Abstract
Cytochrome P-450, family 2, subfamily c, polypeptide 44 (Cyp2c44) epoxygenase metabolizes arachidonic acid (AA) to epoxyeicosatrienoic acids (EETs) in kidney and vascular tissues. In the present study, we used real-time quantitative PCR techniques to examine the effect of high salt or high K+ (HK) intake on the expression of Cyp2c44, a major Cyp2c epoxygenase in the mouse kidney. We detected Cyp2c44 in the proximal convoluted tubule, thick ascending limb, distal convoluted tubule (DCT)/connecting tubule (CNT), and collecting duct (CD). A high-salt diet increased the expression of Cyp2c44 in the thick ascending limb and DCT/CNT but not in the proximal convoluted tubule and CD. In contrast, an increase in dietary K+ intake augmented Cyp2c44 expression only in the DCT/CNT and CD. Neither high salt nor HK intake had a significant effect on the blood pressure (BP) of wild-type mice. However, HK but not high salt intake increased BP in CD-specific, Cyp2c44 conditional knockout (KO) mice. Amiloride, an epithelial Na+ channel (ENaC) inhibitor, normalized the BP of KO mice fed HK diets, suggesting that lack of Cyp2c44 in the CD enhances ENaC activity and increases Na+ absorption in KO mice fed HK diets. This notion was supported by metabolic cage experiments demonstrating that renal Na+ excretion was compromised in KO mice fed HK diets. Also, patch-clamp experiments demonstrated that 11,12-EET, a major Cyp2c44 product, but not AA inhibited ENaC activity in the cortical CD of KO mice. We conclude that Cyp2c44 in the CD is required for preventing the excessive Na+ absorption induced by HK intake by inhibition of ENaC and facilitating renal Na+ excretion.
Keywords: epoxyeicosatrienoic acid; epithelial Na+ channel; renal sodium excretion; hypertension; cytochrome P-450, family 2, subfamily c, polypeptide 44
the main cytochrome P-450 (Cyp)2c epoxygenase expressed in the renal tubule is Cyp2c polypeptide 44 (Cyp2c44), which converts arachidonic acid (AA) to epoxyeicosatrienoic acids (EETs) (3, 8, 21). Previous studies have demonstrated that 11,12-EET accounts for >60% of total renal EETs (7) and that 11,12-EET inhibits the epithelial Na+ channel (ENaC) in the cortical collecting duct (CCD) (40). The role of Cyp2c44 in the regulation of renal Na+ transport through ENaC was demonstrated in studies with Cyp4A10 knockout (KO) mice. Although Cyp4A10 does not metabolize AA to EETs, disruption of the Cyp4A10 gene downregulated renal Cyp2c44 expression and EET biosynthesis (22). Hence, whereas in Cyp4a10 KO mice AA failed to inhibit ENaC, 11,12-EET did. Consequently, Cyp4a10 KO mice develop salt-sensitive hypertension, and amiloride normalizes the blood pressures (BPs) of Cyp4A10 KO mice fed high salt. A recent study (2) performed in global Cyp2c44−/− mice convincingly demonstrated that Cyp2c44 was required for the inhibition of ENaC by AA and that disruption of the Cyp2c44 gene caused salt-sensitive hypertension. Cyp2c44 is also responsible for mediating the high K+ (HK) intake-induced antihypertensive effect. We have previously demonstrated that lack of Cyp2c44 epoxygenase impaired the renal ability to excrete Na+ in response to HK intake. Thus, an increase in dietary K+ intake caused hypertension in global Cyp2c44 KO mice, an effect that was also abolished by amiloride (36). Two lines of evidence suggested that Cyp2c44 expressed in the CCD may be responsible for enhancing renal Na+ excretion during increasing dietary K+ intake (36). First, an increase in dietary K+ intake significantly augmented Cyp2c44 expression in the CCD. Second, HK intake increased EET generation in the CCD of wild-type (WT) but not Cyp2c44−/− mice. Thus, the aim of the present study was to use collecting duct (CD)-specific Cyp2c44 conditional KO mice to test the hypothesis that during increased dietary K+ intake, Cyp2c44 plays a key role in stimulating renal Na+ excretion in the CCD by inhibition of ENaC.
METHODS
Generation of CD-specific Cyp2c44 KO mice.
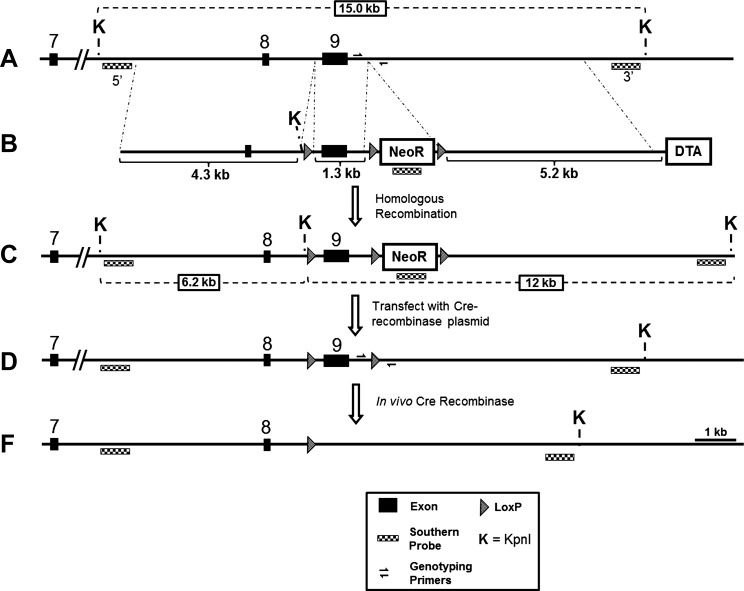
Figure 1 shows the scheme illustrating the vector construction targeting stratagem for the CD-specific Cyp2c44 conditional KO mouse. Briefly, CD-specific Cyp2c44 conditional KO mice (in C57Bl6 backgrounds) were generated by mating mice in which exon 9 of the Cyp2c44 gene was flanked by loxP sites [Cyp2c44loxP(+/+)] with isogenic mice expressing Cre recombinase under the control of the HoxB7 promoter (HoxB7-Cre mice; a gift from Dr. Roy Zent, Vanderbilt University) to generate HoxB7-Cre+/+, Cyp2c44flx+/+ (CD-Cyp2c44-KO mice) in C57Bl backgrounds. In these mice, HoxB7 promoter-driven Cre recombinase in the CD has excised exon 9 of the Cyp2c44 gene (coding for the cysteine heme ligand), leading to nonfunctional transcripts. CD-Cyp2c44-KO mice were identified by PCR amplification of a 300-bp fragment using tail DNAs using the following Cre- and HoxB7-specific primers: 5′-GGTCACGTGGTCAGAAGAGG-3′ (Cre forward primer) and 5′-CTCATCACTCGTTGCATCGA-3′ (HoxB7 reverse primer). The use of HoxB7-Cre mice for CD-selective gene disruption has been previously documented (13, 43). Isogenic Cyp2c44loxP(+/+) mice were used as controls. Animal use protocols were approved by an independent animal user committee at the National Institute of Environmental Health Sciences, Vanderbilt University, and New York Medical College.
Fig. 1.
Vector construction and targeting strategy for the cytochrome P-450, family 2, subfamily c, polypeptide 44 (Cyp2c44) conditional knockout (KO) mouse. A: wild-type (WT) mouse Cyp2c44 locus. B: targeting vector generated via the conventional recombineering technique from mouse BAC RP23-103P24. The targeting vector was linearized with the NotI restriction enzyme before electroporation. The targeting scheme introduced three LoxP sites: two LoxP sites flanking exon 9 and one LoxP site 3′ of the positive selection marker [neomycin resistance (NeoR)]. C: targeted Cyp2c44 allele identified by Southern blot analysis with 5′ and 3′ probes generated by PCR from WT genomic DNA (targeting efficiency: 4 of 192 ES clones). D: floxed Cyp2c44 allele generated by transient expression of Cre recombinase in correctly targeted ES clones to remove the LoxP-flanked NeoR cassette but not LoxP flanked exon 9 (desired recombination event: 5 of 96 ES clones). PCR genotyping across the region of the 3′ LoxP site was used to differentiate the WT (418 bp) and floxed (550 bp) allele. Forward primer: 5′-CAATTCCCAGCAACCACACCACA-3′; reverse primer: 5′-GCTCTGTCTTTACAGTCCGCTCATTTC-3′. DTA, diptheria toxin A.
Dissection of the CCD and patch-clamp experiments.
For the patch-clamp experiments, we used mice on a HK diet for 3 days to increase the density of ENaC. Mice were euthanized by cervical dislocation, and both kidneys were removed immediately. Several thin slices of the kidney (<1 mm) were cut and placed in ice-cold Ringer solution. The CCD was dissected under a microscope, and the isolated CCD was placed on a 5 × 5-mm coverglass coated with polylysine. The coverglass was then transferred to a chamber (1,000 μl) mounted on an inverted Nikon microscope. The CCD was cut open with a sharpened micropipette to expose the apical membrane and superfused with HEPES-buffered NaCl solution. A borosilicate glass (1.7-mm outer diameter) was used to make patch-clamp pipettes, which were pulled with a Narishege electrode puller. An Axon200B patch-clamp amplifier was used to record the channel current. Currents were low-pass filtered at 50 Hz and digitized by an Axon interface (Digidata 1322). Data were analyzed using pCLAMP software system 9 (Axon). Channel activity (NPo) was calculated from data samples of 60-s duration in the steady state as follows: NPo = Σ(t1 + 2t2 +…iti), where ti is the fractional open time spent at each of the observed current levels. The pipette solution for studying Na+ channels contained (in mM) 140 NaCl, 1.8 MgCl2, 1.8 CaCl2, and 5 HEPES (pH 7.4). The bath solution for single channel patch-clamp experiments contained (in mM) 135 NaCl, 5 KCl, 1.8 CaCl2, 1.8 MgCl2, 2 glucose, and 10 HEPES (pH 7.4).
Real-time PCR to quantify Cyp2c44 expression.
Proximal convoluted tubule (PCT), thick ascending limb (TAL), distal convoluted tubule (DCT)/connecting tubule (CNT), and CD segments, including the CCD and outer medullary collecting duct (OMCD), were isolated under a microscope from mice on a control diet (1% K+ + 0.3% Na+), HK diet (5% K+), or high-Na+ diet (4% Na+) for 3 days. The HK diet (TD10866) and high-Na+ diet (TD 92034) were purchased from Harlan Laboratories (Madison, WI). Each nephron segment (3–4 tubules for 1 sample) was lysed in 10 μl SideStep lysis and stabilization buffer (Stratagene), tapped gently, and mixed completely. To set up the RT reaction for cDNA synthesis, 1 μl of lysate was mixed with 1 μl of oligo(dT) primer (10 μM), 2 μl of 10× RT reaction buffer, and 14 μl of distilled H2O and incubated at 65°C for 5 min. Samples were then cold down to room temperature to allow the primer anneal with RNA. We added 1 μl of dNTP (10 mM each) and 1 μl of AffinityScript RT enzyme to the above mixture (20-μl total reaction volume). Samples were then incubated at 60°C for 30 min, and the amplification program was started and finalized by 5 min of incubation at 85°C to inactivate the RT enzyme. This generated cDNAs that were used for real-time PCR. Cyp2c44 primers (2.5 nM) (sense: 5′-TTCATCCTGGCCTGTGCTCC-3′ and antisense: 5′-GGCACCACACGGAGTTCAC-3′) were mixed with 2 μl of cDNA (200 ng) and 12.5 μl of 2× SYBR Green master. The sense primer and antisense primer bind to Cyp2c44 nucleotide position 279 (forward) and 457 (reverse), respectively. MxPro3000 (Stratagene) was used for the real-time PCR experiments, and we used the 2−ΔΔCT method (where CT is threshold cycle) to analyze the comparative expression level of Cyp2c44. GAPDH was used as a normalization control.
Measurements of BP.
We measured BP using a carotid artery catheter or noninvasive tail-cuff methods (Kent Scientific, Torrington, CT) between 9 and 11 AM. For catheter insertion, mice were anesthetized with Nembutal (75 mg/kg body wt), and the carotid artery was separated from the vagus nerve and muscle. After cutting open the carotid artery, we inserted a polyethylene-10 catheter into the vessel. Catheters were connected to a remote pressure sensor. After animals became familiar with the environment, their BPs were measured with a Digi-Med Blood Pressure Analyzer (Micro-Med, Louisville, KY). The BP of each animal was monitored continuously for at least 40 min at an ambient temperature of 23°C, and >20 such measurements were performed to obtain the mean value. For the tail-cuff method, mice were trained for 1–2 wk before the start of experiments. Systolic BP measurements were recorded after 5 cycles of acclimatization, and 7–15 measurements was taken as the representative pressure for each mouse. Because we performed patch-clamp and metabolic cage experiments in mice on a HK diet for 3 days, we measured the BP of mice just for 3 days.
Metabolic cage experiments.
We followed a previously described protocol for metabolic cage experiments (1). Two mice were kept in one cage, and we recorded their daily Na+ intakes, body weights, and urinary volume excretions at the same time of the day. After 3–4 days of training, mice were fed with normal K+ diet for 3 days and a HK diet for an additional 3 days. We measured urine Na+ concentrations, and these values were used to calculate the urinary Na+ excretion rate (UNa). Urinary Na+ and K+ concentrations were measured by Antech Diagnostics (Morrisville, NC), and daily urinary Na+ and K excretion+ were presented as equivalent micromolars (μeqm) per 24 h.
Materials and statistics.
AA and 11,12-EET were purchased from Cayman Chemicals (Ann Arbor, MI). Both high-salt (4% NaCl) and HK (5% KCl) diets were purchased from Harlan Laboratories.
Data are presented as means ± SE. We used either Student's t-test or one-way ANOVA to determine statistical significance. P values of ≤0.05 were considered significant.
RESULTS
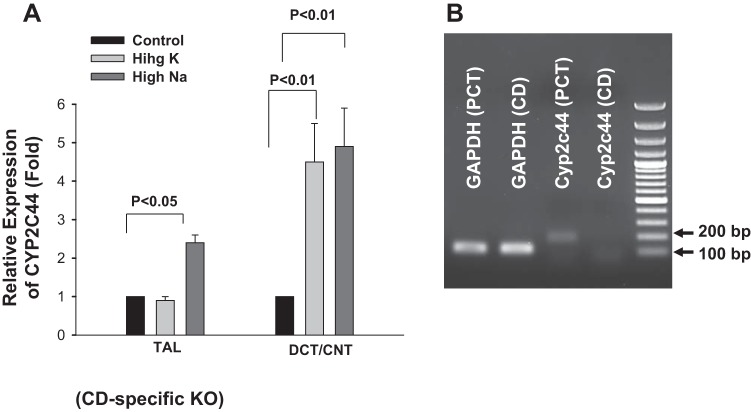
We first used quantitative PCR techniques to examine the expression of Cyp2c44 along the nephron segments (3, 8). The results are shown in Fig. 2 and demonstrate that Cyp2c44 was expressed in the PCT, TAL, DCT/CNT, and CD, including the CCD/OMCD. Since previous studies (22, 36) have indicated a role for Cyp2c44 in the inhibition of renal Na+ transport during increasing Na+ and K+ intake, we next investigated the effect of increasing Na+ or K+ intake on the relative expression of Cyp2c44 in the above nephron segments. As shown in Fig. 2, high Na+ intake significantly increased Cyp2c44 expression in the TAL (95 ± 20%) and DCT/CNT (295 ± 100%, n = 5) but not in the PCT and CCD/OMCD. On the other hand, HK intake increased Cyp2c44 expression in the DCT/CNT and CCD/OMCD by 395 ± 100% and 196 ± 50% (n = 4), respectively, but not in the PCT and TAL segments. Thus, HK intake but not high Na intake stimulated Cyp2c44 expression in the CCD/OMCD. Moreover, in CD-specific Cyp2c44 conditional KO mice, high Na+ intake still increased Cyp2c44 expression in the TAL by 140 ± 20% (n = 4) and in the DCT/CNT by 350 ± 100% (n = 4), respectively (Fig. 3A). Also, HK intake increased Cyp2c44 expression only in the DCT/CNT (340 ± 100%, n = 3; Fig. 3A), whereas no Cyp2c44 expression was detected in the CD of KO mice under the presented experimental conditions (Fig. 3B).
Fig. 2.
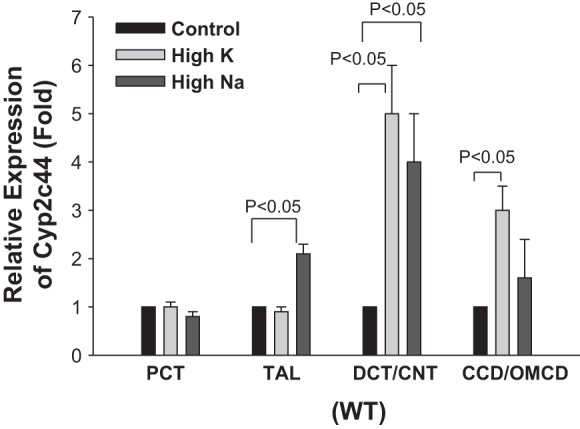
Relative expression of Cyp2c44 in the proximal convoluted tubule (PCT), thick ascending limb (TAL), distal convoluted tubule (DCT)/connecting tubule (CNT), and cortical collecting duct (CCD)/outer medullary collecting duct (OMCD) from mice on a normal salt diet (0.3% NaCl and 1.0% KCl), high-salt diet (4% NaCl), or high-K+ (HK) diet (5% KCl) for 3 days. n = 5 mice.
Fig. 3.
A: relative expression of Cyp2c44 in the TAL and DCT/CNT from collecting duct (CD)-specific Cyp2c44 conditional KO mice on a normal salt diet (0.3% NaCl and 1.0% KCl), high-salt diet (4% NaCl), or HK diet (5% KCl) for 3 days. n = 3–4 mice. B: agarose gel showing the expression of mouse Cyp2c44 (179 bp) amplified with real-time PCR (30 cycles) in the PCT and CD of KO mice. GAPDH mRNA isolated from the corresponding tubules was used as a control.
To determine the effect of Cyp2c44 expression in the CD on the BP of mice on high-Na+ or HK diets, we measured BPs in age-matched WT and CD-specific Cyp2c44 KO mice using the carotid artery catheter or noninvasive tail-cuff methods. Since the results were similar, we pooled the data. KO mice had normal body weights compared with WT mice (Table 1), and the mean systolic BP of 3-day measurements was 126 ± 5 mmHg (n = 9 mice), which was not significantly different from control WT mice (123 ± 5 mmHg, n = 16 mice). Moreover, neither high Na+ intake (Fig. 4A) nor HK intake (Fig. 4B) for 3 days had a significant effect on BP in WT mice. Mean systolic BPs of 3-day measurements for WT mice on high-Na+ and HK diets were 127 ± 5 and 125 ± 4 mmHg, respectively (Fig. 4). Whereas the mean systolic BP of CD-specific Cyp2c44 KO mice was not significantly different from that of WT mice under control conditions, an increase in dietary K+ intake significantly increased systolic BP of CD-specific Cyp2c44 KO mice, and the mice remained hypertensive for 3 days during increased K+ intake (HK day 1: 144 ± 4 mmHg, HK day 2: 146 ± 5 mmHg, and HK day 3: 148 ± 5 mmHg, n = 8 mice; Fig. 4B). In contrast, an increase in dietary Na+ intake for 3 days did not significantly affect BP in KO mice (high Na+ day 1: 130 ± 5 mmHg, high Na+ day 2: 122 ± 3 mmHg, and high Na+ day 3: 125 ± 5 mmHg, n = 8 mice; Fig. 4A). Also, neither heart rate nor diastolic BP was altered by dietary K+ or Na+ intake in both WT and KO mice (data not shown). Thus, HK intake but not a high Na+ intake increased systolic BP in CD-specific Cyp2c44 KO mice but not WT mice. This finding recapitulated previous experiments in which HK intake raised BP in global Cyp2c44 KO mice (36).
Table 1.
Body weights of wild-type and knockout mice on 1% and 5% K+ diets
| Diet | Wild-Type Mice | Knockout Mice |
|---|---|---|
| Day 1 (1% K+ diet) | 25.5 ± 0.4 | 26.4 ± 0.5 |
| Day 2 (1% K+ diet) | 25.4 ± 0.3 | 26.5 ± 0.4 |
| Day 3 (1% K+ diet) | 25.6 ± 0.4 | 26.6 ± 0.3 |
| Day 4 (5% K+ diet) | 25 ± 0.3 | 26.9 ± 0.3 |
| Day 5 (5% K+ diet) | 24.6 ± 0.2 | 27.8 ± 0.4* |
| Day 6 (5% K+ diet) | 24.9 ± 0.4 | 27.3 ± 0.3 |
Values are means ± SE of body weight (in g).
Value is significantly high compared with any other groups (P < 0.05).
Fig. 4.
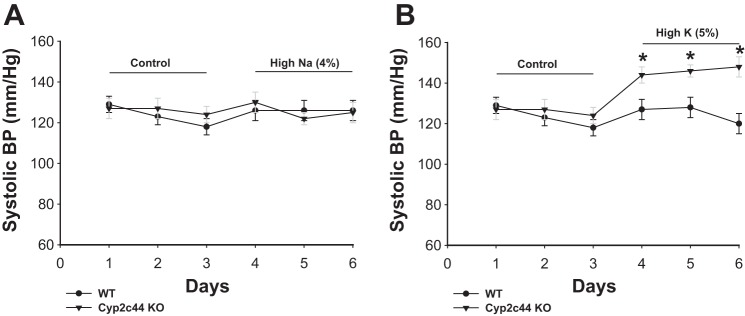
A: line graph showing the effect of increasing Na+ on systolic blood pressure (BP) in WT and KO mice. Mice were fed normal salt or high-salt (4% NaCl) diets for 3 days. BP was measured with carotid artery catheter and tail-cuff methods. n = 8 mice. B: line graph showing the effect of increasing K+ intake on systolic BP in WT and CD-specific Cyp2c44 KO mice. Mice were fed normal K+ (1.0% KCl) or HK (5% KCl) diets for 3 days. BP was measured with carotid artery catheter and tail-cuff methods. n = 8 mice. *Significant difference between WT and KO mice.
Because HK-induced hypertension in global Cyp2c44 KO mice was the result of compromised renal ability to excrete Na+ (36), we suspected that CD-specific Cyp2c44 KO mice might also have diminished renal ability to excrete Na+ during increasing dietary K+ intake. To test this hypothesis, we carried out metabolic cage experiments with WT and KO mice, respectively. The results are shown in Fig. 5 and demonstrate that 24-h UNa of WT mice on control diets was similar to that of CD-specific Cyp2c44 KO mice. At day 3, Na+ intake and urinary Na+ excretion were 25.8 ± 0.5 and 17.8 ± 0.8 μeqm/g body wt in WT mice on the control diet (Fig. 5), whereas they were 24.1 ± 0.5 and 17.3 ± 0.7 μeqm/g body wt in KO mice. An increase in dietary K+ intake did not significantly affect the ratio between Na+ intake (HK day 1: 23 ± 0.4 μeqm/g body wt, HK day 2: 22.6 ± 0.5 μeqm/g body wt, and HK day 3: 23.5 ± 0.5 μeqm/g body wt) and UNa (HK day 1: 16.5 ± 0.8 μeqm/g body wt, HK day 2: 17 ± 0.8 μeqm/g body wt, and HK day 3: 17 ± 0.8 μeqm/g body wt) in WT mice (Fig. 5). However, disruption of Cyp2c44 in the CD decreased Na+ excretion in CD-specific Cyp2c44 KO mice on a HK (5% K+) diet compared with those on a 1% K+ diet. As shown in Fig. 5, UNa was reduced from 17.3 ± 0.7 to 6.5 ± 0.6 μeqm/g body wt, whereas Na+ intake was similar in KO mice on the 5% K+ diet (day 1: 22.8 ± 0.4 μeqm/g body wt) compared with the 1% K+ diet (day 1: 24.1 ± 0.5 μeqm/g body wt). Also, the HK diet significantly increased the body weight of CD-specific Cyp2c44 KO mice from 26.6 ± 0.3 to 27.8 ± 0.4 g (HK day 2), whereas it had no significant effect on the body weight of WT mice (Table 1). This suggests that CD-specific Cyp2c44 KO mice may have renal Na+ retention and extracellular volume expansion.
Fig. 5.
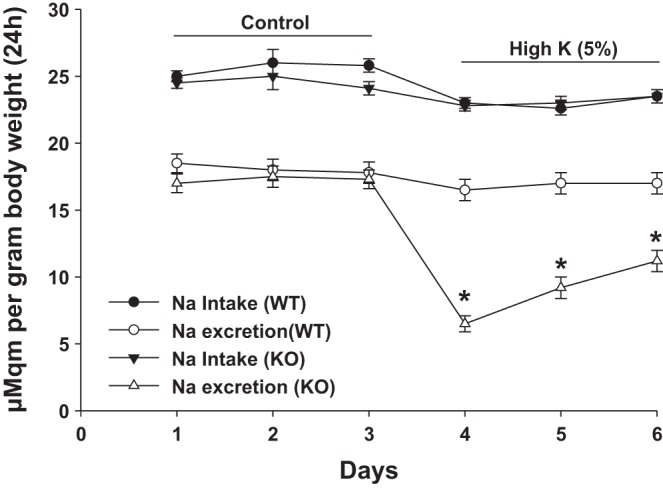
Line graph showing dietary Na+ intake and urinary Na+ excretion rate (UNa) for 24 h in WT and CD-specific Cyp2c44 KO mice on normal K+ (control) or HK diets. *Significant difference between WT and KO mice. Two mice were placed in a metabolic cage, and 24-h food intake and UNa were recorded. Mice were kept on an normal K+ diet for 3 days before being switched to the HK diet for an additional 3 days. n = 8 mice.
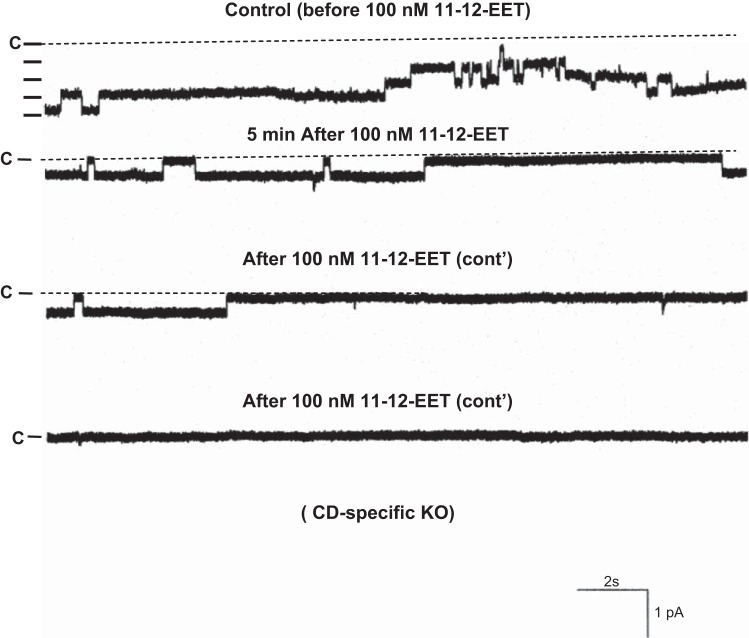
The observation that the ability to excrete Na+ was compromised in CD-specific Cyp2c44 KO mice strongly suggested the possibility that disruption of Cyp2c44 may increase ENaC activity. Therefore, we used the patch-clamp technique to examine the effect of AA on apical ENaC activity in the split-open CCD of WT and KO mice (Fig. 6). We confirmed the previous finding that AA inhibited ENaC in the CCD of WT mice on a HK diet and decreased NPo from 1.2 ± 0.2 to 0.3 ± 0.1 (n = 6). In contrast, AA failed to inhibit ENaC in CD-specific Cyp2c44 KO mice on a HK diet (control: 2.6 ± 0.4 and AA: 2.5 ± 0.4, n = 7). However, the addition of 100 nM 11,12-EET was still able to inhibit ENaC in the CCD of KO mice. Figure 7 shows a recording demonstrating the effect of 100 nM 11,12-EET on ENaC in a cell-attached patch of the CCD dissected from KO mice. As shown in Fig. 7, the addition of 100 nM 11,12-EET decreased ENaC activity from 2.5 ± 0.4 to 0.2 ± 0.1 (n = 7). These findings are similar to what was reported in global Cyp2c44−/− mice in which HK intake increased ENaC activity in the CCD compared with WT mice (36). This indicates that Cyp2c44-dependent metabolites suppressed the effect of aldosterone on ENaC in an aldosterone-sensitive distal nephron (ASDN) such as the CCD. This view was also supported by the previous finding that low Na+ intake for 3 days increased ENaC activity higher in the CCD of Cyp4a10−/− mice compared with WT mice (22). Thus, our results indicate that Cyp2c44 was responsible for mediating the inhibitory effect of AA on ENaC and that lack of functional Cyp2c44 epoxygenase increased ENaC activity in the CCD. The possibility that the upregulated ENaC activity was responsible for the HK-induced hypertension in CD-specific Cyp2c44 KO mice was also suggested by experiments in which the effect of HK intake on the BP of KO mice treated with amiloride was examined. The results are shown in Fig. 8 and demonstrate that the addition of amiloride in the drinking water of CD-specific Cyp2c44 KO mice abolished HK intake-induced hypertension and reduced systolic BP from 146 ± 5 to 117 ± 3 mmHg, whereas amiloride had no effect on BP in WT mice (n = 5 mice).
Fig. 6.
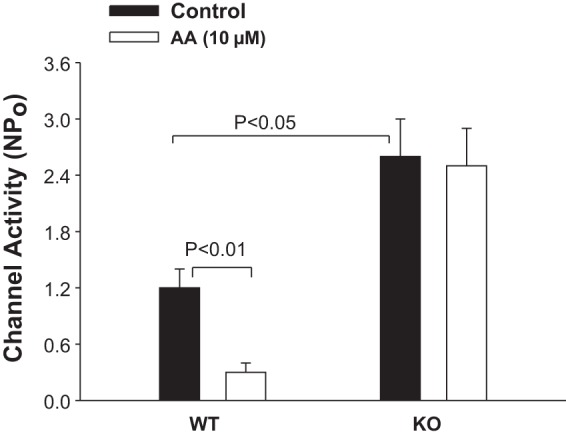
Bar graph summarizing the experiments in which the effect of 10 μM arachidonic acid (AA) on the epithelial Na+ channel (ENaC) in the CCD was examined. n = 11–12 patches from 5 WT or KO mice. Mice were fed with a 5% K+-containing diet for 3 days. Experiments were performed in cell-attached patches. Pipette and bath solutions contained 140 mM K+ and 140 mM Na+-5 mM K+, respectively. Channel activity (NPo) was measured with the cell holding potential at −60 mV.
Fig. 7.
Representative channel traces demonstrating channel activity in the CCD of CD-specific Cyp2c44 KO mice on a HK diet in the absence of 100 nM 11,12-EET (top trace) and in the presence of 100 nM 11,12-EET (bottom three traces). The gap between the top and bottom three traces is 5 min. Experiments were performed in cell-attached patches, and the holding potential was −60 mV. n = 6 patches from 4 mice.
Fig. 8.
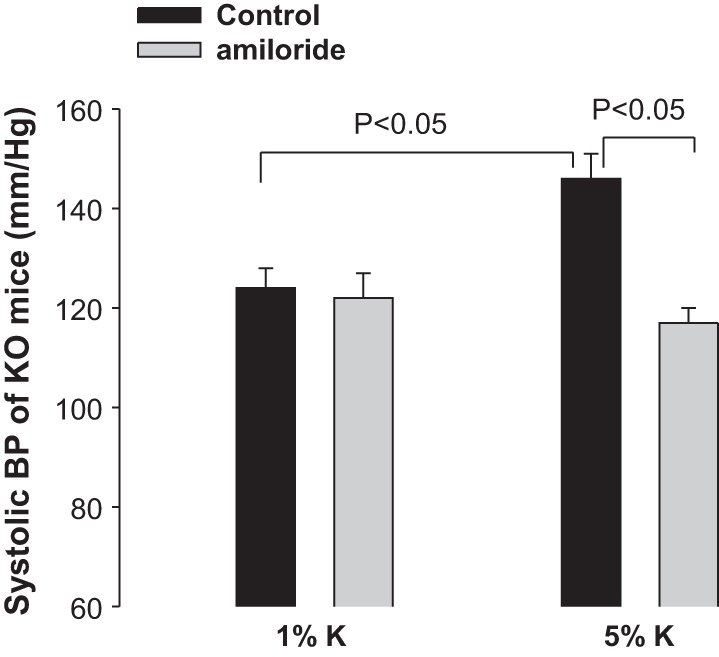
Effect of amiloride application on systolic BP of CD-specific Cyp2c44 KO mice on 1% K+ or 5% K+ diets. n = 5 mice. Amiloride (0.5 mg/100 g body wt) was administered through drinking water. BP was measured with the tail-cuff method.
DISCUSSION
A large body of evidence has suggested that Cyp2c epoxygenase-dependent AA metabolism plays a role in the regulation of renal Na+ transport, in the prevention of salt-sensitive hypertension, and in the modulation of vascular tissue activity (6, 7, 22, 40, 44). It has been reported that high Na+ intake stimulated the renal expression of Cyp2c44 in mice or CYP2C23 in rats (the homolog of mouse Cyp2c44) and increased urinary excretion of EET, an indication of enhanced Cyp epoxygenase activity (22, 35, 37). Moreover, inhibition of Cyp epoxygenase caused salt-sensitive hypertension in rats (7, 17). Thus, these results strongly indicated that Cyp epoxygenase-dependent AA metabolites were involved in the inhibition of renal Na+ transport during increasing Na+ intake. Cyp epoxygenases convert AA to EET, such as 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET (3, 28, 42). We and others (26, 36, 37, 40) have demonstrated that 11,12-EET inhibits ENaC. ENaC plays a key role in mediating Na+ absorption in the ASDN by providing a Na+ entry pathway across the apical membrane (5, 16, 24, 25, 27), and it is the rate-limiting step of transepithelial Na+ transport in the ASDN (14). An increase in either ENaC number or channel open probability augments the apical Na+ conductance and leads to increases in Na+ absorption. For instance, Liddle's syndrome, an inherited form of hypertension, is the result of increasing apical ENaC expression in the ASDN (32, 33).
In addition to high Na+ intake, a previous study (36) has demonstrated that an increase in dietary K+ intake augments the expression of Cyp2c44 or its rat homolog. An increase in dietary K+ intake has been shown to prevent high salt intake-induced hypertension in both human and rats (15, 19, 20). The guidelines published in the Dietary Approaches to Stop Hypertension recommend all healthy adults to double their daily K+ intake to prevent salt-sensitive hypertension; however, the underlying mechanisms by which HK intake decreases BP are not completely understood. Factors such as kallikrein (9) and prostaglandins (23) have been suggested to play a role in decreasing BP and increasing renal Na+ excretion. Because increased dietary K+ intake suppresses the renin and angiotensin II pathway (30, 31), it is also possible that inhibition of the renin-angiotensin system is responsible for the HK-induced stimulation of renal Na+ excretion. HK intake significantly decreases the generation of superoxide anions by inhibition of NADPH oxidase activity in cardiovascular tissues (12, 18). Also, dietary K+ intake has been shown to alter NADPH oxidase activity and superoxide production in the kidney (1). Excessive generation of superoxide anions might be partially responsible for salt-induced hypertension by increasing Na+ absorption (10, 11, 38, 41).
The role of Cyp2c44 in mediating the effect of HK intake on renal Na+ excretion has been suggested by our previous experiments in which global disruption of the Cyp2c44 gene increased ENaC activity and decreased renal Na+ excretion (36). However, since ENaC is expressed in the late DCT, CNT, CCD, and OMCD (29), it is not clear whether the DCT/CNT or CCD/OMCD is the main target for suppression of Na+ absorption during increasing dietary K+ intake. The finding that lack of functional Cyp2c44 epoxygenase in the CCD/OMCD impairs renal Na+ excretion and causes hypertension during increased dietary K+ intake strongly indicates a role of Cyp2c44 in the CCD/OMCD in the regulation of ENaC activity and renal Na+ excretion during HK intake. This conclusion is supported by the following three lines of evidence: 1) feeding CD-specific Cyp2c44 KO mice with a HK diet recapitulated the hypertension phenotype observed in global Cyp2c44 KO mice, 2) HK intake stimulated the expression of Cyp2c44 in the DCT/CNT and CD, and 3) administration of amiloride normalized BPs in CD-specific Cyp2c44 KO mice. Thus, inhibition of Na+ absorption in the CD by Cyp2c44 epoxygenase-dependent metabolites is critically involved in mediating the HK-induced antihypertension effect.
The second important finding of the present study is that high salt intake for 3 days failed to cause hypertension in CD-specific Cyp2c44 conditional KO mice. Because high salt caused hypertension in global Cyp2c44 KO mice (2, 22), this suggests the possibility that Cyp2c44 activity in the CD might not be responsible for inhibition of Na+ transport during increasing Na+ intake. The fact that administration of amiloride restored normal BP in global Cyp2c44 KO mice indicates that the salt-sensitive hypertension in these mice was due to hyperactive ENaC in the late DCT and CNT rather than the CD (2, 22). This conclusion is also supported by the finding that high Na+ intake significantly increased Cyp2c44 expression in the TAL and DCT/CNT but not in the CD. The observation that Cyp2c44 expression in the TAL and DCT/CNT was upregulated by high Na+ intake suggests that Cyp2c44 in the TAL and DCT/CNT rather than in the CD may play a role in the inhibition of Na+ absorption during increasing dietary Na+ intake. High Na+ intake is expected to suppress aldosterone secretion and ENaC activity in the CD. Accordingly, the role of Cyp2c44-dependent AA metabolites in the inhibition of Na+ absorption in the CD is expected to be minimal in CD-specific Cyp2c44 KO mice. In contrast, a HK diet would increase aldosterone and ENaC expression in the CD (4, 39). Moreover, HK intake has been shown to decrease Na+-Cl− cotransporter (NCC) activity by the dephosphorylation of NCC and to reduce NCC expression in the DCT (4, 34). A decrease in NCC activity is expected to increase Na+ delivery to the CCD, thereby stimulating Na+ absorption through ENaC. Because HK intake also stimulates the expression of Cyp2c44 homologs in the CD, high expression of Cyp2c44 in the CD would prevent excessive Na+ absorption in the CD. Thus, a disruption of Cyp2c44 in the CD would enhance Na+ absorption in the CD, thereby increasing BP in mice on a HK diet. However, we need further experiments to examine the role of Cyp2c44 in the suppression of Na+ transport in the CCD of mice on long-term high salt intake. In addition, it is possible that EETs generated in the CD could also influence the membrane transport in a neighboring tubule, such as the medullary thick ascending limb, through a paracrine pathway. Thus, the disruption of Cyp2c44 in the CD not only increases ENaC activity in the CD but also enhances Na+ transport in neighboring tubules.
During balanced Na+ intake and excretion, Cyp2c epoxygenases contribute to the maintenance of Na+ homeostasis, at least partially, by regulating ENaC activity along the ASDN. Increased dietary Na+ or K+ intake facilitates Na+ excretion by inducing Cyp2c44 epoxygenase and EET biosynthesis in the early ASDN (DCT/CNT) or late ASDN (CCD/OMCD), respectively. These Cyp2c44-mediated increases in EET biosynthesis inhibit ENaC in the corresponding nephron segments, leading to increased Na+ excretion and stable systemic BPs. Impairments in Cyp2c44 expression along the ASDN increase ENaC activity, reduce urinary Na+ excretion, and result in Na+ retention and dietary Na+- or K+-sensitive hypertension. We conclude that Cyp2c44 in the CD plays a key role in stimulating renal Na+ excretion during increasing dietary K+ intake and that Cyp epoxygenase is required for adequate Na+ excretion in the CD and the antihypertensive effect induced by HK intake.
GRANTS
The work was supported by National Institutes of Health (NIH) Grant DK-38226 (to J. H. Capdevila) and P01-HL-34300 (W.-H. Wang). This work was also supported, in part, by the Intramural Research Program of the NIH under NIH Grant Z01-ES-025034 (to D. C. Zeldin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.-H.W. and J.H.C. conception and design of research; W.-H.W., C.Z., D.L., L.W., J.P.G., and J.H.C. performed experiments; W.-H.W., C.Z., D.L., L.W., J.P.G., D.C.Z., and J.H.C. analyzed data; W.-H.W., C.Z., D.L., L.W., J.P.G., D.C.Z., and J.H.C. interpreted results of experiments; W.-H.W. and D.L. prepared figures; W.-H.W. drafted manuscript; W.-H.W. and J.H.C. edited and revised manuscript; W.-H.W., C.Z., D.L., L.W., J.P.G., D.C.Z., and J.H.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Artiom Gruzdev and the National Institute of Environmental Health Sciences Knockout Core for helpful suggestions.
REFERENCES
- 1.Babilonia E, Lin D, Zhang Y, Wei Y, Yue P, Wang WH. Role of gp91phox-containing NADPH oxidase in mediating the effect of K restriction on ROMK channels and renal K excretion. J Am Soc Nephrol 18: 2037–2045, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capdevila JH, Pidkovka N, Mei S, Gong Y, Falck JR, Imig JD, Harris RC, Wang W. The Cyp2c44 epoxygenase regulates epithelial sodium channel activity and the blood pressure responses to increased dietary salt. J Biol Chem 289: 4377–4386, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLozier TC, Tsao CC, Coulter SJ, Foley J, Bradbury JA, Zeldin DC, Goldstein JA. CYP2C44, a new murine CYP2C that metabolizes arachidonic acid to unique stereospecific products. J Pharmacol Exp Ther 310: 845–854, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Frindt G, Palmer LG. Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299: F890–F897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garty H, Palmer LG. Epithelial sodium channel: function, structure, and regulation. Physiol Rev 77: 359–396, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Hirt DL, Capdevila J, Falck JR, Breyer MD, Jacobson HR. Cytochrome P450 metabolites of arachidonic acid are potent inhibitors of vasopressin action on rabbit cortical collecting duct. J Clin Invest 84: 1805–1812, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holla VR, Makita K, Zaphiropoulos PG, Capdevila JH. The kidney cytochrome P450 2C23 arachidonic acid epoxygenase is upregulated during dietary salt loading. J Clin Invest 104: 751–760, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imaoka S, Wedlund PJ, Ogawa H, Kimura S, Gonzalez FJ, Kim HY. Identification of CYP2C23 expressed in rat kidney as an arachidonic acid epoxygenase. J Pharmacol Exp Ther 267: 1012–1016, 1993 [PubMed] [Google Scholar]
- 9.Jin L, Chao L, Chao J. Potassium supplement upregulates the expression of renal kallikrein and bradykinin B2 receptor in SHR. Am J Physiol Renal Physiol 276: F476–F484, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol 288: F982–F987, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Juncos R, Hong NJ, Garvin JL. Differential effects of superoxide on luminal and basolateral Na+/H+ exchange in the thick ascending limb. Am J Physiol Regul Integr Comp Physiol 290: R79–R83, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kido M, Ando K, Onozato ML, Tojo A, Yoshikawa M, Ogita T, Fujita T. Protective effect of dietary potassium against vascular injury in salt-sensitive hypertension. Hypertension 51: 225–231, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132: 2809–2823, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Koeppen BM, Stanton BA. Sodium chloride transport. In: The Kidney: Physiology and Pathophysiology, edited by Seldin DW, Giebisch G. New York: Raven, 1992, p. 2003–2039 [Google Scholar]
- 15.Lawton WJ, Fitz AE, Anderson EA, Sinkey CA, Coleman RA. Effect of dietary potassium on blood pressure, renal function, muscle sympathetic nerve activity, and forearm vascular resistance and flow in normotensive and borderline hypertensive humans. Circulation 81: 173–184, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Light DB, McCann FV, Keller TM, Stanton BA. Amiloride-sensitive cation channel in apical membrane of inner medullary collecting duct. Am J Physiol Renal Physiol 255: F278–F286, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Makita K, Takahashi K, Kerara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest 94: 2414–2420, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui H, Shimosawa T, Uetake Y, Wang H, Ogura S, Kaneko T, Liu J, Ando K, Fujita T. Protective effect of potassium against the hypertensive cardiac dysfunction: association with reactive oxygen species reduction. Hypertension 48: 225–231, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Mervaala EM, Himberg JJ, Laakso J, Tuomainen P, Karppanen H. Beneficial effects of a potassium- and magnesium-enriched salt alternative. Hypertension 19: 535–540, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Morris RC, Jr, Sebastian A, Forman A, Tanaka M, Schmidlin O. Normotensive salt sensitivity: effects of race and dietary potassium. Hypertension 33: 18–23, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Muller DN, Theuer J, Shagdasuren E, Kaergel E, Honeck H, Park JK, Markovic M, Barbosa-Sicard E, Dechend R, Wellner M, Kirsch T, Fiebeler A, Rothe M, Haller H, Luft FC, Schunck WH. A peroxisome proliferator-activated receptor-alpha activator induces renal CYP2C23 activity and protects from angiotensin II-induced renal injury. Am J Pathol 164: 521–532, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price EJ, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt sensitive hypertension is associated with a dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest 116: 1696–1702, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasjletti A, Erman A, Cagen LM, Brooks DP, Crofton JT, Share L, Baer PG. High potassium intake selectively increases urinary PGF2α excretion in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 248: F382–F388, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Palmer LG, Frindt G. Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting duct. Proc Natl Acad Sci USA 83: 2767–2770, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer LG, Frindt G. Effect of cell Ca and pH on Na channels from rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 253: F333–F339, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Pavlov TS, Ilatovskaya DV, Levchenko V, Mattson DL, Roman RJ, Staruschenko A. Effects of cytochrome P-450 metabolites of arachidonic acid on the epithelial sodium channel (ENaC). Am J Physiol Renal Physiol 301: F672–F681, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pochynyuk O, Tong Q, Medina J, Vandewalle A, Staruschenko A, Bugaj V, Stockand JD. Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation of the epithelial Na+ channel. J Gen Physiol 130: 399–413, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roman RJ. P450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, Carroll T, McMahon A, Hummler E, Rossier BC. Collecting duct-specific gene inactivation of αENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest 112: 554–565, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saikaley A, Bichet D, Kucharczyk J, Peterson LN. Neuroendocrine factors mediating polydipsia induced by dietary Na, Cl, and K depletion. Am J Physiol Regul Integr Comp Physiol 251: R1071–R1077, 1986 [DOI] [PubMed] [Google Scholar]
- 31.Sealey JE, Clark I, Bull MB, Laragh JH. Potassium balance and the control of renin secretion. J Clin Invest 49: 2119–2127, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimkets RA. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Snyder PM, Price MP, McDonald FJ, Adams CM, Volk KA, Zeiher BG, Stokes JB, Welsh MJ. Mechanism by which Liddle's syndrome mutations increase activity of a human epithelial Na+ channel. Cell 83: 969–978, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Sun P, Lin DH, Wang T, Babilonia E, Wang ZJ, Jin Y, Kemp R, Nasjletti A, Wang WH. Low Na intake suppresses expression of CYP2C23 and arachidonic acid-induced inhibition of ENaC. Am J Physiol Renal Physiol 291: F1192–F1200, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Sun P, Antoun J, Lin DH, Yue P, Gotlinger KH, Capdevila J, Wang WH. Cyp2c44 epoxygenase is essential for preventing the renal sodium absorption during increasing dietary potassium intake. Hypertension 59: 339–347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun P, Lin DH, Yue P, Jiang H, Gotlinger KH, Schwartzman ML, Falck JR, Goli M, Wang WH. High potassium intake enhances the inhibitory effect of 11,12-EET on ENaC. J Am Soc Nephrol 21: 1667–1677, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor NE, Cowley AW., Jr Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol 289: R1573–R1579, 2005 [DOI] [PubMed] [Google Scholar]
- 39.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl− cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Wei Y, Lin DH, Kemp R, Yaddanapudi GSS, Nasjletti A, Falck JR, Wang WH. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol 124: 719–727, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem 276: 36059–36062, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Mernaugh G, Yang DH, Gewin L, Srichai MB, Harris RC, Iturregui JM, Nelson RD, Kohan DE, Abrahamson D, F+ñssler R, Yurchenco P, Pozzi A, Zent R. +¦1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development 136: 3357–3366, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension 41: 709–714, 2003 [DOI] [PubMed] [Google Scholar]