosis on day 14 after FA injection. D: quantitative RT-PCR analysis for Fgα, Fgβ, and Fgγ after treatment with IL-6 and dexamethasone (Dex) or STAT3 transfection in NRK-49F cells. HepG2 cells served as positive controls. Data represent 3 independent experiments and are presented as means ± SE of fold changes from HepG2 cells transfected with control small interfering (si)RNA (Ctrl cells) or cells transfected with pRC/CMV (EV cells), respectively; HepG2 cells were normalized to Ctrl cells. *P < 0.05 compared with Ctrl cells; #P < 0.05 compared with EV cells. E: Western blot analyses for pSTAT3, STAT3, Fg, and α-tubulin in NRK-49F cells after IL-6 and Dex treatment and/or Stattic inhibitor. F: Fgγ luciferase assay in NRK-49F cells after IL-6 and Dex treatment or STAT3 transfection as well as HepG2 cells transfected with STAT3 siRNA. Data represent 3 independent experiments and are presented as means ± SE of fold change from Ctrl or EV cells, respectively; HepG2 cells were normalized to Ctrl cells. *P < 0.05 compared with Ctrl cells; #P < 0.05 compared with EV cells; @P < 0.05 compared with Ctrl siRNA. G: chromatin immunoprecipitation assay for STAT3 kidney tissue binding on Fgα, Fgβ, and Fgγ promoters. Data are presented as means ± SE of fold changes from contralateral kidneys (for UUO kidney samples collected at 7 and 14 days) or normal kidneys (for FA samples collected at 1 and 14 days); n = 3 mice/group. *P < 0.05 compared with contralateral kidneys; #P < 0.05 compared with normal kidneys. RNA polymerase (RP) and mouse IgG were used as positive and negative control antibodies, respectively, in only one 14-day UUO sample, and results were normalized to contralateral kidneys.
To more directly test if the IL-6/STAT3 pathway controls kidney Fg expression, we performed in vitro experiments using rat kidney fibroblasts (NRK-49F cells). In these cells, IL-6 and dexamethasone treatment [primary mediators of Fg expression in the liver and other tissues (50)] or STAT3 transfection resulted in significantly increased Fgα, Fgβ, and Fgγ mRNA (Fig. 3D) and protein expression (Fig. 3E). A hepatocellular carcinoma cell line (HepG2 cells) served as a positive control. Furthermore, specific STAT3 inhibition by Stattic resulted in decreased Fg expression (Fig. 3E). Increased mRNA expression by IL-6 + dexamethasone treatment or STAT3 transfection in NRK-49F cells was further confirmed by the Fgγ luciferase assay (Fig. 3F). Additionally, constitutive HepG2 Fgγ expression was inhibited by siRNA-mediated STAT3 knockdown.
For in vivo testing of this mechanism, we performed kidney lysate chromatin immunoprecipation analysis, and significantly higher STAT3 binding to Fg promoters was detected both after UUO and FA injection (Fig. 3G, with RNA polymerase and IgG as positive and negative controls, respectively). Binding coincided temporally with increased mRNA expression, as previously reported for both models (Figs. 1 and 2). Taken together, the results shown in Fig. 3 indicate the involvement of IL-6-driven STAT3 activation in kidney Fg chain expression after injury.
Fg induces fibroblast proliferation synergistically with TGF-β1 but not activation.
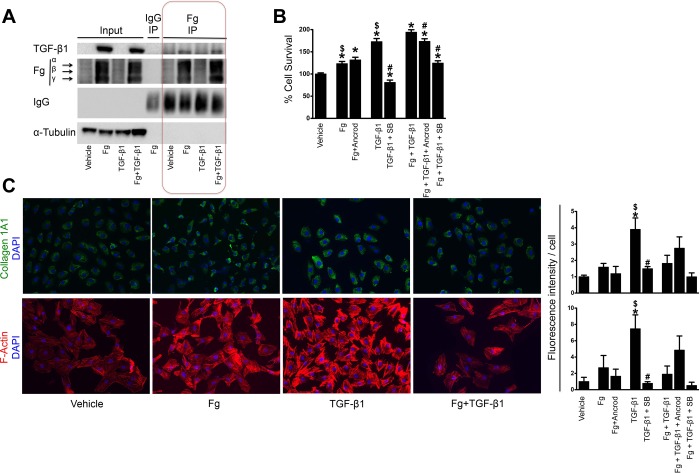
Fg physically interacts with TGF-β1 (45), and this could be important for latent TGF-β1 activation (53). In our in vitro system, Fg immunoprecipation showed binding to TGF-β1 in cells treated with Fg alone but even more in those with combined Fg and TGF-β1 (Fig. 4A). Actually, as shown in input columns, Fg is a potent inducer of TGF-β1 expression in fibroblasts. Fibroblasts showed increased proliferation when treated with Fg alone, and, while TGF-β1 had a stronger effect, they acted synergistically in combination, with an even higher increase (Fig. 4B). While Ancrod-induced fibrinogenolysis did not affect Fg-only treatment, specific TGF-β1 inhibition was very effective in reducing proliferation. In combined treatment, both inhibitors reduced proliferation, with a more pronounced effect for specific TGF-β1 inhibition again. By immunofluorescence, markers of fibroblast activation (collagen 1A1 and F-actin) showed significantly increased expression only for TGF-β1 (Fig. 4C), suggesting that the main effect of Fg is on fibroblast proliferation as opposed to activation.
Fig. 4.
Fg treatment leads to transforming growth factor (TGF)-β1 expression and proliferation of cultured fibroblasts. A: immunoprecipation with anti-Fg antibody in NRK-49F cells after treatment with Fg, TGF- β1, or the combination of both. B: cell survival as evaluated by MTT assay in NRK-49F cells after treatment with Fg, TGF-β1, or the combination of both. The effects of additional Fg inhibition (Ancrod) and TGF-β1 inhibition [SB-431542 (SB)] on these treatments were also evaluated. n = 12 replicates/group (6 replicates for each condition from 2 experiments). *P < 0.05 compared with vehicle; #P < 0.05 compared with treatment without inhibitor; $P < 0.05 compared with Fg + TGF-β1 treatment. C: representative immunostaining of Col1A1- and phalloidin-based staining of F-actin in NRK-49F cells after treatment with Fg, TGF-β1, or the combination of both. DAPI, 4′,6-diamidino-2-phenylindole. Fluorescence quantification results are shown for these as well as combination treatments with Fg and TGF-β1 inhibitors (data represent 2 independent experiments with 2 replicates each for a total of 4 replicates; 5 images/replicate were taken, and the results were averaged). Data are presented as means ± SE of fold changes from vehicle. *P < 0.05 compared with vehicle; #P < 0.05 compared with treatment without inhibitor; $P < 0.05 compared with Fg + TGF-β1 treatment.
Genetic Fg depletion protects mice from kidney fibrosis.
Considering the increased Fg expression in kidney fibrosis mouse models and the functional correlation between Fg and TGF-β1, we hypothesized that Fg could serve as a therapeutic target to reduce fibrosis. To test interventions aimed at reducing Fg levels, we first used mice that constitutively express less Fg [disrupted Fgα chain results in no fully assembled functional Fg hexamer and eventually Fgβ and Fgγ are also degraded (57)]. We tested the development of kidney fibrosis in these mice after FA injection. On day 1, there was no indication that a reduction or complete absence of Fg in heterozygous and KO mice, respectively (confirmed by native gel immunoblot; Fig. 5A), protects from AKI in this model. All three genotypes had histologically similar mild acute tubular injury and also similar increases in BUN and serum creatinine (Fig. 5B). As expected, kidneys expressed high mRNA levels for all three Fg chains compared with normal mice except for Fgα in KO mice (with both alleles of this chain disrupted; Fig. 5C). Kidney protein expression showed similar trends (Fig. 5D). By 14 days, heterozygous mice with reduced but not completely abolished Fg expression (shown by native gel immunoblot; Fig. 5E) were significantly protected from the development of kidney fibrosis. By quantification of kidney Masson's trichrome staining, Fg heterozygous mice had, on average, 3% of the section area affected compared with 24% in WT mice, whereas KO mice had 14% (Fig. 5F). BUN and serum creatinine levels returned toward normal by this time point. The improved pathological picture was not matched by fibrosis markers kidney mRNA and protein levels, which showed similar levels for all Fg genotypes (Fig. 5, G and H).
Pharmacological fibrinogenolysis through Ancrod protects from kidney fibrosis.
We then tested a fibrinogenolytic pharmaceutical intervention, Ancrod administration, in the context of FA-induced fibrosis. The treatment (continuous subcutaneous Ancrod infusion in normal saline, 3 IU/day, delivered by miniosmotic pumps) started 3 days before FA injection and successfully reduced circulating Fg levels 1 day after FA (Fig. 6A). Treatment was administered until day 11, and, 3 days after its interruption (day 14), mice reverted to circulating Fg levels similar to saline-treated control mice. As for genetic depletion, pharmacological Fg reduction did not significantly alter the extent of AKI as indicated by histopathological analysis and BUN and serum creatinine levels on day 1 (Fig. 6B). Although it did not affect kidney Fg chain mRNA expression (Fig. 6C), the treatment reduced protein levels of Fgα in half at this time point (Fig. 6D). By day 14, mice that received Ancrod developed significantly less kidney fibrosis. As evidenced by Masson's trichrome staining, the fibrotic area was more than threefold smaller in mice that received treatment (9% vs. 28% in saline-treated control mice; Fig. 6F). Expression of fibrosis markers matched the pathological picture, with significant reductions in fibronectin (5-fold) and collagen 1A1 (9-fold) mRNA expression in Ancrod-treated animals (Fig. 6G). Kidney α-SMA and collagen 1A1 protein levels were also significantly reduced in this group (Fig. 6H).
Fg depletion reduces TGF-β1 signaling pathway activation in vivo.
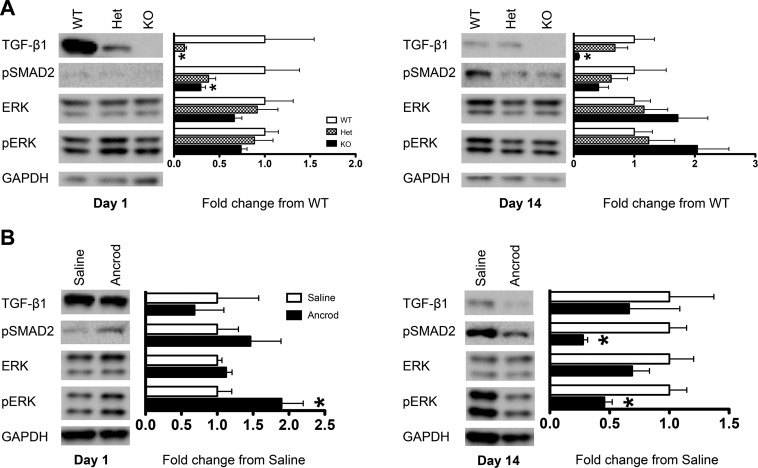
Since we established Fg/TGF-β1 interactions in vitro (Fig. 4), we next tested if the protective effects of reduced Fg levels in vivo could result from a dampened TGF-β1 signaling pathway. Genetic Fg depletion in KO mice resulted in a pronounced reduction of TGF-β1 and signaling through pSMAD2 in kidneys both in acute injury on day 1 and established fibrosis on day 14 (Fig. 7A). The reduction of Fg levels in heterozygous mice decreased TGF-β1 and pSMAD2 levels, but not as significantly. pERK, an alternative signaling molecule activated by TGF-β1 (41), was not as affected by Fg levels on day 1. By day 14, decreased Fg resulted in slight pERK increases. Ancrod treatment did not result in acute TGF-β1 and pSMAD2 changes but showed significant pERK increases on day 1 (Fig. 7B). By day 14, however, animals that received Ancrod had significantly lower pSMAD2 (almost 4-fold lower than saline) and pERK (more than 2-fold lower). Taken together, these results indicate that TGF-β1/pSMAD2 signaling is responsible at least partially for the beneficial effects of reductions in Fg levels on the development of kidney fibrosis.
Fig. 7.
Genetic and pharmacological Fg depletion leads to decreased TGF-β1 signaling in the kidney after FA injection in mice. A: representative immunoblots and quantification of protein expression of TGF-β1 and downstream signaling molecules pSMAD2 and pERK in the kidney indicating that genetic depletion of fibrinogen results in early, day 1 downregulation of TGF-β1 expression and signaling through pSMAD2 after FA injection in mice. This was maintained by day 14. Western blot data were normalized to levels measured for GAPDH and are presented as means ± SE of fold changes from WT mice; n = 3–4 mice/group. *P < 0.05 compared with WT mice. B: significant downregulation of the TGF-β1 signaling pathway in Ancrod-treated mice by day 14 after FA injection. Western blot data were normalized to levels measured for GAPDH and are presented as means ± SE of fold changes from saline treatment; n = 5–6 mice/group. *P < 0.05 compared with saline treatment.
DISCUSSION
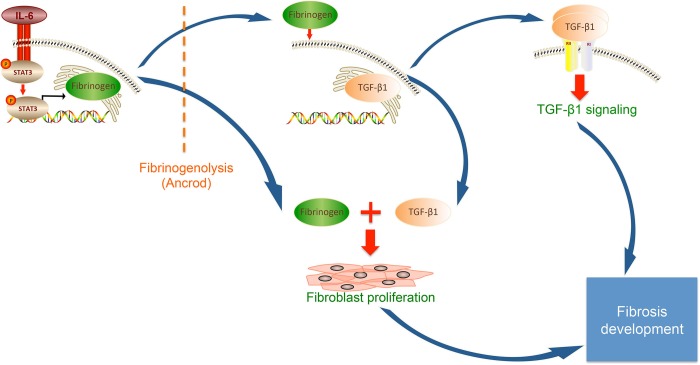
Renal fibrosis is the outcome of concerted actions of various cellular signaling pathways controlling myofibroblasts proliferation, activation, and ECM component deposition (23). Building on previous reports showing fibrin(ogen) deposition in a range of clinical and experimental fibrotic kidney disorders (11, 19, 49), our work suggests the following model for its involvement in fibrosis development (Fig. 8): injury of kidney tissue triggers IL-6-driven activation of STAT3 followed by binding of this transcription factor to Fg chain promoters, Fg upregulation triggers fibroblast TGF-β1 expression and proliferation, TGF-β1 signaling pathway activation increases ECM deposition and fibrosis, and fibrinogenolysis can prevent fibrosis by diminishing both fibroblast proliferation and TGF-β1 signaling. This study sheds more light on the major role of Fg in kidney fibrosis pathophysiology and, by repurposing a drug that efficiently reduces its levels, indicates the clinical potential for interventions to reduce fibrosis development.
Fig. 8.
Schematic of the involvement of Fg in the development of kidney fibrosis.
Animal models of kidney injury seem to indicate that kidney Fg expression and urinary excretion are more a reflection of acute injury and would not perform well as fibrosis biomarkers. While our previous studies (34, 38) showed this after rodent ischemia-reperfusion injury and cisplatin-induced nephrotoxicity, the data presented here show that after murine FA nephropathy, kidney Fg expression and urinary excretion are high early, when AKI is present, but not at 14 days, when tubulointerstitial fibrosis develops. Urinary levels reflecting the injured kidney cannot be measured after UUO, but kidney expression of Fg was increased throughout time points in fibrosis development. While primarily used as a tubulointerstitial fibrosis model, a previous report (25) has indicated persistent acute tubular injury after UUO. Kidney expression for another established AKI marker, Kim-1, was similarly increased only acutely after FA nephropathy and throughout time points after UUO. Taken together, these results strengthen kidney Fg expression and urinary secretion as markers of acute tubular injury. Detection in CKD patients would most probably indicate superimposed AKI, with the former condition being a significant risk factor for the development of the latter (35, 59).
Previous reports (29, 55) have indicated plasma and kidney Fg upregulation after UUO. Contrary to suggestions that Fg increases after injury merely reflect extravasation of hepatic sourced protein (17), we show here that kidney mRNA expression of all Fg chains is upregulated after UUO and FA nephropathy. Our previous studies (34, 38) showed this to be true for other AKI models induced by ischemia-reperfusion and cisplatin. We wanted to find a mechanism for this increased kidney expression. IL-6 is the main mediator of liver acute-phase Fg expression (60). Promoters of all three Fg genes contain IL-6-responsive elements (27), and the results of our study indicate that STAT3 binds to them in vivo in mouse kidney fibrosis models. In vitro studies have more directly suggested that this binding results in gene expression and that, with the use of a STAT3 inhibitor (Stattic), this expression could be blocked in kidney fibroblasts. This suggests STAT3 inhibition as a possible option to reduce fibrosis, decreasing the induction of genes responsive to inflammatory signals, as previously observed (51). As previously discussed for Fg and Kim-1 expression, the profile of increased IL-6 and pSTAT3 expression matches the presence of AKI, indicating that perhaps such treatment would be most effective when acute injury and fibrosis development coexist. Measuring urinary Fg could be the biomarker for detecting such situations. The fact that AKI leads to CKD is drawing increasing attention (12, 13, 30), and the reduction in Fg expression seems to provide preventive opportunities.
Our results indicate that a mechanism for Fg involvement in kidney fibrosis is increased TGF-β1 expression, the central fibrosis mediator (26). While previously suggested in fibrotic disease by increased TGF-β1 production by Fg-stimulated macrophages isolated from a Duchenne muscular dystrophy mouse model (61), our study has explored the role of Fg on TGF-β1 production in the kidney for the first time. While we tested this in vitro using kidney fibroblasts [the main precursors of myofibroblasts, which are responsible for disregulated ECM production characteristic of fibrosis (26)], all kidney cells can secrete this cytokine (48). Fg depletion, particularly through genetic manipulation, resulted in a global kidney TGF-β1 reduction from day 1 after FA injury (Fig. 7A). It has been recently shown that TGF-β1 directly injures tubular epithelial cells (28), and the protection after Fg depletion could partially be attributed to this mechanism. Although histological and functional analyses did not reveal differences between groups on day 1, it is possible that this becomes apparent subsequently, as AKI peaks on day 2 and persists through day 3 after FA injection. Additionally, Fg synergized with TGF-β1 in inducing kidney fibroblast proliferation in vitro. As previously reported (55), Fg did not induce fibroblast activation, but this is probably achieved indirectly through increased TGF-β1 expression. It was shown that Fg directly interacts with many growth factors, including TGF-β1 (45), and we detected this in vitro by IP. TGF-β1 signaling inhibition has been the main focus of antifibrotic therapy testing, but global inhibition adversely affects immunnity and tumor suppression (7, 26). Targeting Fg as a TGF-β1 inducer in kidney injury could specifically inhibit the pathway only in organs developing fibrosis.
While many preclinical and clinical trials have been performed, there are currently no effective fibrosis treatments for any organ, including the kidney (26). The coagulation and fibrinolytic systems have also been recognized as important, and, particularly, reductions in plasminogen activator inhibitor-I (52) or thrombin-activated fibrinolysis inhibitor (8) have shown promise. Our study and a previous report (44) on protection from kidney UUO fibrosis in genetically depleted mice have indicated an important role for Fg potentially independent of fibrin formation.
Snake venoms contain many enzymes with fibrin(ogen)olytic acitivty (58). One such protease, Ancrod, derived from Malayan pit viper venom, cleaves Fgα and rapidly decreases plasma Fg levels (22). It effectively limited infarct size in acute stroke animal models (21), and two of three randomized controlled clinical trials involving acute stroke patients showed positive results (8a, 54). While lack of beneficial effect, increased mortality, and symptomatic intracranial hemorrhage in one study (33) completely stopped drug production, post hoc analysis suggested that more carefully supervising dosing regimens and patient stratification could have achieved efficacy and reduced hemorrhagic events (40). Higher dose treatments (4–5 IU·kg−1·h−1) effectively reduce murine Fg levels and were protective in axonal injury models (5, 6). In the present study, we used this regimen in the FA nephropathy mouse model and found it safe (no mortality), efficient (reducing both plasma Fg and kidney levels for its direct target, Fgα), and beneficial (significantly reducing TGF-β1 signaling, kidney ECM component deposition, and histologically detectable kidney tubulointerstitial fibrosis). While provided in a preventive manner, the treatment achieved its proof-of-principle goal of identifying Fg as a pharmacologically targetable molecule for potential new fibrosis treatments. Due to limited drug availability, followup experiments with administration in already established kidney fibrosis could not be performed. At the moment, there are no other fibrinogenolytic drugs commercially available.
The fibrosis-driven gradual renal excretory function degradation seen in CKD represents a major public health problem, both in the United States and globally, affecting 10–16% of adults (14, 39, 46). While some estimates have indicated that the lifetime risk of developing CKD will balloon to >50% (31), even at current levels CKD has high socioeconomic impact, with 27.6% of total Medicare costs being used to treat it (third only to diabetes and heart failure) (56). Generally, kidney fibrosis is considered untreatable at the moment. Our results identified kidney Fg as a key fibrosis development regulator, providing information on upstream events leading to its local expression (IL-6/STAT3 signaling) and downstream consequences (TGF-β1 synthesis, signaling, and fibroblast proliferation) and, importantly, showing that fibrinogenolytics like Ancrod can reduce the extent of fibrosis, at least in a preventive manner. In conclusion, we indicated the mechanistic involvement of Fg in kidney fibrosis and showed its potential as a therapeutic target.
GRANTS
This work was supported by National Institute of Environmental Health Sciences Outstanding New Environmental Scientist Award ES-017543.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: F.L.C., A.K.A., D.H., and V.S.V. conception and design of research; F.L.C., A.K.A., D.H., J.S., and S.L.F. performed experiments; F.L.C., A.K.A., D.H., V.B., and V.S.V. analyzed data; F.L.C., A.K.A., D.H., and V.S.V. interpreted results of experiments; F.L.C., A.K.A., and D.H. prepared figures; F.L.C., A.K.A., and J.S. drafted manuscript; F.L.C., A.K.A., D.H., J.S., S.L.F., V.B., B.D.H., and V.S.V. edited and revised manuscript; F.L.C., A.K.A., D.H., J.S., S.L.F., V.B., B.D.H., and V.S.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Brock Matthias (Center of Experimental Rheumatology and Working Group of Pulmonary Hypertension, University Hospital Zurich, Zurich, Switzerland) for the pGL3-Basic-FGG and pRL-GAPDH plasmid constructs, Dr. David A. Frank (Department of Medical Oncology, Dana Farber Cancer Institute, Boston, MA) for the pRC/CMV and pRC/CMVSTAT3 plasmid constructs, and Dr. Jay L. Degen (Children's Hospital Research Foundation, Cincinnati, OH) for providing breeding pairs of mice with a mutated Fgα chain.
REFERENCES
- 1.Abraham AP, Ma FY, Mulley WR, Ozols E, Nikolic-Paterson DJ. Macrophage infiltration and renal damage are independent of matrix metalloproteinase 12 in the obstructed kidney. Nephrology (Carlton) 17: 322–329, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Ajay AK, Kim TM, Ramirez-Gonzalez V, Park PJ, Frank DA, Vaidya VS. A bioinformatics approach identifies signal transducer and activator of transcription-3 and checkpoint kinase 1 as upstream regulators of kidney injury molecule-1 after kidney injury. J Am Soc Nephrol 25: 105–118, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajay AK, Saikumar J, Bijol V, Vaidya VS. Heterozygosity for fibrinogen results in efficient resolution of kidney ischemia reperfusion injury. PLOS ONE 7: e45628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akassoglou K, Kombrinck KW, Degen JL, Strickland S. Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J Cell Biol 149: 1157–1166, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akassoglou K, Yu WM, Akpinar P, Strickland S. Fibrin inhibits peripheral nerve remyelination by regulating Schwann cell differentiation. Neuron 33: 861–875, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 11: 790–811, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Ancrod Stroke Study Investigators. Ancrod for the treatment of acute ischemic brain infarction. The Ancrod Stroke Study Investigators. Stroke 25: 1755–1759, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Atkinson JM, Pullen N, Johnson TS. An inhibitor of thrombin activated fibrinolysis inhibitor (TAFI) can reduce extracellular matrix accumulation in an in vitro model of glucose induced ECM expansion. Matrix Biol 32: 277–287, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Baumann H, Isseroff H, Latimer JJ, Jahreis GP. Phorbol ester modulates interleukin 6- and interleukin 1-regulated expression of acute phase plasma proteins in hepatoma cells. J Biol Chem 263: 17390–17396, 1988 [PMC free article] [PubMed] [Google Scholar]
- 10.Becker GJ, Hewitson TD. Animal models of chronic kidney disease: useful but not perfect. Nephrol Dial Transplant 28: 2432–2438, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Bouissou F, Barthe P, Pierragi M. Severe idiopathic nephrotic syndrome with tubular dysfunction (report of nine pediatric cases). Clin Nephrol 14: 135–176, 1980 [PubMed] [Google Scholar]
- 12.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol 34: 43–62, 2012 [DOI] [PubMed] [Google Scholar]
- 16.de Giorgio-Miller A, Bottoms S, Laurent G, Carmeliet P, Herrick S. Fibrin-induced skin fibrosis in mice deficient in tissue plasminogen activator. Am J Pathol 167: 721–753, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Docherty NG, Godson C. Fibrinogen as a damage-associated mitogenic signal for the renal fibroblast. Kidney Int 80: 1014–1016, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Doolittle RF. Fibrinogen and fibrin. Annu Rev Biochem 53: 195–229, 1984 [DOI] [PubMed] [Google Scholar]
- 19.Drew A, Tucker H, Liu H, Witte D, Degen J, Tipping P. Crescentic glomerulonephritis is diminished in fibrinogen-deficient mice. Am J Physiol Renal Physiol 281: F1157–F1163, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Eddy AA, Lopez-Guisa JM, Okamura DM, Yamaguchi I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr Nephrol 27: 1233–1247, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elger B, Hornberger W, Schwarz M, Seega J. MRI study on delayed ancrod therapy of focal cerebral ischaemia in rats. Eur J Pharmacol 336: 7–14, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Ewart MR, Hatton MW, Basford JM, Dodgson KS. The proteolytic action of Arvin on human fibrinogen. Biochem J 118: 603–609, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farris AB, Colvin RB. Renal interstitial fibrosis: mechanisms and evaluation. Curr Opin Nephrol Hypertens 21: 289–300, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Floege J, Hackmann B, Kliem V, Kriz W, Alpers C, Johnson R, Kühn KW, Koch K, Brunkhorst R. Age-related glomerulosclerosis and interstitial fibrosis in Milan normotensive rats: a podocyte disease. Kidney Int 51: 230–273, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Forbes MS, Thornhill BA, Minor JJ, Gordon KA, Galarreta CI, Chevalier RL. Fight-or-flight: murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis. Am J Physiol Renal Physiol 303: F120–F129, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med 5: 167sr1, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Fuller GM, Zhang Z. Transcriptional control mechanism of fibrinogen gene expression. Ann NY Acad Sci 936: 469–479, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Gentle ME, Shi S, Daehn I, Zhang T, Qi H, Yu L, D'Agati VD, Schlondorff DO, Bottinger EP. Epithelial cell TGFβ signaling induces acute tubular injury and interstitial inflammation. J Am Soc Nephrol 24: 787–799, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannakis E, Samuel C, Hewitson T, Boon WM, Macris M, Reeve S, Lawrence J, Ian Smith A, Tregear G, Wade J. Aberrant protein expression in plasma and kidney tissue during experimental obstructive nephropathy. Proteomics Clin Appl 3: 1211–1235, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Goldberg R, Dennen P. Long-term outcomes of acute kidney injury. Adv Chronic Kidney Dis 15: 297–307, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3–5 in the United States. Am J Kidney Dis 62: 245–252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hattori N, Degen J, Sisson T, Liu H, Moore B, Pandrangi R, Simon R, Drew A. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest 106: 1341–1391, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hennerici MG, Kay R, Bogousslavsky J, Lenzi GL, Verstraete M, Orgogozo JM. Intravenous ancrod for acute ischaemic stroke in the European Stroke Treatment with Ancrod Trial: a randomised controlled trial. Lancet 368: 1871–1878, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann D, Bijol V, Krishnamoorthy A, Gonzalez VR, Frendl G, Zhang Q, Goering PL, Brown RP, Waikar SS, Vaidya VS. Fibrinogen excretion in the urine and immunoreactivity in the kidney serves as a translational biomarker for acute kidney injury. Am J Pathol 181: 818–828, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW, Tonelli M. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet 376: 2096–2103, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Kant JA, Fornace AJ, Jr, Saxe D, Simon MI, McBride OW, Crabtree GR. Evolution and organization of the fibrinogen locus on chromosome 4: gene duplication accompanied by transposition and inversion. Proc Natl Acad Sci USA 82: 2344–2348, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S, Nadel J. Fibrinogen binding to ICAM-1 promotes EGFR-dependent mucin production in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 297: L174–L183, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnamoorthy A, Ajay AK, Hoffmann D, Kim TM, Ramirez V, Campanholle G, Bobadilla NA, Waikar SS, Vaidya VS. Fibrinogen β-derived Bβ15-42 peptide protects against kidney ischemia/reperfusion injury. Blood 118: 1934–1942, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G. Chronic kidney disease as a global public health problem: approaches and initiatives–a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Levy DE, Trammel J, Wasiewski WW. Ancrod for acute ischemic stroke: a new dosing regimen derived from analysis of prior ancrod stroke studies. J Stroke Cerebrovasc Dis 18: 23–27, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Hernandez FJ, Lopez-Novoa JM. Role of TGF-β in chronic kidney disease: an integration of tubular, glomerular and vascular effects. Cell Tissue Res 347: 141–154, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Novoa JM, Rodriguez-Pena AB, Ortiz A, Martinez-Salgado C, Lopez Hernandez FJ. Etiopathology of chronic tubular, glomerular and renovascular nephropathies: clinical implications. J Transl Med 9: 13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luyendyk JP, Kassel KM, Allen K, Guo GL, Li G, Cantor GH, Copple BL. Fibrinogen deficiency increases liver injury and early growth response-1 (Egr-1) expression in a model of chronic xenobiotic-induced cholestasis. Am J Pathol 178: 1117–1125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makino H, Theng L, Kumagai I, Takatori K, Ota Z, Yoshifusa H. [Progressive glomerular sclerosis and renal failure in rat with 7/8 nephrectomy model]. Nihon Jinzo Gakkai Shi 32: 127–163, 1990 [PubMed] [Google Scholar]
- 45.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci USA 110: 4563–4568, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsushita K, Ballew SH, Astor BC, Jong PE, Gansevoort RT, Hemmelgarn BR, Levey AS, Levin A, Wen CP, Woodward M, Coresh J; Chronic Kidney Disease Prognosis Consortium. Cohort profile: the Chronic Kidney Disease Prognosis Consortium. Int J Epidemiol 42: 1660–1668, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet 365: 331–340, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Meng XM, Chung AC, Lan HY. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond) 124: 243–254, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Nagy J, Brasch H, Deák G, Sámik J, Säle T, Trinn C, Burger T. IgA glomerulonephritis: light microscopic and immunohistological studies. Acta Morphol Hung 32: 143–197, 1984 [PubMed] [Google Scholar]
- 50.Nguyen MD, Simpson-Haidaris PJ. Cell type-specific regulation of fibrinogen expression in lung epithelial cells by dexamethasone and interleukin-1β. Am J Respir Cell Mol Biol 22: 209–217, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, Chin YE, Yan H, Dworkin LD, Zhuang S. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 78: 257–268, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Ruppert C, Markart P, Wygrecka M, Preissner KT, Gunther A. Role of coagulation and fibrinolysis in lung and renal fibrosis. Hamostaseologie 28: 30–32, 34–36, 2008 [PubMed] [Google Scholar]
- 53.Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-β after vascular damage. J Neurosci 30: 5843–5854, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherman DG, Atkinson RP, Chippendale T, Levin KA, Ng K, Futrell N, Hsu CY, Levy DE. Intravenous ancrod for treatment of acute ischemic stroke: the STAT study: a randomized controlled trial. Stroke Treatment with Ancrod Trial. JAMA 283: 2395–2403, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Sorensen I, Susnik N, Inhester T, Degen JL, Melk A, Haller H, Schmitt R. Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int 80: 1035–1044, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J Intern Med 268: 456–467, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Suh TT, Holmback K, Jensen NJ, Daugherty CC, Small K, Simon DI, Potter S, Degen JL. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev 9: 2020–2033, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Swenson S, Markland FS., Jr Snake venom fibrin(ogen)olytic enzymes. Toxicon 45: 1021–1039, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16: 162–168, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Verschuur M, de Jong M, Felida L, de Maat MP, Vos HL. A hepatocyte nuclear factor-3 site in the fibrinogen β promoter is important for interleukin 6-induced expression, and its activity is influenced by the adjacent −148C/T polymorphism. J Biol Chem 280: 16763–16771, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Vidal B, Serrano A, Tjwa M, Suelves M, Ardite E, De Mori R, Baeza-Raja B, Martínez de Lagrán M, Lafuste P, Ruiz-Bonilla V, Jardí M, Gherardi R, Christov C, Dierssen M, Carmeliet P, Degen J, Dewerchin M, Muñoz-Cánoves P. Fibrinogen drives dystrophic muscle fibrosis via a TGFβ/alternative macrophage activation pathway. Genes Dev 22: 1747–1799, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Pratt JR, Hartley B, Evans B, Zhang L, Sacks SH. Expression of tissue type plasminogen activator and type 1 plasminogen activator inhibitor, and persistent fibrin deposition in chronic renal allograft failure. Kidney Int 52: 371–377, 1997 [DOI] [PubMed] [Google Scholar]
- 63.Wynn T. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117: 524–533, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1α. Am J Pathol 163: 2289–2301, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]