Abstract
While disruption of energy production is an important contributor to renal injury, metabolic alterations in sepsis-induced AKI remain understudied. We assessed changes in renal cortical glycolytic metabolism in a mouse model of sepsis-induced AKI. A specific and rapid increase in hexokinase (HK) activity (∼2-fold) was observed 3 h after LPS exposure and maintained up to 18 h, in association with a decline in renal function as measured by blood urea nitrogen (BUN). LPS-induced HK activation occurred independently of HK isoform expression or mitochondrial localization. No other changes in glycolytic enzymes were observed. LPS-mediated HK activation was not sufficient to increase glycolytic flux as indicated by reduced or unchanged pyruvate and lactate levels in the renal cortex. LPS-induced HK activation was associated with increased glucose-6-phosphate dehydrogenase activity but not glycogen production. Mechanistically, LPS-induced HK activation was attenuated by pharmacological inhibitors of the EGF receptor (EGFR) and Akt, indicating that EGFR/phosphatidylinositol 3-kinase/Akt signaling is responsible. Our findings reveal LPS rapidly increases renal cortical HK activity in an EGFR- and Akt-dependent manner and that HK activation is linked to increased pentose phosphate pathway activity.
Keywords: lipopolysaccharide, acute kidney injury, hexokinase, EGFR, pentose phosphate pathway
acute kidney injury (aki) is defined as an abrupt decline in renal function which occurs over a period of hours to days (7). A number of stimuli may lead to the development of AKI, including sepsis, ischemia-reperfusion (I/R) injury, trauma, or exposure to nephrotoxic agents. Among the leading causes of AKI, sepsis is thought to be the most common contributor (∼50% of all cases) in the intensive care unit (ICU) setting (64). Despite increased understanding of the pathophysiology underlying AKI, mortality from the disease remains near 40% and has not changed over several decades (27, 60, 65). AKI in the setting of sepsis increases this mortality by almost twofold (48, 65). Unfortunately, currently available treatments for sepsis-induced AKI include supportive care and renal replacement therapy. A better understanding of the mechanisms leading to renal damage as well as recovery in septic AKI is essential for development of therapeutic strategies to improve outcomes in this disease.
The pathophysiology of sepsis-induced AKI is widely recognized as multifactorial, involving microvascular, immunological, and tubular components that contribute to renal dysfunction. The microvascular component is characterized by reduced capillary flow, leading to local areas of hypoperfusion (29, 30, 66). Infiltration of both macrophages and neutrophils also exposes the septic kidney to a diverse array of proinflammatory factors (56, 72). Finally, tubular damage characterized by tubular cell vacuolization, mild tubular dilatation, and mitochondrial swelling has been noted in septic AKI (57, 62). Although histopathology in sepsis-induced AKI appears modest, preservation of renal function depends heavily on the proximal tubule for reabsorption and secretion of solutes, including sodium, glucose, and amino acids, from the glomerular filtrate via active transport processes. Therefore, any pathological condition resulting in reduced ATP levels may contribute to tubular deenergization and subsequent loss of renal function characteristic of AKI (51, 55).
Our laboratory demonstrated that I/R- and glycerol-induced AKI leads to rapid and sustained mitochondrial dysfunction in the renal cortex, characterized by suppressed expression of mitochondrial biogenesis markers and electron transport chain components at the mRNA and protein levels (16). Disruption of normal mitochondrial homeostasis in these models was closely associated with proximal tubule cell injury and loss of renal function (16). Tran et al. (62) recently reported similar findings following systemic exposure to LPS (a component of gram-negative bacterial cell walls), a well-established model of sepsis-induced AKI. In particular, LPS administration resulted in a marked reduction in cytochrome c oxidase activity and protein levels as well as downregulation of a number of mitochondrial genes in the renal cortex. Suppression of mitochondrial mRNAs correlated with renal function as measured by blood urea nitrogen (BUN) (62). LPS-induced mitochondrial dysfunction results in a decline (∼50%) in renal ATP content within 5 h of exposure in rodents, likely contributing to changes in renal function in this model of septic AKI (32). Disruption of renal mitochondrial function and ATP production has also been reported in the cecal ligation and puncture model (CLP) of sepsis (40). What remains understudied are metabolic alterations which may facilitate both proximal tubule cell energy production and transport function in the presence of mitochondrial suppression.
Under normal conditions in vivo, renal proximal tubule cells (RPTC) primarily utilize oxidative phosphorylation via mitochondria to generate ATP and have very low glycolytic capacity. Early work characterizing renal metabolism demonstrated the proximal tubule possesses the lowest activities of glycolytic enzymes, including hexokinase (HK), phosphofructokinase (PFK), and pyruvate kinase (PK) (the 3 major regulatory steps), along the entire nephron segment (19, 70). These findings have led to considerable debate concerning the ability of the proximal tubule to induce glycolytic metabolism as an alternate means of ATP generation. However, both in vitro and in vivo work indicate that a variety of stressors including ischemia-hypoxia, nephrotoxicants, elevated intracellular calcium, and inhibition of mitochondrial respiration may increase glycolytic flux in the proximal tubule (4, 14, 20). Glycolytic induction appears to play an important role in maintaining tubular cell viability, producing ATP, and preserving transport processes (14, 47, 72). In addition, recent studies have demonstrated that glycolytic metabolism is rapidly increased in the cortex in response to renal ischemia in vivo (75). These findings indicate that glycolysis may provide an alternate means of energy generation in response to tubular insult.
We hypothesized that glycolysis would be rapidly induced in the renal cortex in response to LPS-induced renal injury. To test this hypothesis, an extensive characterization of renal cortical glycolytic enzyme activity and expression after LPS challenge was performed. We report herein that LPS exposure results in a rapid (within 3 h) and specific induction of HK activity mediated by the EGF receptor (EGFR)/phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway. Surprisingly, HK activation by LPS was not sufficient to enhance glycolysis in the renal cortex but was associated with upregulation of the pentose phosphate pathway.
METHODS
Animal model.
Male C57BL/6 mice (6–8 wk of age, 20–25 g body wt) were obtained from the National Institutes of Health National Cancer Institute (Bethesda, MD). Mice received an intraperitoneal injection of 10 mg/kg LPS from Escherichia coli O111:B4 (catalog no. L4130, lot no. 052M4016V, Sigma-Aldrich) as previously described (62). Control mice were injected intraperitoneally (ip) with an equal volume of 0.9% saline vehicle. Mice were euthanized at 1, 3, 6, and 18 h after LPS injection, and serum and kidneys were collected for biochemical analysis. All experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animal use was approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina.
Administration of gefitinib and MK-2206.
For studies examining the role of EGFR and PI3K/Akt signaling in LPS-induced renal injury, the EGFR inhibitor gefitinib and the Akt inhibitor MK-2206 were obtained from Selleckchem Chemicals (Houston, TX) (12, 23). Mice were randomly assigned to four groups: 1) saline+vehicle control, 2) LPS+vehicle control, 3) LPS+gefitinib (100 mg/kg), or 4) LPS+MK-2206 (100 mg/kg), and inhibitors were administered ip 1 h before LPS administration. Dosing of gefitinib and MK-2206 was based on previous studies demonstrating inhibition of EGFR and Akt in mouse models (21, 33).
Routine biochemical assays.
Blood urea nitrogen (BUN) was measured using a QuantiChrom Urea Assay Kit (BioAssay Systems, Hayward, CA) according to the manufacturer's protocol. Values are expressed as BUN concentration in milligrams per deciliter.
Renal cortical HK, PFK, PK, and glucose-6-phosphate dehydrogenase (G6PDH) activities and glucose, pyruvate, lactate, and glycogen contents were determined using kits from BioVision (Milpitas, CA) according to the manufacturer's protocol. Briefly, renal cortical tissue was homogenized and centrifuged at 14,000 g for 10 min to clear debris. Values were normalized to protein content (determined by BCA assay) or wet tissue weight as described in each figure.
Analysis of mRNA expression.
Total RNA was isolated from renal cortex samples using the TRIzol reagent (Life Technologies, Grand Island, NY). A cDNA library was generated from 2 μg RNA by reverse transcription reaction using an iScript Advanced cDNA Synthesis Kit for RT-qPCR according to the manufacturer's protocol (Bio-Rad, Hercules, CA). Quantitative real-time PCR was then performed with cDNA using the SsoAdvanced SYBR Green Supermix (Bio-Rad). mRNA expression of genes of interest was calculated by the 2−ΔΔCt method and normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) as previously described (67). Primer sequences used for mRNA analysis are shown in Table 1.
Table 1.
Primers used in mRNA analysis
| Gene | Primer Sequence | Accession No. |
|---|---|---|
| HPRT | ||
| Sense | 5′-GCTTACCTCACTGCTTTCCG-3′ | NM_013556 |
| Antisense | 5′-ATCATCGCTAATCACGACGC-3′ | |
| HK1 | ||
| Sense | 5′-GGGACTATGACGCTAACATT-3′ | NM_001146100 |
| Antisense | 5′-CCAGTGCCAATGATCAGG-3′ | |
| HK2 | ||
| Sense | 5′-GGTACAGAGAAAGGAGACTTC-3′ | NM_013820 |
| Antisense | 5′-TCTTGTTATGCATCTCTACGC-3′ | |
| HK3 | ||
| Sense | 5′-CACTTAACCAATCTCGGAGT-3′ | NM_001033245 |
| Antisense | 5′-AGGCTATCACTTTCGATCTC-3′ | |
| GLUT1 (Slc2a1) | ||
| Sense | 5′-AGATGAAAGAAGAGGGTCGG-3′ | NM_011400 |
| Antisense | 5′-AGAACACAGCATTGATACCC-3′ | |
| PFKL | ||
| Sense | 5′-GATGTCTACCGTAAAGGGCG-3′ | NM_008826 |
| Antisense | 5′-ACTACCTTCTTCCGCAGGC-3′ | |
| PFKM | ||
| Sense | 5′-CAATCTGCAAGAAAGCAGCG-3′ | NM_001163487 |
| Antisense | 5′-GCAGCATTCATACCTTGGGC-3′ | |
| PGK1 | ||
| Sense | 5′-CTGTGGTACTGAGAGCAGCAAGA-3′ | NM_008828 |
| Antisense | 5′-CAGGACCATTCCAAACAATCTG-3′ | |
| PKM | ||
| Sense | 5′-GGTGTTTGCATCTTTCATCCG-3′ | NM_001253883 |
| Antisense | 5′-AGATCTCATCAAACCTGCGG-3′ | |
| LDHA | ||
| Sense | 5′-CTTAATGAAGGACTTGGCGG-3′ | NM_010699 |
| Antisense | 5′-TGGTGTTTTAAGGAAGAGGC-3′ | |
| PDK1 | ||
| Sense | 5′-ATCTCATCGAAAGCACATTGGA-3′ | NM_172665 |
| Antisense | 5′-CCGCCTAGCGTTCTCATAGC-3′ |
Immunoblot analysis.
Tissue samples from the renal cortex were lysed in RIPA buffer (50 mM Tris·HCl, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, pH 7.4) containing protease inhibitor cocktail (1:100), 1 mM sodium orthovanadate, and 10 mM sodium fluoride (Sigma-Aldrich). Total protein content was determined by BCA protein assay. Equal amounts of protein (50–100 μg) were resolved on 4–15% SDS-PAGE gels (Bio-Rad). Proteins were then transferred to nitrocellulose membranes and blocked in 2.5% BSA for 1 h. Membranes were incubated with primary antibody overnight at 4°C. Primary antibodies included HK1, HK2, PFKP, PKM1/2, PDH, phospho-Akt (Ser 473), total Akt (1:1,000, all from Cell Signaling Technology, Danvers, MA), VDAC/porin (1:1,000, Abcam, Cambridge, MA), and β-actin (1:10,000, Santa Cruz Biotechnology, Dallas, TX). Membranes were then incubated in horseradish peroxidase (HRP)-conjugated secondary for 1 h at room temperature. Proteins were visualized using enhanced chemiluminescence reagents (Thermo Scientific, Waltham, MA) and the GE ImageQuant LAS4000 digital imaging system. National Institutes of Health ImageJ software (v. 1.46) was used to measure optical density.
Mitochondrial fractionation.
Mitochondria were isolated from whole kidneys by differential centrifugation as previously described (3). The resulting mitochondrial pellet was resuspended in RIPA buffer, and mitochondrial localization of HK1 and HK2 was determined by immunoblot analysis as indicated above. The purity of mitochondrial fractions was confirmed by immunoblots for nuclear (lamin B1), cytosolic (α-tubulin), and mitochondrial (VDAC) markers.
Statistical analysis.
Data are presented as means ± SE. Statistical comparisons between two groups were performed using an unpaired, two-tailed t-test. For multiple comparisons, a one-way ANOVA was performed followed by a Holm-Sidak post hoc test. A P value <0.05 was considered statistically significant. All statistical analysis was completed using GraphPad Prism software.
RESULTS
LPS increases renal cortical HK activity.
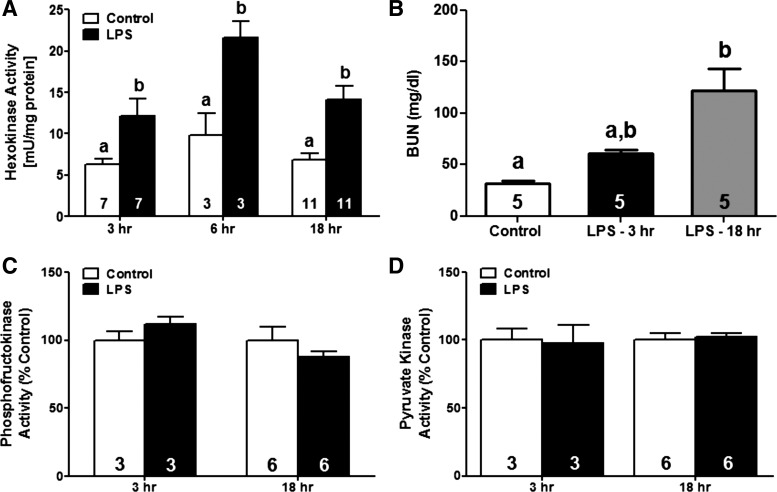
Because suppression of both mitochondrial function and gene expression has been reported in multiple animal models of AKI, including sepsis-induced AKI, we hypothesized that glycolytic metabolism would increase in the renal cortex following LPS exposure (16, 62). We first measured activities of the three major rate-limiting enzymes in the glycolytic pathway, HK, PFK, and PK, in the renal cortex at 3, 6, and 18 h after LPS exposure. Renal cortical HK activity increased approximately twofold as early as 3 h post-LPS and remained elevated for at least 18 h (Fig. 1A). However, PFK and PK activities were unchanged through 18 h (Fig. 1, C and D). BUN progressively increased from ∼32 mg/dl in vehicle-treated mice to 61 and 122 mg/dl at 3 and 18 h after LPS administration, respectively (Fig. 1B). Taken together, these findings indicate that HK is specifically and rapidly activated in the renal cortex of mice subjected to LPS-induced AKI.
Fig. 1.
Hexokinase (HK) activation and renal dysfunction after LPS exposure. Male C57BL/6 mice were injected with LPS (10 mg/kg ip) or saline vehicle and euthanized at 3, 6, and 18 h. Renal cortical tissue was isolated and activities of HK (A), phosphofructokinase (PFK; C), and pyruvate kinase (PK; D) were measured and normalized to total protein content. Renal function at 3 and 18 h post-LPS was assessed by measuring blood urea nitrogen (BUN; B). The number of animals used for analysis in each group is denoted in the bar. Data are expressed as means ± SE for each group. Different superscripts above bars indicate statistically significant differences (P < 0.05) compared with time-point controls. Bars with multiple superscripts (a, b) are results not significantly different from other groups.
LPS-induced HK activation occurs independently of isoform expression.
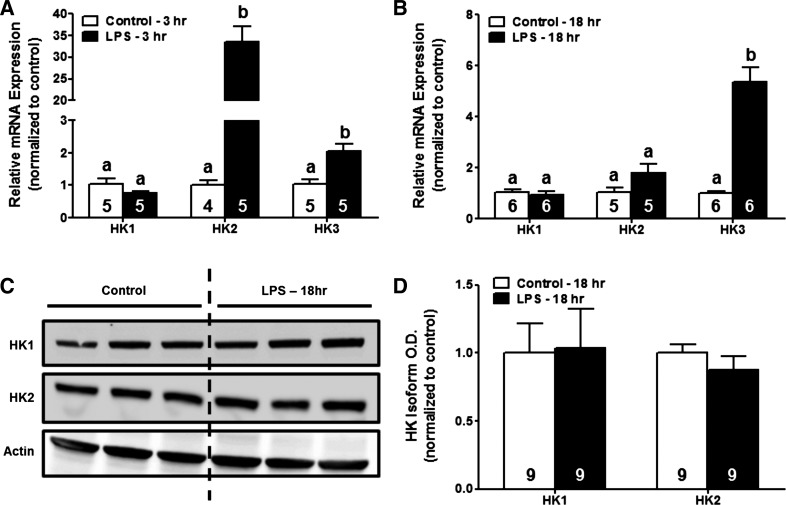
Because HK activity in the renal cortex increased following LPS exposure, we determined the effects of LPS on the expression of HK isoforms at both the mRNA and protein level via qPCR and immunoblot analysis at 3 and 18 h post-LPS. HK1 mRNA levels did not change in response to LPS exposure at any time point. HK2 gene expression increased ∼33-fold at 3 h in LPS-treated mice but returned to baseline levels by 18 h post-LPS. LPS administration also resulted in increases in HK3 mRNA levels at both 3 (∼2-fold) and 18 h (∼5-fold) (Fig. 2, A and B). Although increased gene expression of HK2 and HK3 was observed in LPS-treated mice, immunoblot analysis revealed no changes in protein levels of HK1 or HK2 at 18 h after LPS administration (Fig. 2, C and D). These data demonstrate that rapid HK activation in the renal cortex of LPS-treated mice is independent of changes in HK isoform expression.
Fig. 2.
Systemic LPS exposure does not alter HK isoform expression at the protein level. RNA and protein were isolated from the renal cortex of mice treated with LPS or vehicle control. Relative expression of HK1, HK2, and HK3 at the transcript level at 3 (A) and 18 h (B) after LPS administration was determined by RT-qPCR. Protein expression of HK1 or HK2 isoforms at the 18-h time point was determined by immunoblot analysis (C and D). Data were normalized to fold-change in expression compared with vehicle controls and are presented as means ± SE for each group. Superscripts above bars indicate statistically significant differences between groups. (P < 0.05).
LPS exposure does not alter mitochondrial localization of HK1 or HK2.
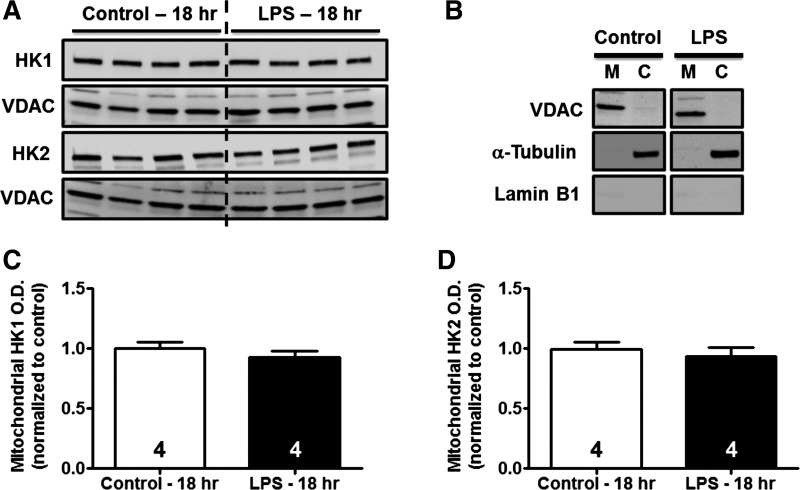
Both HK1 and HK2 isoforms possess mitochondrial binding domains which allow for their association with the outer mitochondrial membrane (45, 68). Because mitochondrial binding of HK has been demonstrated in the kidney and may result in increased HK activity, we determined the effects of LPS administration on HK1 and HK2 localization to mitochondria. Mitochondria were isolated by differential centrifugation from kidneys of vehicle- and LPS-treated mice at 18 h. Protein levels of HK1 and HK2 in the mitochondrial fraction were determined by immunoblot analysis using VDAC as a mitochondrial loading control. No differences were noted in the mitochondrial localization of either HK1 or HK2 following LPS administration (Fig. 3, A, C, and D). The purity of mitochondrial fractions was confirmed by immunoblot analysis for VDAC, α-tubulin, and lamin B1 (Fig. 3B). These findings demonstrate that LPS-induced increases in renal cortical HK activity cannot be attributed to mitochondrial localization.
Fig. 3.
Effects of LPS on mitochondrial localization of HK1 and HK2. Whole kidneys were harvested from mice treated as described above, and the mitochondrial fraction was isolated by differential centrifugation. Immunoblot analysis was performed to examine mitochondrial localization of HK isoforms. Mitochondrial VDAC was used as a loading control. A: representative immunoblots of mitochondrial-associated HK1 or HK2 isoforms 18 h after LPS exposure. B: purity of mitochondrial (M) and cytosolic (C) fractions was assessed by immunoblotting for VDAC (mitochondrial marker), α-tubulin (cytosolic marker), and lamin B1 (nuclear marker). C and D: densitometric analysis revealed no change in mitochondrial localization of HK1 or HK2.
LPS administration does not alter expression of other glycolysis-related enzymes in the renal cortex.
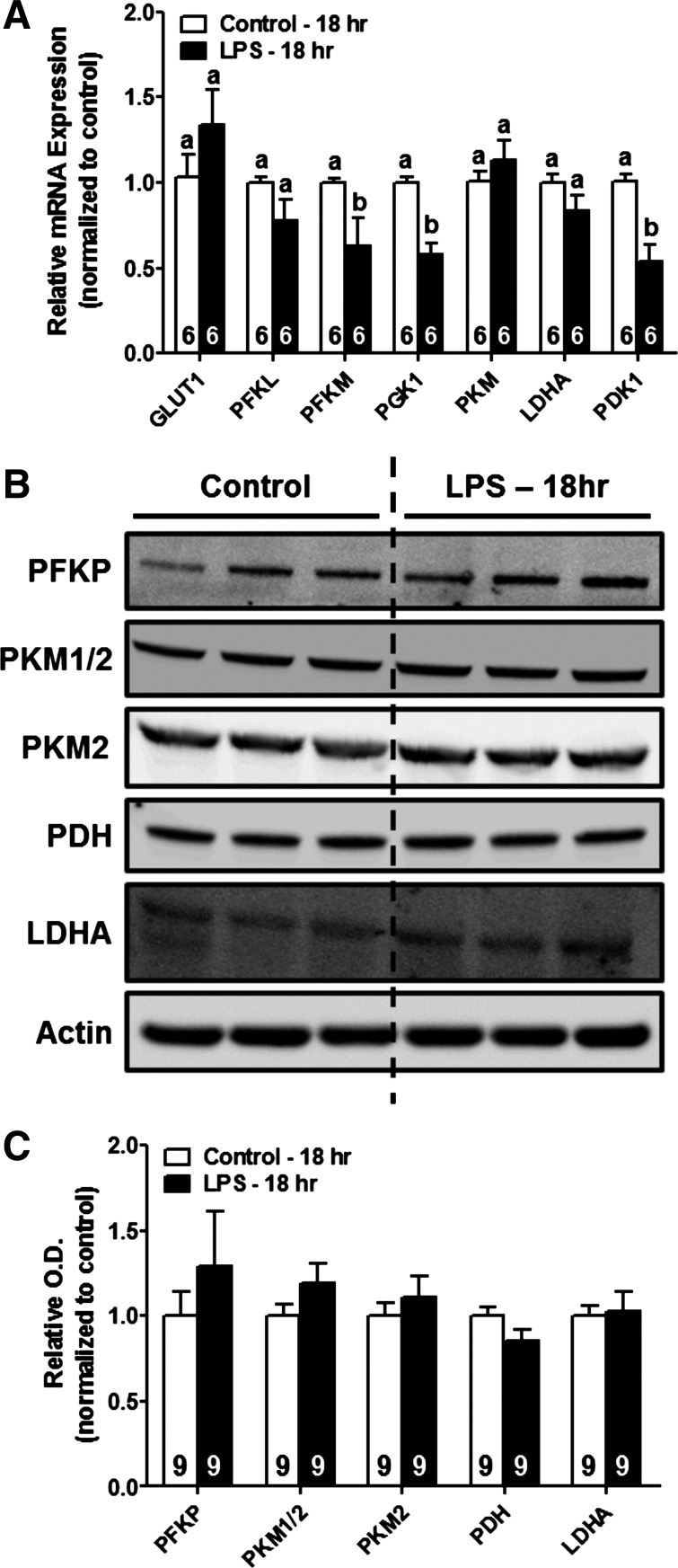
We also examined expression of a number of other glycolysis-related enzymes in the renal cortex of mice exposed to LPS. In particular were genes and proteins involved in cellular glucose uptake (GLUT1/Slc2a1), glycolytic metabolism (PFKP, PFKL, PFKM, PGK1, PKM), pyruvate conversion into lactate (LDHA), and pyruvate entry into the TCA cycle (PDK1, PDH). No increases were noted in gene expression of glycolysis-related enzymes at 18 h post-LPS (Fig. 4A). Transcript levels of the glycolytic enzymes PFK, muscle type (PFKM), and phosphoglycerate kinase 1 (PGK1) were significantly decreased (∼40%) following LPS exposure (Fig. 4A). In addition, no changes in protein expression of glycolysis-related enzymes were observed in mice treated with LPS (Fig. 4, B and C). Taken together, these data indicate that LPS exposure does not result in changes in gene or protein expression to facilitate glycolytic metabolism in the renal cortex.
Fig. 4.
LPS administration increases expression of other glycolysis-related enzymes in the renal cortex. RNA and protein were harvested from the renal cortex of mice treated with saline vehicle or LPS as described above. A: expression of GLUT1, PFKL, PFKM, PGK1, PKM, LDHA, and PDK1 mRNAs was determined by RT-qPCR analysis. B and C: representative immunoblots demonstrating no change in expression of PFKP, PKM1/2, PDH, or LDHA 18 h after systemic LPS administration. Data were normalized to fold-change in expression relative to control and are expressed as means ± SE for each group. Different superscripts above bars indicate statistically significant differences (P < 0.05).
LPS-induced HK activation does not enhance glycolytic flux in the renal cortex.
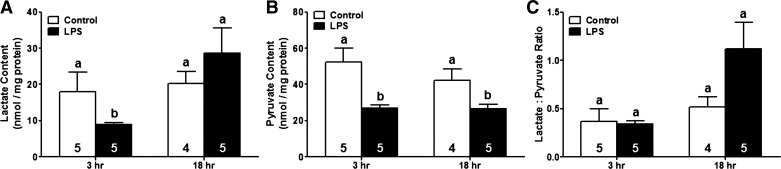
To determine whether LPS-induced activation of HK was sufficient to increase glycolytic metabolism, we next measured end products of glycolysis. Mice were treated with LPS or vehicle control and kidneys were harvested at 3 and 18 h after injection to determine pyruvate and lactate in renal cortical tissue. LPS exposure decreased pyruvate content (∼50%) at both the 3 and 18-h time points (Fig. 5A). Cortical lactate levels were also reduced to ∼50% of control levels at 3 h post-LPS. However, lactate content returned to baseline levels by the 18-h time point (Fig. 5B). The changes observed in both pyruvate and lactate levels did not result in an increase in the lactate-to-pyruvate ratio at 18 h post-LPS (Fig. 5C). These findings demonstrate that, although LPS exposure results in activation of HK in the renal cortex, increased HK activity is not adequate to increase flux of glucose through the glycolytic pathway.
Fig. 5.
LPS-induced HK activation does not increase renal cortical glycolytic flux. Lactate and pyruvate were measured in renal cortices of mice treated with either saline or LPS. A and B: renal cortical pyruvate (A) and lactate (B) levels at 3- and 18-h time points. C: no significant changes were noted in the lactate-to-pyruvate ratio after LPS. Data are presented as means ± SE for each group. Superscripts above bars indicate statistically significant differences between groups (P < 0.05) compared with time point controls.
Increased HK activity after LPS exposure does not promote glycogen synthesis.
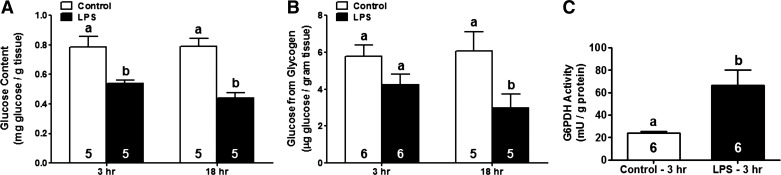
Because increased HK activity due to LPS exposure in the renal cortex did not result in enhanced glycolytic flux, we sought to determine whether HK activation in this setting has an alternate physiological role. Conversion of glucose to glucose-6-phosphate by HK has been described as a rate-limiting step in glycogen synthesis in a variety of model systems, including rodent skeletal muscle (10, 41, 50). Thus we measured glucose and glycogen levels following LPS exposure to determine whether HK activation in this model may result in glycogenesis. Glucose levels in the renal cortex were reduced 3 h after LPS administration and remained lower at 18 h (Fig. 6A). Measurement of glycogen content revealed no change in LPS-treated mice at 3 h and a ∼60% reduction at 18 h (Fig. 6B). Our results demonstrate that LPS-induced HK activation does not stimulate glycogen synthesis.
Fig. 6.
LPS-induced increases in HK activity do not promote glycogen synthesis in the renal cortex but are associated with increased glucose-6-phosphate dehydrogenase (G6PDH) activity. Glucose (A) and glycogen content (B) and G6PDH activity (C) were evaluated in the renal cortex at different time points following systemic LPS administration in mice and were normalized to wet tissue weight (glucose, glycogen) or protein content (G6PDH activity). Data are shown as means ± SE for each group. Different superscripts above bars indicate statistically significant differences (P < 0.05) compared with time point controls.
HK activation after LPS exposure increased pentose phosphate pathway activity.
We next questioned whether renal cortical HK activation following systemic LPS exposure might facilitate glucose flux through the pentose phosphate pathway (PPP). G6PDH activity, the rate-limiting step of the PPP, was measured (53). Renal cortical G6PDH activity increased ∼3.5-fold at 3 h after LPS administration (Fig. 6C). Thus HK activation following LPS exposure is associated with an increase in G6PDH activity.
LPS-induced HK activity in the renal cortex is mediated by EGFR and Akt.
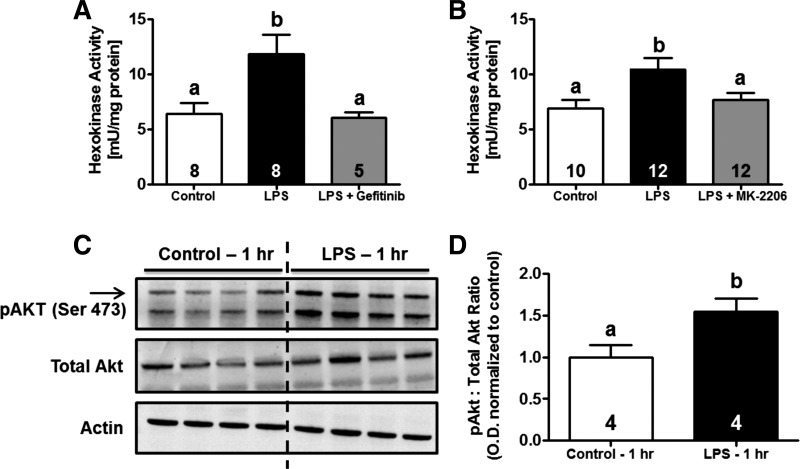
To understand the mechanism by which LPS exposure increases HK activation, we investigated the role of EGFR. Earlier studies from our laboratory and others have shown that EGFR ligands (EGF, HB-EGF) stimulate HK activity in RPTC (9, 36, 46, 54). LPS is also known to induce EGFR transactivation in a variety of cell types, including epithelial cells and renal medullary collecting duct cells (15, 24, 28, 34, 61). One hour before LPS administration, mice were treated with the EGFR inhibitor gefitinib (100 mg/kg) or vehicle control and kidneys were harvested 3 h post-LPS. This dose has previously been reported to inhibit EGFR signaling following AKI in mice (21). LPS-induced HK activity was completely blocked by gefitinib, suggesting an essential role for EGFR in this process (Fig. 7A).
Fig. 7.
HK activation following LPS exposure is mediated through EGF receptor (EGFR)/Akt signaling. One hour before LPS administration, as described above, mice were treated with a single dose of either gefitinib (100 mg/kg ip; A), MK-2206 (100 mg/kg; B), or vehicle control. HK activity was determined in the renal cortex at 3 h after systemic LPS administration. Immunoblot analysis revealed increased activation/phosphorylation of Akt 1 h after LPS exposure (C and D). Levels of phosphorylated Akt were normalized to total protein expression and are presented as fold-change vs. control animals. Data are expressed as means ± SE for each experimental group. Superscripts above bars indicate statistically significant differences between groups (P < 0.05).
Because EGFR is known to activate PI3K/Akt signaling, we determined whether Akt is responsible for EGFR-mediated HK activation. To demonstrate Akt activation following systemic LPS exposure, mice were treated with LPS or vehicle control and kidneys were harvested 1 h later for immunoblot analysis of phosphorylated and total Akt. LPS exposure resulted in a ∼1.5-fold increase in expression of phospho-Akt without a change in total Akt (Fig. 7, C and D). Based on these findings, another group of mice was pretreated with the pan-Akt inhibitor MK-2206 (100 mg/kg) 1 h before LPS injection and kidneys were harvested 3 h post-LPS. MK-2206 is an allosteric inhibitor of Akt and exhibits high selectivity over other kinases because it requires the pleckstrin homology domain for its activity (74). LPS-induced HK activity was attenuated following Akt inhibition by MK-2206 (Fig. 7B). These data indicate that the EGFR/PI3K/Akt signaling axis is responsible for activation of renal cortical HK in LPS-induced AKI.
DISCUSSION
Mitochondrial dysfunction is an important contributor to the pathophysiology of multiple forms of AKI (16, 52). Although histological changes in sepsis-induced AKI are limited, post mortem and experimental studies have demonstrated mitochondrial swelling in the relative absence of overt tubular cell death (57, 62, 63). On the molecular level, sepsis-induced AKI results in suppression of mitochondrial genes which is associated with functional decline (62). Patil et al. (40) recently demonstrated reduced renal electron transport chain complex I and complex II/III activity occurring as early as 6 h after cecal ligation and puncture in mice. Furthermore, a reduction of renal ATP levels has been observed in rodent models of sepsis-induced AKI which correlated well with renal dysfunction (32, 40, 49). Taken together, these data provide strong evidence that mitochondrial dysfunction plays a central role in the pathophysiology of sepsis-induced AKI. To this point, therapies that restore mitochondrial function and/or reduce oxidative stress have proven beneficial in experimental models (40, 66).
We hypothesized that RPTC might respond to mitochondrial dysfunction by increasing glycolytic flux to generate ATP. Although the proximal tubule has been viewed as having very low glycolytic capacity, studies have revealed that glycolysis may be induced in RPTC in response to a cellular insult (5, 19, 70). Dickman and Mandel (14) demonstrated that proximal tubules in vitro increase glycolytic metabolism in response to hypoxia, mitochondrial uncoupling, and inhibition of complex I of the electron transport chain. Inhibition of the Na+-K+-ATPase and glycolysis in proximal tubules following complex I inhibition indicated that glycolytic induction serves to promote cellular function and survival (14). In vivo data also reveal that anaerobic glycolysis is increased in the renal cortex during ischemia but rapidly returns to baseline levels after reperfusion (75). These data provide strong evidence that glycolytic metabolism can be activated for energy production in the proximal tubule.
We performed a comprehensive evaluation of the glycolytic pathway to examine activities of the major regulatory enzymes, mRNA and protein levels of key components of glucose metabolism, and end products of glycolysis (pyruvate and lactate). We observed an increase in renal cortical HK activity as early as 3 h after LPS exposure in mice that was maintained for at least 18 h without an upregulation of any other glycolytic enzymes nor an increase in flux through the glycolytic pathway as measured by renal cortical pyruvate and lactate levels.
To our knowledge, this is the first demonstration of rapid, specific activation of HK in the renal cortex after systemic LPS administration. Three high-affinity HK isoforms (Km values in the micromolar range) are expressed in the mammalian kidney (HK1, HK2, and HK3) (18, 45). HK1 is constitutively expressed/active and accounts for ∼70% of total renal HK activity under normal conditions. In contrast, HK2 appears to be regulated in response to a variety of stimuli (37, 43, 58). Little is known about the regulation of HK3 expression/activity. Together, HK2 and HK3 account for the remainder of renal HK activity (∼30%) (9).
The experimental method used to measure HK activity in this study does not distinguish which HK isoform is activated following systemic LPS exposure. Therefore, we measured HK isoforms at both the mRNA and protein levels in the renal cortex. Although early changes were noted in mRNA levels of HK2, no changes were seen in HK1 or HK2 isoforms at the protein level following LPS exposure. HK3 mRNA expression increased at both 3- and 18-h time points. We were not able to measure HK3 protein due to the lack of a validated antibody with reactivity to mouse HK3. An anabolic role (PPP or glycogen synthesis) has been proposed for HK2 and HK3 since these isoforms are subjected to inhibition by glucose-6-phosphate and Pi, whereas HK1 is thought to mainly facilitate glycolytic metabolism (69).
Given that HK isoform expression did not change after LPS exposure, we investigated whether HK activation might be attributed to increased mitochondrial localization. Recent evidence revealed that mitochondrial localization of HK isoforms may serve a number of physiological roles, including direct coupling of glucose phosphorylation to the intramitochondrial ATP pool, reducing feedback inhibition by glucose-6-phosphate (G-6-P), and preventing initiation of apoptosis by proapoptotic members of the Bcl-2 family (2, 8, 38, 39, 68). However, we did not observe any changes in mitochondrial localization of either HK1 or HK2 in response to systemic LPS exposure. In contrast to HK1 and HK2, HK3 does not possess a mitochondrial localization sequence and is thought to be predominantly perinuclear in location (42). Taken together, these data suggest that renal cortical HK activation in endotoxin-induced AKI is likely due to a posttranslational modification (i.e., phosphorylation) which regulates HK activity (35, 44).
Although HK activity rapidly increased and was sustained for up to 18 h after LPS exposure, we observed no changes in pyruvate and lactate content indicative of increased glycolysis. For the purposes of this study, glycolytic flux was defined as the conversion of glucose to pyruvate as well as downstream generation of lactate. Lactate-to-pyruvate ratios were compared under different experimental conditions as an indicator of anaerobic glycolysis. Under anaerobic conditions, LDH would be expected to convert pyruvate to lactate, resulting in increased lactate with a corresponding equimolar reduction in pyruvate. Pyruvate content was decreased approximately twofold at both 3 and 18 h, whereas lactate levels trended toward a decrease at 3 h but returned to baseline levels by 18 h post-LPS. These findings are in agreement with recent reports of renal cortical pyruvate depletion in both I/R and glycerol-induced AKI in mice (75). The same study also reported glycolytic induction in the renal cortex only occurs during the ischemic period and is reversed after reperfusion (75). However, it should be noted that some of the analyses performed here, including pyruvate and lactate measurements, are not sufficiently powered to detect small changes between groups. Thus it is possible that there are minimal changes in glycolytic flux in the renal cortex up to 18 h after LPS exposure that we were not able to distinguish.
Using a combination of microultrasound and blood oxygen level-dependent MRI, Tran et al. (62) demonstrated that although renal perfusion is markedly decreased in LPS-treated mice, there is minimal change in tissue oxygenation (62). Thus the lack of renal hypoxia may explain why we did not detect increases in lactate following systemic LPS administration. However, the importance of hypoperfusion/hypoxia in renal pathophysiology remains unclear. Changes in renal blood flow following endotoxin exposure are local and dynamic at the dose used in this study. Wu et al. (71) reported the disruption in cortical peritubular capillary flow at 10 h after LPS administration in mice. Peritubular capillary dysfunction observed in this study was correlated with an increase in tubular NAD(P)H autofluorescence, suggesting that local hypoxia may contribute to cellular injury after LPS (71). In light of these findings, it is important to note that our analyses are limited to only two time points (3 and 18 h) after endotoxin exposure.
Our analysis of glycolytic enzyme activities was restricted to the major rate-limiting enzymes in the glycolysis pathway (HK, PFK, PK). In addition, we measured mRNA and protein expression of other enzymes involved in glucose metabolism in the renal cortex following LPS exposure (e.g., GLUT1, PGK, LDHA, PDH, PDK1). There was no evidence of increased mRNA or protein expression of any of these components, indicating that LPS-induced AKI does not result in early activation of a transcriptional program to facilitate glycolysis in the renal cortex. We cannot rule out the possibility that activities of one or more of these enzymes are increased after endotoxin exposure without an associated increase in expression. However, the data presented here demonstrating minimal changes in the lactate-to-pyruvate ratio, specific HK activation, and an absence of increases in expression of other glycolytic components provide considerable evidence indicating that glycolysis is minimally activated at early time points (3 and 18 h) following endotoxin-induced AKI. Further studies are necessary to determine whether glycolytic metabolism might be activated in the chronic phase.
In addition to glycolysis, G-6-P has multiple fates that include flux through the PPP to produce NADPH and nucleotide/amino acid precursors, glycogen synthesis, and the HK biosynthetic pathway. Zager et al. (75) reported that pyruvate depletion in the renal cortex following ischemic and nephrotoxic forms of AKI was partially attributed to an increase in gluconeogenesis and glycogen synthesis. Interestingly, generation of G-6-P by HK is thought to be a rate-limiting step in glycogen synthesis in a number of tissues and has been associated with glycogen supercompensation in rat skeletal muscle (10, 25, 41, 50). In contrast to changes observed in other mouse models of AKI, we observed a decrease in both renal cortical glucose and glycogen content. Together, these data indicate that gluconeogenesis and glycogen content are not upregulated in the septic kidney. These findings are consistent with the reduction in renal glucose and gluconeogenic enzymes in the kidney after endotoxin administration in rats (1, 22).
G-6-P generated following HK activation in the renal cortex may also be utilized by the PPP. Through the PPP, G-6-P is further metabolized by the rate-limiting enzyme G6PDH to generate NADPH and biosynthetic precursors (45). Reduced NADPH levels in G6PDH-deficient mice were associated with increased renal oxidative stress, inflammation, and dysfunction, indicating an important role for NADPH in antioxidant defense in the kidney (73). Given that oxidative stress rapidly develops in the proximal tubule following systemic LPS exposure, these findings suggest an alternate hypothesis that HK activation may contribute to PPP activity (26). Interestingly, G6PDH activity was increased (∼3.5 fold) in the renal cortex of mice exposed to LPS. These findings provide strong evidence that G-6-P formed as a result of HK activation is selectively metabolized via the PPP. The importance of the PPP in NADPH production and thus antioxidant defense following AKI has received little attention.
Our laboratory previously demonstrated that activation of EGFR signaling in primary rabbit proximal tubule cells lead to a rapid increase in both glycolysis and PPP activity (36). Further studies demonstrated that EGFR ligands are capable of increasing HK activity in multiple renal cell types, including proximal tubule cells and mesangial cells (45, 46). A link between LPS and EGFR signaling has also been established in studies demonstrating that TLR4 rapidly transactivates the EGFR via protease-mediated EGFR ligand shedding (28, 34). In addition, EGFR activation can contribute to the pathology and recovery of multiple forms of AKI (59). Results presented here demonstrate that EGFR signaling is required for HK activation in LPS-induced AKI.
We next focused on Akt as a downstream mediator of EGFR-induced HK activation in LPS-treated mice based on extensive evidence indicating that Akt modulates both HK activity and localization through phosphorylation (17, 35). Site-directed mutagenesis has further revealed that HK2 is phosphorylated by Akt on threonine 473 within an Akt consensus sequence in cardiomyocytes in vitro (44). We examined signaling changes in the renal cortex that might precede HK activation and noted increased activation of Akt as early as 1 h after LPS administration, which is consistent with other reports (11, 31). Inhibition of Akt by MK-2206 attenuated increases in HK activity following LPS exposure. Taken together, these findings provide strong evidence that the EGFR/Akt signaling pathway is responsible for LPS-mediated HK activation in the renal cortex.
In conclusion, the present study reports the novel finding of rapid activation of renal cortical HK activity in a mouse model of sepsis-induced AKI. HK was activated following LPS-induced AKI via an EGFR/Akt-dependent signaling mechanism. Surprisingly, the increase in HK activity observed in this model was associated with minimal changes in glycolysis and glycogen synthesis and was strongly linked with an increase in G6PDH, the rate-limiting enzyme in the PPP. The production of reducing equivalents (i.e., NADPH) may be key in preserving oxidant defense pathways.
GRANTS
This material is based on work supported in part by National Institute of General Medical Sciences Grants GM084147 (to R. G. Schnellmann) and P20GM103542–02 (to South Carolina COBRE in Oxidants, Redox Balance, and Stress Signaling); National Center for Research Resources Grant UL1-RR029882; the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs [Grant 5I01 BX-000851 (to R. G. Schnellmann)], and the South Carolina Clinical and Translational Research Institute at the Medical University of South Carolina. Animal facilities were funded by National Center for Research Resources Grant C06-RR015455.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.A.S., L.J.S., and R.G.S. provided conception and design of research; J.A.S. and L.J.S. performed experiments; J.A.S. and R.G.S. analyzed data; J.A.S. and R.G.S. interpreted results of experiments; J.A.S. prepared figures; J.A.S. drafted manuscript; J.A.S. and R.G.S. edited and revised manuscript; J.A.S., L.J.S., and R.G.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The contents of this manuscript do not represent the views of the Department of Veteran Affairs or the United States Government.
REFERENCES
- 1.Ardawi MS, Khoja SM, Newsholme EA. Metabolic regulation of renal gluconeogenesis in response to sepsis in the rat. Clin Sci 79: 483–490, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Arora KK, Pedersen PL. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem 263: 17422–17428, 1988 [PubMed] [Google Scholar]
- 3.Arrington DD, Van Vleet TR, Schnellmann RG. Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol 291: C1159–C1171, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Ash SR, Cuppage FE. Shift toward anaerobic glycolysis in the regenerating rat kidney. Am J Pathol 60: 385–402, 1970 [PMC free article] [PubMed] [Google Scholar]
- 5.Bagnasco S, Good D, Balaban R, Burg M. Lactate production in isolated segments of the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 248: F522–F526, 1985 [DOI] [PubMed] [Google Scholar]
- 6.Bellomo R, Kellum JA, Pinsky MR. Transvisceral lactate fluxes during early endotoxemia. Chest 110: 198–204, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 380: 756–766, 2012 [DOI] [PubMed] [Google Scholar]
- 8.BeltrandelRio H, Wilson JE. Hexokinase of rat brain mitochondria: relative importance of adenylate kinase and oxidative phosphorylation as sources of substrate ATP, and interaction with intramitochondrial compartments of ATP and ADP. Arch Biochem Biophys 286: 183–194, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Bryson JM, Coy PE, Gottlob K, Hay N, Robey RB. Increased hexokinase activity, of either ectopic or endogenous origin, protects renal epithelial cells against acute oxidant-induced cell death. J Biol Chem 277: 11392–11400, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Chase JR, Rothman DL, Shulman RG. Flux control in the rat gastrocnemius glycogen synthesis pathway by in vivo 13C/31P NMR spectroscopy. Am J Physiol Endocrinol Metab 280: E598–E607, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Shetty S, Zhang P, Gao R, Hu Y, Wang S, Li Z, Fu J. Aspirin-triggered resolvin D1 down-regulates inflammatory responses and protects against endotoxin-induced acute kidney injury. Toxicol Appl Pharmacol 277: 118–123, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, De Placido S, Bianco AR, Tortora G. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res 6: 2053–2063, 2000 [PubMed] [Google Scholar]
- 13.Devalaraja-Narashimha K, Padanilam BJ. PARP-1 inhibits glycolysis in ischemic kidneys. J Am Soc Nephrol 20: 95–103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickman KG, Mandel LJ. Differential effects of respiratory inhibitors on glycolysis in proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1608–F1615, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Finzi L, Shao MX, Paye F, Housset C, Nadel JA. Lipopolysaccharide initiates a positive feedback of epidermal growth factor receptor signaling by prostaglandin E2 in human biliary carcinoma cells. J Immunol 182: 2269–2276, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol 302: F853–F864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev 15: 1406–1418, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossbard L, Schimke RT. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem 241: 3546–3560, 1966 [PubMed] [Google Scholar]
- 19.Guder WG, Ross BD. Enzyme distribution along the nephron. Kidney Int 26: 101–111, 1984 [DOI] [PubMed] [Google Scholar]
- 20.Gullans SR, Brazy PC, Soltoff SP, Dennis VW, Mandel LJ. Metabolic inhibitors: effects on metabolism and transport in the proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 243: F133–F140, 1982 [DOI] [PubMed] [Google Scholar]
- 21.He S, Liu N, Bayliss G, Zhuang S. EGFR activity is required for renal tubular cell dedifferentiation and proliferation in a murine model of folic acid-induced acute kidney injury. Am J Physiol Renal Physiol 304: F356–F366, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heemskerk AE, Huisman E, van Lambalgen AA, van den Bos GC, Hennekes M, Thijs LG, Tangelder GJ. Renal function and oxygen consumption during bacteraemia and endotoxaemia in rats. Nephrol Dial Transplant 12: 1586–1594, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther 9: 1956–1967, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Hsu D, Fukata M, Hernandez YG, Sotolongo JP, Goo T, Maki J, Hayes LA, Ungaro RC, Chen A, Breglio KJ, Xu R, Abreu MT. Toll-like receptor 4 differentially regulates epidermal growth factor-related growth factors in response to intestinal mucosal injury. Lab Invest 90: 1295–1305, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irimia JM, Rovira J, Nielsen JN, Guerrero M, Wojtaszewski JF, Cusso R. Hexokinase 2, glycogen synthase and phosphorylase play a key role in muscle glycogen supercompensation. PloS One 7: e42453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalakeche R, Hato T, Rhodes G, Dunn KW, El-Achkar TM, Plotkin Z, Sandoval RM, Dagher PC. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J Am Soc Nephrol 22: 1505–1516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly KJ, Molitoris BA. Acute renal failure in the new millennium: time to consider combination therapy. Semin Nephrol 20: 4–19, 2000 [PubMed] [Google Scholar]
- 28.Kuper C, Beck FX, Neuhofer W. Toll-like receptor 4 activates NF-κB and MAP kinase pathways to regulate expression of proinflammatory COX-2 in renal medullary collecting duct cells. Am J Physiol Renal Physiol 302: F38–F46, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Legrand M, Almac E, Mik EG, Johannes T, Kandil A, Bezemer R, Payen D, Ince C. l-NIL prevents renal microvascular hypoxia and increase of renal oxygen consumption after ischemia-reperfusion in rats. Am J Physiol Renal Physiol 296: F1109–F1117, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Legrand M, Bezemer R, Kandil A, Demirci C, Payen D, Ince C. The role of renal hypoperfusion in development of renal microcirculatory dysfunction in endotoxemic rats. Intensive Care Med 37: 1534–1542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li XJ, Zhang GX, Sun N, Sun Y, Yang LZ, Du YJ. Protective effects of erythropoietin on endotoxin-related organ injury in rats. J Huazhong Univ Sci Technolog Med Sci 33: 680–686, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Liaudet L, Fishman D, Markert M, Perret C, Feihl F. l-canavanine improves organ function and tissue adenosine triphosphate levels in rodent endotoxemia. Am J Respir Crit Care Med 155: 1643–1648, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23: 854–867, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McElroy SJ, Hobbs S, Kallen M, Tejera N, Rosen MJ, Grishin A, Matta P, Schneider C, Upperman J, Ford H, Polk DB, Weitkamp JH. Transactivation of EGFR by LPS induces COX-2 expression in enterocytes. PloS One 7: e38373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ 15: 521–529, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Nowak G, Schnellmann RG. Integrative effects of EGF on metabolism and proliferation in renal proximal tubular cells. Am J Physiol Cell Physiol 269: C1317–C1325, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Osawa H, Printz RL, Whitesell RR, Granner DK. Regulation of hexokinase II gene transcription and glucose phosphorylation by catecholamines, cyclic AMP, and insulin. Diabetes 44: 1426–1432, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr 40: 171–182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem 277: 7610–7618, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Patil NK, Parajuli N, Macmillan-Crow LA, Mayeux PR. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria targeted antioxidant mitigates injury. Am J Physiol Renal Physiol 306: F734–F743, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preller A, Wilson CA, Quiroga-Roger D, Ureta T. Hexokinase and not glycogen synthase controls the flux through the glycogen synthesis pathway in frog oocytes. FEBS Lett 587: 2825–2831, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Preller A, Wilson JE. Localization of the type III isozyme of hexokinase at the nuclear periphery. Arch Biochem Biophys 294: 482–492, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Riddle SR, Ahmad A, Ahmad S, Deeb SS, Malkki M, Schneider BK, Allen CB, White CW. Hypoxia induces hexokinase II gene expression in human lung cell line A549. Am J Physiol Lung Cell Mol Physiol 278: L407–L416, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Roberts DJ, Tan-Sah VP, Smith JM, Miyamoto S. Akt phosphorylates HK-II at Thr-473 and increases mitochondrial HK-II association to protect cardiomyocytes. J Biol Chem 288: 23798–23806, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25: 4683–4696, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Robey RB, Ma J, Santos AV, Noboa OA, Coy PE, Bryson JM. Regulation of mesangial cell hexokinase activity and expression by heparin-binding epidermal growth factor-like growth factor: epidermal growth factors and phorbol esters increase glucose metabolism via a common mechanism involving classic mitogen-activated protein kinase pathway activation and induction of hexokinase II expression. J Biol Chem 277: 14370–14378, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Schley G, Klanke B, Schodel J, Forstreuter F, Shukla D, Kurtz A, Amann K, Wiesener MS, Rosen S, Eckardt KU, Maxwell PH, Willam C. Hypoxia-inducible transcription factors stabilization in the thick ascending limb protects against ischemic acute kidney injury. J Am Soc Nephrol 22: 2004–2015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrier RW, Wang W. Acute renal failure and sepsis. New Engl J Med 351: 159–169, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Shimahara Y, Kono Y, Tanaka J, Ozawa K, Sato T, Jones RT, Cowley RA, Trump BF. Pathophysiology of acute renal failure following living Escherichia coli injection in rats: high-energy metabolism and renal functions. Circ Shock 21: 197–205, 1987 [PubMed] [Google Scholar]
- 50.Shulman RG, Bloch G, Rothman DL. In vivo regulation of muscle glycogen synthase and the control of glycogen synthesis. Proc Natl Acad Sci USA 92: 8535–8542, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soltoff SP. ATP and the regulation of renal cell function. Annu Rev Physiol 48: 9–31, 1986 [DOI] [PubMed] [Google Scholar]
- 52.Stallons LJ, Funk JA, Schnellmann RG. Mitochondrial homeostasis in acute organ failure. Curr Pathobiol Rep 1: 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanton RC. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 64: 362–369, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanton RC, Seifter JL. Epidermal growth factor rapidly activates the hexose monophosphate shunt in kidney cells. Am J Physiol Cell Physiol 254: C267–C271, 1988 [DOI] [PubMed] [Google Scholar]
- 55.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol 22: 1041–1052, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi K, Mizukami H, Kamata K, Inaba W, Kato N, Hibi C, Yagihashi S. Amelioration of acute kidney injury in lipopolysaccharide-induced systemic inflammatory response syndrome by an aldose reductase inhibitor, fidarestat. PloS One 7: e30134, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 187: 509–517, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taneja N, Coy PE, Lee I, Bryson JM, Robey RB. Proinflammatory interleukin-1 cytokines increase mesangial cell hexokinase activity and hexokinase II isoform abundance. Am J Physiol Cell Physiol 287: C548–C557, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int 83: 804–810, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. New Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 61.Thuringer D, Hammann A, Benikhlef N, Fourmaux E, Bouchot A, Wettstein G, Solary E, Garrido C. Transactivation of the epidermal growth factor receptor by heat shock protein 90 via Toll-like receptor 4 contributes to the migration of glioblastoma cells. J Biol Chem 286: 3418–3428, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121: 4003–4014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trump BF, Valigorsky JM, Jones RT, Mergner WJ, Garcia JH, Cowley RA. The application of electron microscopy and cellular biochemistry to the autopsy. Observations on cellular changes in human shock. Human Pathol 6: 499–516, 1975 [DOI] [PubMed] [Google Scholar]
- 64.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Beginning, and ending supportive therapy for the kidney. I. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Wang Z, Holthoff JH, Seely KA, Pathak E, Spencer HJ, 3rd, Gokden N, Mayeux PR. Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am J Pathol 180: 505–516, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, Schnellmann RG. The beta2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther 342: 106–118, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson JE. Hexokinases. Rev Physiol Biochem Pharmacol 126: 65–198, 1995 [DOI] [PubMed] [Google Scholar]
- 69.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol 206: 2049–2057, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Wirthensohn G, Guder WG. Renal substrate metabolism. Physiol Rev 66: 469–497, 1986 [DOI] [PubMed] [Google Scholar]
- 71.Wu L, Tiwari MM, Messer KJ, Holthoff JH, Gokden N, Brock RW, Mayeux PR. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol 292: F261–F268, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Wu X, Guo R, Wang Y, Cunningham PN. The role of ICAM-1 in endotoxin-induced acute renal failure. Am J Physiol Renal Physiol 293: F1262–F1271, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Xu Y, Zhang Z, Hu J, Stillman IE, Leopold JA, Handy DE, Loscalzo J, Stanton RC. Glucose-6-phosphate dehydrogenase-deficient mice have increased renal oxidative stress and increased albuminuria. FASEB J 24: 609–616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, Baird RD, Delgado L, Taylor A, Lupinacci L, Riisnaes R, Pope LL, Heaton SP, Thomas G, Garrett MD, Sullivan DM, de Bono JS, Tolcher AW. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol 29: 4688–4695, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Zager RA, Johnson AC, Becker K. Renal cortical pyruvate depletion during AKI. J Am Soc Nephrol 25: 998–1012, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]