Abstract
Aryl hydrocarbon receptor nuclear translocator (ARNT) is a transcription factor that binds to partners to mediate responses to environmental signals. To investigate its role in the innate immune system, floxed ARNT mice were bred with lysozyme M-Cre recombinase animals to generate lysozyme M-ARNT (LAR) mice with reduced ARNT expression. Myeloid cells of LAR mice had altered mRNA expression and delayed wound healing. Interestingly, when the animals were rendered diabetic, the difference in wound healing between the LAR mice and their littermate controls was no longer present, suggesting that decreased myeloid cell ARNT function may be an important factor in impaired wound healing in diabetes. Deferoxamine (DFO) improves wound healing by increasing hypoxia-inducible factors, which require ARNT for function. DFO was not effective in wounds of LAR mice, again suggesting that myeloid cells are important for normal wound healing and for the full benefit of DFO. These findings suggest that myeloid ARNT is important for immune function and wound healing. Increasing ARNT and, more specifically, myeloid ARNT may be a therapeutic strategy to improve wound healing.
Keywords: aryl hydrocarbon receptor nuclear translocator, hypoxia-inducible factor-1α, deferoxamine
the innate immune system functions as the first line of defense against infection. Mononuclear phagocytes and neutrophils of the myeloid lineage are key effector cells of the innate immune system that function in the elimination of pathogens, modulation of the adaptive immune system, and tissue repair. Aryl hydrocarbon receptor (AhR) nuclear translocator (ARNT) is a transcription factor of the basic helix-loop-helix Per/ARNT/Sim (bHLH-PAS) family (19). ARNT heterodimerizes with class II bHLH/PAS family members, including hypoxia-inducible factor (HIF)-1α, HIF-2α, and AhR, to form active transcription complexes that regulate genes involved in hypoxic responses, cell survival, proliferation, glycolysis, angiogenesis, inflammation, and response to xenobiotics (13, 22, 39, 51).
HIF-1α and HIF-2α are regulated by cellular oxygen content; at normal oxygen concentrations, these proteins are rapidly degraded by the ubiquitin-proteasome pathway (15). HIF-1α can be stabilized by inflammation, transforming growth factor (TGF), platelet-derived growth factor (PDGF), epidermal growth factor, insulin-like growth factor I, and interleukin (IL)-1β (7, 19, 37, 56) and in human fibroblasts, and in diabetic animals its stability and activity are reduced by high glucose concentrations (1, 29, 43). Hypoxia-induced HIF-1α protein stabilization leads to differential gene transcription compared with that induced by Toll-like receptor agonists, showing that the combination of stimuli leading to HIF-1α stabilization is important in determining subsequent actions (18).
AhR is activated by ligand binding, which leads to its binding to ARNT, translocation to the nucleus, and gene regulation (19). Exogenous ligands are numerous and include the aromatic hydrocarbons, including 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and benzo[a]pyrene (8, 9). There is disagreement regarding the endogenous ligands for AhR: bile acids and cAMP are candidates, as are breakdown products from cruciferous vegetables (26, 35, 57). Importantly, different ligands have different effects; for example, TCDD and 6-formylindolo[3,2-b]carbazole produce opposing effects in experimental autoimmune encephalomyelitis (8, 38, 49).
The importance of HIF-1α in myeloid cell function was shown by Cramer et al. (7), who reported decreased immune function. HIF-1α regulates apoptosis, phagocytosis, and bacterial killing in macrophages and neutrophils (7, 36, 50). Imtiyaz et al. (16) showed that HIF-2α also regulates immune responses in a similar direction to HIF-1α, with myeloid cell knockout animals and isolated macrophages displaying reduced inflammatory cytokine production and decreased inflammation in models of immune function.
In contrast, deletion of AhR in macrophages leads to increased production of inflammatory cytokines after lipopolysaccharide (LPS) treatment (21), and AhR knockout animals are sensitive to LPS-induced shock (40). The role of macrophage and neutrophil ARNT in models of immune function and wound healing has not been examined. Since ARNT inactivation would block the effects of HIF-1α, HIF-2α, and AhR, it was not possible to predict the outcomes from the preexisting studies. To investigate its role in the innate immune system, floxed ARNT mice were bred with lysozyme M (LysM)-Cre recombinase (Cre) animals to generate LysM-ARNT (LAR) mice with reduced ARNT.
These studies demonstrate altered mRNA expression of cytokines and genes involved in wound healing in myeloid cells of LAR mice. LAR mice also had delayed wound healing. When the animals were rendered diabetic, the difference in wound healing between the LAR mice and their littermate controls disappeared.
MATERIALS AND METHODS
Animal Studies
All animals received humane care according to the criteria outlined in the “Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.” All procedures were approved by the Garvan Animal Ethics Committee. Floxed ARNT mice were created as previously described (13, 46) and bred with LysM-Cre mice (5) to produce LAR mice with reduced ARNT and floxed-control (FC) offspring. All mice were on an inbred C57BL/6 background for ≥12 generations.
Investigation of Effect of ARNT Depletion on Myeloid Cell Function
To obtain thioglycollate-stimulated macrophages, male mice were euthanized by cervical dislocation 4 days after peritoneal injection of 2 ml of 3% thioglycollate (Difco, Melbourne, Australia). Macrophages were isolated by injection of 10 ml of sterile ice-cold PBS into the peritoneum. Cells were cultured in RPMI medium (Gibco, Melbourne, Australia) with 10% FCS and 1% l-glutamine. After 2 h, the cells were washed twice with PBS, and the cells remaining were used in experiments. Cells were stimulated with LPS (LPS:B4, 100 ng/ml; Sigma, Castle Hill, Australia). After 24 h, the cells were lysed for RNA (n = 5/group). For Western blot analysis, macrophages were cultured for 72 h (n = 4/group).
Investigation of Effect of ARNT Depletion on Inflammatory Response
Skin irritation study.
Mice were anesthetized, and hair was removed. After 3 days of rest, 5% sodium dodecyl sulfate (SDS) was painted onto the skin twice daily for 5 days, and the animals were euthanized (n = 5–6/group). Skin was fixed in formalin for histology, and the inflammatory response was scored by two independent observers blinded to genotype using a semiquantitative scale of inflammation that included signs of infiltrate, edema, and epidermal proliferation. A scale of 0–4, with 0 being no skin inflammation and 4 being maximal inflammation, was used.
Skin transplant study.
Mice were anesthetized and shaved. Skin grafts were collected from Balb/c mice (H-2d) and transplanted onto LAR or FC (C57BL/6, H-2b) mice. Grafts were sex-matched but full-major histocompatibility complex-mismatched. The survival curve of 16 grafts onto FC mice and 13 grafts onto LAR female mice is shown. Transplant performance was scored by observers blinded to mouse genotype until complete rejection.
Wound healing.
Female mice at 20 wk of age were anesthetized and shaved, and hair was removed. On the next day, the mice were again anesthetized, and 0.5 × 0.5 cm wounds were created in the superficial skin layer. Wounds were covered with a sterile bandage and checked daily. Day 0 was taken as the day following wounding, and bandages were removed from the mice at day 6 (n = 20). Wound tissue was obtained from a second cohort of mice (n = 6) at day 4 for RNA and histological studies. For RNA collection, the wound center was collected using a 4-mm biopsy punch, and samples were homogenized in RLT buffer (Qiagen, Valencia, CA). For determination of tensile strength, a third cohort of LAR mice (n = 6) were euthanized at day 18 postwounding, and the tissue containing the wound was excised for assessment of tensile strength and calculation of Young's modulus, as previously described (45).
Diabetes was induced by streptozotocin injection, as previously reported (41). Wound experiments were initiated once mice had random blood glucose levels of >15 mmol/l for 3 consecutive days. Because of differences in blood glucose levels between FC and LAR mice, only animals with blood glucose levels ≥28 mmol/l and no animals with blood glucose levels <15 mmol/l, before insulin, were included (n = 12–20/group).
For DFO experiments, 10-wk-old female mice were rendered diabetic by alloxan injection (41), and wounding experiments were commenced once mice had a random blood glucose level of >15 mmol/l for 3 consecutive days (n = 8–10). Twenty microliters of 0.0125 μM DFO were added drop-wise to wounds from day 0 every 2nd day until wounds were healed.
Gene Expression
Total RNA was isolated and cDNA was synthesized as described elsewhere (3, 24). Real-time PCR was performed using specific primers and SYBR Green PCR master mix (Applied Biosystems, Melbourne, Australia), and amplification was performed using a light cycler (model 7900, Applied Biosysems). Results were corrected for expression of the housekeeping gene TATA box-binding protein (Tbp), which did not differ between groups (data not shown). Primers are shown in Table 1.
Table 1.
Real-time PCR primers
| mRNA | Forward Primer | Reverse Primer |
|---|---|---|
| Mouse | ||
| Tbp | atgatgactgcagcaaatcg | tatcactcctgccacaccag |
| Arnt | tctccctcccagatgatgac | caatgttgtgtcgggagatg |
| Elastin | atcctcttgctcaacctcct | gcccctggataatagactcc |
| Mmp9 | gaaggcaaaccctgtgtgtt | agagtactgcttgcccagga |
| Timp-1 | aggtggtctcgttgatttct | gtaaggcctgtagctgtgcc |
| Col1a1 | taggccattgtgtatgcagc | acatgttcagctttgtggacc |
| α-Sma | gagaagcccagccagtcg | ctcttgctctgggcttca |
| Tnf-α | ccagaccctcactagatca | cacttggtggtttgctacgac |
| Mcp-1 | ggtccctgtcatgcttctgg | cctgctgctggtgatcctct |
| Tgf-β1 | tgagtggctgtcttttgacg | ggttcatgtcatggatggtg |
| Il-6 | ccagagatacaaagaaatgatgg | actccagaagaccagaggaaat |
| Cxcl1 | tggctgggattcacctcgaa | tatgacttcggtttgggtgcag |
| F4/80 | ctttggctatgggcttccagtc | gcaaggaggacagagtttatcgtg |
| Human | ||
| ARNT | aacctcacttcgtggtggtc | caatgttgtgtcgggagatg |
| TBP | gttgagttgcagggtgtgg | ctcaaaccaacttgtcaacagc |
Tbp, TATA box-binding protein; Arnt, aryl hydrocarbon receptor nuclear translocator; Mmp9, matrix metallopeptidase 9; Timp-1, tissue inhibitor of matrix metallopeptidase; Col1a1, collagen type 1α1; α-Sma, α-smooth muscle actin; Mcp-1, monocyte chemoattractant protein 1; Tgf-β1, transforming growth factor-β1; Cxcl1, chemokine (C-X-C motif) ligand 1.
Western Immunoblotting
Sonicated protein (25 μg) was run on a 10% SDS-polyacrylamide gel in Western SDS running buffer using a Bio-Rad Protein 3 apparatus, as previously described (13). A 1:500 dilution of ARNT (BD Bioscience Pharmingen, Australia) or a 1:1,000 dilution of α-tubulin (Abcam, San Francisco, CA) primary antibody was used. α-Tubulin was used as a loading control, and signal intensity was quantitated by densitometry using ImageJ to calculate ARNT protein level relative to α-tubulin level.
Histology
Tissue was dissected from FC and LAR mice and fixed in 10% buffered formalin. Sections (5 μm) were stained with hematoxylin and eosin or Milligan's trichrome according to standard protocols, as previously described (3).
Human Monocyte Isolation
Informed consent was obtained from all participants. The study was approved by the Human Ethics Committee of Royal Prince Alfred Hospital. Blood (30 ml) was obtained from patients with a >10-yr duration of diabetes attending the Diabetes Centre of Royal Prince Alfred Hospital and from nondiabetic subjects (n = 9). Mononuclear cells were isolated using OptiPrep (Sigma, St. Louis, MO). The yield was 4–726 × 104 peripheral blood mononuclear cells/ml. Flow cytometry using CD14 as a monocyte marker showed >75% purity (53). A total of 2 μg of RNA from each sample were transcribed to cDNA using oligo(dT)18 (25 pmol) and SuperScript III RNase H− reverse transcriptase (Invitrogen). Gene expression analysis was performed as described elsewhere (13). Within the diabetic group, the majority of the patients had type 2 diabetes (T2D) and one patient had type 1 diabetes. Average hemoglobin A1c was 7.7 ± 0.2. The age of patients was significantly different: 52 ± 3 (control) vs. 67 ± 2 (diabetic) yr. There was no significant difference in weight (75 ± 5 vs. 82 ± 3 kg) or body mass index (27 ± 2 vs. 30 ± 1).
Human Cytokine Assays
The circulating concentrations of proinflammatory cytokines and chemokines [IL-6, IL-8, tumor necrosis factor-α (TNF-α), and monocyte chemoattractant protein-1 (MCP-1)] were measured using the BioPlex Pro Assay (Bio-Rad, Hercules, CA). Intra- and interassay coefficients of variation were 7–10% for all assays.
Statistics
Significance was calculated with Excel or Prism (version 5). Student's 2-tailed t-test was used unless otherwise specified. For time courses, repeated-measure ANOVA (rmANOVA) was used. Log-rank (Mantel-Cox) test was used to assess graft survival. Pearson's correlations (r) were calculated, and P values from two-tailed analysis are shown. Values are means ± SE. P < 0.05 was considered significant.
RESULTS
Thioglycollate-Isolated LAR Macrophages Had Reduced Arnt and Decreased Cytokine mRNA After LPS Treatment
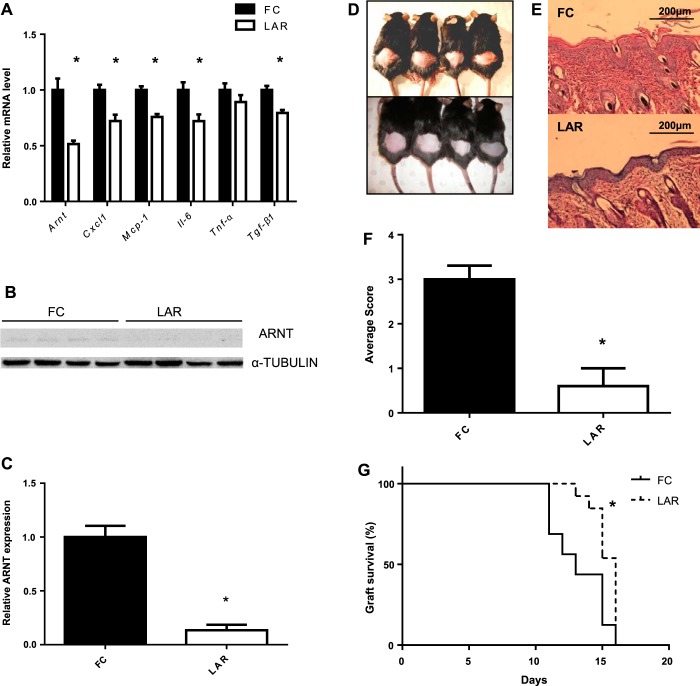
The number of macrophages isolated after thioglycollate stimulation from LAR mice was similar to controls: 14.7 × 106 and 14.3 × 106 from FC and LAR mice, respectively (P > 0.9). Arnt mRNA was reduced by 50% in macrophages from LAR mice after 24 h of LPS treatment (Fig. 1A). This relatively modest deletion efficiency is consistent with the percentage of enhanced green fluorescent protein (EGFP) expression in peritoneal macrophages isolated from lysozyme-EGFP mice (60%) and blood monocytes isolated from LysM-Cre × Rosa26-stop flox EGFP mice (55–75%) in previous studies (10, 17). Macrophages from LAR mice had significantly reduced expression of chemokine (C-X-C motif) ligand 1 (Cxcl1), Mcp-1, Tgf-β1, and Il-6 (P < 0.005 for all). There was no change in Tnf-α mRNA (P = 0.2; Fig. 1A). To further investigate the efficiency of ARNT reduction in macrophages, we extracted protein from thioglycollate-elicited macrophages after 72 h of treatment with LPS and assessed ARNT protein by Western immunoblotting (Fig. 1B). We found after LPS treatment that ARNT protein was significantly reduced to 13.5% of FC levels (P = 0.0012; Fig. 1C).
Fig. 1.
Acute inflammatory response. A: aryl hydrocarbon receptor (AhR) nuclear translocator (ARNT) and cytokine mRNA expression in thioglycollate-elicited macrophages after 24 h of culture in RPMI medium containing 100 ng/ml LPS in floxed-control (FC) and lysozyme M-ARNT (LAR) animals. B: Western blot of ARNT in thioglycollate-elicited macrophages from FC and LAR mice after 72 h of LPS treatment. α-Tubulin is shown as a loading control. C: densitometry of ARNT protein expression in thioglycollate-elicited macrophages after 72 h of LPS treatment. D: FC (top) and LAR (bottom) mice treated with SDS. E: hematoxylin-stained section of skin from FC and LAR mice after SDS study. Magnification ×100. F: histological inflammation score in FC and LAR mice treated with SDS. Values are means ± SE. *P < 0.05 by Student's t-test (A, C, and F). G: survival curve of Balb/c grafts on FC and LAR. *P < 0.05 by log-rank (Mantel-Cox) test (G).
Cutaneous Inflammatory Response Was Reduced in LAR Animals Following SDS Treatment
Macrophages play an important role in the inflammatory responses in the skin, including response to irritation induced by SDS, so the response of LAR mice to this model was investigated (44). As expected, FC animals showed erythema and keratosis (Fig. 1D, top). These responses were markedly reduced in LAR animals (Fig. 1D, bottom). Inflammatory changes in FC animals were marked by vasodilation, epidermal hyperproliferation, and edema and were reduced in LAR animals (Fig. 1E, top and bottom). Histological scores of inflammation averaged from results of two observers blinded to genotype are shown in Fig. 1F. Scores were lower in LAR than FC mice, indicating impaired response to skin irritants (P = 0.0013).
LAR Mice Had Delayed Skin Transplant Rejection
Transplant rejection is immune-mediated, and mononuclear phagocytes and neutrophils are important for this process (23, 42, 55). Skin graft survival after transplantation was studied. There was a significant delay in rejection of Balb/c skin transplants (full major histocompatibility complex mismatch, LAR and FC mice are C57BL/6) by LAR mice compared with FC mice (P < 0.006 by Mantel-Cox test; Fig. 1G).
LAR Mice Had Delayed Wound Healing
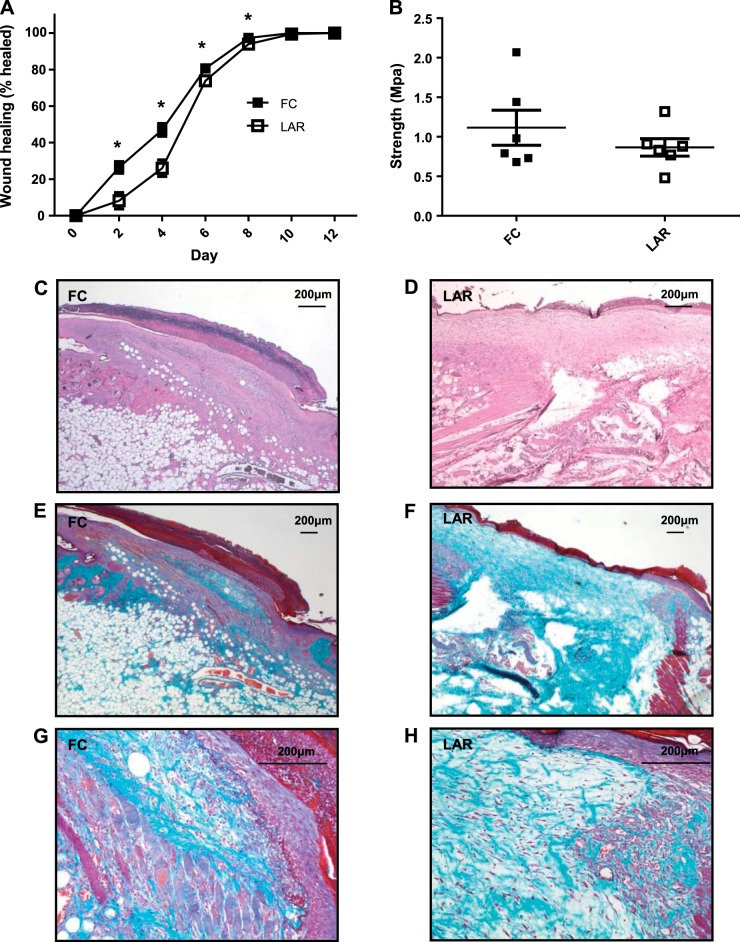
Mononuclear phagocytes and neutrophils are important to wound healing in nonsterile conditions (12, 31, 33). Given the observation that skin healing after graft rejection was delayed in LAR animals, wound healing was formally assessed. LAR mice had significantly delayed wound healing [P = 0.0017 (overall) by rmANOVA, P = 0.0023 at day 2, P < 0.001 at day 4, P = 0.027 at day 6, and P = 0.0038 at day 8; Fig. 2A]. Final median tensile strength was not significantly altered at day 18 (Fig. 2B). Hematoxylin-eosin and Milligan's trichrome staining of wounds at day 4 showed no obvious histological changes (Fig. 2, C–H).
Fig. 2.
A: wound healing in FC and LAR mice. B: tensile strength of FC and LAR wounds (day 18). Values are means ± SE. *P < 0.05 by Student's t-test. C and D: hematoxylin-eosin-stained sections of FC and LAR wounds at day 4. Magnification ×40. E and F: Milligan's trichrome-stained sections of FC and LAR wounds. Magnification ×25. G and H: Milligan's trichrome-stained sections of FC and LAR wounds. Magnification ×100 at wound edge.
ARNT Deletion Altered Gene Expression in Wounds
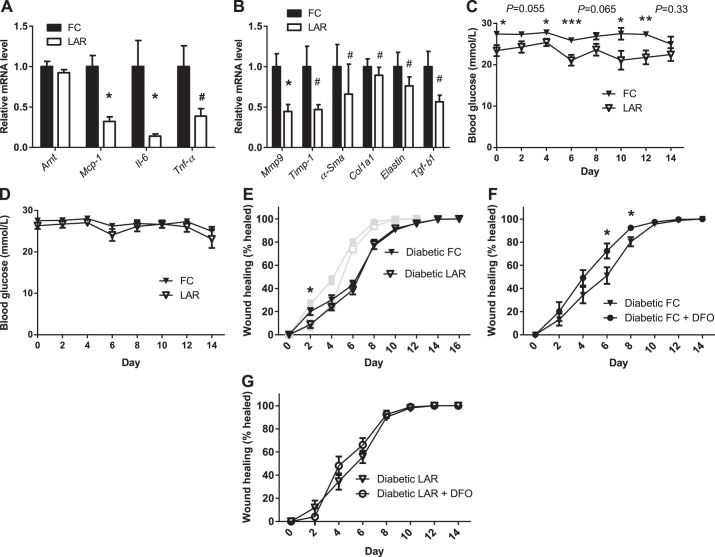
In a separate cohort of mice, wound tissue was collected at day 4 of healing to measure gene expression. Cytokine expression in LAR animals was reduced. Mcp-1 mRNA was reduced to 32% (P < 0.0001), Il-6 was reduced to 14% (P = 0.02), and the key tissue-remodeling gene matrix metallopeptidase 9 (Mmp9) was reduced to 44% (P = 0.004). Tnf-α was decreased to 39% of control, and Tgf-β1, collagen type 1α1 (Col1a1), α-smooth muscle actin (α-Sma), tissue inhibitor of metallopeptidase 1 (Timp-1), and elastin were also decreased (all nonparametrically distributed, P < 0.05; Fig. 3, A and B). No significant change in macrophage staining was evident. This was confirmed by real-time PCR for F4/80 mRNA (data not shown). Together with unchanged numbers of thioglycollate-elicited macrophages, this suggests normal tissue migration of LAR macrophages.
Fig. 3.
Wound mRNA expression and wound healing in diabetic mice. A: cytokine mRNA expression in FC and LAR wounds at day 4. B: tissue-remodeling mRNA expression in FC and LAR wounds at day 4. C: blood glucose levels after streptozotocin injection in LAR and FC mice. D: blood glucose after streptozotocin treatment in LAR and FC mice included in the final analysis of wound healing. E: wound healing in diabetic FC and LAR mice. F and G: wound healing in diabetic FC and LAR mice compared with FC and LAR mice receiving DFO treatment. Values are means ± SE. *P < 0.05, **P < 0.01, ***P < 0.005 by Student's t-test. #P < 0.05 by Wald-Wolfowitz test.
Diabetic LAR and FC Mice Had Equivalent Wound Healing
Because it has been reported that HIF-1α decreases in diabetic wounds and that this contributes to impaired wound healing, wound healing was investigated in LAR and control mice that were rendered diabetic (1, 2, 29, 43). Unexpectedly, overall, LAR mice had significantly reduced blood glucose levels compared with controls (Fig. 3C). For that reason, only animals with at least two blood glucose levels >28 mmol/l (506 mg/dl) and none with levels <15 mmol/l (270 mg/dl) before insulin treatment (required by the Garvan Animal Ethics Committee for animals with levels >20 mmol/l) were included in the study (Fig. 3D).
Interestingly, the differences in wound healing between LAR and control mice (Fig. 2A) were no longer significant in diabetic mice (P = 0.3 by rmANOVA; Fig. 3E). That is, the controls deteriorated much more than the LAR mice. This suggests that decreased function of ARNT-containing dimers in myeloid cells may contribute to the impaired wound healing observed in diabetes. We previously demonstrated decreased ARNT in the islets and liver of diabetic patients (13, 52). HIF-1α expression has also been found to be decreased in human diabetic ulcers, while activity is reduced at high glucose concentrations in human fibroblasts and diabetic animals (1, 2, 29).
DFO Treatment Does Not Significantly Improve Wound Healing in Diabetic LAR Mice
DFO is an iron chelator that increases HIFs (48). HIFs require ARNT for function. To delineate whether part of the mechanism of DFO in improving healing in diabetic mice (1) is increasing activity of the ARNT/HIF-1α complex in myeloid cells, the response of diabetic FC and LAR mice to DFO was investigated (Fig. 3, F and G). DFO treatment improved (i.e., decreased) wound size of FC animals at days 6 and 8 (P = 0.045 and P = 0.03, P = 0.046 by rmANOVA). In contrast, DFO treatment failed to significantly improve wound healing in LAR animals (P = 0.4735 by rmANOVA). This indicated that activity of the ARNT/HIF dimer in myeloid cells may be required for the beneficial effects of DFO on wound healing.
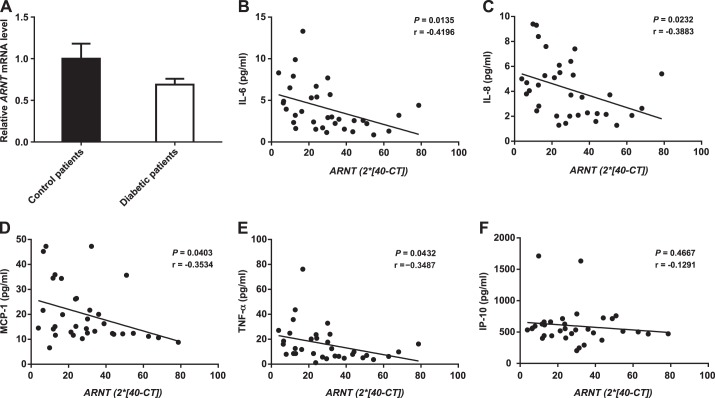
Quantification of Monocyte ARNT mRNA in Control and Diabetic Patients
We previously reported that ARNT mRNA is reduced in the liver and islets of T2D patients and that ARNT protein is reduced in the liver of T2D patients (13, 52). To test whether ARNT mRNA was decreased in circulating human myeloid cells from diabetes patients, levels were measured in human blood monocytes by real-time PCR. There was very wide interindividual variability, and no difference in ARNT mRNA was observed (P = 0.3; Fig. 4A). Monocyte ARNT mRNA was, however, correlated with serum levels of various cytokines. Decreased ARNT mRNA expression correlated with increased IL-6 and IL-8 (P = 0.0135 and P = 0.0232; Fig. 4, B and C), MCP-1 (P = 0.0403; Fig. 4D), and TNF-α (P = 0.0432; Fig. 4E). No significant correlation was found for interferon-γ-induced protein 10 (P = 0.4667; Fig. 4F) or for interferon-γ or macrophage inflammatory protein 1β (data not shown).
Fig. 4.
ARNT expression in human monocytes, expressed as estimated relative fold change using the following formula: ARNT = 2 ∗ (40 − CT), where CT is cycle threshold. A: average human monocyte ARNT mRNA expression in control subjects and diabetic patients. Values are means ± SE. B–F: correlation of IL-6, IL-8, MCP-1, TNF-α, and interferon-γ-induced protein 10 (IP-10) to ARNT level. Pearson's correlations (r) were calculated using PRISM software.
DISCUSSION
Mice with reduced ARNT in myeloid cells displayed altered cytokine transcription, skin inflammation, skin transplant rejection, and wound healing. Wound healing was similarly impaired in diabetic LAR and diabetic control mice, suggesting that decreased myeloid cell ARNT/HIF-1α function may contribute to impaired wound healing in diabetes. DFO administration failed to significantly improve wound healing in LAR mice, suggesting that ARNT/HIF-1α induction in myeloid cells may also be a component of the mechanism of action of DFO in improving wound healing (1, 43).
LAR granulocytes displayed altered expression of cytokine mRNA. Interestingly, ARNT deletion in Kupffer cells using the Mx-Cre promoter system has also been shown to prevent the upregulation of Pdgf-β, vascular endothelial growth factor (Vegf), angiopoietin-1, and Mcp-1 in hypoxia in vitro (6). Overall, the phenotype of myeloid cells lacking ARNT in response to acute stimulation appears most consistent with the combined phenotype of HIF-1α and HIF-2α knockout animals, with impairment of acute inflammatory response (7, 16), rather than the phenotype of myeloid cells lacking AhR (21, 40).
Within wound biopsies we found significantly reduced Mcp-1, Il-6, and Tnf-α expression, suggesting that myeloid cell ARNT deletion led to an impaired acute inflammatory response to wounding in vivo. We did not assess mRNA changes at multiple time points; however, the decreased Il-6 and Mcp-1 are consistent with the reduced cytokine mRNA observed in isolated macrophages. Indirect effects on nonmyeloid cells may also play a role. MCP-1 and IL-6 have roles in wound healing, with those knockout animals displaying delayed healing (11, 27). Reduction of these cytokines may contribute to the delay in wound healing in our model. Although macrophages are not required for effective wound healing in sterile conditions in the absence of neutrophils (30), the importance of macrophage function in wound healing when neutrophils are present has been shown in a number of studies (12, 20, 25, 28, 33). These studies have demonstrated that macrophages play an important role in neutrophil clearance, angiogenesis, epithelialization, and collagen deposition. Reduction in the mRNAs for Col1α1, Mmp9, and Timp-1 were found and suggest altered collagen deposition and altered wound remodeling, although tensile strength at day 18 was equivalent. DFO was also shown to significantly decrease wound size in diabetic FC animals, but not in mice lacking myeloid ARNT, suggesting a role for myeloid cell HIF-1α in mediating the effects of DFO on wound healing in diabetes.
Expression of ARNT in circulating human monocytes was highly variable. Ideally, future studies would also involve measurement of monocyte ARNT in wounds and blood in patients with sepsis and impaired wound healing. A novel finding was that ARNT mRNA in human monocytes correlated negatively with serum IL-6, IL-8, MCP-1, and TNF-α. Although a cause-and-effect relationship is speculative, a recent study reported increased allergic responses in mice lacking HIF-1α in myeloid cells (47). This study suggests that myeloid ARNT/HIF-1α may dampen some forms of excess inflammation.
Although ARNT was previously considered to be constitutively expressed, it has now been shown to be regulated in specific circumstances by reactive oxygen species, LPS, and curcumin (4, 34, 54). We recently reported that, in T2D islets, increasing HIF-1α with DFO increased ARNT expression to near-normal levels (3). Increasing myeloid cell ARNT may be a therapeutic strategy to improve wound healing.
GRANTS
J. E. Gunton was supported by the National Health and Medical Research Council. C. Scott was supported by a University of Sydney Postgraduate Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.S., S.T.G., S.T., S.M., and J.E.G. are responsible for conception and design of the research; C.S., J.B., D.M., P.B., R.S., K.M.C., S.N.W., K.M., and F.S. performed the experiments; C.S., D.M., P.B., R.S., S.N.W., K.M., S.M., and J.E.G. analyzed the data; C.S., J.B., P.B., K.M., S.T., S.M., and J.E.G. interpreted the results of the experiments; C.S. and J.E.G. prepared the figures; C.S. drafted the manuscript; C.S., D.M., R.S., K.M., F.S., S.T., S.M., and J.E.G. edited and revised the manuscript; C.S., J.B., D.M., P.B., R.S., S.N.W., K.M., F.S., S.T.G., S.T., S.M., and J.E.G. approved the final version of the manuscript.
REFERENCES
- 1.Botusan IR, Sunkari VG, Savu O, Catrina AI, Grunler J, Lindberg S, Pereira T, Yla-Herttuala S, Poellinger L, Brismar K, Catrina SB. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA 105: 19426–19431, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1α protein stability and function. Diabetes 53: 3226–3232, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Cheng K, Ho K, Stokes R, Scott C, Lau SM, Hawthorne WJ, O'Connell PJ, Loudovaris T, Kay TW, Kulkarni RN, Okada T, Wang XL, Yim SH, Shah Y, Grey ST, Biankin AV, Kench JG, Laybutt DR, Gonzalez FJ, Kahn CR, Gunton JE. Hypoxia-inducible factor-1α regulates beta cell function in mouse and human islets. J Clin Invest 120: 2171–2183, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi H, Chun YS, Kim SW, Kim MS, Park JW. Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Mol Pharmacol 70: 1664–1671, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8: 265–277, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Copple BL, Bai S, Moon JO. Hypoxia-inducible factor-dependent production of profibrotic mediators by hypoxic Kupffer cells. Hepatol Res 40: 530–539, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112: 645–657, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43: 309–334, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol 30: 447–454, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 96: 719–726, 2000 [PubMed] [Google Scholar]
- 11.Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, Luster MI. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J 14: 2525–2531, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Goren I, Allmann N, Yogev N, Schurmann C, Linke A, Holdener M, Waisman A, Pfeilschifter J, Frank S. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol 175: 132–147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, Roberson RS, Ricordi C, O'Connell PJ, Gonzalez FJ, Kahn CR. Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122: 337–349, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA 95: 7987–7992, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest 120: 2699–2714, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med 205: 2839–2850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jantsch J, Wiese M, Schodel J, Castiglione K, Glasner J, Kolbe S, Mole D, Schleicher U, Eckardt KU, Hensel M, Lang R, Bogdan C, Schnare M, Willam C. Toll-like receptor activation and hypoxia use distinct signaling pathways to stabilize hypoxia-inducible factor 1α (HIF1A) and result in differential HIF1A-dependent gene expression. J Leukoc Biol 90: 551–562, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol 36: 189–204, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLos One 5: e9539, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med 206: 2027–2035, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak KR, Abbott BD, Hankinson O. ARNT-deficient mice and placental differentiation. Dev Biol 191: 297–305, 1997 [DOI] [PubMed] [Google Scholar]
- 23.LaRosa DF, Rahman AH, Turka LA. The innate immune system in allograft rejection and tolerance. J Immunol 178: 7503–7509, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau SL, Gunton JE, Athayde NP, Byth K, Cheung NW. Serum 25-hydroxyvitamin D and glycated haemoglobin levels in women with gestational diabetes mellitus. Med J Aust 194: 334–337, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol 78: 71–100, 1975 [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147: 629–640, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, DiPietro LA. Wound healing in MIP-1α−/− and MCP-1−/− mice. Am J Pathol 159: 457–463, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol 184: 3964–3977, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Mace KA, Yu DH, Paydar KZ, Boudreau N, Young DM. Sustained expression of Hif-1α in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen 15: 636–645, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Martin P, D'Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr Biol 13: 1122–1128, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 15: 599–607, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 175: 2454–2462, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oda Y, Nakajima M, Mohri T, Takamiya M, Aoki Y, Fukami T, Yokoi T. Aryl hydrocarbon receptor nuclear translocator in human liver is regulated by miR-24. Toxicol Appl Pharmacol 260: 222–231, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Oesch-Bartlomowicz B, Oesch F. Role of cAMP in mediating AHR signaling. Biochem Pharmacol 77: 627–641, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1α expression regulates the bactericidal capacity of phagocytes. J Clin Invest 115: 1806–1815, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9: 677–684, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453: 65–71, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Salceda S, Beck I, Caro JF. Absolute requirement of aryl hydrocarbon receptor nuclear translocator protein for gene activation by hypoxia. Arch Biochem Biophys 334: 389–394, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, Igarashi K, Gonzalez FJ, Ikuta T, Kawajiri K, Fujii-Kuriyama Y. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol Cell Biol 29: 6391–6400, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stokes RA, Cheng K, Deters N, Lau SM, Hawthorne WJ, O'Connell PJ, Stolp J, Grey S, Loudovaris T, Kay TW, Thomas H, Gonzalez FJ, Gunton JE. Hypoxia-inducible factor 1α (HIF-1α) potentiates β-cell survival after islet transplantation of human and mouse islets. Cell Transplant 22: 253–266, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tashiro-Yamaji J, Maeda S, Ikawa M, Okabe M, Kubota T, Yoshida R. Macrophage MHC and T-cell receptors essential for rejection of allografted skin and lymphoma. Transplantation 96: 251–257, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Thangarajah H, Yao D, Chang EI, Shi Y, Jazayeri L, Vial IN, Galiano RD, Du XL, Grogan R, Galvez MG, Januszyk M, Brownlee M, Gurtner GC. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci USA 106: 13505–13510, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thepen T, van Vuuren AJ, Kiekens RC, Damen CA, Vooijs WC, van De Winkel JG. Resolution of cutaneous inflammation after local elimination of macrophages. Nat Biotechnol 18: 48–51, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Thomson SE, McLennan SV, Hennessy A, Boughton P, Bonner J, Zoellner H, Yue DK, Twigg SM. A novel primate model of delayed wound healing in diabetes: dysregulation of connective tissue growth factor. Diabetologia 53: 572–583, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Tomita S, Sinal CJ, Yim SH, Gonzalez FJ. Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1α. Mol Endocrinol 14: 1674–1681, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Toussaint M, Fievez L, Drion PV, Cataldo D, Bureau F, Lekeux P, Desmet CJ. Myeloid hypoxia-inducible factor 1α prevents airway allergy in mice through macrophage-mediated immunoregulation. Mucosal Immunol 6: 485–497, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Triantafyllou A, Liakos P, Tsakalof A, Georgatsou E, Simos G, Bonanou S. Cobalt induces hypoxia-inducible factor-1α (HIF-1α) in HeLa cells by an iron-independent, but ROS-, PI-3K- and MAPK-dependent mechanism. Free Radic Res 40: 847–856, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453: 106–109, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER. Hypoxia-induced neutrophil survival is mediated by HIF-1β-dependent NF-κB activity. J Exp Med 201: 105–115, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang XL, Suzuki R, Lee K, Tran T, Gunton JE, Saha AK, Patti ME, Goldfine A, Ruderman NB, Gonzalez FJ, Kahn CR. Ablation of ARNT/HIF1β in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab 9: 428–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong J, McLennan SV, Molyneaux L, Min D, Twigg SM, Yue DK. Mitochondrial DNA content in peripheral blood monocytes: relationship with age of diabetes onset and diabetic complications. Diabetologia 52: 1953–1961, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Wu R, Cui X, Dong W, Zhou M, Simms HH, Wang P. Suppression of hepatocyte CYP1A2 expression by Kupffer cells via AhR pathway: the central role of proinflammatory cytokines. Int J Mol Med 18: 339–346, 2006 [PubMed] [Google Scholar]
- 55.Wyburn KR, Jose MD, Wu H, Atkins RC, Chadban SJ. The role of macrophages in allograft rejection. Transplantation 80: 1641–1647, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Yu DH, Mace KA, Hansen SL, Boudreau N, Young DM. Effects of decreased insulin-like growth factor-1 stimulation on hypoxia inducible factor 1α protein synthesis and function during cutaneous repair in diabetic mice. Wound Repair Regen 15: 628–635, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Zollner G, Wagner M, Trauner M. Nuclear receptors as drug targets in cholestasis and drug-induced hepatotoxicity. Pharmacol Ther 126: 228–243, 2010 [DOI] [PubMed] [Google Scholar]