Abstract
Pulmonary artery smooth muscle cell (PASMC) proliferation plays a fundamental role in the vascular remodeling seen in pulmonary hypertensive diseases associated with hypoxia. Arginase II, an enzyme regulating the first step in polyamine and proline synthesis, has been shown to play a critical role in hypoxia-induced proliferation of human PASMC (hPASMC). In addition, there is evidence that patients with pulmonary hypertension have elevated levels of arginase in the vascular wall. Resveratrol, a natural polyphenol found in red wine and grape skins, has diverse biochemical and physiological actions including antiproliferative properties. Furthermore, resveratrol has been shown to attenuate right ventricular and pulmonary artery remodeling, both pathological components of pulmonary hypertension. The present studies tested the hypothesis that resveratrol would prevent hypoxia-induced pulmonary artery smooth muscle cell proliferation by inhibiting hypoxia-induced arginase II expression. Our data indicate that hypoxia-induced hPASMC proliferation is abrogated following treatment with resveratrol. In addition, the hypoxic induction of arginase II was directly attenuated by resveratrol treatment. Furthermore, we found that the inhibitory effect of resveratrol on arginase II in hPASMC was mediated through the PI3K-Akt signaling pathway. Supporting these in vitro findings, resveratrol normalized right ventricular hypertrophy in an in vivo neonatal rat model of chronic hypoxia-induced pulmonary hypertension. These novel data support the notion that resveratrol may be a potential therapeutic agent in pulmonary hypertension by preventing PASMC arginase II induction and proliferation.
Keywords: pulmonary hypertension, pulmonary vasculature, phosphatidylinositol 3-kinase, vascular smooth muscle cells, stilbene
pulmonary hypertension (PH) is a disease characterized by increased pulmonary vascular resistance leading to the elevation of right ventricular afterload, and eventual right heart failure and death. Based on the World Health Organization Classification (28), there are five updated clinical categories of PH, each associated with high morbidity and mortality. Despite being etiologically diverse, cellular proliferation and vascular remodeling as a response to lung injury, systemic disease, or stimuli such as hypoxia, drugs, or toxins are the common hallmark pathogenic features of PH (12, 20). Currently, treatment options available for PH are limited to vasodilators that are only supportive with no curative therapy (8). Therefore, new strategies are needed for the prevention and treatment of the pathogenic vascular remodeling and vascular smooth muscle cell proliferation characteristic of PH.
We have previously shown that hypoxia-induced proliferation in human pulmonary artery smooth muscle cells (hPASMC) depends on the induction of arginase II (2). Arginase, of which there are two described isoforms, has been shown to be important in proliferation of different cell types. Both isoforms are expressed in the lung and metabolize the conversion of l-arginine to l-ornithine and urea. This is the first step in polyamine and proline synthesis, which are critical for cell proliferation, differentiation, tissue repair, and growth (14, 15).
Resveratrol (3,5,4′-trihydroxystilbene) is a natural polyphenol compound found in many plant species, including red grapes and berries, which exerts significant antioxidant, anti-inflammatory, and endothelial protective effects in the systemic circulation (24). Recent data have shown that stilbenic derivatives inhibited, in a dose-dependent manner, arginase activity in kidney and liver lysates isolated from mice (33). In addition, the same stilbenic derivative resulted in decreased arginase activity and increased intracellular concentration of nitric oxide in human umbilical vein endothelial cells (33). Although there have been relatively little data on the effects of resveratrol in the pulmonary circulation, it has been shown to prevent monocrotaline-induced pulmonary hypertension in animal models and inhibit proliferation of pulmonary arterial smooth muscle cells (7). In the present study, we hypothesized that resveratrol would prevent hypoxia-induced hPASMC proliferation via inhibition of arginase II induction. Furthermore, we provide evidence that the mechanism of action of resveratrol's inhibition of arginase II induction is at the level of transcription via PI3K-Akt-dependent cell signaling.
MATERIALS AND METHODS
Cell culture.
Human PASMC (Lonza, Walkersville, MD) were grown in 21% O2, 5% CO2, balance N2 at 37°C in smooth muscle growth media (SmGM, Lonza), which includes smooth muscle basal medium (Lonza), 5% fetal bovine serum, 0.5 ng/ml human recombinant epidermal growth factor, 2 ng/ml human recombinant fibroblast growth factor, 5 μg/ml insulin, and 50 μg/ml of gentamicin. Human PASMC from at least two donors were used between the fifth and eighth passages. The cells were grown to ∼80% confluence and washed, and fresh medium was placed on the cells. Varying concentrations of resveratrol (Acros Organics, part of Thermo Fisher Scientific, Geel, Belgium; 40, 80, or 100 μM), LY294002 (Calbiochem, Billerica, MA; 10 μM), or vehicle was added to the cells and were incubated in 21% O2, 5% CO2, balance N2 (normoxia) or 1% O2, 5% CO2, balance N2 (hypoxia) for 30 min, 24, 48, or 120 h. In addition, hPASMC were exposed to hypoxia for 5, 10, 15, 20, or 30 min to confirm Akt activation. For the 30-min time points, resveratrol or LY294002 were added to the cells for 30 min prior to hypoxia exposure. After completion of exposure, cells were isolated for RNA or protein as described below.
RNA isolation, reverse transcription, and quantitative real time-PCR.
Human PASMC were denatured with 4 M guanidine thiocyanate, mixed sequentially with 2 M sodium acetate (pH 4), extracted with phenol and phenol/chloroform/isoamyl alcohol, and then precipitated with isopropanol. RNA pellet was washed with 75% ethanol and then dissolved in diethylpyrocarbonate water. The RNA was reverse transcribed and quantitative real-time PCR was performed as previously described (2, 3). Relative arginase I and II mRNA amounts were normalized to 18S mRNA expression by the ΔΔCt method (18). Samples were analyzed in triplicate.
Protein isolation.
Protein was isolated from hPASMC as previously described (22). Briefly, hPASMC were washed with phosphate-buffered saline (PBS), and 50–150 μl of lysis buffer (0.2 M NaOH, 0.2% SDS) was added to each plate or each well of a six-well plate. Before use, the following protease inhibitors were added to each milliliter of lysis buffer: 1 μl aprotinin [10 mg/ml double distilled (dd) H2O], 1 μl leupeptin (10 mg/ml ddH2O), and 1 μl of phenylmethylsulfonyl fluoride (34.8 mg/ml methanol). The lysis buffer solution was added to each plate or each well of a six-well plate and placed on ice for 30 min. The hPASMC were scraped, pipetted into sterile centrifuge tubes. The cell lysates were centrifuged at 12,000 g for 10 min. The supernatant was stored in 1.5-ml tubes at −80°C. Total protein concentration was determined by the Bradford method (1) using a commercially available assay (Bio-Rad, Hercules, CA).
Western blot.
The lysed hPASMC were assayed for phosphorylated Akt (p-Akt), total Akt, arginase I, arginase II, and cleaved caspase 3 protein by Western blot analysis as previously described (21, 22). Aliquots of cell lysate were diluted 4:1 with NuPAGE LDS sample buffer (Life Technologies, Grand Island, NY), heated to 70°C for 10 min, and then quickly spun down at room temperature. Aliquots of the supernatant were used for SDS-PAGE. The proteins were transferred to polyvinylidene difluoride membranes and blocked for 2 h in PBS with 0.1% Tween (PBS-T) containing 5% nonfat dried milk. The membranes were then incubated with primary antibody, p-Akt (1:1,500; Cell Signaling Technology, Danvers, MA), total Akt (1:1,000; Cell Signaling), arginase I (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), arginase II (1:500, Santa Cruz), or cleaved caspase 3 (1:1,000, Cell Signaling) overnight and then washed three times with PBS-T. The membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:15,000, Bio-Rad) or sheep anti-mouse secondary antibody (1:15,000, GE Healthcare, Piscataway Township, NJ) for 1 h and then washed three times with PBS-T. The protein bands were visualized with enhanced chemiluminescence (Luminata Classico/Forte Western HRP Substrate; EMD Millipore, Billerica, MA) and quantified by densitometry (Sigma Gel, Jandel Scientific, San Rafael, CA or UVP BioImaging Systems, Upland, CA). To control for protein loading, the blots were stripped by use of a stripping buffer containing 62.5 mM Tris·HCl (pH 6.8), 2% SDS, and 100 mM 2-β-mercaptoethanol, and the blots were reprobed for β-actin (1:10,000; Sigma) as described above.
Arginase activity.
Arginase activity was measured in lysed hPASMC as previously described (2). Briefly, for one experiment, 16 10-cm-diameter cell culture plates were used to yield 200 μg protein for each test tube. The cell lysate samples were added to 10% (vol/vol) MnCl2 with 0.2 M Tris buffer (pH 9.0), vortexed, and incubated at 55°C for 5 min. Increasing concentrations of l-arginine (0, 0.03, 0.1, 0.3, 1, 3, 10, and 30 mM) were added to the heat-activated lysates in each test tube, vortexed, and incubated at 37°C for 1 h. The samples were colorimetrically assayed in duplicate for urea as previously described (2, 22). Each sample was added to 1.5 ml chromogenic reagent [5 mg thiosemicarbazide, 250 mg diacetyl monoxime, and 25 mg FeCl3 in 150 ml 16.7% (vol/vol) H2SO4, and 13.3% (vol/vol) H3PO4]. The mixtures were vortexed, boiled at 100°C for 5 min, then cooled to room temperature. Each sample with the added chromogenic reagent was pipetted into a microplate in duplicate and the difference in absorbance at 530 nm was compared with a urea standard curve. Arginase activity was calculated as amount of urea produced (μmol) over time normalized to protein amount [μmol·mg protein−1·min−1]. The reaction velocity was plotted against the substrate concentration and the Vmax and Km values were determined by use of the Michaelis-Menten equation (Sigmaplot 11.0, Jandel Scientific, Carlsbad, CA).
The effects of resveratrol on arginase activity in hPASMC were assessed by adding either vehicle or resveratrol (100 μM) to the cells prior to incubation in normoxia or hypoxia for 48 h, and the assay was performed as described above.
Transfection of Akt-siRNA.
Human PASMC were seeded at 7 × 104 in six-well plates and were cultured in normoxia and grown to 80% confluence prior to transfection with 30 nM Akt siRNA (Invitrogen, Life Technologies) or scramble siRNA control by using DharmaFECT transfection reagent (Thermo Fisher) according to the manufacturer's protocol. After 24 h the transfection medium was removed, cells washed, and smooth muscle growth medium added. Cells were then incubated in normoxia or hypoxia for 48 h and were collected in lysis buffer as previously described.
Viable cell number assay.
To determine viable cell numbers, hPASMC (Lonza) were equally seeded in six-well plates (104 cells per well) in SmGM and incubated in hypoxia for 120 h as previously described (2, 3). Adherent cells were trypsinized, and viable cells were counted by use of a hemacytometer and Trypan blue exclusion. To determine the role of resveratrol in the hypoxia-induced proliferative response in hPASMC, resveratrol (40, 80, or 100 μM) or vehicle was added to the media and viable cell numbers were determined.
Animal studies.
All protocols employed in this study were reviewed and approved by the Institutional Animal Care and Use Committees of The Research Institute at Nationwide Children's Hospital. Neonatal Sprague-Dawley rats (on day 2 of life; Harlan Industries, Indianapolis, IN) were housed with their birthing dams and placed into hypobaric hypoxic chamber with barometric pressure maintained at 435 ± 5 Torr for 14 days. The neonatal rats received daily subcutaneous injections of resveratrol [diluted in propylene glycol (80%); 100 mg/kg; Sigma] or vehicle. The animals were provided with fresh food, water, and clean bedding three times per week. Animals were removed from the hypoxic chamber and immediately placed in a hypoxic (12% O2-88% N2) gas mixture to maintain hypoxic exposure until the time of death. Age-matched normoxic, vehicle-treated controls were housed in ambient barometric pressure. All animals were housed on a 12:12-h light-dark cycle.
Assessment of right ventricular hypertrophy.
After 14 days of treatment, determination of right ventricular hypertrophy (RVH) was performed as previously described (25). Briefly, the atria and major vessels were removed from the fixed hearts, and the right ventricle (RV) wall was dissected from the left ventricle (LV) and the septum (S). The RV and the LV+S were weighed, and the RV/(LV+S) calculations were used to assess the degree of RVH.
Statistical analysis.
Values are given as means ± SE. Unpaired t-test or two-way ANOVA was used to compare the data between groups. Two-way ANOVA was used with treatment concentrations and oxygen exposure as independent variables. Individual differences were detected by multiple comparisons, if significance was determined by two-way ANOVA (Prism, GraphPad Software, La Jolla, CA). Differences were considered significant when P < 0.05.
RESULTS
Resveratrol prevents hypoxia-induced arginase II protein expression.
To determine the effects of resveratrol on arginase protein expression, hPASMC treated with resveratrol or vehicle were incubated in either normoxia or hypoxia for 48 h and analyzed by Western blot. Overall, consistent with previous results (2, 3), hPASMC exposed to hypoxia had substantially (P < 0.0001) greater arginase II protein levels at 48 h than did hPASMC exposed to normoxia (Fig. 1, B and C). The addition of resveratrol for 48 h prevented hypoxia-induced arginase II protein expression (P < 0.001; Fig. 1, B and C), such that there was no difference in arginase II expression in the resveratrol-treated, hypoxia group compared with the vehicle-treated, normoxia controls. Consistent with previous results (2, 3), hypoxia did not have any significant effects on arginase I protein expression (Fig. 2, B and C). Similarly, resveratrol did not effect arginase I protein levels in either normoxia or hypoxia (Fig. 2, B and C).
Fig. 1.
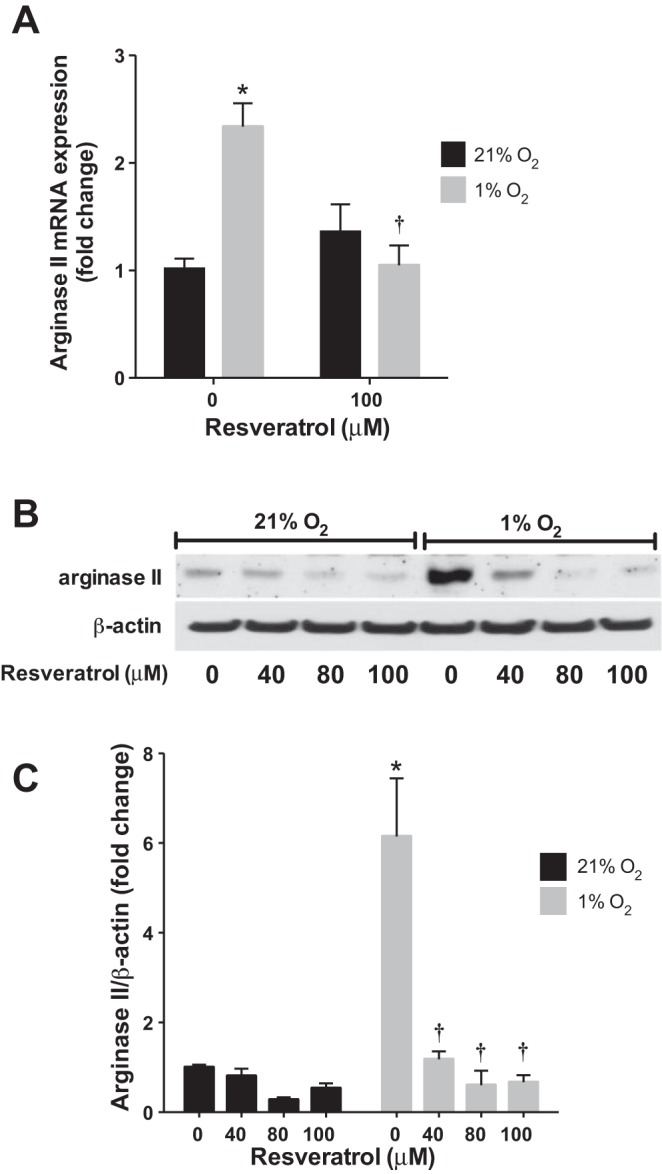
Resveratrol prevents hypoxia-induced arginase II expression. Human pulmonary artery smooth muscle cell (PASMC) were incubated with vehicle or resveratrol, then exposed to 21% O2 or 1% O2 for 24 or 48 h. A: arginase II mRNA levels were analyzed by quantitative real-time PCR and normalized to 18S expression. B: representative Western blots are shown for arginase II and β-actin. C: Western blot data summarized and shown as fold change ± SE relative to vehicle-treated, normoxia controls. *Different from normoxia, vehicle treated, P < 0.0001; †different from hypoxia, vehicle treated, P < 0.001 (n = 3–16).
Fig. 2.
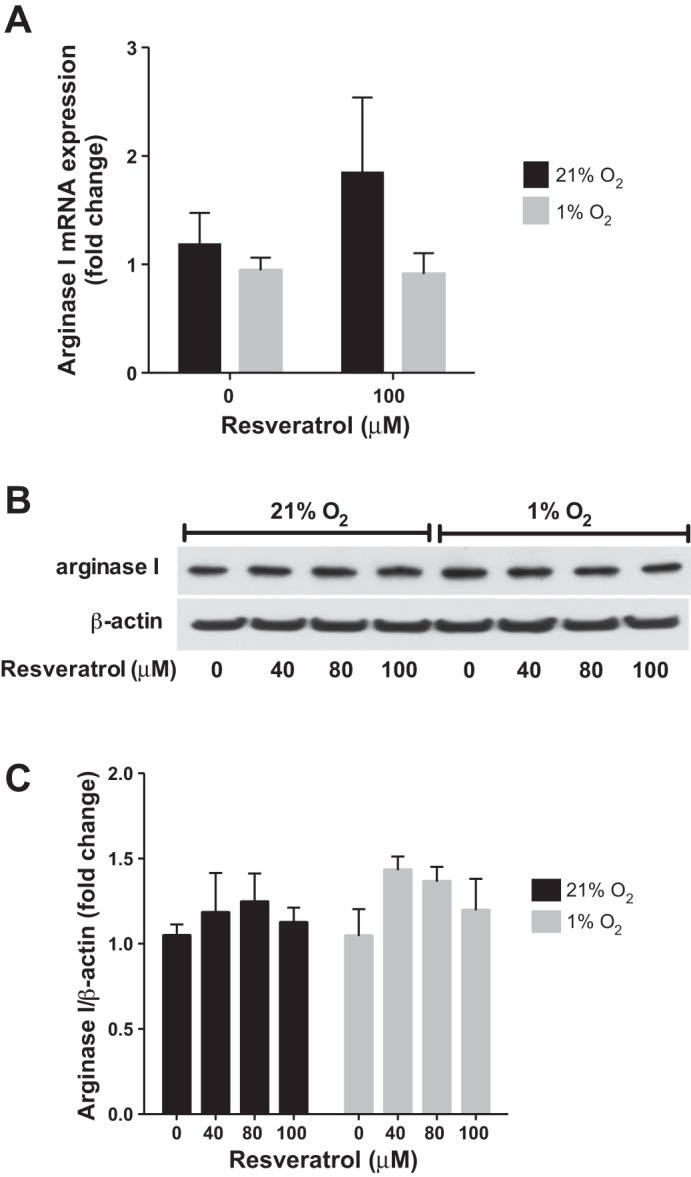
Resveratrol has no effect on arginase I expression. Human PASMC were incubated with vehicle or resveratrol, then exposed to 21% O2 or 1% O2 for 24 or 48 h. A: arginase I mRNA levels were analyzed by quantitative real-time PCR and normalized to 18S expression. B: representative Western blots are shown for arginase I and β-actin. C: Western blot data summarized and shown as fold change ± SE relative to vehicle-treated, normoxia controls (n = 3–9).
Resveratrol prevents hypoxia-induced arginase II mRNA expression.
To determine whether resveratrol exerted its effects on arginase II at the level of transcription, hPASMC treated with resveratrol or vehicle were incubated in normoxia or hypoxia for 24 h and analyzed by quantitative real-time PCR. Hypoxia increased arginase II mRNA expression after 24 h (P < 0.001). Treatment with resveratrol prevented the hypoxia-induced arginase II mRNA expression (P < 0.001), such that there was no difference in arginase II expression in the resveratrol-treated, hypoxia group compared with the vehicle-treated, normoxia controls. Hypoxia did not have any significant effects on arginase I mRNA expression (Fig. 2A). Similarly, resveratrol did not affect arginase I mRNA expression in normoxia or hypoxia (Fig. 2A).
Resveratrol inhibits arginase activity with no effect on nitrite levels.
To determine the effects of resveratrol on arginase activity, hPASMC were treated with resveratrol or vehicle and incubated in normoxia or hypoxia for 48 h. The cell lysates were incubated with increasing concentrations of l-arginine and urea production was determined. Hypoxia resulted in a significantly greater Vmax for arginase than in vehicle-treated, normoxia control cells (Fig. 3; Vmax 0.32 ± 0.01 μmol·mg protein−1·min−1, hypoxia vs. 0.15 ± 0.01 μmol·mg protein−1·min−1, normoxia; P < 0.0001). The arginine Km for arginase was not different between the two groups (1.11 ± 0.48 mM, hypoxia vs. 1.56 ± 0.62 mM, normoxia). The addition of resveratrol to hPASMC during the 48-h incubation period had no significant effect on the Vmax for arginase in normoxic hPASMC (Fig. 3). However, resveratrol completely inhibited the hypoxia-induced increase in Vmax (Fig. 3; Vmax 0.10 ± 0.01 μmol·mg protein−1·min−1, resveratrol; P < 0.0001) to levels not significantly different from the baseline Vmax in normoxic, vehicle-treated cells. The arginine Km for arginase was not different between the hypoxic, vehicle-treated and hypoxic, resveratrol-treated hPASMC (1.56 ± 0.62 mM, vehicle vs. 4.88 ± 3.12 mM, resveratrol).
Fig. 3.
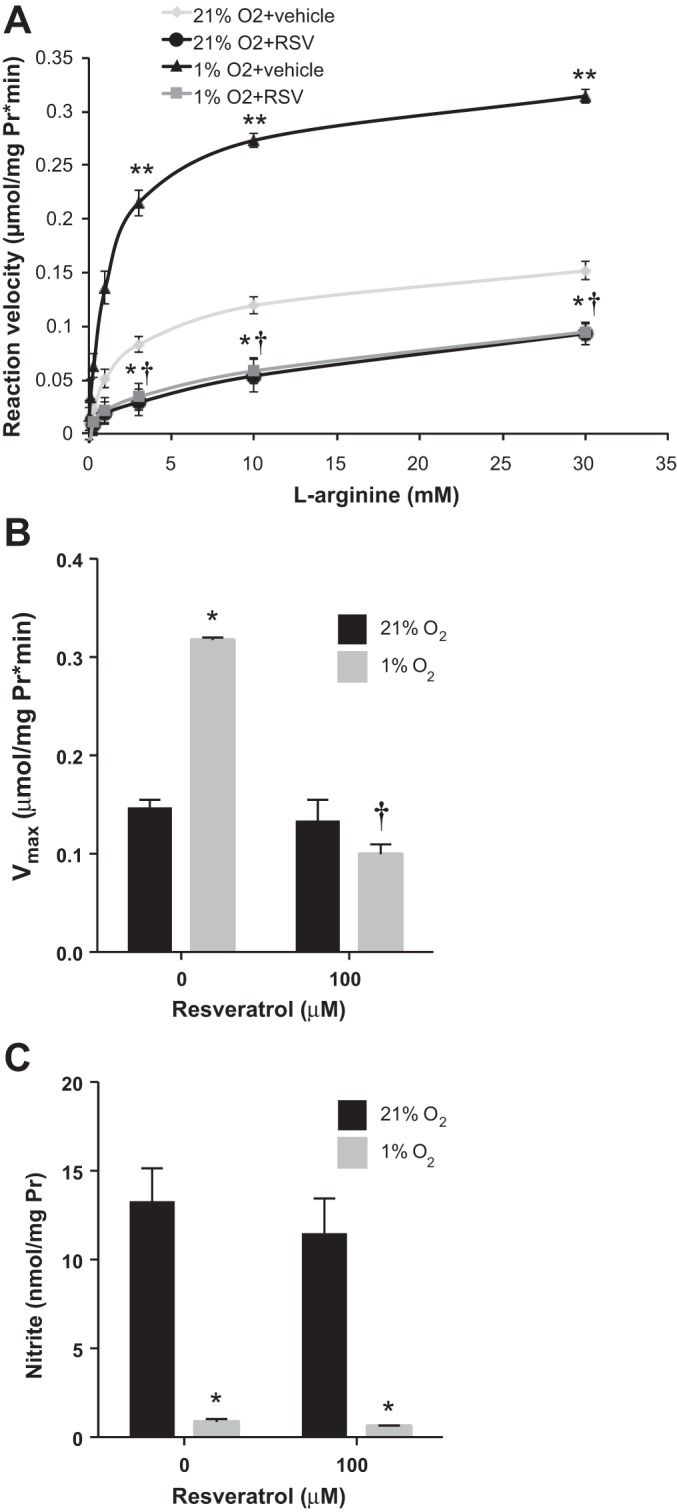
Resveratrol inhibits hypoxia-induced arginase activity with no effects on nitrite levels. Human PASMC were incubated with vehicle or resveratrol (RSV), then exposed to 21% O2 or 1% O2 for 48 h. Cell lysates were incubated with increasing concentrations of l-arginine and urea production was measured. The reaction velocity was calculated as μmol·mg protein−1·min−1. A: data were fit by using the Michaelis-Menten equation to determine Vmax and Km values. B: data are shown as a bar graph representing Vmax for arginase. C: nitrite levels were measured in 21% O2 or 1% O2 after resveratrol or vehicle treatment. *Different from normoxia, vehicle treated, P < 0.0001; **different from normoxia, resveratrol treated, P < 0.0001; †different from hypoxia, vehicle treated, P < 0.0001 (n = 3).
Nitrite levels were measured from the media of normoxic and hypoxic hPASMC treated with resveratrol. Hypoxia significantly decreased nitrite levels, relative to normoxia (P < 0.1; Fig. 3C). Interestingly, resveratrol had no measurable effect on nitrite levels (Fig. 3C).
Resveratrol prevents hypoxia-induced hPASMC proliferation with no evidence of apoptosis.
To determine the role of resveratrol on hypoxia-induced cell proliferation, viable cell numbers were determined by Trypan blue exclusion after resveratrol treatment of hPASMC (seeded at 104 cells/well) in normoxia and hypoxia for 120 h. Data are shown as viable cell numbers as a percentage change from number of seeded cells. Overall, hypoxia resulted in significantly greater viable cell numbers (Fig. 4A). In both normoxia and hypoxia, resveratrol decreased the viable number of hPASMC at all of the tested concentrations (Fig. 4A). Indeed, the viable cell numbers in the highest resveratrol concentration group in both normoxia and hypoxia were less than the number of cells seeded.
Fig. 4.
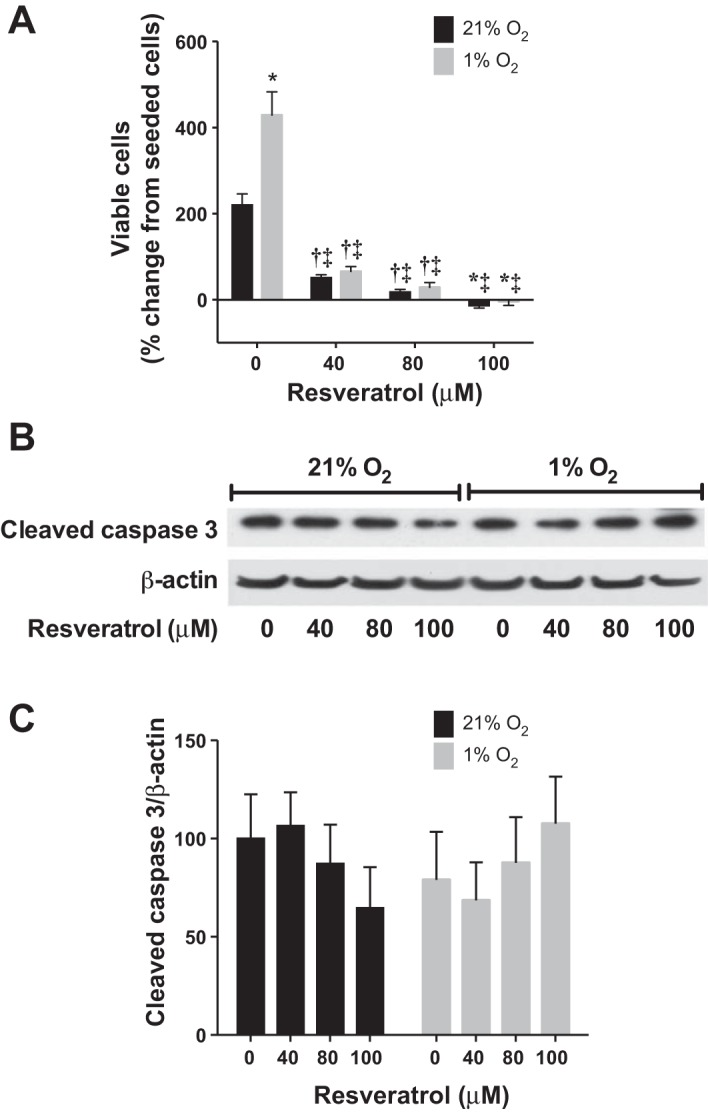
Resveratrol prevents hypoxia-induced hPASMC proliferation with no evidence of apoptosis. Human PASMC were seeded at equal densities (104 cells/well) in 6-well plates, incubated with vehicle or resveratrol, then exposed to 21% O2 or 1% O2 for 120 h. A: data are shown as % change in viable cell numbers from seeded cells (n = 6–12). Human PASMC were incubated with vehicle or resveratrol, then exposed to 21% O2 or 1% O2 for 48 h. B: representative Western blots are shown for cleaved caspase 3 and β-actin. C: data are summarized and shown normalized to β-actin ± SE relative to vehicle-treated, normoxia controls (n = 5); *different from normoxia, vehicle treated, P < 0.0001; †different from normoxia, vehicle treated, P < 0.05; ‡different from hypoxia, vehicle treated, P < 0.0001.
hPASMC were evaluated for apoptosis by measuring cleaved caspase 3 by Western blot analyses. There was no difference in cleaved caspase 3 protein expression in normoxia or hypoxia with or without resveratrol treatment (Fig. 4, B and C).
Hypoxia activates Akt.
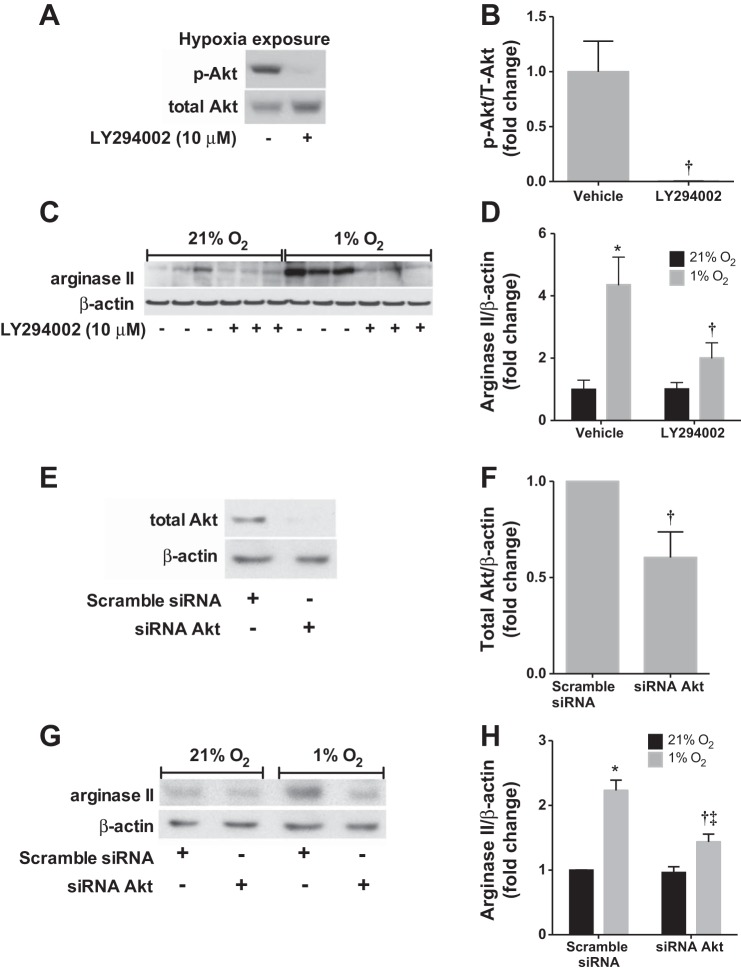
The PI3K-Akt pathway has been implicated in hypoxia-induced PASMC proliferation (13) and our group has previously shown Akt activation by hypoxia in hPASMC (4). We used Western blot analysis to assess activation of Akt by hypoxia in hPASMC. Phosphorylated Akt levels were barely detectable at 0 min, were dramatically elevated, and were highest at 30 min following hypoxic exposure (figure not shown). The PI3K inhibitor LY294002 was utilized to confirm inhibition of hypoxia-induced Akt activation at 30 min (Fig. 5, A and B).
Fig. 5.
Inhibition of Akt prevents hypoxia-induced arginase II protein expression. Human PASMC were treated with LY29002 or siRNA targeting Akt, then exposed to 21% O2 or 1% O2 for 0.5 or 48 h. A: representative Western blots are shown for phosphorylated Akt (p-Akt) and total Akt after hypoxia exposure. B: data are shown as fold change of p-Akt/total Akt ± SE relative to hypoxia, vehicle treated. C: representative Western blots are shown for arginase II and β-actin. D: data are shown as fold change of arginase II/β-actin ± SE relative to normoxia, vehicle treated. E: validation of Akt knockdown after siRNA treatment. Representative Western blots are shown for total Akt and β-actin. F: data are shown as fold change of total Akt/β-actin ± SE relative to hypoxia, scramble siRNA. G: representative Western blots are shown for arginase II and β-actin after siRNA knockdown of Akt. H: data are shown as fold change of arginase II/β-actin ± SE relative to normoxia, vehicle treated. *Different from normoxia, vehicle treated, P < 0.01; †different from hypoxia, vehicle treated, P < 0.05; ‡different from hypoxia, siRNA Akt treated, P < 0.05 (n = 5–6).
Hypoxia induces arginase II expression via Akt-dependent signaling.
To determine the effects of PI3K-Akt inhibition on arginase II protein expression, hPASMC were treated with LY294002 or vehicle, incubated in either normoxia or hypoxia for 48 h, and analyzed by Western blot. The PI3K-Akt inhibitor LY294002 had no appreciable effect on arginase II protein levels in normoxia (Fig. 5, C and D). However, LY294002 completely prevented hypoxia-induced arginase II protein expression in hPASMC (P < 0.05; Fig. 5, C and D), such that there was no difference between arginase II protein levels in LY294002-treated hypoxic cells and vehicle-treated normoxic cells.
To more specifically delineate the role of Akt in hypoxia-induced arginase II expression, transfection of Akt-siRNA into hPASMC was performed. Akt expression decreased by ∼40% after siRNA transfection, which was confirmed by Western blot (Fig. 5, E and F). Scramble-siRNA transfection resulted in greater arginase II protein levels in hypoxia than normoxia (Fig. 5, G and H). In normoxia, the arginase II protein expression in the Akt-siRNA-transfected cells was not different from scramble siRNA-transfected cells (Fig. 5, G and H). In hypoxia, the Akt-siRNA attenuated the hypoxia-induced increase in arginase II protein levels (P < 0.001; Fig. 5, G and H).
Resveratrol prevents Akt phosphorylation.
To determine whether the inhibitory effect of resveratrol on arginase II was Akt dependent, hPASMC treated with resveratrol were incubated in either normoxia or hypoxia and analyzed by Western blot. We found that Akt activation was inhibited by resveratrol at all concentrations studied (Fig. 6, A and B).
Fig. 6.
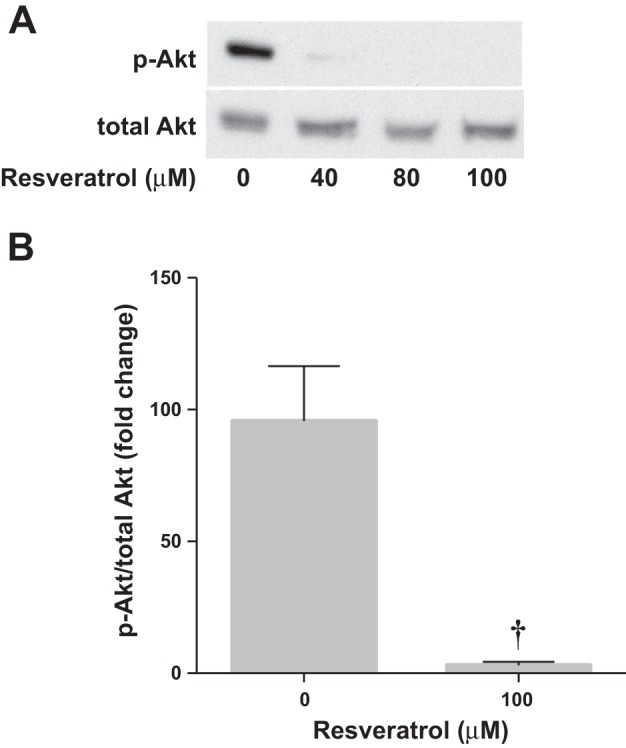
Resveratrol prevents Akt phosphorylation. Human PASMC were incubated with vehicle or resveratrol, then exposed to 1% O2 for 0.5 h. A: representative Western blots are shown for p-Akt and total Akt. B: data are shown as fold change of p-Akt/total Akt ± SE relative to hypoxia, vehicle treated. †Different from hypoxia, vehicle treated, P < 0.02 (n = 3).
Resveratrol normalizes RVH in chronic hypoxia-induced pulmonary hypertension in neonatal rats.
Neonatal Sprague-Dawley rats were treated with subcutaneous resveratrol or vehicle and exposed to chronic hypobaric hypoxia or ambient room air. Hypobaric hypoxia exposure for 14 days increased the RV/(LV+S) ratio, thereby worsening RVH. Treatment with 14 days of resveratrol normalized the chronic hypoxia-induced RVH in the neonatal rats (Fig. 7).
Fig. 7.
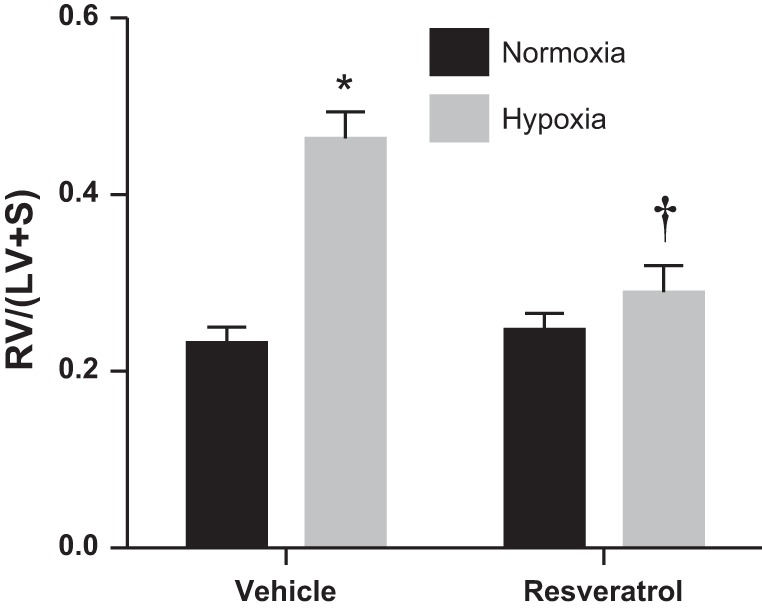
Resveratrol attenuates chronic hypoxia-induced right ventricular hypertrophy in neonatal rats. Neonatal rats were treated with resveratrol and exposed to normoxia or hypobaric hypoxia for 14 days. Right ventricle (RV) and left ventricle + septum (LV+S) weights were measured. Data are shown as RV/(LV+S) ratios. *Different from normoxia, vehicle treated, P < 0.0001; †different from hypoxia, vehicle treated, P < 0.001 (n = 8–12).
DISCUSSION
The main objective of the present study was to investigate the effects of resveratrol on hypoxia-induced arginase II expression, arginase activity, and hypoxia-induced proliferation in hPASMC. The major findings of this study were that 1) consistent with our previous findings (2, 3), hypoxia increased arginase activity, arginase II mRNA and protein expression, and proliferation in hPASMC; 2) resveratrol prevented the hypoxia-induced increase in arginase II mRNA and protein expression and arginase activity; 3) resveratrol prevented hypoxia-induced hPASMC proliferation; 4) Akt was activated by hypoxia and resveratrol prevented hypoxia-induced Akt activation; 5) inhibition of Akt prevented hypoxia-induced arginase II protein expression; and 6) resveratrol attenuated chronic hypoxia-induced RVH in neonatal rats as evidenced by normalization of RV/(LV+S) ratios. Taken together, these findings support our hypothesis that resveratrol prevents hypoxia-induced hPASMC proliferation via Akt-dependent inhibition of arginase II induction.
Vascular smooth muscle cell proliferation and resultant vascular remodeling are important pathogenic features of pulmonary hypertensive diseases; therefore, understanding mechanistic pathways involved in smooth muscle cell proliferation may isolate novel targets for drug therapies that may be able to reverse vascular remodeling. Arginase II has been demonstrated to be elevated in the pulmonary vasculature of patients with pulmonary hypertension (34). In addition to greater arginase expression, the authors also found a decrease in NO synthesis in the pulmonary artery endothelial cells in the same patients. This is not surprising because l-arginine is the substrate for both NO synthase (NOS) and arginase and an increase in arginase activity may decrease the bioavailability of l-arginine for NOS (5). Furthermore, NO tends to be antiproliferative; thus in conditions where arginase activity is increased and NO production is decreased there will be a net proliferative phenotype as well as decreased production of an important pulmonary vasodilator. Consistent with these findings, in our cell culture model in both hPASMC and human pulmonary microvascular endothelial cells, hypoxia-induced cellular proliferation is dependent on arginase II (2, 30), with a concomitant decrease in nitrite levels (Fig. 3C).
The natural dietary polyphenolic compound resveratrol exerts significant antioxidant, anti-inflammatory, and endothelial protective effects in the systemic circulation (9, 16). It has also been shown to have an antiproliferative effect on both rat and cultured human aortic smooth muscle cells (32, 36). More recently in the pulmonary circulation, resveratrol and other stilbene derivatives have been shown to inhibit rat PASMC proliferation (10). The treatment of isolated carotid arteries and aortic rings, as well as chronic treatment of rats with resveratrol, stimulates endothelium-dependent relaxation in both normotensive and hypertensive rat models (26, 29, 31). In addition, resveratrol prevented monocrotaline-induced pulmonary hypertension in rats (7). In the present studies, we found a striking inhibition of the hypoxia-induced arginase II protein expression and activity in hPASMC. Upon further analysis, we determined that the inhibitory effect of resveratrol occurs at the level of transcription. Despite an inhibitory effect of resveratrol on arginase expression, nitrite production did not change. We speculate that this may be due to low levels of NO synthase in the PASMC. Furthermore, resveratrol prevented the hypoxia-induced increase in viable cell numbers and even induced cell death at the highest tested concentration. Thus the potential for resveratrol as a therapeutic option for pulmonary hypertension and other cardiovascular diseases exists.
The PI3K-Akt signaling pathway plays a critical role in regulating cellular processes including cellular growth, proliferation, migration, and survival. Pterostilbene, a natural dimethylated analog of resveratrol, has been previously shown to have inhibitory effects on cell proliferation in vascular smooth muscle cells by blocking Akt-dependent pathways (23). More specifically, the PI3K-Akt pathway has been implicated in the molecular mechanisms involved in smooth muscle cell proliferation in the pulmonary vasculature (4, 11, 35). Indeed, this pathway has been shown to be involved in PASMC proliferation induced by hypoxia (4, 19). Consistent with these studies we found that resveratrol completely prevented hypoxia-induced Akt activation. Furthermore, the PI3K inhibitor LY294002, which prevents downstream Akt phosphorylation, as well as siRNA knockdown of Akt, prevented hypoxia-induced arginase II protein expression. The idea that resveratrol has been shown to prevent Akt phosphorylation in vascular smooth muscle cells is not novel (23, 27), but to our knowledge this is the first report of not only resveratrol's inhibitory action on arginase II expression in PASMC, but also evidence directly linking Akt phosphorylation to downstream positive effects on arginase II expression. Although one study did not find evidence for an effect of resveratrol on Akt activity (6), the variation in resveratrol concentrations used in cell culture experiments may explain these differing effects. It is reasonable to consider that the target effects and/or mechanism of action of resveratrol may be dependent on concentrations achieved in the cell.
We have previously shown in our cell culture model that arginase II is required for hypoxia-induced hPASMC proliferation (2, 3). The molecular mechanism(s) involved in hypoxic induction of arginase II have been elusive; however, we now show that the PI3K-Akt signaling pathway plays a critical role. We demonstrate that inhibition of Akt by resveratrol prevents not only the hypoxic induction of arginase II, but also hPASMC proliferation induced by hypoxia. Interestingly, the viable cells counted at the highest concentration of resveratrol (100 μM) were actually fewer than the number of cells seeded. There is a balance between cell proliferation and cell death, and in hypoxia this balance normally favors cell proliferation, i.e., a substantial increase in viable cell numbers through 120 h. Our findings suggest that by preventing proliferation, the highest doses of resveratrol tip the balance toward cell death such that there are fewer viable cells at 120 h than there were at 0 h. This is consistent with previous data in which Gao et al. (10) showed increased apoptosis in rat PASMC following what they considered to be intermediate concentrations of resveratrol (100 μM), compared with low concentrations of resveratrol (50 μM). Although we did not find evidence for apoptosis by caspase 3 activation, other cell cycle regulatory mechanisms were not evaluated in the present study that may be responsible for the cell death seen at the higher resveratrol doses.
Supporting our findings in vitro, we provide evidence that right ventricular hypertrophy is improved following treatment with resveratrol in a chronic hypoxia-induced neonatal rat model of pulmonary hypertension. Although changes in vessel remodeling are not specifically shown in the present study, Liu et al. (17) reveal that in a similar model of chronic hypoxia-induced rat model of pulmonary hypertension the ratio of vessel wall thickness to total external diameter, ratio of wall area to total vessel area, right ventricular systolic pressures, and right ventricular hypertrophy were all attenuated following resveratrol treatment.
There are several limitations to the present study that should be noted. Our studies use commercially available hPASMC that are isolated from main pulmonary artery segments. The PASMC proliferation seen in more proximal arteries may potentially contribute to arterial stiffening, given that increased arginase leads to an increase in proline, which is responsible for collagen synthesis; however, this was not tested in the present study. Although we do not use the resistance-level pulmonary arteries that are classically affected in pulmonary hypertensive diseases, we chose these cells, in part, because they are human cells. We acknowledge that these findings may need to be validated in resistance-level PASMC. Secondly, we use perhaps more severe levels of hypoxia of 1% O2 to induce PASMC proliferation. Based on numerous trials in our laboratory utilizing various conditions, which include different oxygen levels, our cell culture model has proven consistently to be a reliable and reproducible model (2–4). Additionally, our findings are consistent with those in the literature that show increased Akt phosphorylation after exposure to even less severe levels of hypoxia (11, 35).
In conclusion, our data indicate that hypoxia induces arginase II mRNA and protein expression and arginase activity resulting in increased viable cell numbers demonstrating proliferation in hPASMC and that this hypoxia-induced effect on arginase II is via the PI3K-Akt pathway. We show that resveratrol attenuates chronic hypoxia-induced RV/(LV+S) changes in neonatal rats and may play a role in preventing hypoxia-induced hPASMC proliferation by inhibition of arginase II induction. Furthermore, we provide evidence that targeting the PI3K-Akt signaling pathway may provide novel therapies in the prevention or treatment of the pathological PASMC proliferation associated with hypoxia.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant 5K08HL105677 (B. Chen) and internal grant support from The Research Institute at Nationwide Children's Hospital.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.C. and L.G.C. conception and design of research; B.C., J.X., and A.E.C. analyzed data; B.C. and L.G.C. interpreted results of experiments; B.C., J.X., and A.E.C. prepared figures; B.C. drafted manuscript; B.C. and L.G.C. edited and revised manuscript; B.C., J.X., X.M., J.L.S., A.E.C., and L.G.C. approved final version of manuscript; J.X., X.M., J.L.S., and A.E.C. performed experiments.
ACKNOWLEDGMENTS
The authors thank Zhaosheng Han and Elizabeth Natale for technical assistance in gathering the animal data.
REFERENCES
- 1.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 2.Chen B, Calvert AE, Cui H, Nelin LD. Hypoxia promotes human pulmonary artery smooth muscle cell proliferation through induction of arginase. Am J Physiol Lung Cell Mol Physiol 297: L1151–L1159, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Chen B, Calvert AE, Meng X, Nelin LD. Pharmacologic agents elevating cAMP prevent arginase II expression and proliferation of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 47: 218–226, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen B, Nelin VE, Locy ML, Jin Y, Tipple TE. Thioredoxin-1 mediates hypoxia-induced pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 305: L389–L395, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 287: L60–L68, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Choi KH, Kim JE, Song NR, Son JE, Hwang MK, Byun S, Kim JH, Lee KW, Lee HJ. Phosphoinositide 3-kinase is a novel target of piceatannol for inhibiting PDGF-BB-induced proliferation and migration in human aortic smooth muscle cells. Cardiovasc Res 85: 836–844, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 54: 668–675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferns SJ, Wehrmacher WH, Serratto M. Pediatric pulmonary arterial hypertension—a review. Compr Ther 35: 81–90, 2009 [PubMed] [Google Scholar]
- 9.Frombaum M, Le Clanche S, Bonnefont-Rousselot D, Borderie D. Antioxidant effects of resveratrol and other stilbene derivatives on oxidative stress and *NO bioavailability: potential benefits to cardiovascular diseases. Biochimie 94: 269–276, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Gao G, Wang X, Qin X, Jiang X, Xiang D, Xie L, Hu J, Gao J. Effects of trimethoxystilbene on proliferation and apoptosis of pulmonary artery smooth muscle cells. Cell Biochem Biophys 64: 101–106, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, Krymskaya VP. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283: L354–L363, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir K, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: 13S–24S, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt TP, Sanders KA, Kennedy TP, Hoidal JR. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of TGF-β1 and IGFBP-3. Am J Physiol Lung Cell Mol Physiol 296:(3) L489–L499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janne J, Alhonen L, Leinonen P. Polyamines: from molecular biology to clinical applications. Ann Med 23: 241–259, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Li H, Meinginger CJ, Hawker JR, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab 280: E75–E80, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Li H, Xia N, Forstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 26: 102–110, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Liu B, Luo XJ, Yang ZB, Zhang JJ, Li TB, Zhang XJ, Ma QL, Zhang GG, Hu CP, Peng J. Inhibition of NOX/VPO1 pathway and inflammatory reaction by trimethoxystilbene in prevention of cardiovascular remodeling in hypoxia-induced pulmonary hypertensive rats. J Cardiovasc Pharmacol 63: 567–576, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Luo C, Yi B, Bai L, Xia Y, Wang G, Qian G, Feng H. Suppression of Akt1 phosphorylation by adenoviral transfer of the PTEN gene inhibits hypoxia-induced proliferation of rat pulmonary arterial smooth muscle cells. Biochem Biophys Res Commun 397: 486–492, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20–S31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelin LD, Krenz GS, Chicoine LG, Dawson CA, Schapira RM. l-Arginine uptake and metabolism following in vivo silica exposure in rat lungs. Am J Respir Cell Mol Biol 26: 348–355, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Nelin LD, Nash HE, Chicoine LG. Cytokine treatment increases arginine metabolism and uptake in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 281: L1232–L1239, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Park ES, Lim Y, Hong JT, Yoo HS, Lee CK, Pyo MY, Yun YP. Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking Akt-dependent pathway. Vascul Pharmacol 53: 61–67, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal 11: 2851–2897, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Resta TC, Chicoine LG, Omdahl JL, Walker BR. Maintained upregulation of pulmonary eNOS gene and protein expression during recovery from chronic hypoxia. Am J Physiol Heart Circ Physiol 276: H699–H708, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Rush JW, Quadrilatero J, Levy AS, Ford RJ. Chronic resveratrol enhances endothelium-dependent relaxation but does not alter eNOS levels in aorta of spontaneously hypertensive rats. Exp Biol Med (Maywood) 232: 814–822, 2007 [PubMed] [Google Scholar]
- 27.Schreiner CE, Kumerz M, Gesslbauer J, Schachner D, Joa H, Erker T, Atanasov AG, Heiss EH, Dirsch VM. Resveratrol blocks Akt activation in angiotensin II- or EGF-stimulated vascular smooth muscle cells in a redox-independent manner. Cardiovasc Res 90: 140–147, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54: S43–S54, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Soylemez S, Sepici A, Akar F. Resveratrol supplementation gender independently improves endothelial reactivity and suppresses superoxide production in healthy rats. Cardiovasc Drugs Ther 23: 449–458, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Toby IT, Chicoine LG, Cui H, Chen B, Nelin LD. Hypoxia-induced proliferation of human pulmonary microvascular endothelial cells depends on epidermal growth factor receptor tyrosine kinase activation. Am J Physiol Lung Cell Mol Physiol 298: L600–L606, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106: 1652–1658, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Chen Y, Labinskyy N, Hsieh TC, Ungvari Z, Wu JM. Regulation of proliferation and gene expression in cultured human aortic smooth muscle cells by resveratrol and standardized grape extracts. Biochem Biophys Res Commun 346: 367–376, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Woo A, Min B, Ryoo S. Piceatannol-3′-O-beta-d-glucopyranoside as an active component of rhubarb activates endothelial nitric oxide synthase through inhibition of arginase activity. Exp Mol Med 42: 524–532, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Yi B, Cui J, Ning JN, Wang GS, Qian GS, Lu KZ. Over-expression of PKGIalpha inhibits hypoxia-induced proliferation, Akt activation, and phenotype modulation of human PASMCs: the role of phenotype modulation of PASMCs in pulmonary vascular remodeling. Gene 492: 354–360, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Zghonda N, Yoshida S, Araki M, Kusunoki M, Mliki A, Ghorbel A, Miyazaki H. Greater effectiveness of epsilon-viniferin in red wine than its monomer resveratrol for inhibiting vascular smooth muscle cell proliferation and migration. Biosci Biotechnol Biochem 75: 1259–1267, 2011 [DOI] [PubMed] [Google Scholar]