Abstract
This study identified a distinctive pattern of expression and activity of adenylyl cyclase (AC) and phosphodiesterase (PDE) isoforms in mouse colonic longitudinal smooth muscle cells and determined the changes in their expression and/or activity in response to proinflammatory cytokines (IL-1β and TNF-α) in vitro and 2,4,6 trinitrobenzene sulphonic acid (TNBS)-induced colonic inflammation in vivo. AC5/6 and PDE4D5, expressed in circular muscle cells, were also expressed in longitudinal smooth muscle. cAMP formation was tightly regulated via feedback phosphorylation of AC5/6 and PDE4D5 by PKA. Inhibition of PKA activity by myristoylated PKI blocked phosphorylation of AC5/6 and PDE4D5 and enhanced cAMP formation. TNBS treatment in vivo and IL-1β and TNF-α in vitro induced inducible nitric oxide synthase (iNOS) expression, stimulated ERK1/2 activity, caused iNOS-mediated S-nitrosylation and inhibition of AC5/6, and induced phosphorylation of PDE4D5 and stimulated its activity. The resultant decrease in AC5/6 activity and increase in PDE4D5 activity decreased cAMP formation and smooth muscle relaxation. S-nitrosylation and inhibition of AC5/6 activity were reversed by the iNOS inhibitor 1400W, whereas phosphorylation and activation of PDE4D5 were reversed by the phosphatidylinositol 3-kinase inhibitor LY294002 and the ERK1/2 inhibitor PD98059. The effects of IL-1β or TNF-α on forskolin-stimulated cAMP formation and smooth muscle relaxation reflected inhibition of AC5/6 activity and activation of PDE4D5 and were partly reversed by 1400W or PD98059 and completely reversed by a combination of the two inhibitors. The changes in the cAMP/PKA signaling and smooth muscle relaxation contribute to colonic dysmotility during inflammation.
Keywords: phosphodiesterases, protein kinase A, S-nitrosylation, inflammation, muscle relaxation
relaxant neurotransmitters released by the enteric nervous system initiate signaling cascades that lead to generation of cGMP (nitric oxide and carbon monoxide), cAMP (peptide histidine isoleucine), or both cAMP and cGMP (vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide) (31). The levels of cAMP and cGMP are tightly regulated within narrow limits by the balance of cyclase and phosphodiesterase (PDE) activity, which, in turn, are regulated via feedback phosphorylation of cyclases and PDEs by cAMP- and/or cGMP-dependent protein kinases (5, 6, 8, 16, 18, 22, 26, 27, 46).
PDEs catalyze the cyclic phosphate bond in cAMP and cGMP to yield the inactive products 5′-AMP and 5′-GMP. Eleven PDE families, derived from 20 genes and classified on the basis of their regulatory and catalytic properties, have been identified. Various isoforms of PDE3 and PDE4 account for the greater part of cAMP-hydrolyzing activity in various tissues (5, 8,16, 46). PDE3A and PDE3B are products of different genes; they exhibit greater affinity for cAMP but are differentially regulated (9, 46). The large PDE4 family is the product of four genes (PDE4A, -B, -C, and -D) that encode ∼25 isozymes classified into long and short forms on the basis of the presence of one or both conserved regulatory domains known as “upstream conserved regions” 1 and 2 (UCR1 and UCR2) (16–18, 26–30, 42). Long isoforms possess a sequence on UCR1 for stimulatory serine phosphorylation by PKA and a sequence located within the COOH terminus of the catalytic unit for inhibitory serine phosphorylation by extracellular signal-regulated kinases1/2 (ERK1/2) (13–15, 27–30). Some studies have reported stimulatory phosphorylation of PDE4D3 and PDE4D5 via a protein kinase C-ERK1/2 pathway (2, 3). PDEs are usually localized to subcellular compartments by binding or recruitment to various lipid rafts or anchoring proteins, mainly A-kinase-anchoring protein (AKAP) and β-arrestin (16). Selective recruitment and absence of functional redundancy among PDE4 isoenzymes suggest that even splice variants of PDE4 may have specific functions. PDE4D3 and PDE4D5 appear to be the main cAMP-specific long isoform of PDE4 expressed in vascular and visceral smooth muscle (2, 27, 37, 38).
Ten mammalian genes encode nine membrane-bound and one soluble adenylyl cyclase (AC) (6, 47, 48). The 10 isoforms are divided into 5 distinct groups based on amino acid sequence and functional similarities. AC5 and AC6, expressed mainly in excitable cells, are activated by Gαs and inhibited by Gαi, capacitative Ca2+ entry, and PKA-dependent phosphorylation (6, 34). AC2, AC4, and AC7 are activated by Gαs and synergistically by Gβγ subunits derived from Gi and inhibited by PKA-dependent phosphorylation (6, 47, 48). AC1, AC3, and AC8 are activated by Gαs and by Ca2+-calmodulin upon Ca2+ entry via voltage-gated or capacitative Ca2+ channels and inhibited by Gαo (present abundantly in brain tissue), Gβγ subunits derived from Gi, and PKA-dependent phosphorylation (6). Membrane-bound AC9 and soluble AC are insensitive to forskolin; soluble AC is highly expressed in sperm cells and insensitive also to G proteins (11, 49).
Earlier studies have shown that AC type V/VI (AC5/6) and both PDE3A and PDE4D5 are the main determinants of cAMP levels in the circular smooth muscle layer of the intestine (34, 37, 38). AC5/6 activity was inhibited by Gi-coupled agonists and by Ca2+ influx, independently of whether influx occurred via ligand-gated or capacitative Ca2+ channels. PDE4D5 activity was regulated via stimulatory phosphorylation by PKA and indirectly via PKC (37, 38). The latter involved PKC-mediated inhibition of protein phosphatase 2A (PP2A) and underlies a cross-regulatory mechanism whereby contractile agonists that activate PKC attenuate cAMP levels by enhancing PDE4D5 activity (38).
Expression and activity of AC and PDE isoforms in intestinal longitudinal smooth muscle have not been explored. In the present study, we identified expression of AC and PDE isoforms in normal intestinal longitudinal smooth muscle and in smooth muscle treated with proinflammatory cytokines (IL-1β and TNF-α) in vitro and exposed to inflammation in vivo. AC5/6 and PDE4D5 were expressed in normal longitudinal smooth muscle. PDE4D5 underwent stimulatory phosphorylation by phosphatidylinositol 3-kinase (PI3-kinase)/ERK1/2 pathway in response to cytokines and during inflammation. Concurrently, AC5/6 activity was decreased as a result of S-nitrosylation via NOS-III [inducible nitric oxide synthase (iNOS)]. The combined effects of ERK1/2-mediated phosphorylation of PDE4D5 and iNOS-mediated S-nitrosylation of AC5/6 decreased cAMP formation and longitudinal smooth muscle relaxation during inflammation.
MATERIALS AND METHODS
Materials.
[125I]cAMP and [α-32P]ATP, and [3H]cAMP were obtained from PerkinElmer Life Sciences (Boston, MA); collagenase and soybean trypsin inhibitor from Worthington Biochemical (Freehold, NJ); Western blot, chromatography material, and protein assay kit from Bio-Rad Laboratories (Hercules, CA); and antibody to AC5/6 from Santa Cruz Biotechnology (Santa Cruz, CA), this antibody does not distinguish between AC5 and AC6. Antibody to PDE3A and PDE4D5 and phospho-antibody to PDE4D5 (Ser126) were obtained from FabGennix (Frisco, TX); S-nitrosylation detection kit from Cayman (Ann Arbor, MI); and cAMP, Crotalus atrox snake venom, and all other chemicals from Sigma Chemical (St. Louis, MO).
All animal treatments were performed according to a protocol approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Induction of colonic inflammation and preparation of dispersed colonic smooth muscle cells.
Colonic inflammation in mice was induced with 2,4,6 trinitrobenzene sulphonic acid (TNBS) as described previously (1, 12, 39, 41). Adult C57BL/6J male mice (6–8 wk old) were anesthetized, and 100 μl of TNBS [2.5% in 50% ethanol (vol/vol)] were instilled intrarectally via a catheter advanced to 3 cm proximal to the anus via 1-ml syringe fitted with a catheter, and mice were euthanized 3 days after the induction of inflammation. Age-matched control mice were treated with vehicle. Colonic tissue from mice treated with TNBS exhibited typical histological characteristics of colitis. Distal colonic segments of 2- to 3-cm long were obtained, and muscle cells from longitudinal muscle layer were obtained as described previously (1, 12, 39, 41). Briefly, strips of longitudinal muscle from mice colon were dissected and incubated at 31°C for 30 min in HEPES medium containing 120 mM NaCl, 4 mM KCl, 2.6 mM KH2PO4, 0.6 mM MgCl2, 25 mM HEPES, 14 mM glucose, 2.1% Eagle's essential amino acid mixture, 0.1% collagenase, and 0.1% soybean trypsin inhibitor. After the partly digested strips were washed twice with 50 ml of enzyme-free medium, the muscle cells were allowed to disperse spontaneously for 30 min. The smooth muscle cells (SMCs) were harvested by filtration through 500-μm Nitex (Tetko, Briarcliff Manor, NY) and centrifuged twice at 350 g for 10 min. For some experiments, muscle cells were cultured in DMEM containing 10% fetal bovine serum until they attained confluence and were then passaged once for use.
Assay for AC activity.
AC activity was measured by using [α-32P]ATP as substrate as described previously (34, 37). Homogenates of SMCs were incubated for 15 min at 37°C in 50 mM Tris·HCl (pH 7.4), 2 mM cAMP, 0.1 mM ATP, 1 mM isobutylmethyl xanthine (IBMX), 5 mM MgCl2, 100 mM NaCl, 5 mM creatine phosphate, 50 U/ml of creatine phosphokinase, and 0.5 mM [α-32P]ATP (0.2 μCi). The reaction was terminated by addition of 2% sodium dodecyl sulfate, 45 mM ATP, and 1.5 mM cAMP. [32P]cAMP was separated from [32P]ATP by sequential chromatography on Dowex AG50W-4X and alumina columns. The results were expressed as picomoles of cAMP per milligrams of protein per minute.
Assay for PDE4 activity.
PDE4 activity was measured in immunoprecipitates of PDE4D5 as described previously (37, 38). One-millimeter aliquots (3 × 106 cells/ml) were incubated with forskolin for 5 min. Immunoprecipitates were washed in a medium containing 50 mM Tris (pH 7.5), 200 mM NaCl, and 5 mM EDTA and then incubated for 15 min at 30°C in a medium containing 100 mM MES (pH 7.5), 10 mM EDTA, 0.1 M Mg acetate, 0.9 mg/ml BSA, 20 μM cAMP, and [3H]cAMP. The samples were boiled for 3 min, chilled for 3 min, and then incubated at 30°C for 10 min in 20 mM Tris (pH 7.5) medium containing 10 μl of Crotalus atrox snake venom (10 μg/μl). The samples were added to DEAE-Sephacel A-25 columns, and the radioactivity in the effluent was counted. The results were expressed as counts per minute per milligrams of protein.
Phosphorylation of PDE4D5 by PKA.
Phosphorylation of PDE4D5 was measured by immunoblot analysis using phospho-specific antibody (Ser126) as described previously (38). One-milliliter aliquots (3 × 106 cells/ml) were incubated with forskolin for 5 min, and the reaction was terminated with an equal volume of lysis buffer and placed on ice for 30 min. The cell lysates were separated from the insoluble material by centrifugation at 13,000 g for 15 min at 4°C, precleared with 40 μl of protein A-Sepharose, and incubated with polyclonal PDE4D5 for 2 h at 4°C and with 40 μl of protein A-Sepharose for another 1 h. The immunoprecipitates were washed five times with 1 ml of wash buffer (0.5% Triton X-100, 150 mM NaCl, and 10 mM Tris-HCl pH 7.4), extracted with Laemmli sample buffer, boiled for 15 min, and then separated on 10% SDS-PAGE followed by transfer to polyvinylidine difluoride membranes. The membranes were incubated for 12 h with phospho-specific antibodies to PDE4D5 (Ser126) and then for 1 h with a horseradish peroxidase-conjugated secondary antibody. The bands were identified by enhanced chemiluminescence (ECL).
Phosphorylation of AC5/6 and PDE4D5.
Phosphorylation of AC5/6 and PDE4D5 was determined from the amount of 32P incorporated into the enzyme after immunoprecipitation with specific antibody (37). SMCs (3 × 106 cell/ml) prelabeled with 0.5 Ci/ml of [32P]Pi for 3 h were homogenized in medium containing 1% Triton X-100, 0.5% SDS, 10 mM EDTA, 1 mM PMSF, 10 μg/ml leupeptin, 100 μg/ml aprotinin, 10 mM sodium pyrophosphate, 50 mM NaF, and 0.2 mM sodium azide. The cell lysates were centrifuged at 13,000 g for 15 min at 4°C, precleared with 40 μl of protein A/G PLUS agarose beads, and incubated with antibody to AC5/6 or PDE4D5 for 3 h at 4°C followed by another 1 h of incubation with 40 μl of protein A/G PLUS agarose beads. The immunoprecipitates were washed with medium containing 10 mM Tris·HCl (pH 7.4), 150 mM NaCl, and 0.5% Triton X-100 and extracted with sample buffer. The samples were resolved by gel electrophoresis, 32P-labeled AC5/6 and PDE4D5 were visualized by autoradiography, and the amount of radioactivity in the band was counted.
Measurement of ERK1/2 activity.
ERK1/2 activity was determined in cell extracts by immunokinase assay as described previously (36). Cell pellets obtained from dispersed SMCs were solubilized, and equal amounts of protein extracts were incubated with ERK1/2 antibody plus protein A/G agarose overnight at 4°C. The immunoprecipitates were washed and incubated for 5 min on ice with 5 μg of myelin basic protein. The assay was initiated by the addition of 10 μCi of [32P]ATP (3,000 Ci/mmol) and 20 μM ATP. 32P-labeled myelin basic protein was absorbed onto phosphocellulose disks, and the amount of radioactivity was measured by liquid scintillation.
Assay for S-nitrosylation.
The S-nitrosylated AC5/6 was detected using a detection assay kit (Cayman, Ann Arbor, MI). In brief, AC5/6 was immunoprecipitated with anti-AC5/6 conjugated with protein A/G plus agarose beads. Immunoprecipitated AC5/6 was released by boiling the beads for 5 min, the free thiols were blocked, and then S-NO bonds were cleaved. The proteins were labeled with biotin by biotinylation of the newly formed SH groups. The labeled proteins were then analyzed by SDS-PAGE and transferred to PVDF membranes (Invitrogen) for Western blot.
Radioimmunoassay for cAMP.
cAMP production was measured by radioimmunoassay as described previously (34, 35, 36, 38). Briefly, muscle cells (3 × 106 cells) were treated with forskolin for 5 min and the reaction was terminated with 10% trichloroacetic acid. After extraction with water-saturated diethyl ether, the lyophilized aqueous phase was reconstituted in 500 μl of 50 mM Na acetate (pH 6.2). The samples were acetylated with triethylamine/acetic anhydride (2:1) for 30 min and cAMP was measured in duplicate using 100 μl aliquots. The results were expressed as picomoles per milligrams of protein.
Statistical analysis.
All values are expressed as means ± SE; n represents the number of animal studies. Regression analysis was performed using GraphPad Prism 5. Statistical analysis was performed by unpaired t-test, and P < 0.05 was considered statistically significant.
RESULTS
Expression of AC and PDE isoforms in mouse colonic longitudinal smooth muscle.
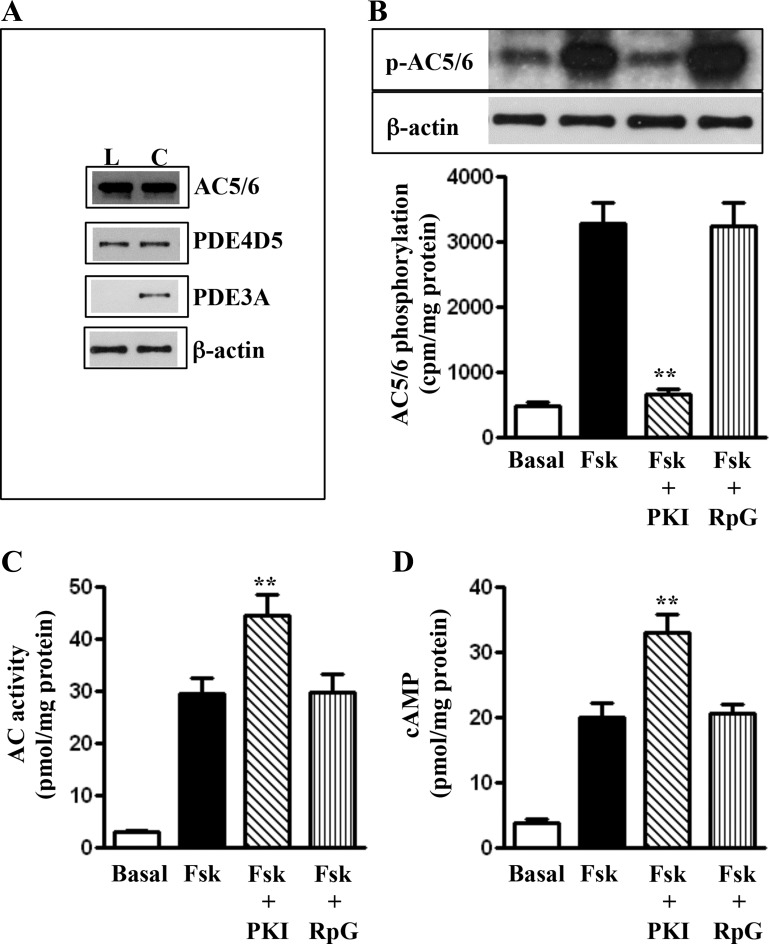
Western blot analysis in dispersed SMCs of colonic longitudinal muscle detected the presence of AC5/6 and PDE4D5 but not PDE3A (Fig. 1A). Earlier studies using gastric circular SMCs detected the presence of AC5/6 but not AC2, AC3, or AC4; the studies detected also the presence of PDE4D5 and PDE3A but not PDE3B (35, 37, 38).
Fig. 1.
Expression of adenylyl cyclase (AC) and phosphodiesterases (PDE) in colonic circular and longitudinal smooth muscle cells (SMCs) and PKA-dependent phosphorylation of adenylyl cyclase 5/6 (AC5/6) and feedback inhibition of AC5/6 activity in longitudinal smooth muscle cells. A: expression of AC5/6 and PDE4D5 and PDE3A were analyzed in freshly prepared dispersed circular (C) and longitudinal (L) SMCs of colon. Muscle cell lysates containing equal amounts of total proteins were separated with SDS-PAGE, and expression of AC5/6 and PDE3A and PDE4D5 was analyzed using selective antibody. Membranes were reblotted to measure β-actin. Protein bands were visualized with enhanced chemiluminescence. B: longitudinal muscle cells labeled with 32P were incubated with forskolin (Fsk; 10 μM) in the presence or absence of myristoylated PKI (1 μM) or Rp-cGMPS (RpG; 1 μM). Immunoprecipitates using antibody to AC5/6 were separated on SDS-PAGE. [32P]AC5/6 (p-AC5/6) was identified by autoradiography. Measured radioactivity in the bands is expressed as counts/min, and immunoblots (50 μg protein) of the bands are shown for a loading control. Values are means ± SE of 3 experiments. **Significant inhibition of forskolin-induced phosphorylation. C: longitudinal muscle cells were treated with forskolin (10 μM) in the presence or absence of PKA inhibitor myristoylated PKI (1 μM). AC activity was measured as described in materials and methods. Results are expressed as pmol cAMP/mg protein. Values are means ± SE of 4 experiments. **Significant augmentation of forskolin-induced AC activity. D: cAMP was measured in dispersed muscle cells treated with forskolin (1 μM) in the presence of isobutylmethyl xanthine (IBMX; 100 μM) for 5 min by radioimmunoassay (RIA). **Significant increase in cAMP formation in the presence of PKI compared with cAMP formation by forskolin alone.
Feedback regulation of AC5/6 activity by PKA.
Treatment of mouse colonic longitudinal SMCs with 10 μM forskolin stimulated AC5/6 phosphorylation (measured in immunoprecipitates of 32P-labeled cells using AC5/6 antibody); phosphorylation was blocked by myristoylated PKI but not by Rp-cGMPS implying that it was mediated by PKA (Fig. 1B). Treatment of SMCs with 10 μM forskolin for 5 min caused a ∼10-fold increase in AC5/6 activity, which was enhanced in the presence of the PKA inhibitor myristoylated PKI (1 μM) but not the PKG inhibitor Rp-cGMPS (1 μM), suggesting feedback inhibition of AC5/6 activity by PKA (Fig. 1C). This is consistent with AC5/6 phosphorylation by PKA. cAMP formation in response to forskolin (measured after pretreatment with 100 μM IBMX) increased significantly in the presence of myristoylated PKI but not Rp-cGMPS implying that phosphorylation of AC5/6 by PKA inhibited AC5/6 activity (Fig. 1D).
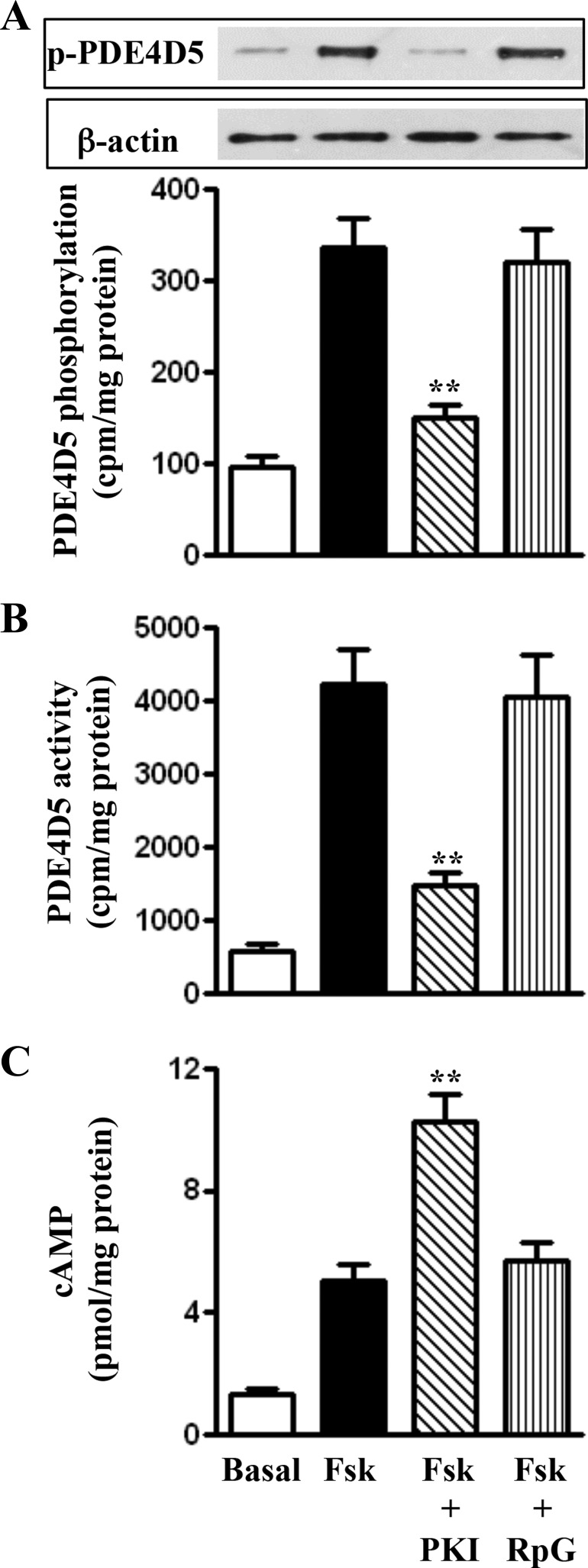
Regulation of PDE4D5 activity by PKA.
Treatment of mouse colonic longitudinal SMCs with 10 μM forskolin for 5 min caused an approximately threefold increase in PDE4D5 phosphorylation accompanied by an approximately six-fold increase in PDE4D5 activity. Both PDE4D5 phosphorylation and activity were blocked in the presence of myristoylated PKI but not Rp-cGMPS (Fig. 2, A and B), suggesting that feedback phosphorylation of PDE4D5 by PKA enhanced PDE4D5 activity. The effect of PDE4D5 was reflected in measurements of cAMP formation in the absence of IBMX; the increase in cAMP formation induced by 10 μM forskolin was significantly increased in the presence of myristoylated PKI but not Rp-cGMPS (Fig. 2C).
Fig. 2.
PKA-dependent phosphorylation of phosphodiesterase 4D5 (PDE4D5) and feedback stimulation of PDE4D5 activity. A: longitudinal muscle cells were treated with forskolin (10 μM) in the presence or absence of myristoylated PKI (1 μM) or Rp-cGMPS (1 μM). PDE4D5 immunoprecipitates were separated on SDS-PAGE and analyzed with phospho-Ser126-specific antibody by Western blotting. Values are means ± SE of 4 experiments. **Significant inhibition of forskolin-induced phosphorylation in the presence of PKI. B: longitudinal muscle cells were treated with forskolin (10 μM) in the presence or absence of PKA inhibitor myristoylated PKI (1 μM). PDE4D5 activity was measured as described in materials and methods. Values are means ± SE of 4 experiments. **Significant decrease in PDE4D5 activity in the presence of PKI compared with activity by forskolin alone. C: cAMP was measured in dispersed muscle cells treated with forskolin (10 μM) in the absence of IBMX (100 μM) for 5 min by RIA. Values are means ± SE of 4 experiments. **Significant increase in cAMP formation in the presence of PKI compared with cAMP formation by forskolin alone.
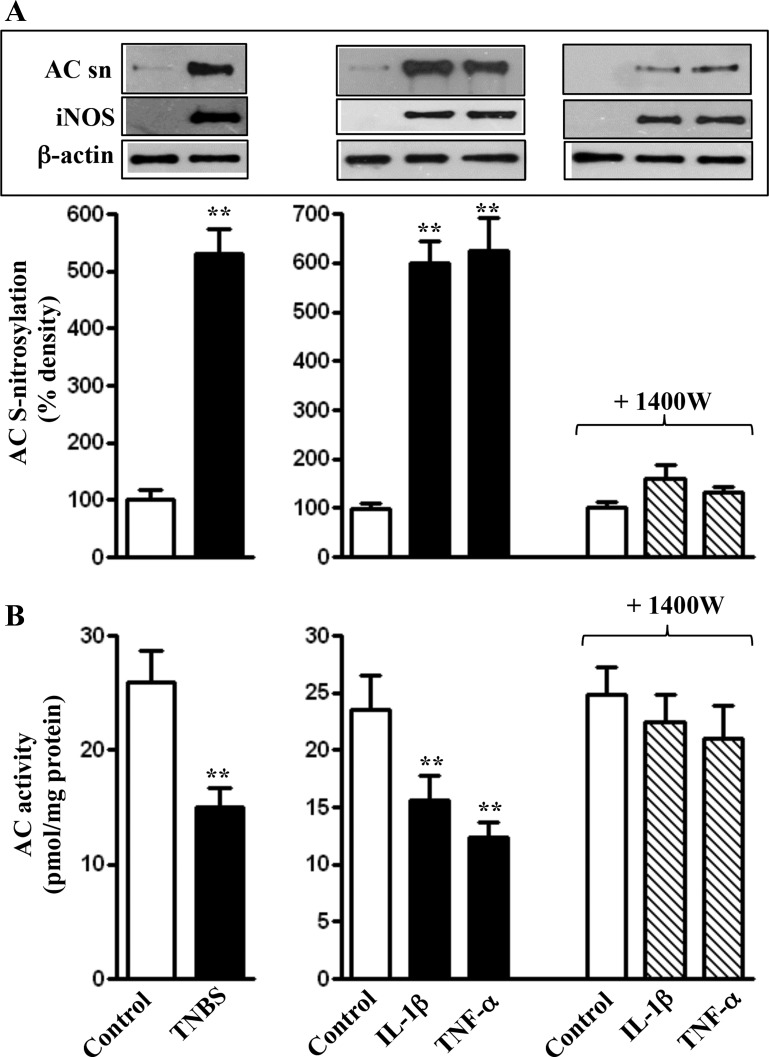
Inhibition of AC5/6 activity by proinflammatory cytokines and during inflammation in vivo.
NOS-III (iNOS) was induced in colonic longitudinal SMCs isolated from the colon of TNBS-treated mice or from control strips treated for 48 h with IL-1β or TNF-α (Fig. 3A). NOS-III induction was accompanied by S-nitrosylation of AC5/6, which was blocked in the presence of the iNOS inhibitor 1400W; the latter had no effect on iNOS expression (Fig. 3A).
Fig. 3.
Suppression of forskolin-induced AC5/6 activity via inducible nitric oxide synthase (iNOS)-mediated S-nitrosylation of AC5/6. Longitudinal muscle cells isolated from colon of control and 2,4,6 trinitrobenzene sulphonic acid (TNBS)-treated mice or from muscle strips cultured in the presence of IL-1β (10 ng/ml) or TNF-α (1 nM) plus iNOS inhibitor 1400W (10 μM) for 48 h were used to measure S-nitrosylation (AC sn) as described in materials and methods. Values are means ± SE of 4 experiments. **Significant increase in S-nitrosylation. Inset: iNOS expression in muscle cells isolated from colon of control and TNBS-treated mice or from muscle strips cultured in the presence of IL-1β (10 ng/ml) or TNF-α (1 nM) plus iNOS inhibitor 1400 W (10 μM) for 48 h was measured by Western blot. B: longitudinal muscle cells isolated from colon of control and TNBS-treated mice or from muscle strips cultured in the presence of IL-1β (10 ng/ml) or TNF-α (1 nM) plus iNOS inhibitor 1400W (10 μM) for 48 h were treated with 10 μM of forskolin for 5 min and AC activity was measured as described in materials and methods. Basal AC activity was not significantly different between control and TNBS-treated mice (B, left) or between control and cytokine treated muscle strips (B, right). Values are means ± SE of 5 experiments. **P < 0.01, significant inhibition in forskolin-induced AC activity.
S-nitrosylation of AC5/6 was accompanied by a decrease in forskolin-stimulated AC5/6 activity in colonic longitudinal SMCs isolated from the colon of TNBS-treated mice or from control muscle strips treated for 48 h with IL-1β or TNF-α (Fig. 3A). AC5/6 activity was restored in the presence of 1400W, suggesting that S-nitrosylation by iNOS inhibited AC5/6 activity (Fig. 3B).
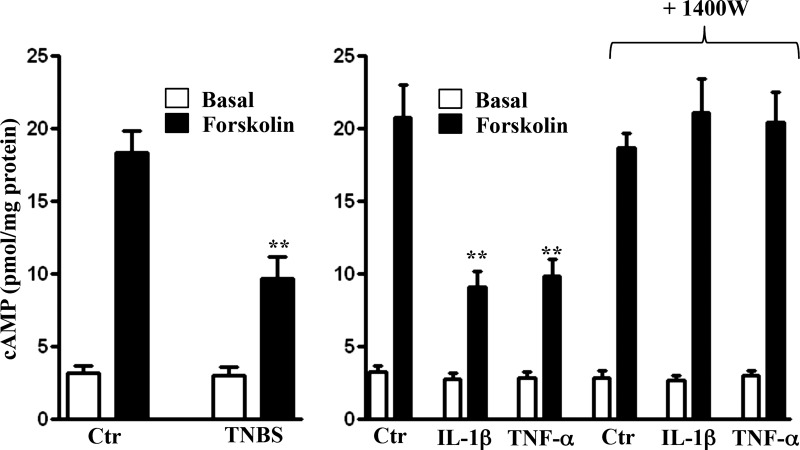
Forskolin-stimulated cAMP formation, measured in the presence of IBMX so as to eliminate the confounding effect of changes in PDE activity, was also inhibited in colonic longitudinal SMCs isolated from the colon of TNBS-treated mice or from control muscle strips treated for 48 h with IL-1β or TNF-α (Fig. 4). The inhibition was reversed in the presence of 1400W, reflecting the inhibition of AC5/6 activity by iNOS (Fig. 4).
Fig. 4.
Suppression of forskolin-induced cAMP formation via iNOS-mediated S-nitrosylation of AC5/6. Longitudinal muscle cells isolated from colon of control and TNBS-treated mice or from muscle strips cultured in the presence of IL-1β (10 ng/ml) or TNF-α (1 nM) plus iNOS inhibitor 1400W (10 μM) for 48 h were treated with 10 μM of forskolin in the presence of 100 μM IBMX for 5 min and cAMP formation was measured by RIA as described in materials and methods. Basal cAMP levels were not significantly different between control (Ctr) and TNBS-treated mice (left) or between control and cytokine-treated muscle strips (right). Values are means ± SE of 5 experiments. **P < 0.01, significant inhibition in forskolin-induced cAMP formation.
Stimulation of PDE4D5 phosphorylation and activity by proinflammatory cytokines and during inflammation.
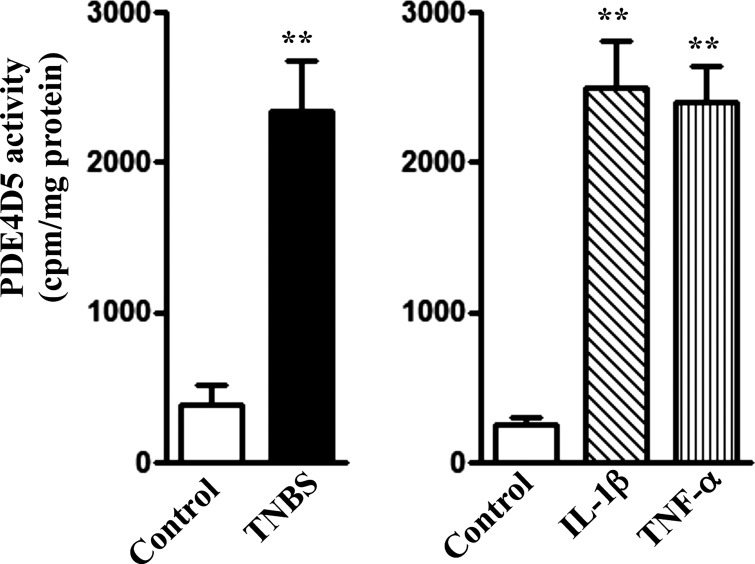
PDE4D5 activity was increased in colonic longitudinal SMCs isolated from the colon of TNBS-treated mice or from control muscle strips treated for 48 h with IL-1β or TNF-α (Fig. 5). PDE4D5 activity was accompanied by increase in PDE4D5 phosphorylation in colonic longitudinal SMCs isolated from control muscle strips treated for 48 h with IL-1β or TNF-α (Figs. 6 and 7).
Fig. 5.
Augmentation of PDE4D5 activity by cytokines. Longitudinal muscle cells isolated from colon of control and TNBS-treated mice (left) or from muscle strips cultured in the presence of IL-1β (10 ng/ml) or TNF-α (1 nM) for 48 h (right) were used to measure PDE4D5 activity as described in materials and methods. Values are means ± SE of 4 experiments. **Significant increase in PDE4D5 activity.
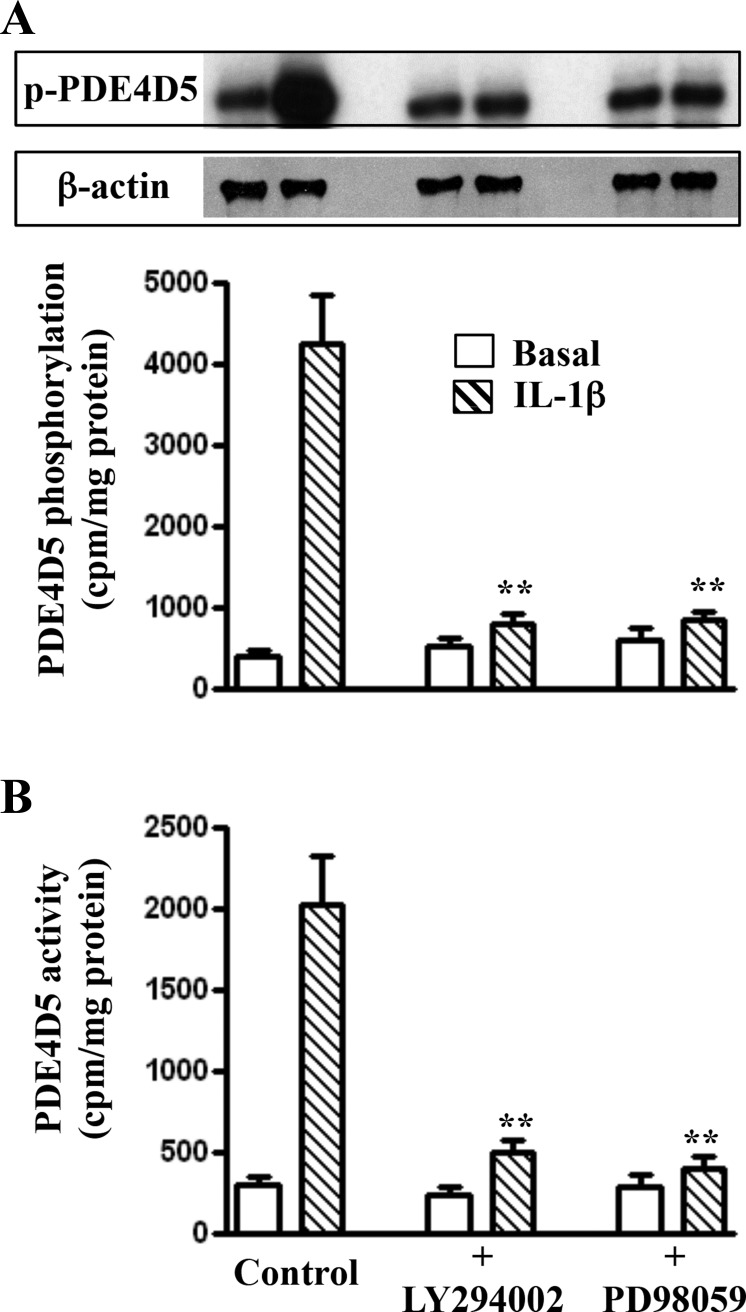
Fig. 6.
Phosphorylation of PDE4D5 and stimulation of its activity by IL-1β via phosphatidylinositol 3-kinase (PI3-kinase) and ERK1/2 pathway. Longitudinal muscle cells isolated from muscle strips cultured in the presence of IL-1β (10 ng/ml) plus PI3-kinase inhibitor LY294002 (10 μM) or ERK1/2 inhibitor PD98059 (10 μM) for 48 h. PDE4D5 phosphorylation (A) was measured in 32P-labeled cells, and PDE4D5 activity (B) was measured in PDE4D5 immunoprecipitates as described in materials and methods. Values are means ± SE of 4–5 experiments. **Significant inhibition of PDE4D5 phosphorylation and activity in the presence of LY294002 or PD98059 compared with control.
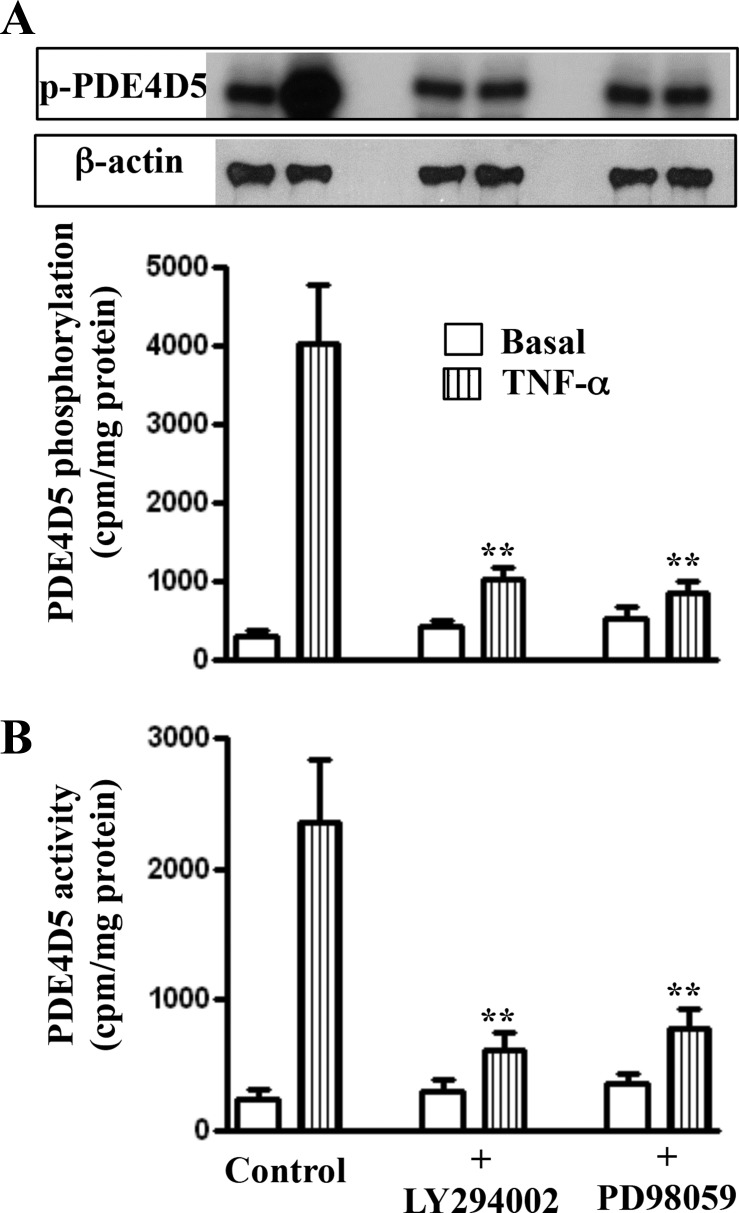
Fig. 7.
Phosphorylation of PDE4D5 and stimulation of its activity by TNF-α via PI3-kinase and ERK1/2 pathway. Longitudinal muscle cells isolated from muscle strips cultured in the presence of TNF-α (1 nM) plus PI3-kinase inhibitor LY294002 (10 μM) ERK1/2 inhibitor PD98059 (10 μM) for 48 h. PDE4D5 phosphorylation (A) was measured in 32P-labeled cells, and PDE4D5 activity (B) was measured in PDE4D5 immunoprecipitates as described in materials and methods. Values are means ± SE of 4–5 experiments. **Significant inhibition of PDE4D5 phosphorylation and activity in the presence of LY294002 or PD98059 compared with control.
Previous studies have shown that activity of PDE4 long isoforms was increased by PI3-kinase and ERK1/2 under oxidative stress (14). The involvement of PI3-kinase and ERK1/2 in the activation of PDE4D5 during inflammation was examined with a selective blocker of PI3-kinase (LY294002) and ERK1/2 (PD98059). The increase in PDE4D5 phosphorylation and activity was blocked in the presence of the LY294002 and PD98059 (Figs. 6 and 7).
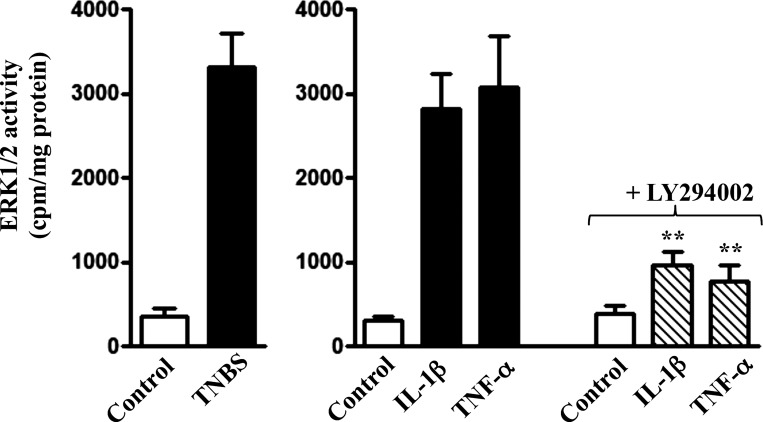
ERK1/2 activity was also increased in colonic longitudinal SMCs isolated from the colon of TNBS-treated mice or from control muscle strips treated for 48 h with IL-1β or TNF-α (Fig. 8). The increase in ERK1/2 activity induced by IL-1β or TNF-α was blocked in the presence of the PI3-kinase inhibitor LY294002, suggesting activation of ERK1/2 was downstream of PI3-kinase activation (Fig. 8).
Fig. 8.
Stimulation of ERK1/2 activity by cytokines via PI3-kinase. Longitudinal muscle cells isolated from muscle strips cultured in the presence of IL-1β (10 ng/ml) or TNF-α (1 nM) plus PI3-kinase inhibitor LY294002 (10 μM). ERK1/2 activity was measured by immunokinase assay as described in materials and methods. Values are means ± SE of 4–5 experiments. **Significant inhibition of ERK1/2 activity in the presence of LY294002 compared with control response to IL-1β or TNF-α.
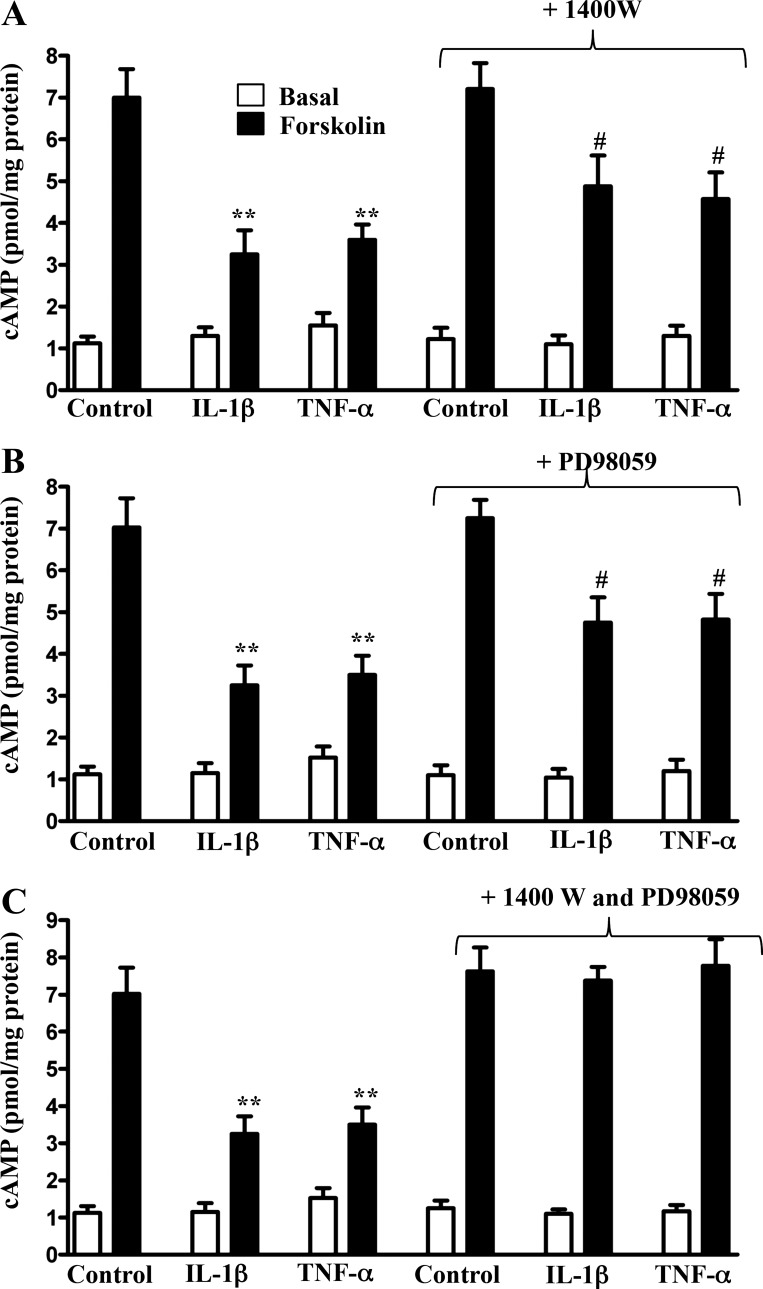
Forskolin-stimulated cAMP formation in longitudinal SMCs isolated from control muscle strips treated for 48 h with IL-1β or TNF-α, measured in the absence of IBMX so as to maintain the effect of changes in PDE activity, was inhibited (Fig. 9). The inhibition was partly reversed in the presence of 1400W or PD98059 and was completely reversed in the presence of both inhibitors (Fig. 9, A–C). The inhibition was also partly reversed in the presence of LY294002 (Fig. 10). The pattern of cAMP inhibition during inflammation in vivo or upon treatment with proinflammatory cytokines in vitro reflected inhibition of AC5/6 upon S-nitrosylation by iNOS and activation of PDE4D5 upon phosphorylation via PI3-kinase/ERK1/2 pathway.
Fig. 9.
Suppression of forskolin-induced cAMP formation by proinflammatory cytokines via ERK1/2-mediated increase in PDE4D5 activity and iNOS-mediated decrease in AC5/6 activity. Longitudinal muscle cells isolated from muscle strips cultured in the presence of IL-1β (10 ng/ml) or TNF-α (1 nM) plus 1400W (10 μM; A), PD98059 (10 μM; B), or both (C) for 48 h were treated with 10 μM of forskolin in the absence of IBMX for 5 min. cAMP formation was measured by RIA as described in materials and methods. Basal cAMP levels were not significantly different between control and cytokine-treated muscle strips. Values are means ± SE of 5 experiments. **P < 0.01, #P < 0.05, significant inhibition in forskolin-induced cAMP formation.
Fig. 10.
Suppression of forskolin-induced cAMP formation by proinflammatory cytokines via PI3-kinase. Longitudinal muscle cells isolated from muscle strips cultured in the presence of IL-1β (10 ng/ml) or TNF-α (1 nM) plus PI3-kinase inhibitor LY294002 for 48 h were treated with 10 μM of forskolin in the absence of IBMX for 5 min. cAMP formation was measured by RIA as described in materials and methods. Basal cAMP levels were not significantly different between control and cytokine-treated muscle strips. Values are means ± SE of 4 experiments. **P < 0.01, #P < 0.05, significant inhibition in forskolin-induced cAMP formation.
Inhibition of forskolin-stimulated relaxation by proinflammatory cytokines and during inflammation in vivo.
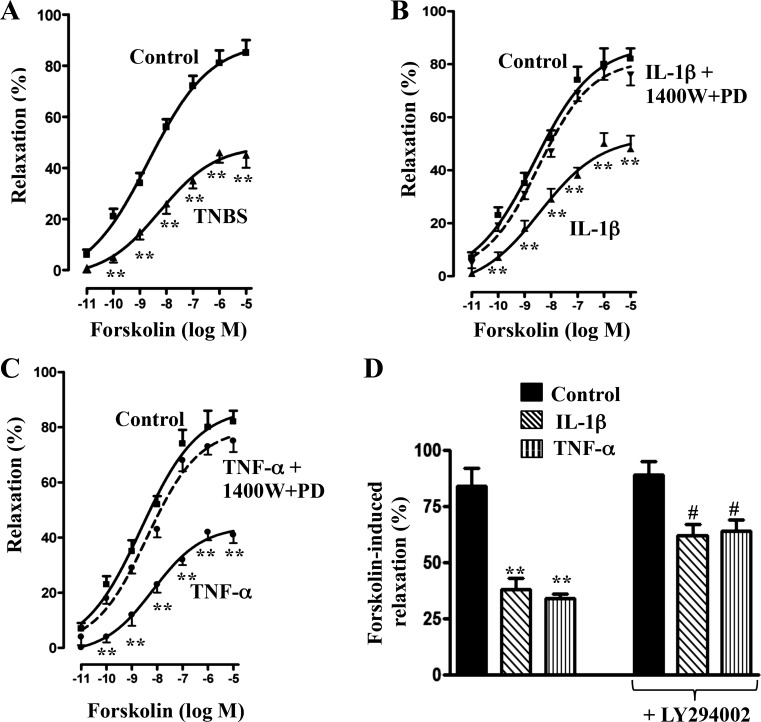
Forskolin-stimulated relaxation, measured in the absence of IBMX, was inhibited in longitudinal SMCs isolated from the colon of TNBS-treated mice or from control muscle strips treated for 48 h with IL-1β or TNF-α (Fig. 11). Maximal relaxation induced by 10 μM forskolin was inhibited by 45–50% (P < 0.05; n = 6). Inhibition of relaxation induced by treatment with IL-1β or TNF-α was completely reversed in the presence of both 1400W and PD98059 (Fig. 11, B and C). Inhibition of relaxation was also partly reversed in the presence of LY294002 (Fig. 11D). The inhibition of relaxation and partial reversal by LY294002 or complete reversal by combination of both 1400W and PD98059 paralleled the changes in cAMP formation.
Fig. 11.
Suppression of forskolin-induced relaxation by proinflammatory cytokines via iNOS and PI3-kinase/ERK1/2 pathway. Longitudinal muscle cells isolated from colon of control and TNBS-treated mice (A) or from muscle strips cultured in the presence of IL-1β (10 ng/ml; B) or TNF-α (1 nM; C) plus 1400W (10 μM) and PD98059 (10 μM) or LY294002 (10 μM; D) for 48 h were treated with different concentrations of forskolin for 5 min and carbachol for 30 s to measure initial Ca2+-dependent contraction. SMC contraction was measured by scanning micrometry, and relaxation is expressed as percent inhibition of carbachol-induced contraction. Values are means ± SE of 5–6 experiments. **P < 0.01, #P < 0.05, significant inhibition in forskolin-induced relaxation.
DISCUSSION
Recent studies have shown that critical components of the signaling cascades mediating contraction of intestinal circular and longitudinal smooth muscle are different (1, 10, 24, 25, 31–33, 36, 39). Contraction in longitudinal smooth muscle is initiated by Ca2+ influx and Ca2+- and cyclic ADP ribose-induced Ca2+ release via RYR-2/Ca2+ channels and terminated predominantly by inactivation of myosin light chain kinase (MLCK) via sequential activation of calmodulin kinase kinase β (CaMKKβ) and AMP kinase, whereas contraction in circular smooth muscle is initiated by IP3-dependent Ca2+ release via IP3R-I/Ca2+ channels and terminated predominantly by inactivation of Gαq via RGS4. Contraction is also sustained differently in the two muscle layers. In longitudinal smooth muscle, contraction is sustained via sequential activation of G12, RhoGEF (LARG)/RhoA and Rho kinase-dependent phosphorylation of MYPT1 and inhibition of MLC phosphatase (MLCP), whereas in circular smooth muscle contraction is sustained via sequential activation of Gq/13 and p115 RhoGEF/RhoA. Activation of RhoA leads to inhibition of MLCP activity via Rho kinase-dependent phosphorylation of MYPT1, the regulatory subunit of MLCP, and PKC-dependent phosphorylation of CPI-17, an endogenous inhibitor of MLCP (1, 7, 31, 36). The signaling components in both types of smooth muscle are targets of inflammatory cytokines acting via transcription factors (e.g., NF-κB and AP-1), inducible enzymes (e.g., iNOS), and various regulatory kinases (ERK1/2, p38 MAP kinase, and Jun kinase) (4, 19–21, 23, 41, 43–45, 50). Distinctive changes in expression and/or activity of specific signaling components (i.e., RGS4 and CPI-17 in circular smooth muscle, and AMP kinase and LARG in longitudinal smooth muscle) underlie the hypocontractility of circular and hypercontractility of longitudinal smooth muscle (19–21, 39–41).
This study identified a distinctive pattern of expression and activity of AC and PDE isoforms in intestinal longitudinal smooth muscle and determined the changes in their expression and/or activity in response to inflammatory cytokines (IL-1β and TNF-α) in vitro and TNBS-induced colonic inflammation in vivo. AC5/6 and PDE4D5 were expressed in both circular and longitudinal smooth muscle, whereas PDE3A, which plays a major role in circular smooth muscle, was absent in longitudinal smooth muscle (35, 37, 38). cAMP formation in longitudinal smooth muscle was shown to be tightly regulated via feedback phosphorylation of AC5/6 and PDE4D5 by PKA (Fig. 12). Inhibition of PKA activity by myristoylated PKI blocked AC5/6 phosphorylation and significantly enhanced AC5/6 activity and cAMP formation. In the absence of the PDE inhibitor IBMX, inhibition of PKA activity by myristoylated PKI blocked PDE4D5 phosphorylation and activity and significantly enhanced cAMP formation.
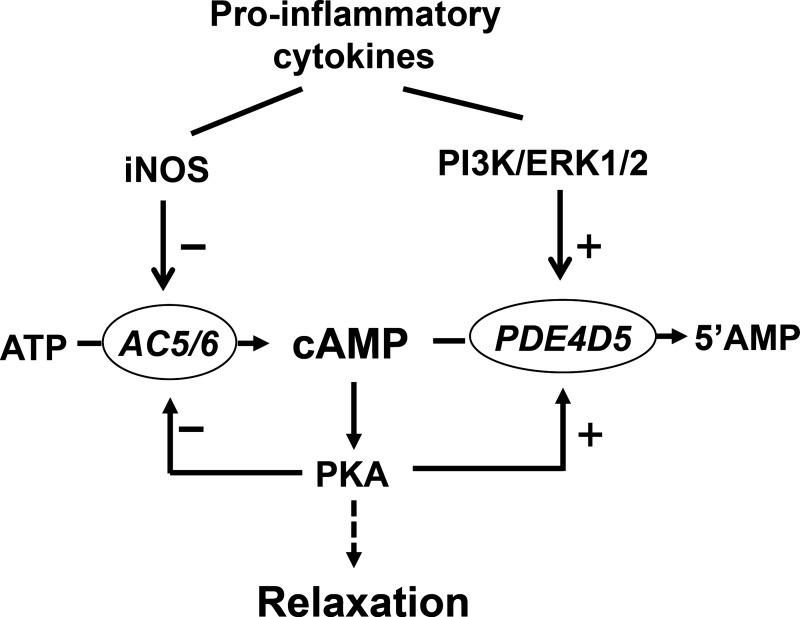
Fig. 12.
Schematic diagram demonstrating the effects of proinflammatory cytokines on pathways that regulate cAMP levels in colonic longitudinal SMCs. In longitudinal SMCs, cAMP levels are regulated by the feedback inhibitory phosphorylation of AC5/6 and stimulatory phosphorylation of PDE4D5 via PKA. Proinflammatory cytokines (IL-1β or TNF-α) inhibited AC5/6 activity via iNOS-mediated S-nitrosylation of AC5/6 and stimulated PDE4D5 activity via PI3-kinase/ERK1/2-mediated phosphorylation of PDE4D5. Inhibition of AC5/6 activity and stimulation of PDE4D5 activity lead to inhibition of cAMP formation and muscle relaxation during inflammation.
Exposure of longitudinal smooth muscle to inflammation in vivo or proinflammatory cytokines (IL-1β and TNF-α) in vitro induced expression of iNOS and caused S-nitrosylation of AC5/6 and stimulated phosphorylation of PDE4D5. The resultant increase in PDE4D5 activity and decrease in AC5/6 activity caused a decrease in cAMP formation and smooth muscle relaxation (Fig. 12). The effects of TNBS-induced inflammation in vivo on AC5/6 and PDE4D5 closely mimicked those elicited by exposure to either cytokine in vitro.
The S-nitrosylation of AC5/6 and inhibition of AC5/6 activity elicited by exposure of longitudinal smooth muscle to IL-1β and TNF-α were fully reversed by the iNOS inhibitor 1400W, whereas the increases in PDE4D5 phosphorylation and activity were reversed by the PI3-kinase inhibitor LY294002 and the ERK1/2 inhibitor PD98059. The effects of IL-1β or TNF-α on forskolin-stimulated cAMP levels (measured in the absence of IBMX) reflected the inhibition of AC5/6 activity and stimulation of PDE4D5 activity and were partly reversed by 1400W or PD98059 and completely reversed by a combination of the two inhibitors.
Inhibition of forskolin-stimulated relaxation in longitudinal SMCs isolated from TNBS-treated mice was similar to that of SMCs isolated from muscle strips treated for 48 h with IL-1β or TNF-α. Inhibition of relaxation in cells treated with either cytokine paralleled the changes in cAMP and was reversed by a combination of 1400W and PD98059.
A notable aspect of this study is the apparent stimulatory phosphorylation and activation of the long isoform of PDE4, PDE4D5 by ERK1/2. Hoffmann et al. (15) have shown that phosphorylation of Ser579, a residue located in an ERK consensus site in the COOH-terminal end of the catalytic domain of PDE4D3, leads to profound inhibition of PDE4D3 activity and elevation of cAMP levels. All long isoforms PDE4B, PDE4C, and PDE4D (but not PDE4A) are phosphorylated by ERK1/2 at a single Ser residue that is cognate to Ser579 site in PDE4D3, leading to inhibition of PDE4 activity (2, 3, 13, 14, 17, 29, 30). In contrast, phosphorylation within the UCR1 region by PKA leads to activation of PDE4D5 and inhibition of cAMP (8, 16, 18, 42). This raises the possibility that an initial increase in cAMP resulting from inhibition of PDE4D5 activity by ERK1/2 could lead to PKA-mediated phosphorylation and activation of PDE4D5, an effect that is susceptible to blockade by PD98059. A variant of this notion has been reported for ERK-mediated stimulatory phosphorylation of PDE4D5, the sole long PDE4 isoform expressed in human aortic smooth muscle, where activation of ERK by phorbol 12-myristate 13-acetate initiated a cascade that led to generation of prostaglandin E2 and stimulation of cAMP; this, in turn, led to phosphorylation of PDE4D5 by PKA at Ser126 and abrogated the effect of inhibitory phosphorylation of PDE4D5 by ERK at Ser651 (2). The cascade that resulted in stimulatory phosphorylation and activation of PDE4D5 was susceptible to blockade by PD98059.
Finally, consideration should be given to the possibility that activation of PDE4D5 could result from dual phosphorylation by ERK and a protein kinase downstream of PI3-kinase. Hill et al. (14) have shown that dual phosphorylation of PDE4D3 (or PDE4D5) upon challenge by H2O2 increased the activity of both isoforms; the effect of H2O2 was blocked by N-acetyl cysteine and NADPH oxidase inhibitors. For PDE4D3, phosphorylation occurred at the established ERK site (Ser579) as well as a distinct site (Ser239) located at the NH2 terminus of the catalytic domain (13). Phosphorylation at Ser239 switched the functional outcome of ERK1/2 phosphorylation from inhibition to activation. Phosphorylation at Ser239 reflected the action of an unknown protein kinase downstream of PI3-kinase and could be blocked by wortmannin. PDE4D3 activity required dual phosphorylation and was blocked by PD98059 or wortmannin (13). In the context of inflammation where an increase in the levels of reactive oxygen species is the norm, dual phosphorylation would lead to activation of PDE4D5 that is susceptible to blockade by PD98059.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-15564 (to K. S. Murthy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.M., D.P.K., W.H., J.F.K., J.R.G., and K.S.M. conception and design of research; S.M., A.D.N., D.P.K., W.H., and K.S.M. performed experiments; S.M., D.P.K., and K.S.M. analyzed data; S.M., A.D.N., J.R.G., and K.S.M. interpreted results of experiments; S.M. and D.P.K. prepared figures; S.M. drafted manuscript; S.M., A.D.N., W.H., J.F.K., J.R.G., and K.S.M. edited and revised manuscript; S.M., A.D.N., D.P.K., W.H., J.F.K., J.R.G., and K.S.M. approved final version of manuscript.
REFERENCES
- 1.Al-Shboul O, Nalli AD, Kumar DP, Mahavadi S, Kuemmerle JF, Grider JR, Murthy KS. Jun kinase-induced overexpression of leukemia-associated RhoGEF (LARG) mediates sustained hypercontraction of longitudinal smooth muscle in inflammation. Am J Physiol Cell Physiol 306: C1129–C1141, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baillie GS, MacKenzie SJ, Houslay MD. Phorbol 12-myristate 13-acetate triggers the protein kinase A-mediated phosphorylation and activation of the PDE4D5 cAMP phosphodiesterase in human aortic smooth muscle cells through a route involving extracellular signal regulated kinase (ERK). Mol Pharmacol 60: 1100–1111, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Baillie GS, MacKenzie SJ, McPhee I, Houslay MD. Sub-family selective actions in the ability of Erk2 MAP kinase to phosphorylate and regulate the activity of PDE4 cyclic AMP-specific phosphodiesterases. Br J Pharmacol 131: 811–819, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao W, Vrees MD, Potenti FM, Harnett KM, Fiocchi C, Pricolo VE. Interleukin 1β-induced production of H2O2 contributes to reduced sigmoid colonic circular smooth muscle contractility in ulcerative colitis. J Pharmacol Exp Ther 311: 60–70, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Conti M, Jin SL. The molecular biology of cyclic nucleotide phosphodiesterases. Prog Nucleic Acid Res Mol Biol 63: 1–38, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J 375: 517–529, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Godoy MA, Rattan S. Role of rho kinase in the functional and dysfunctional tonic smooth muscles. Trends Pharmacol Sci 32: 384–393, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekholm D, Belfrage P, Manganiello V, Degerman E. Protein kinase A-dependent activation of PDE4 (cAMP-specific cyclic nucleotide phosphodiesterase) in cultured bovine vascular smooth muscle cells. Biochim Biophys Acta 1356: 64–70, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog Nucleic Acid Res Mol Biol 65: 1–52, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Grider JR, Makhlouf GM. Contraction mediated by Ca2+ release in circular and Ca2+ influx in longitudinal intestinal muscle cells. J Pharmacol Exp Ther 244: 432–437, 1988 [PubMed] [Google Scholar]
- 11.Hacker BM, Tomlison JE, Wayman GA, Sultana R, Villacres E, Disteche C, Storm DR. Cloning, chromosomal mapping and regulatory properties of human type 9 adenylyl cyclase (ADCY9). Genomics 50: 97–104, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Hazelgrove KB, Flynn RS, Qiao LY, Grider JR, Kuemmerle JF. Endogenous IGF-I and αvβ3 integrin ligands regulate increased smooth muscle growth in TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 296: G1230–G1237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill EV, Houslay MD, Baillie GS. Investigation of extracellular signal-regulated kinase 2 mitogen-activated protein kinase phosphorylation and regulation of activity of PDE4 cyclic adenosine monophosphate-specific phosphodiesterases. Methods Mol Biol 307: 225–237, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Hill EV, Sheppard CL, Cheung YF, Gall I, Krause E, Houslay MD. Oxidative stress employs phosphatidyl inositol 3-kinase and ERK signalling pathways to activate cAMP phosphodiesterase-4D3 (PDE4D3) through multi-site phosphorylation at Ser239 and Ser579. Cell Signal 18: 2056–2069, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann R, Baillie GS, MacKenzie SJ, Yarwood SJ, Houslay MD. The MAP kinase ERK2 inhibits the cyclic AMP-specific phosphodiesterase HSPDE4D3 by phosphorylating it at Ser579. EMBO J 18: 893–903, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J 370: 1–18, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houslay MD, Baillie GS. The role of ERK2 docking and phosphorylation of PDE4 cAMP phosphodiesterase isoforms in mediating cross-talk between the cAMP and ERK signalling pathways. Biochem Soc Trans 31: 1186–1190, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Houslay MD, Kolch W. Cell-type specific integration of cross-talk between extracellular signal-regulated kinase and cAMP signaling. Mol Pharmacol 58: 659–668, 2000 [PubMed] [Google Scholar]
- 19.Hu W, Li F, Mahavadi S, Murthy KS. Upregulation of RGS4 and downregulation of CPI-17 mediate inhibition of colonic smooth muscle contraction by interleukin-1β. Am J Physiol Cell Physiol 293: C1991–C2000, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu W, Li F, Mahavadi S, Murthy KS. Interleukin-1β up-regulates RGS4 through the canonical IKK2/IκBα/NF-κB pathway in rabbit colonic smooth muscle. Biochem J 412: 35–43, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu W, Li F, Mahavadi S, Murthy KS. Upregulation of RGS4 expression by IL-1β in colonic smooth muscle is enhanced by ERK1/2 and p38 MAPK and inhibited by the PI3K/Akt/GSK3β pathway. Am J Physiol Cell Physiol 296: C1310–C1320, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwami G, Kawabe J, Ebina T, Cannon PJ, Homcy CJ, Ishikawa Y. Regulation of adenylyl cyclase by protein kinase A. J Biol Chem 270: 12481–12484, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Khan WI, Collins SM. Gut motor function: immunological control in enteric infection and inflammation. Clin Exp Immunol 143: 389–397, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuemmerle JF, Makhlouf GM. Agonist-stimulated cyclic ADP ribose: endogenous modulator of Ca2+-induced Ca2+-release in intestinal longitudinal muscle. J Biol Chem 270: 25488–25494, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Kuemmerle JF, Murthy KS, Makhlouf GM. Longitudinal smooth muscle of the mammalian intestine: a model for Ca2+ signaling by cADPR. Cell Biochem Biophys 28: 31–44, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Lim J, Pahlke G, Conti M. Activation of the cAMP-specific phosphodiesterase PDE4D3 by phosphorylation. Identification and function of an inhibitory domain. J Biol Chem 274: 19677–19685, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Maurice DH. Phosphorylation-mediated activation and translocation of the cyclic AMP-specific phosphodiesterase PDE4D3 by cyclic AMP-dependent protein kinase and mitogen-activated protein kinases. A potential mechanism allowing for the coordinated regulation of PDE4D activity and targeting. J Biol Chem 274: 10557–10565, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Palmer D, Jimmo SL, Tilley DG, Dunkerley HA, Pang SC, Maurice DH. Expression of phosphodiesterase 4D (PDE4D) is regulated by both the cyclic AMP-dependent protein kinase and mitogen-activated protein kinase signaling pathways. A potential mechanism allowing for the coordinated regulation of PDE4D activity and expression in cells. J Biol Chem 275: 26615–26624, 2000 [DOI] [PubMed] [Google Scholar]
- 29.MacKenzie SJ, Baillie GS, McPhee I, Bolger GB, Houslay MD. ERK2 mitogen-activated protein kinase binding, phosphorylation, and regulation of the PDE4D cAMP-specific phosphodiesterases. The involvement of COOH-terminal docking sites and NH2-terminal UCR regions. J Biol Chem 275: 16609–16617, 2000 [DOI] [PubMed] [Google Scholar]
- 30.MacKenzie SJ, Baillie GS, McPhee I, MacKenzie C, Seamons R, McSorley T, Millen J, Beard MB, van Heeke G, Houslay MD. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in upstream conserved region 1 (UCR1). Br J Pharmacol 136: 421–433, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Murthy KS, Grider JR, Makhlouf GM. InsP3-dependent Ca2+ mobilization in circular but not longitudinal muscle cells of intestine. Am J Physiol Gastrointest Liver Physiol 261: G937–G944, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Murthy KS, Kuemmerle JF, Makhlouf GM. Release of arachidonic acid by agonist-mediated activation of PLA2 initiates Ca2+ mobilization in intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 269: G93–G102, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Murthy KS, Makhlouf GM. Regulation of adenylyl cylase type V/VI in smooth muscle: interplay of inhibitory G protein and Ca2+ influx. Mol Pharmacol 54: 122–128, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Murthy KS, Makhlouf GM. Differential coupling of muscarinic m2 and m3 receptors to adenylyl cyclases V/VI in smooth muscle. Concurrent M2-mediated inhibition via Gαi3 and m3-mediated stimulation via Gβ. J Biol Chem 272: 21317–21324, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. Differential signaling by m3 and m2 receptors in smooth muscle: m2-mediated inactivation of MLCK via Gi3, Cdc42/Rac1, and PAK1, and m3-mediated MLC20 phosphorylation via Rho kinase/MYPT1 and PKC/CPI-17 pathways. Biochem J 374: 145–155, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murthy KS, Zhou H, Makhlouf GM. PKA-dependent inactivation of PDE3A and PDE4 and inhibition of adenylyl cyclase V/VI in smooth muscle. Am J Physiol Cell Physiol 282: C508–C517, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Murthy KS, Sriwai W. Stimulatory phosphorylation of cAMP-specific PDE4D5 by contractile agonists is mediated by PKC-dependent inactivation of protein phosphatase 2A. Am J Physiol Gastrointest Liver Physiol 294: G327–G335, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Nalli AD, Kumar DP, Mahavadi S, Alshboul O, Alkhatani R, Kuemmerle JF, Grider JR, Murthy KS. Hypercontractility of intestinal longitudinal smooth muscle induced by cytokines is mediated by NFκB/AMP-kinase/MLC kinase pathway. J Pharmacol Exp Therap 350: 89–98, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1β attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem 278: 48794–48804, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Ohama T, Hori M, Ozaki H. Mechanism of abnormal intestinal motility in inflammatory bowel disease: how smooth muscle contraction is reduced. J Smooth Muscle Res 43: 43–54, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J Biol Chem 271: 16526–16534, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Shea-Donohue T, Notari L, Sun R, Zhao A. Mechanisms of smooth muscle responses to inflammation. Neurogastroenterol Motil 24: 802–811, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi XZ, Lindholm PF, Sarna SK. NF-kappa B activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterology 124: 1369–1380, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Shi XZ, Sarna SK. Transcriptional regulation of inflammatory mediators secreted by human colonic circular smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 289: G274–G284, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol 12: 174–179, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Sunahara RK, Dessauer CW, Gliman AG. Complexity and diversity of mammalian adenylyl cylcases. Annu Rev Pharmacol Toxicol 36: 461–480, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Taussig R, Gliman AG. Mammalian membrane-bound adenylyl cyclases. J Biol Chem 270: 1–4, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, Gossen JA, Esposito G, van Dulin M, Conti M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol 296: 33–362, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Yang Z, Sun R, Grinchuk V, Fernandez-Blanco JA, Notari L, Bohl JA, McLean LP, Ramalingam TR, Wynn TA, Urban JF, Jr, Vogel SN, Shea-Donohue T, Zhao A. IL-33-induced alterations in murine intestinal function and cytokine responses are MyD88, STAT6, and IL-13 dependent. Am J Physiol Gastrointest Liver Physiol 304: G381–G389, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]