Abstract
Individuals with functional lower urinary tract disorders including interstitial cystitis (IC)/bladder pain syndrome (BPS) and overactive bladder (OAB) often report symptom (e.g., urinary frequency) worsening due to stress. One member of the transient receptor potential ion channel vanilloid family, TRPV4, has recently been implicated in urinary bladder dysfunction disorders including OAB and IC/BPS. These studies address the role of TRPV4 in stress-induced bladder dysfunction using an animal model of stress in male rats. To induce stress, rats were exposed to 7 days of repeated variate stress (RVS). Quantitative PCR data demonstrated significant (P ≤ 0.01) increases in TRPV4 transcript levels in urothelium but not detrusor smooth muscle. Western blot analyses of split urinary bladders (i.e., urothelium and detrusor) showed significant (P ≤ 0.01) increases in TRPV4 protein expression levels in urothelial tissues but not detrusor smooth muscle. We previously showed that RVS produces bladder dysfunction characterized by decreased bladder capacity and increased voiding frequency. The functional role of TRPV4 in RVS-induced bladder dysfunction was evaluated using continuous, open outlet intravesical infusion of saline in conjunction with administration of a TRPV4 agonist, GSK1016790A (3 μM), a TRPV4 antagonist, HC067047 (1 μM), or vehicle (0.1% DMSO in saline) in control and RVS-treated rats. Bladder capacity, void volume, and intercontraction interval significantly decreased following intravesical instillation of GSK1016790A in control rats and significantly (P ≤ 0.01) increased following administration of HC067047 in RVS-treated rats. These results demonstrate increased TRPV4 expression in the urothelium following RVS and that TRPV4 blockade ameliorates RVS-induced bladder dysfunction consistent with the role of TRPV4 as a promising target for bladder function disorders.
Keywords: micturition, TRPV4, stress, bladder, Q-PCR, Western blotting
under hostile conditions, there is a coordinated reaction known as the stress response that is activated to enhance survival. Symptom exacerbation due to stress is prevalent in many disease states, including functional disorders of the urinary bladder such as overactive bladder (OAB) and interstitial cystitis (IC)/bladder pain syndrome (BPS) (26, 44, 52). The prevalence of micturition disorders is high among people with anxiety disorders, and various stressors often increase levels of anxiety (8). Symptom exacerbation during times of stress may be partly due to disruption of the hypothalamic-pituitary-adrenal (HPA) axis. However, the pathophysiology underlying the effects of stress on micturition reflex function remains unknown.
Previous studies in our laboratory have examined stress-induced effects on micturition reflex function (37). A repeated variate stress (RVS) paradigm (17) that lacks habituation was used, in which a different stressor was presented every day for 7 days (d). RVS altered micturition reflex function by causing a decrease in bladder capacity and void volume and an increase in urinary frequency in rats exposed to the RVS paradigm (37). We also observed an increase in nerve growth factor (NGF) protein expression in the urinary bladders of rats exposed to RVS. The role of the neurotrophin NGF has been well established in urinary bladder function (7, 9), urinary bladder inflammation (45, 50), increased voiding frequency (21, 54), and functional urinary tract disorders including IC/BPS (34, 41), OAB (25, 33), and bladder outlet obstruction (32). Prior work in the laboratory has demonstrated the pleiotropic effects of NGF overexpression to modulate other proteins, including other neurotrophins/receptors, neuropeptides/receptors, and transient receptor potential (TRP) channels (36).
The TRP protein family is a group of nonspecific cation channels that are involved in sensory transduction in a variety of cellular processes. Members of the vanilloid (V) family, specifically TRPV4, are expressed in urothelial cells and lumbosacral dorsal root ganglia (5, 12, 16) and may act as sensors of stretch and/or chemical irritation in the lower urinary tract (31). The TRPV4 channel has been implicated in bladder diseases such as OAB and BPS (40). The role of TRPV4 in bladder function has been heavily studied in animal models. TRPV4 knockout (KO) mice exhibit an abnormal urine voiding pattern including a decreased frequency of voiding contractions and an increased frequency of nonvoiding contractions, intermicturition interval, bladder capacity, and total urine volume per micturition (13, 14). In addition to the TRPV4 KO mouse model, pharmacological manipulation of the channel has been used to study its role in bladder function. TRPV4 activation in rodents results in decreases in bladder capacity and increases in voiding frequency (2, 47), whereas TRPV4 blockade in animal models of cystitis decreases voiding frequency (13, 51), making the channel a promising target for bladder function disorders (2, 23).
The aim of the current study is to investigate the role of one member of the TRPV family, TRPV4, as a sensory transducer in stress-induced urinary bladder dysfunction. Bladder function was assessed following RVS using continuous, intravesical infusion of saline in conscious, unrestrained male rats with an open outlet both before and after TRPV4 blockade or activation. We also evaluated urinary bladder TRPV4 transcript levels and protein content in both the urothelium and detrusor smooth muscle following RVS.
MATERIALS AND METHODS
Animals
Adult, male Wistar rats (300–350 g) were purchased from Charles River Laboratories (Wilmington, MA) and housed one or two per cage and maintained in standard laboratory conditions with free access to food and water. The University of Vermont Institutional Animal Care and Use Committee (IACUC) approved all animal use procedures (Protocol 08-085). Animal experimentation was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Repeated Variate Stress
Rats were assigned to control or RVS groups based on body weight. Rats assigned to the RVS group were exposed to 7 d of stress where rats were administered a single stressor on each day as described previously (17). Control rats were left in home cages in the animal facility following weight measurement.
Oscillation stress.
Rats were placed inside a plastic chamber 28 × 17 ×13 cm [length (L) × width (W) × height (H)] that was secured to a clinical rotator (Fisher Scientific, Morris Plains, NJ) and oscillated at low to medium speed for 30 min.
Forced swim.
Rats were placed in a cylindrical container 29 × 37 cm [ depth (D) × H] that was filled with room temperature water to a depth that prevented the tail from touching the bottom of the container. After 5 min of monitored swimming, rats were placed in a holding chamber for 30 min before being returned to their home cage.
Electrical footshock.
Rats were placed inside a Plexiglas conditioning chamber (Med Associates, St. Albans, VT) 30 × 25 × 35 cm (L × W × H). After a 5-min acclimation period, two 1.0-mA 5 s (s) scrambled footshocks were delivered through the grid floor with a 1-min intertrial interval.
Restraint.
Rats were placed in a cylindrical restraining device 9 × 15 cm (D × H) for 60 min.
Pedestal.
Rats were placed on an elevated platform 20 × 20 cm (L × W) that was 60 cm from the floor for 30 min.
Real-Time Quantitative RT-PCR for TRPV4
Rats (n = 6/group) from both experimental groups (RVS and control) were euthanized 24 h following the last stressor by being deeply anesthetized with isoflurane (3–4%) and then a thoracotomy was performed. The urinary bladders (95–150 mg) were quickly dissected under RNAse-free conditions. The bladders were cut open along the midline and pinned to a Sylgard-coated dish, and the urothelium was removed with the aid of fine forceps and a dissecting microscope. Tissues were snap-frozen on dry ice and then transferred into −80°C conditions until time of experimentation (3). Total RNA was extracted using the STAT-60 total RNA/mRNA isolation reagent (Tel-Test “B”, Friendswood, TX) under RNAse-free conditions as previously described (15, 28). RNA sample was used to synthesize complementary DNA using M-MLV reverse transcriptase and random hexamer primers (Promega). The quantitative (Q)-PCR standards for all transcripts were prepared with the amplified cDNA products ligated directly into pCR2.1 TOPO vector using the TOPO TA cloning kit (Invitrogen). The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Facility).
Complementary DNA templates, diluted 10-fold to minimize the inhibitory effects of the reverse transcription reaction components, were assayed using HotStart-IT SYBR Green qPCR Master Mix (USB, Cleveland, OH). Rat TRPV4 primer (upper: 5′-ACTGGCAAGATCGGGGTCTT-3′; lower: 5′-GAGGAGAGGTCGTAGAGAGAAGAAT-3′) was designed with the upper primer bridging an intron/exon boundary to exclude DNA amplification. Primer sequences for the ribosomal protein L32, used as a reference gene in this study, have been previously reported (28).
Q-PCR was performed on an Applied Biosystems 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA). The amplified product from these amplification parameters was subjected to SYBR Green I melting analysis. A single DNA melting profile was observed under these dissociation assay conditions demonstrating amplification of a single unique product free of primer dimers or other anomalous products.
For data analysis, a standard curve was constructed by amplification of serially diluted plasmids containing the target sequence. Data were analyzed at the termination of each assay using the Sequence Detection Software (v. 1.3.1; Applied Biosystems, Norwalk, CT). In standard assays, default baseline settings were selected. The increase in SYBR Green I fluorescence intensity (ΔRn) was plotted as a function of cycle number, and the threshold cycle was determined by the software as the amplification cycle at which the ΔRn first intersects the established baseline. All data are expressed as the relative quantity of the gene of interest normalized to the relative quantity of the reference gene L32.
Western Blotting for TRPV4
Following euthanasia, whole urinary bladders (10–50 mg, urothelium; 100–200 mg, detrusor) from control and RVS groups were dissected and homogenized separately in tissue protein extraction agent (T-PER; Roche, Indianapolis, IN), a mild zwitterionic dialyzable detergent in 25 mM bicine, and 150 mM sodium chloride (pH 7.6) containing a protease inhibitor mix (Sigma-Aldrich; 16 μg/ml benzamidine and 2 μg/ml pepstatin A), and aliquots were removed for protein assay. In some instances, the bladder was cut open along the midline and pinned to a Sylgard-coated dish and the urothelium was removed with the aid of fine forceps and a dissecting microscope. Samples (35 μg) were suspended in sample buffer for fractionation on gels and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose membranes, and efficiency of transfer was evaluated. Membranes were blocked for 1 h at room temperature in a solution of 5% donkey serum albumin in Tris-buffered saline with 0.1% Tween. Membranes were incubated in anti-TRPV4 (1:500; Osenses, Fisher Scientific, Pittsburgh, PA; catalog no. OSR00136W) in 5% donkey serum albumin in Tris-buffered saline with 0.1% Tween overnight at 4°C. Washed membranes were incubated in species-specific secondary antibodies for 2 h at room temperature for enhanced chemiluminescence detection (Pierce, Rockford, IL). Blots were exposed to Biomax film (Kodak, Rochester, NY) and developed. Blots were analyzed using the Versa Doc 4000 MP Imaging System (Bio-Rad, Hercules, CA). The adjusted volume of each band was analyzed, and background intensities were subtracted using Quantity One software (Bio-Rad; Vermont Cancer Center DNA Analysis Facility). Images were scanned with a flatbed scanner, the contrast was corrected, and the images ere imported and figures ere assembled with Adobe Photoshop (San Jose, CA). Western blot analysis of actin (1:1,000; Santa Cruz Biotechnologies; catalog no. sc1616) in samples was used as a loading control. When TRPV4−/− mouse tissues were used in Western blotting procedures, we did not demonstrate a band (16).
Intravesical Catheter Implant
A lower midline abdominal incision was made while the animals were under general anesthesia with 2–3% isoflurane using aseptic techniques (29, 48). One end of polyethylene tubing (PE-50; Clay Adams, Parsippany, NJ) was flared with a flame and inserted in the dome of the bladder and secured in place with a 6–0 nylon purse-string suture (6, 48). The distal end of the tubing was tunneled subcutaneously to the back of the neck where it was buried in an incision in the back of the neck, out of the animal's reach (29, 48). Rats received buprenorphine (0.05 mg/kg sc) starting at the time of surgery and then every 8–12 h postoperatively for a total of four doses. Animals were maintained for 72 h after surgery before conscious cystometry was initiated to ensure complete recovery.
Conscious Cystometry with Continuous Intravesical Infusion of Saline and Manipulation of TRPV4 Channels
The effects of TRPV4 on bladder function in control rats and rats exposed to 7 d of RVS were evaluated with both a TRPV4 agonist (GSK1016790A; 3 μM) and a TRPV4 antagonist (HC067047; 1 μM) using conscious cystometry and continuous infusion of intravesical saline (n = 4–6/group). Previous studies have shown no effects of either agent in TRPV4−/− mice, thus establishing specificity of their agonist and antagonist actions (11, 46; Nelson MT, personal communication). In addition, in pilot in vivo and ex vivo studies evaluating the roles of TRPV4 in bladder function in other animal models of bladder dysfunction, GSK agonist effects are blocked with HC067047 using the concentrations reported in the current experiments (unpublished observations). During cystometry, unrestrained and conscious rats were placed in a recording cage over a scale and pan to collect and measure voided urine. To elicit repetitive bladder contractions, room temperature saline was infused at a constant rate (10 ml/h). After an initial stabilization period (25–30 min), at least six reproducible micturition cycles were recorded. Intravesical pressure changes were recorded using a small Animal Cystometry System (Med Associates) (6, 29). Baseline resting pressure, pressure threshold for voiding, maximal voiding pressure, and intercontraction interval were measured. Nonvoiding bladder contractions (NVCs), defined as rhythmic intravesical pressure increases 7 cmH2O above baseline without the release of fluid from the urethra (38), were also determined per voiding cycle. Bladder capacity was measured as the amount of saline infused in the bladder at the time when micturition commenced (20).
To evaluate the effects of TRPV4 on bladder function, rats were anesthetized (1–2% isoflurane) and vehicle [0.1% DMSO (Sigma-Aldrich) in saline], GSK1016790A (3 μM), or HC067047 (1 μM) was intravesically infused for 30 min. The concentrations selected for agonist and antagonist evaluation were based on a dose-response curve (data not shown) and previous studies (13), respectively. Before intravesical drug infusion, the bladder was manually emptied using the Credé maneuver. Bladders were then infused with ∼1 ml (less than bladder capacity so that a bladder contraction is not elicited resulting in expulsion of instillate) of vehicle, GSK1016790A, or HC067047 as previously described (6, 29). Rats remained anesthetized during the 30-min infusion to depress the micturition reflex and prevent expulsion of the drug from the bladder. To avoid potential variation resulting from circadian rhythms, experiments were conducted at similar times of the day (10). At the conclusion of the study, rats were euthanized as described above. Micturition function before and after vehicle, GSK1016790A, or HC067047 intravesical instillation was evaluated in the same rats (control and RVS groups).
Exclusion Criteria
Rats were removed from the study when adverse events occurred that included 20% reduction in body weight postsurgery, a significant postoperative event, lethargy, pain, or distress not relieved by our IACUC-approved regimen of postoperative analgesics or hematuria in control rodents (6, 29). In the present study, no rats were excluded from the study. In addition, behavioral movements such as grooming, standing, walking, and defecation rendered bladder pressure recordings during these events unusable.
Materials
GSK1016790A (produced and distributed by GlaxoSmithKline, Dr. Kevin Thorneloe, contact) and HC067047 (Tocris Biosciences; catalog no. 4100) were prepared as stock solutions in DMSO, aliquoted, and stored at −20°C until use. Aliquots were diluted with saline to achieve final concentration at time of experimentation.
Statistical Analyses
All values represent means ± SE. Data from Q-PCR were compared using ANOVA, and Western blot studies were compared with unpaired t-tests (whole urinary bladder) and ANOVA (split urinary bladder, i.e., urothelium, detrustor). Cystometry data for vehicle groups were compared using repeated-measures ANOVA, where each animal served as its own control, and drug-treated groups were compared using two-way ANOVA. Animals, processed and analyzed on the same day, were tested as a block in the ANOVA. When F ratios exceeded the critical value (P ≤ 0.05), the Newman-Keuls or Bonferroni post hoc tests were used to compare group means. The significance level (P ≤ 0.05) presented is for a two-tailed test.
RESULTS
TRPV4 mRNA and Protein Expression in the Urinary Bladder of Rats Following RVS
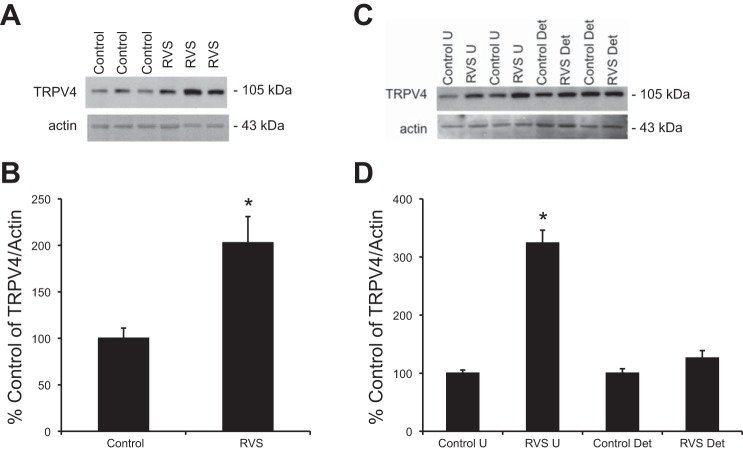
Q-PCR demonstrated a significant (P ≤ 0.01) change in TRPV4 mRNA in urothelium from rats exposed to 7 d of RVS compared with controls (Fig. 1A). No significant change was detected in TRPV4 mRNA relative expression in detrusor smooth muscle from RVS and control groups (P = 0.12; Fig. 1B). TRPV4 protein levels from whole bladder homogenates showed a significant change (P ≤ 0.01) following 7 d of RVS compared with controls as determined with Western blot analysis (Fig. 2, A and B). Western blot analyses of split urinary bladder tissues (i.e., urothelium and detrusor) demonstrated a significant change in TRPV4 protein expression in urothelial tissue, but not in detrusor smooth muscle. (Fig. 2, C and D).
Fig. 1.
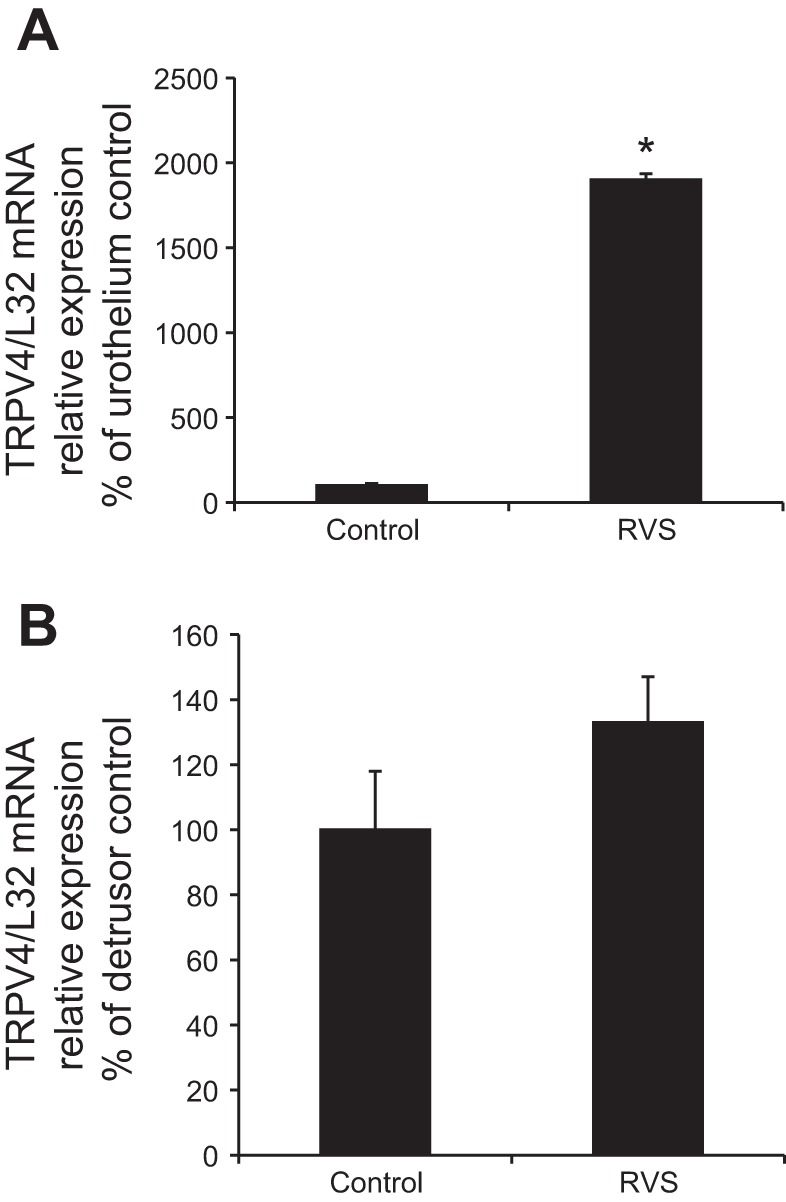
Increased the transient receptor potential ion channel vanilloid 4 (TRPV4) mRNA expression in urothelium following 7 days (d) of repeated variate stress (RVS) as detected by quantitative PCR. TRPV4 mRNA transcript relative expression normalized to the relative expression of the reference gene, L32 (represented as percentage of control) increased significantly (P ≤ 0.01) in the urothelium but not in detrusor smooth muscle, following 7 d of RVS compared with control. Statistical analyses were performed on raw data using ANOVA as described in Statistical Analyses. Values are means ± SE. Sample sizes are n of 6; *P ≤ 0.01.
Fig. 2.
Increased TRPV4 expression in whole urinary bladders (A and B) and in urothelium (U; C and D) following 7 d of RVS as detected by Western blotting techniques. Representative Western blots (A and C) and summary histograms (B and D) of TRPV4 expression in whole urinary bladders (35 μg) and urothelium and detrusor smooth muscle from control rats and RVS-treated rats. Actin expression was used as a loading control. Relative TRPV4 band density in each group was normalized to actin expression and expressed as a percentage (%) of control in the same samples. RVS significantly (P ≤ 0.01) increased TRPV4 expression compared with control in whole urinary bladder and urothelial tissue but not detrusor smooth muscle. Statistical analyses were performed on raw data using Student's t-test (whole bladder) or ANOVA (split bladder, i.e., urothelium and detrusor) as described in Statistical Analyses. Values are means ± SE. Sample sizes are n of 7; *P ≤ 0.01.
Effects of TRPV4 Agonist and Antagonist on Bladder Function
Previous work in our laboratory established that RVS produces biological markers of stress similar to other studies (17, 18, 24, 43) including a robust and reproducible reduction (10%) in body weight gain during stressor exposure (37). We also demonstrated that RVS produces bladder dysfunction in rats characterized by decreased bladder capacity, intercontraction interval, and void volume (37). These results were confirmed in the present experiments (Table 1). To establish the role of TRPV4 in stress-induced bladder dysfunction, we performed intravesical instillation (30 min) of GSK1016790A (3 μM), a potent and selective TRPV4 agonist, as well as HC067047 (1 μM), a potent and selective TRPV4 antagonist, and examined urodynamics in both control rats and in rats exposed to 7 d of RVS. Vehicle (0.1% DMSO) controls were evaluated in both control and RVS-treated rats. There were no significant differences in urodynamic parameters (bladder capacity, intercontraction interval, and void volumes) or bladder pressures (baseline pressure, micturition pressure, and threshold pressure) following intravesical instillation of the vehicle in either control rats or RVS-treated rats (Table 1).
Table 1.
Mean bladder pressures and urodynamic parameters during open outlet, conscious cystometry in control rats and those exposed to RVS before and after intravesical infusion of a TRPV4 agonist or antagonist
| Group Size | Threshold Pressure, kPa | Micturition Pressure, kPa | Baseline Pressure, kPa | Intercontraction Interval, s | Infused Volume, ml | Void Volume, ml | |
|---|---|---|---|---|---|---|---|
| Control | |||||||
| Pre-DMSO | 6 | 1.4 ± 0.2 | 3.9 ± 0.5 | 1.0 ± 0.1 | 388.6 ± 9.3 | 1.1 ± 0.1 | 1.2 ± 0.1 |
| Post-DMSO | 6 | 1.6 ± 0.2 | 4.8 ± 0.7 | 1.0 ± 0.1 | 494.7 ± 27.9 | 1.4 ± 0.1 | 1.3 ± 0.1 |
| Preagonist | 4 | 1.4 ± 0.1 | 4.0 ± 0.7 | 1.0 ± 0.1 | 386.8 ± 49.8 | 1.1 ± 0.1 | 1.1 ± 0.2 |
| Postagonist | 4 | 1.3 ± 0.1 | 4.8 ± 0.8 | 1.2 ± 0.1 | 104.6 ± 11.4§ | 0.3 ± 0.1§ | 0.3 ± 0.1‡ |
| Preantagonist | 5 | 1.3 ± 0.1 | 3.9 ± 0.3 | 1.1 ± 0.1 | 376.0 ± 33.7 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Postantagonist | 5 | 1.5 ± 0.1 | 4.2 ± 0.3 | 1.1 ± 0.1 | 502.8 ± 28.5* | 1.4 ± 0.1* | 1.3 ± 0.1 |
| RVS | |||||||
| Pre-DMSO | 4 | 1.0 ± 0.1 | 3.0 ± 0.5 | 0.9 ± 0.1 | 198.2 ± 28.6 | 0.6 ± 0.1 | 0.5 ± 0.1 |
| Post-DMSO | 4 | 1.1 ± 0.1 | 3.5 ± 0.7 | 0.8 ± 0.2 | 267.5 ± 16.3 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| Preagonist | 4 | 1.0 ± 0.1 | 2.7 ± 0.1 | 0.9 ± 0.1 | 113.0 ± 14.6 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Postagonist | 4 | 1.3 ± 0.1 | 3.7 ± 0.3* | 1.2 ± 0.1 | 85.3 ± 8.4 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Preantagonist | 6 | 2.0 ± 0.7 | 4.4 ± 0.8 | 2.0 ± 0.6 | 147.7 ± 18.9 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Postantagonist | 6 | 1.5 ± 0.2 | 4.0 ± 0.6 | 1.3 ± 0.2 | 319.7 ± 19.4‡ | 0.9 ± 0.1‡ | 0.9 ± 0.1† |
Values are means ± SE; n = 4–6/group. Threshold, micturition, and baseline pressures (kPa) during conscious cystometry for control and repeated variate stress (RVS)-treated (7 days) rats are shown. Urodynamic parameters including intercontraction interval (s), infused volume (ml), and void volume (ml) are also shown. For each group (control and RVS), values before and after instillation of 3 pharmacological treatments [vehicle (0.1% DMSO), agonist (GSK1016790A), and antagonist (HC067047)] are presented. TRPV4, transient receptor potential ion channel vanilloid 4. Statistical analyses were performed using repeated-measures ANOVA for vehicle-treated groups and two-way ANOVA with Bonferroni multiple (6) comparisons as described in Statistical Analyses.
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001;
P ≤ 0.0001.
Effects of Intravesical Instillation of a TRPV4 Agonist on Urinary Bladder Function
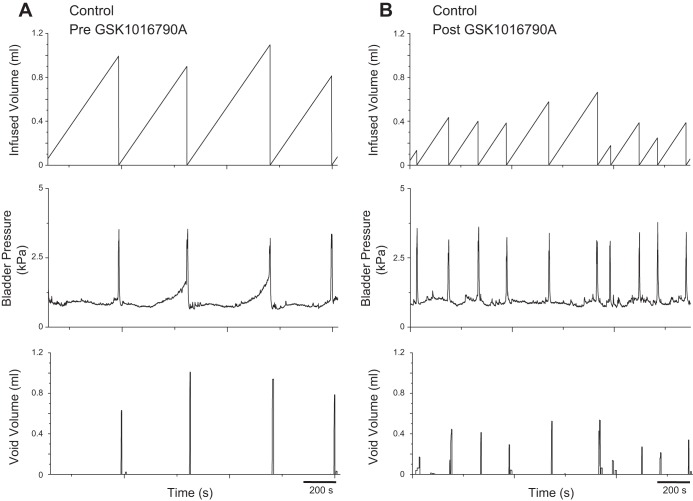
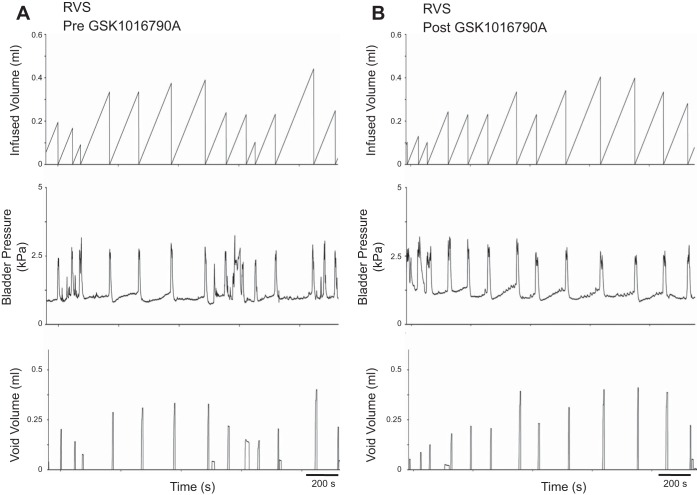
Intravesical instillation (30 min) of GSK1016790A (3 μM) produced a significant (P ≤ 0.0001) change in bladder capacity (3.7-fold), void volume (4.3-fold) and intercontraction interval (3.7-fold) in control rats (Table 1; Fig. 3), consistent with previous findings (2). In fact, there were no significant differences in bladder capacity, void volume, or intercontraction interval between control rats with TRPV4 agonist administration and rats exposed to RVS, implying that the TRPV4 agonist produces stress-like bladder dysfunction in normal rats. In addition, there were no significant differences in these parameters in rats exposed to RVS before or after agonist exposure (Table 1; Fig. 4), indicating that the drug causes no additional changes in bladder function in RVS-treated rats. There were no significant differences in threshold or baseline pressure following intravesical instillation of the agonist (Table 1). However, a significant (P ≤ 0.05) change in micturition pressure was observed (Table 1; Fig. 4). NVCs were not observed in either control or RVS-treated rats following intravesical instillation of the agonist (data not shown). Residual volumes were not affected by agonist administration and were comparable (<10 μl) in both control and RVS groups.
Fig. 3.
Decreased bladder capacity and void volume in control rat following intravesical administration of a TRPV4 agonist. Representative cystometrogram recordings of effects of a TRPV4 agonist (GSK1016790A) in a control (no RVS) rat using continuous intravesical infusion of saline. Pre-GSK1016790A (A) and post-GSK1016790A (B) bladder function traces in a control rat that has not been exposed to 7 d of RVS are shown. TRPV4 receptor activation with intravesical infusion of GSK1016790A (3 μM) decreased both bladder capacity (measured as the amount of saline infused in the bladder at the time when micturition commenced) and void volume compared with pretreatment conditions. Infused volume (ml, top), bladder pressure (kPa, middle), and void volume (ml, bottom) are shown in A and B, which were recorded from the same rat.
Fig. 4.
Intravesical administration of a TRPV4 agonist does not change bladder function in rats exposed to 7 d of RVS. Representative cystometrogram recordings of effects of a TRPV4 agonist (GSK1016790A) in a rat that has been exposed to 7 d of RVS using continuous intravesical infusion of saline. Pre-GSK1016790A (A) and post-GSK1016790A (B) bladder function traces in an RVS treated are shown. TRPV4 receptor activation with intravesical infusion of GSK1016790A (3 μM) did not change bladder capacity or void volume compared with pretreatment conditions. Infused volume (ml, top), bladder pressure (kPa, middle), and void volume (ml, bottom) are shown in A and B, which were recorded from the same rat.
Effects of Intravesical Instillation of a TRPV4 Antagonist on Urinary Bladder Function
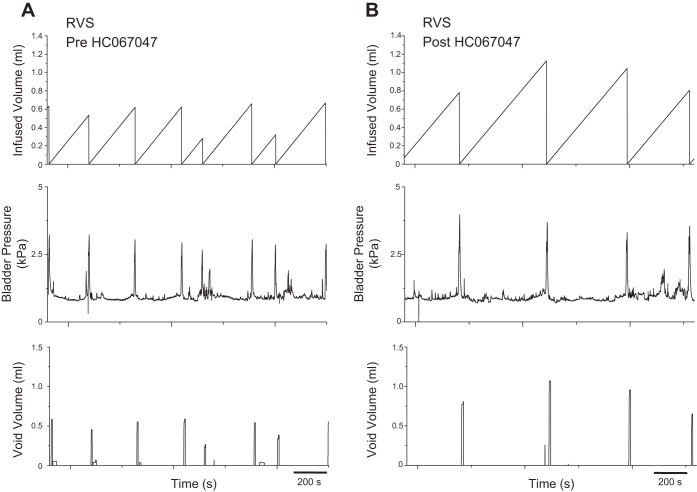
Intravesical instillation (30 min) of HC067047 (1 μM) produced a significant (P ≤ 0.001) change in bladder capacity (2.3-fold) and intercontraction interval (2.3-fold) and a significant (P ≤ 0.01) change in void volume (2.2-fold) in rats exposed to 7 d of RVS compared with the predrug state (Table 1; Fig. 5). There were no significant differences in bladder capacity, void volume, or intercontraction interval between control rats before drug instillation and rats exposed to RVS following TRPV4 antagonist administration (Table 1); administration of the antagonist rescued the phenotype to that of the control. Furthermore, administration of the TRPV4 antagonist produced significant (P ≤ 0.05) changes in bladder capacity (1.4-fold) and intercontraction interval (1.4-fold) in control rats, supporting a previous suggestion (13, 14, 47) that TRPV4 is important for normal micturition function. There were no significant differences in bladder pressure (threshold, baseline, and micturition) following intravesical instillation of the TRPV4 antagonist (Table 1). NVCs were occasionally observed in RVS-treated rats, but no significant changes in the number of NVCs were detected following intravesical instillation of the TRPV4 antagonist (data not shown).
Fig. 5.
Increased bladder capacity and void volume following intravesical administration of a TRPV4 antagonist to rats exposed to 7 d of RVS. Representative cystometrogram recordings of effects of a TRPV4 antagonist (HC067047) in a rat that has been exposed to 7 d of RVS using continuous intravesical infusion of saline. Pre-HC067047 (A) and post-HC067047 (B) bladder function traces in an RVS-treated rat are shown. TRPV4 receptor blockade with intravesical infusion of HC067047 (1 μM) increased both bladder capacity (measured as the amount of saline infused in the bladder at the time when micturition commenced) and void volume compared with pretreatment conditions. Infused volume (ml, top), bladder pressure (kPa, middle), and void volume (ml, bottom) are shown in A and B, which were recorded from the same rat.
DISCUSSION
The present studies confirmed our recent description (37) that RVS in male rats produces urinary bladder dysfunction characterized by decreased bladder capacity and void volume and increased voiding frequency. Furthermore, these studies demonstrated several novel findings with respect to TRPV4 channel expression in the urinary bladder and the effects of pharmacological manipulation following RVS on urinary bladder function. First, we demonstrated that RVS produces increases in TRPV4 mRNA transcript levels and protein expression in urothelium using Q-PCR and Western blotting techniques, respectively. Second, we established that 1) TRPV4 activation at the level of the urinary bladder decreases bladder capacity and increases voiding frequency in control rats, consistent with a previous study (2); and 2) TRPV4 blockade at the level of the urinary bladder increases bladder capacity and decreases voiding frequency in rats exposed to 7 d of RVS.
Previous studies using the RVS model have focused on its role in anxiety-like behavior and on brain regions involved in mediating fear- and anxiety-like behavior (e.g., the bed nucleus of the stria terminalis) (17, 18). Previously, we extended the use of the RVS model to the study of micturition reflex function and demonstrated RVS-induced changes in urinary bladder function and somatic sensitivity and in the inflammatory milieu of the urinary bladder (37). In the present studies, we focused on RVS-induced urinary bladder dysfunction and pharmacological blockade of the TRPV4 channel as a potential therapeutic target. Patients with functional bladder disorders including IC/BPS often report symptom exacerbation during times of stress (52). Few approved therapies for symptoms associated with IC/BPS exist, and those available may not be effective for all patients given the heterogeneity of this chronic pain syndrome. Therefore, it is important to utilize animal models to identify potential disease mechanisms, although their value has been questioned (52). Because of the fact that these are symptom-based disorders, where chronic pelvic/bladder pain is the cardinal symptom of IC/BPS and OAB is characterized by urinary urgency (1), both of which are impossible to study in a rat, interpretation of findings in animal models of these syndromes deserves careful attention. Therefore, the focus has been on animal models that display multiple, key characteristics of symptoms of human lower urinary tract disorders such as urinary frequency and pelvic/bladder pain (42). The TRPV4 channel has been implicated as a promising target for the IC/BPS and OAB symptom of increased voiding frequency. The current studies extend the potential utility of TRPV4 blockade at the level of the urinary bladder as a therapeutic target by demonstrating improvements in stress-induced urinary bladder dysfunction.
These studies demonstrated increased expression of TRPV4 following RVS at both the transcript and the protein level in the urothelium but not detrusor smooth muscle. The expression of TRPV4 in the lower urinary tract has been widely established; TRPV4 expression was first shown in the urinary bladder urothelium of mice and rats in 2007, where it was mainly found in basal and intermediate urothelial cells (14), and has since been confirmed (4, 30, 39). In the present study, we confirmed the presence of TRPV4 specifically in the urothelium using Q-PCR and Western blotting techniques, and we also demonstrated increased TRPV4 expression in urothelial tissue but not in detrusor smooth muscle following RVS. In rats exposed to RVS, we found that TRPV4 activation by administration of the potent and highly selective agonist GSK1016790A increased voiding frequency with associated decreases in bladder capacity and void volumes. Our results are similar to a previous study (2), which used female rats of a different strain. Additionally, the previous study by Aizawa et al. (2) found that the drug effects were transient. Urodyamic parameters were averaged for 20 min (10–30 and 40–60 min after drug administration), and effects were attenuated 40–60 min after drug instillation. We did not observe transient pharmacologic effects in the present study, and this may be attributed to differences in the route of drug administration. Our results indicate that instillation of GSK1016790A in male Wistar rats significantly reduces bladder capacity and void volume and increases voiding frequency for 1 h following drug administration for 30 min under anesthesia. The lack of additional changes in bladder function parameters (Table 1; Fig. 4) in rats exposed to RVS and treated with TRPV4 agonist may be due to a saturation effect; increased voiding frequency cannot be further increased. Although we did not observe changes in bladder pressures (baseline, peak, and threshold) in all other groups, we did observe an increase in peak micturition pressure in rats exposed to RVS and treated with the TRPV4 agonist. This may be indicative of an effect on efferent mechanisms involved in micturition reflex function in the context of RVS. Future studies are needed to further evaluate this mechanism.
We also found that TRPV4 blockade using intravesical administration of the selective antagonist HC067047 alleviated RVS-induced bladder dysfunction. We showed previously (37) and confirmed in this study (Table 1) that rats exposed to 7 d of RVS exhibit decreased bladder capacity and void volumes and increased voiding frequency. The present studies show that, following TRPV4 blockade, bladder capacities and void volumes are increased and voiding frequency is decreased in these rats. Most notably, there are no statistical differences in urodynamic parameters between these rats and control rats. This suggests that TRV4 blockade using intravesical instillation of the TRPV4 antagonist HC067047 fully recovers the phenotype found in rats that have been exposed to RVS. HC067047 has been used previously in regards to urinary bladder function following cyclophosphamide-induced cystitis (13), which is a well-known animal model of urinary bladder inflammation that induces urinary bladder dysfunction. Following intraperitoneal administration of HC067047, both mice and rats with cyclophosphamide-induced cystitis displayed a decrease in micturition frequency and an increase in bladder capacity using cystometric recordings (13). The present study, using an animal model of repeated stress that reproducibly causes bladder dysfunction in rats, provides further evidence that TRPV4 antagonists may offer a promising means of treating bladder dysfunction resulting from various causes.
The urinary bladder dysfunction induced by RVS is likely regulated by either central nervous system (CNS) or peripheral nervous system mechanisms, or both. CNS modulation of the micturition reflex in response to stress would likely occur through the secretion of stress hormones involved in the HPA axis. Corticotropin-releasing hormone (CRH) is released by the paraventricular nucleus of the hypothalamus in response to stressful stimuli and acts as both a steroid hormone and a neurotransmitter in the stress response. The role of CRH in micturition reflex function is well established. Neurons in Barrington's nucleus, a brain region in the pons that controls the descending limb of the micturition reflex, highly express CRH (26, 49), and animal models of stress increase CRH mRNA expression in this brain region (22, 53). Conflicting evidence suggests either an excitatory (27) or inhibitory (35) role of CRH in the micturition reflex. Corticosterone is another steroid hormone involved in the HPA axis that is secreted by the adrenal gland in rodents during stressor exposure. Several animal models of stress have produced increased plasma corticosterone levels throughout stressor exposure (19). Therefore, it is important to consider and study the role of CNS modulation, particularly the role of corticosterone, in stress-induced bladder dysfunction. Ongoing studies in our laboratory are examining the potential role of circulating corticosterone as well as HPA axis function in our model of RVS-induced bladder dysfunction.
Stress is also known to potentiate the immune response, and we have shown previously that RVS causes an increase in a number of inflammatory mediators in the urinary bladder, including NGF (37). The role of NGF in the inflammatory milieu of the urinary bladder is well established, and NGF has been suggested as a potential biomarker for lower urinary tracts including IC/BPS (34, 41). We have shown previously the ability of NGF to modulate several TRP channels including TRPV4 (16, 36). Therefore, it is possible that increases in NGF in the urinary bladder following RVS at least in part contribute to the increases reported in TRPV4 expression, but future studies are necessary to further address this possibility.
Increased TRPV4 expression in the urothelium may lead to changes in the micturition reflex and urinary bladder dysfunction seen following 7 d of RVS. TRPV4 channels are sensory cation channels found in the urothelium and are activated by stretch (51). Stretch stimulation or TRPV4 activation with the selective TRPV4 agonist 4α-phorbol 12,13-didecanoate evokes intracellular Ca2+ increases in urothelial cells from wild-type mice, and this effect is significantly attenuated in urothelial cells from TRPV4 KO mice (14, 39). Similar results are observed with a cultured rat urothelial cell model (4). In addition, the increases in Ca2+ in wild-type urothelial cells were reduced in the presence of the relatively nonselective TRP channel antagonist ruthenium red (39). It has been reported that activation of TRPV4 in bladder urothelial cells induces Ca2+ influx-evoked ATP release and that released ATP can modulate bladder sensory transduction by subsequent stimulation of P2X3-purinoceptors (4). Therefore, it is possible that increases in TRPV4 expression following RVS cause urinary bladder dysfunction in part through a similar mechanism. Future studies involving the use of the RVS model in TRPV4 KO mice, blockade of P2X3-purinoceptors during cystometry, or the use of a novel ex vivo preparation where urodynamic parameters and afferent nerve recordings are collected simultaneously can begin to address this possibility.
Perspectives and Significance
Many disorders of the urinary bladder, including IC/BPS and OAB, exhibit symptom (i.e., urinary frequency) exacerbation due to stress. Previous studies have characterized the effects of RVS on the inflammatory milieu of the urinary bladder and bladder function, and others have identified the role of TRPV4 in bladder dysfunction disorders. These studies further identify the role of TRPV4 in the urinary bladder in regards to RVS and also provide further support of TRPV4 as a potential target for therapeutic intervention. Increased expression of TRPV4 was detected in the urinary bladder, specifically the urothelium, following exposure to RVS. Further, bladder dysfunction, characterized by decreased bladder capacity and increased voiding frequency, following RVS was improved by TRPV4 blockade using intravesical administration of the TRPV4 antagonist HC067047. Notably, urodynamic parameters following drug administration were not different from parameters of control animals, suggesting that HC067047 can completely ameliorate the deleterious bladder dysfunction phenotype caused by RVS. Future studies will continue to explore underlying mechanisms of the role of TRPV4 in RVS-induced changes in urinary bladder structure and function by examining other components of the micturition reflex pathway (e.g., bladder afferent nerves, DRG, spinal cord, etc.), referred hyperalgesia using somatic sensitivity testing of pelvic and hindpaw regions, or other possible contributions of signaling molecules (e.g., ATP), inflammatory mediators (e.g., NGF), and/or neurochemicals (e.g., pituitary adenylate cyclase-activating peptide).
GRANTS
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-051369, DK-060481, and DK-065989. Division of Research Resources Grant P20-RR-16435 from the COBRE Program of the National Center also provided resource support for the project.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.M. and M.A.V. conception and design of research; L.M. performed experiments; L.M. analyzed data; L.M. and M.A.V. interpreted results of experiments; L.M. and M.A.V. prepared figures; L.M. drafted manuscript; L.M. and M.A.V. edited and revised manuscript; L.M. and M.A.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Beatrice Girard and Susan Malley for technical expertise. We also thank Dr. Sayamwong (Jom) Hammack and Dr. Victor May for advice and guidance with the RVS model and for numerous discussions related to this work.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 187: 116–126, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Aizawa N, Wyndaele JJ, Homma Y, Igawa Y. Effects of TRPV4 cation channel activation on the primary bladder afferent activities of the rat. Neurourol Urodyn 31: 148–155, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 298: F589–F600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birder L, Kullmann FA, Lee H, Barrick S, de Groat W, Kanai A, Caterina M. Activation of urothelial transient receptor potential vanilloid 4 by 4alpha-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharmacol Exp Ther 323: 227–235, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA 98: 13396–13401, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheppudira BP, Girard BM, Malley SE, Dattilio A, Schutz KC, May V, Vizzard MA. Involvement of JAK-STAT signaling/function after cyclophosphamide-induced bladder inflammation in female rats. Am J Physiol Renal Physiol 297: F1038–F1044, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol 165: 975–979, 2001 [PubMed] [Google Scholar]
- 8.Clemens JQ, Brown SO, Calhoun EA. Mental health diagnoses in patients with interstitial cystitis/painful bladder syndrome and chronic prostatitis/chronic pelvic pain syndrome: a case/control study. J Urol 180: 1378–1382, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemow DB, Steers WD, McCarty R, Tuttle JB. Altered regulation of bladder nerve growth factor and neurally mediated hyperactive voiding. Am J Physiol Regul Integr Comp Physiol 275: R1279–R1286, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Dorr W. Cystometry in mice–influence of bladder filling rate and circadian variations in bladder compliance. J Urol 148: 183–187, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Dunn KM, Hill-Eubanks DC, Liedtke WB, Nelson MT. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci USA 110: 6157–6162, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everaerts W, Vriens J, Owsianik G, Appendino G, Voets T, De Ridder D, Nilius B. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol 298: F692–F701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA 107: 19084–19089, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117: 3453–3462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard BM, May V, Bora SH, Fina F, Braas KM. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regul Pept 109: 89–101, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Girard BM, Merrill L, Malley S, Vizzard MA. Increased TRPV4 expression in urinary bladder and lumbosacral dorsal root ganglia in mice with chronic overexpression of NGF in urothelium. J Mol Neurosci 51: 602–614, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 34: 833–843, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci 42: 327–340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez ME, Martinez-Mota L, Salinas C, Marquez-Velasco R, Hernandez-Chan NG, Morales-Montor J, Perez-Tapia M, Streber ML, Granados-Camacho I, Becerril E, Javier BH, Pavon L. Chronic stress induces structural alterations in splenic lymphoid tissue that are associated with changes in corticosterone levels in Wistar-Kyoto rats. Biomed Res Int 2013: 868742, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893–903, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol 173: 1016–1021, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci 11: 585–599, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen DA, Hoenderop JG, Jansen KC, Kemp AW, Heesakkers JP, Schalken JA. The mechanoreceptor TRPV4 is localized in adherence junctions of the human bladder urothelium: a morphological study. J Urol 186: 1121–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen A, Maigaard K, Wortwein G, Hageman I, Henriksen T, Weimann A, Moller P, Loft S, Hau J, Poulsen HE, Jorgensen MB. Chronic restraint stress in rats causes sustained increase in urinary corticosterone excretion without affecting cerebral or systemic oxidatively generated DNA/RNA damage. Prog Neuropsychopharmacol Biol Psychiatry 40C: 30–37, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Kim JC, Park EY, Seo SI, Park YH, Hwang TK. Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol 175: 1773–1776; discussion 1776, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Klausner AP, Steers WD. Corticotropin releasing factor: a mediator of emotional influences on bladder function. J Urol 172: 2570–2573, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Klausner AP, Streng T, Na YG, Raju J, Batts TW, Tuttle JB, Andersson KE, Steers WD. The role of corticotropin releasing factor and its antagonist, astressin, on micturition in the rat. Auton Neurosci 123: 26–35, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Klinger MB, Girard B, Vizzard MA. p75NTR expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol 507: 1379–1392, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F1778–F1789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kullmann FA, Shah MA, Birder LA, de Groat WC. Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol 296: F892–F901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lei Z, Ishizuka O, Imamura T, Noguchi W, Yamagishi T, Yokoyama H, Kurizaki Y, Sudha GS, Hosoda T, Nishizawa O, Andersson KE. Functional roles of transient receptor potential melastatin 8 (TRPM8) channels in the cold stress-induced detrusor overactivity pathways in conscious rats. Neurourol Urodyn 32: 500–504, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor level could be a biomarker in the differential diagnosis of mixed urinary incontinence in women. BJU Int 102: 1440–1444, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur Urol 56: 700–706, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 79: 572–577, 1997 [DOI] [PubMed] [Google Scholar]
- 35.McFadden K, Griffin TA, Levy V, Wolfe JH, Valentino RJ. Overexpression of corticotropin-releasing factor in Barrington's nucleus neurons by adeno-associated viral transduction: effects on bladder function and behavior. Eur J Neurosci 36: 3356–3364, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merrill L, Girard BM, May V, Vizzard MA. Transcriptional and translational plasticity in rodent urinary bladder TRP channels with urinary bladder inflammation, bladder dysfunction, or postnatal maturation. J Mol Neurosci 48: 744–756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merrill L, Malley S, Vizzard MA. Repeated variate stress in male rats induces increased voiding frequency, somatic sensitivity, and urinary bladder nerve growth factor expression. Am J Physiol Regul Integr Comp Physiol 305: R147–R156, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyazato M, Sugaya K, Goins WF, Wolfe D, Goss JR, Chancellor MB, de Groat WC, Glorioso JC, Yoshimura N. Herpes simplex virus vector-mediated gene delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord-injured rats. Gene Ther 16: 660–668, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284: 21257–21264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nilius B. Transient receptor potential (TRP) cation channels: rewarding unique proteins. Bull Mem Acad R Med Belg 162: 244–253, 2007 [PubMed] [Google Scholar]
- 41.Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 161: 438–441; discussion 441–432, 1999 [PubMed] [Google Scholar]
- 42.Parsons BA, Drake MJ. Animal models in overactive bladder research. Handb Exp Pharmacol 15–43, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Rostamkhani F, Zardooz H, Zahediasl S, Farrokhi B. Comparison of the effects of acute and chronic psychological stress on metabolic features in rats. J Zhejiang Univ Sci B 13: 904–912, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff T, Zimmerman B. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology 57: 422–427, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534–R547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropa noyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamid e (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther 326: 432–442, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Uvin P, Everaerts W, Pinto S, Alpizar YA, Boudes M, Gevaert T, Voets T, Nilius B, Talavera K, De Ridder D. The use of cystometry in small rodents: a study of bladder chemosensation. J Vis Exp 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valentino RJ, Wood SK, Wein AJ, Zderic SA. The bladder-brain connection: putative role of corticotropin-releasing factor. Nat Rev Urol 8: 19–28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161: 273–284, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol 75: 1262–1279, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Westropp JL, Buffington CA. In vivo models of interstitial cystitis. J Urol 167: 694–702, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol 296: R1671–R1678, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7: 9, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]