Abstract
Wheezing is a major long-term respiratory morbidity in preterm infants with and without bronchopulmonary dysplasia. We hypothesized that mild vs. severe hyperoxic exposure in neonatal mice differentially affects airway smooth muscle hypertrophy and resultant airway reactivity. Newborn mice were exposed to 7 days of mild (40% oxygen) or severe (70% oxygen) hyperoxia vs. room air controls. Respiratory system resistance (Rrs), compliance (Crs), and airway reactivity were measured 14 days after oxygen exposure ended under ketamine/xylazine anesthesia. Baseline Rrs increased and Crs decreased in both treatment groups. Methacholine challenge dose dependently increased Rrs and decreased Crs in 40% oxygen-exposed mice, whereas Rrs and Crs responses were similar between 70% oxygen-exposed and normoxic controls. Airway smooth muscle thickness was increased in 40%- but not 70%-exposed mice, whereas collagen increased and both alveolar number and radial alveolar counts decreased after 40% and 70% oxygen. These data indicate that severity of hyperoxia may differentially affect structural and functional changes in the developing mouse airway that contribute to longer-term hyperreactivity. These findings may be important to our understanding of the complex role of neonatal supplemental oxygen therapy in postnatal development of airway responsiveness.
Keywords: airway, hyperoxia, hyperreactivity, neonate, oxygen
the great strides made in improving survival of preterm infants have been associated with significant increases in the incidence of neonatal lung injury and resultant bronchopulmonary dysplasia (BPD). The combination of high levels of inspired oxygen with or without mechanical ventilation superimposed on an already immature lung has been most widely implicated in the pathogenesis of BPD. Over the last decade, rigorous attempts have been made to limit extent and duration of supplemental oxygen and ventilatory support; however, preterm infants are still frequently in need of prolonged exposure to supplemental oxygen (10, 18). Many of these infants will not necessarily develop BPD, most commonly defined as a need for supplemental oxygen at a postmenstrual age of 36 wk. However, they remain at high risk for subsequent wheezing disorders in infancy, childhood, and adolescence (8, 9). The underlying mechanisms for this increase in airway reactivity in former preterm infants is unclear (11). It would appear unlikely that the mechanisms of increased airway reactivity are identical to those that contribute to the “new BPD” of the postsurfactant age that involves alveolar and vascular dysmorphogenesis. However, given that wheezing disorders including asthma involve structural and functional changes of the airway, there needs to be better understanding of how supplemental oxygen influences different cell types in the developing airway.
Clinically, there is a trend toward reducing the extent and duration of oxygen exposure while minimizing detrimental effects from insufficient oxygen saturations. However, for the immature lung to maintain oxygen saturations, some level of hyperoxia is needed. What is less clear is the differences in the responses of the immature airway to different levels of hyperoxia. Furthermore, what is not known is whether these effects are long-lasting and contribute to altered airway structure or function later in life. From an in vivo perspective, hyperoxia-exposed neonatal rodents have been widely employed to study the pathophysiology of neonatal lung injury. Initial studies focused on the effects of ∼95% oxygen and documented increased airway reactivity, accompanied by an increase in airway smooth muscle (ASM) layer thickness, both of which resolved several days after cessation of oxygen exposure (7). In contrast, neonatal guinea pigs exposed to 70% oxygen showed a persistent increase in airway response to cholinergic agonist after return to normoxia (17). Other studies have focused on the responses of airway function to more modest levels of hyperoxic exposure. In response to prolonged exposure to 50 or 60% oxygen in newborn rats, there was an increase in contractility of tracheal and bronchial rings, but this was only measured at completion of hyperoxic exposure (2, 3). However, the major clinical problem appears to be that increased airway reactivity is exhibited by former preterm infants long after cessation of hyperoxic exposure. What remains unclear is whether the same detrimental processes initiated by hyperoxia in the early postnatal period continue to exert effects even after withdrawal of hyperoxia, or whether new mechanisms come into play (potentially even triggered by early events).
The aim of this project was to determine the differential effect of two levels of neonatal hyperoxic exposure (severe, i.e., 70%, and modest, i.e., 40%) on airway function in mice beyond cessation of exposure to supplemental oxygen. We have previously documented that exposure to >95% oxygen upregulates epithelial arginase in neonatal rat pups, which would be expected to increase lung collagen content and potentially restrict airway constriction (1). Furthermore, we have shown that levels of supplemental oxygen >60% cause apoptosis in human fetal ASM cells in contrast to the ASM proliferation observed at lower levels of oxygen exposure (6). This is consistent with the inhibition of apoptosis observed in lung cells of neonatal rats exposed to 60% compared with 95% oxygen (23). We therefore sought to test the hypothesis that modest hyperoxic exposure would cause a greater increase in airway reactivity compared with severe hyperoxia and that this response would be sustained beyond completion of hyperoxic exposure. These parameters for oxygen exposure match well with the increasingly adopted clinical protocols of moderate hyperoxia of limited duration for preterm infants.
EXPERIMENTAL METHODS
Time-pregnant mice (C57Bl/6J) were purchased from a commercial vendor and were later observed to give birth in our animal facility. All terminal experiments were performed on 21-day-old mice. All procedures were carried out in accordance with the National Institute of Health (NIH) guidelines for care and use of laboratory animals and were approved by the Animal Care and Use Committee at Case Western Reserve University.
Hyperoxia exposures.
Following the day of birth (P0), the dam and her pups were raised in either normoxia (control; Ctrl), 40% oxygen, or 70% oxygen until 7 days of age; temperature and humidity were monitored and maintained constant during a 12:12-h light-dark cycle. Hyperoxia was achieved by placing the mother and pups inside a Plexiglas chamber (30 × 50 × 28 cm) connected to adjustable rotameters for mixing air and oxygen to the designated level of hyperoxia. Oxygen concentration was monitored (TED 60T, Teledyne Analytical Instruments) and adjusted if necessary to maintain appropriate levels. Airflow through the chambers was maintained at ∼3 l/min. Carbon dioxide (CO2) levels in the airflow exiting the chambers were measured to ensure flow was adequate to prevent CO2 accumulation. Sustained hyperoxia exposure lasted 24 h/day for 7 consecutive days. Dams were cycled between litters exposed to room air and hyperoxia every 24 h to minimize acute oxygen toxicity and to ensure that the normoxic and hyperoxic dams received the same exposure. At the end of the seventh day of exposure, the pups were removed from the chamber and then raised for a further 14 days in normoxia. Cages, water, and food were replaced every 3 days. Normoxic mice received room air for the same time period.
Measurement of respiratory mechanics.
At 21 days of postnatal age the mice were similar in body weight (Ctrl, 11.1 ± 0.4 g; 40% oxygen, 11.3 ± 0.4 g; and 70% oxygen, 9.7 ± 0.8 g). The mice were anesthetized in preparation for measurements of airway responses to inhaled methacholine. Anesthesia was induced by an intraperitoneal injection of ketamine/xylazine (100 mg/kg of ketamine and 10 mg/kg of xylazine); depth of anesthesia was assessed by observing the lack of response to toe pinch and supplemental injections were given approximately every 30 min. Once adequately anesthetized, the mouse was placed supine on a heated stainless steel surgical table to maintain constant body temperature (∼37°C). The trachea was cannulated with an 18-gauge blunt needle. The cannula was inserted through a small ventral incision made in the rostral-most part of the trachea and advanced ∼3 mm caudal to the incision. The cannula was then held securely in place with suture tied around the trachea. The mouse was then mechanically ventilated in 50% oxygen (Teladyne O2 sensor; balance N2) at 150 breaths/min, 10 ml/kg, and 3 cmH2O positive end-expiratory pressure with a computer-controlled, small animal ventilator (flexiVent; SCIREQ). Before measurements of respiratory system resistance (Rrs) and compliance (Crs), the lungs were inflated three times to total capacity to standardize the volume history among animals. Freshly prepared methacholine in saline was delivered through an inline nebulizer in doses of 0, 3, 6.25, 12.5, 25, 50, 100, and 200 mg/ml for 10 s. The direct delivery of methacholine to the lung was timed with inspiration by using the automated software. After each methacholine dose was administered, Rrs and Crs were assessed approximately every 45 s. Four measurements were made at each dose of methacholine in control (n = 10), 40% oxygen (n = 9)-, and 70% oxygen (n = 8)-exposed pups. At the end of the experiment, the pups received a lethal intracardiac injection of urethane anesthesia. Additional control and oxygen-exposed mice were used in each of the following morphological and immunohistochemical analyses.
Immunofluorescence for ASM.
The trachea was cannulated and the lungs were inflation fixed (25 cmH2O) for 10 min with 10% neutral-buffered formalin. The left lung was removed, prepared for immunostaining, and postfixed for 2 days at 4°C. The fixed lobe was dehydrated in graded alcohol and embedded in paraffin. Paraffin-embedded sections were dewaxed in xylenes, rehydrated, and incubated with a monoclonal antibody against mouse anti-α-smooth muscle actin (1:400 dilution, Sigma-Aldrich) overnight at 4°C. Immune complexes were captured with FITC-conjugated donkey anti-mouse secondary antibodies (1:500, Alexa Fluor-488, Invitrogen). Appropriate negative controls were run by omitting the primary antibody to confirm nonspecific staining, which was not observed. Immunostained sections were coverslipped with Vectashield mounting medium (Vector Laboratories) and visualized with a fluorescence microscope. Images of the immunostained sections were captured with a Rolera XR CCD camera (Q-Imaging) mounted on a microscope (Leica Microsystems). Five random images at ×20 magnification were captured from six to seven animals per group by using digital image analysis software with settings for color and size identification (Image Pro Plus 7.0). The area of airways and the green fluorescent areas of α-smooth muscle actin from five airways per animal were measured. The amount of ASM area was expressed as a ratio to the total airway (AW) surface area.
Staining for collagen in ASM.
Masson's trichrome staining was performed in 5-μm thin sections from left lung as per the manufacturer's instructions (Masson's trichrome kit, Sigma-Aldrich, St. Louis, MO). In brief, the paraffin sections were dewaxed, rehydrated, stained, and destained to obtain ideal staining for collagen. The sections were dehydrated, dipped in xylene, and mounted in permanent mounting medium. Five airways from a given mouse were randomly selected and the surface area that was positively stained for collagen was averaged from controls (n = 8), 40% (n = 5), and 70% (n = 5)-exposed pups. For collagen measurement all images were taken at the same camera setting. By use of digital image analysis software, airways were outlined and intensity of blue color was measured by using a cutoff window between 155–255. The amount of collagen area was expressed as a ratio to the total airway area (collagen area/airway area).
Lung histology and morphometry.
Paraffin-embedded sections (5 μm) were prepared and stained with hematoxylin-eosin. Three randomly chosen hematoxylin-eosin areas were photographed with a ×20 objective by using a Rolera XR CCD camera (Q-Imaging) mounted on a microscope (Leica Microsystems). Three airways per animal were analyzed with research-based digital image analysis software (Image-pro Plus 7.0, Media Cybernetics) and a custom macro written for automated assessments of alveolar morphometry in five pups from each group. Air spaces within the image were selected by color segmentation, and incomplete air spaces were excluded from analysis. The macro was used to determine the number of complete air spaces in the image. The extent of alveolarization was determined by the radial alveolar count (RAC) method. Two to three airways per pup were analyzed for radial alveolar count, determined by counting the number of alveolar septa transected by a perpendicular line drawn from the terminal bronchiole to the nearest connective tissue septum.
Statistical analysis.
Statistical comparison of baseline mechanics and lung morphology (ASM area, radial alveolar count, collagen and alveolar numbers) between treatment groups were performed by a one-way ANOVA. Comparison of slopes to methacholine responses between groups were made by two-way, repeated-measures ANOVA. Differences were considered significant at P < 0.05. All values are expressed as means ± SE.
RESULTS
Baseline respiratory mechanics.
Baseline values for respiratory mechanics were measured at 3 wk of age in normoxia (n = 10), 40% oxygen (n = 9), and 70% oxygen (n = 8)-exposed pups. The sex distribution between treatment groups was as follows: Normoxia controls, M = 4, F = 6; 40% oxygen, M = 3, F = 6; 70% oxygen, M = 3, F = 5. Rrs in 40% and 70% oxygen was significantly higher than in controls (Ctrl, 1.27 ± 0.06; 40%, 1.48 ± 0.09; and 70%, 1.54 ± 0.06 cmH2O·ml−1·s−1). There was no difference in Rrs between 40% and 70%-exposed pups (Fig. 1A). Respiratory system compliance (Crs) in 40% and 70%-exposed pups also differed to controls (Ctrl, 0.0150 ± 0.0007; 40%, 0.0124 ± 0.0006; and 70%, 0.0130 ± 0.0010 ml/cmH2O). There was no difference in Crs between 40 vs. 70% oxygen-exposed pups (Fig. 1B).
Fig. 1.
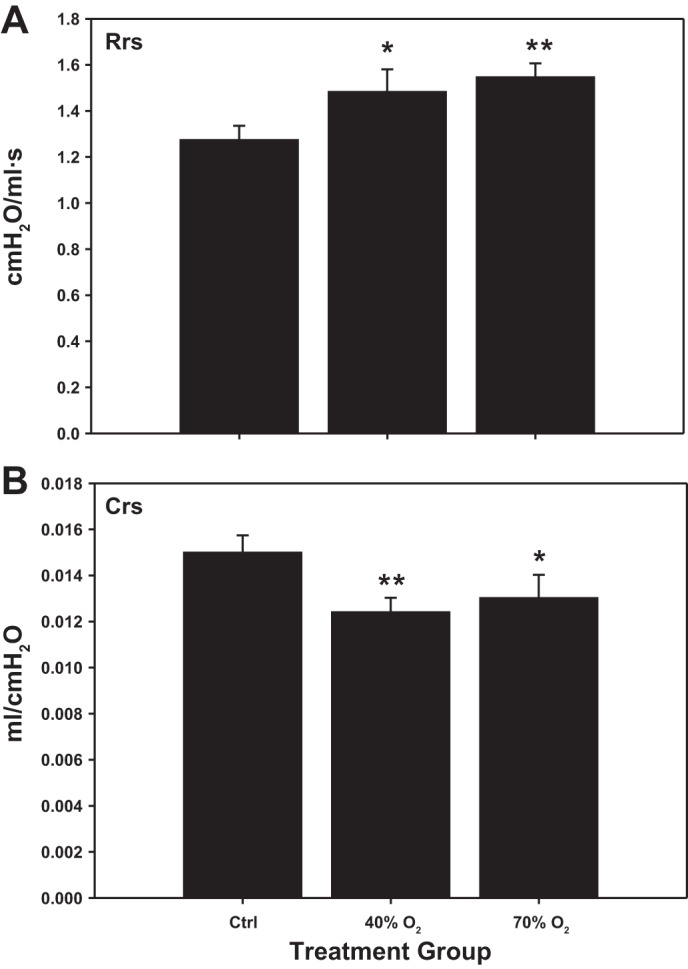
Baseline respiratory system resistance (Rrs; A) and compliance (Crs; B) in 3-wk-old mice exposed to neonatal hyperoxia [40% or 70% oxygen, from birth to postnatal day 7 (P7)] or normoxia (Ctrl). There were statistical differences in baseline Rrs and Crs between both oxygen-exposed groups and controls (*P < 0.05; **P < 0.02).
Dose response to methacholine challenge.
Three-week-old mice exposed to 40% oxygen demonstrated a statistically significant increase in the slope of Rrs and Crs in response to methacholine compared with controls. On the other hand, the response to methacholine in pups exposed to 70% oxygen did not significantly differ from control mice (Fig. 2, A and B).
Fig. 2.
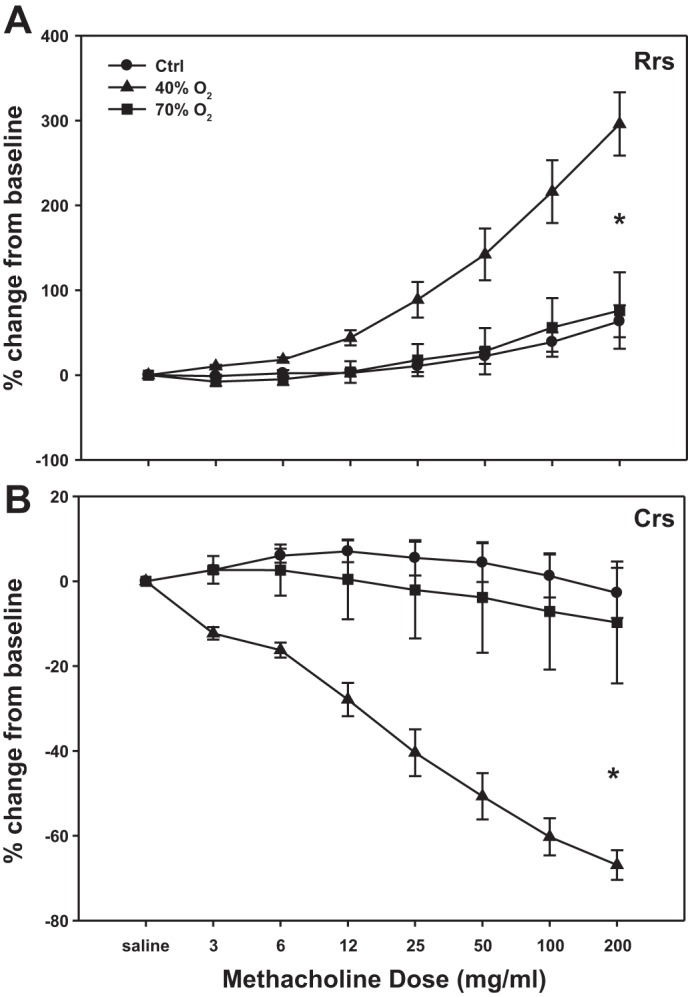
Dose-dependent effects of methacholine on Rrs (A) and Crs (B) in 3-wk-old mice exposed to neonatal hyperoxia (40% or 70% oxygen, from birth to P7) or normoxia (Ctrl). Slope of the responses of Rrs and Crs to methacholine in 40%-exposed mice were significantly different from Ctrl and 70% oxygen-exposed mice. *Slope is significantly difference from control, P < 0.001.
ASM morphology.
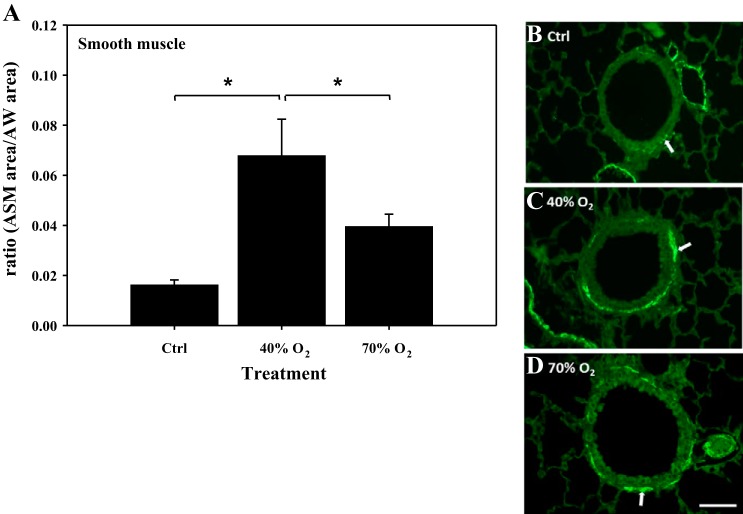
The amount of ASM was quantified by determining the surface area of α-smooth muscle actin-positive staining in lung sections from each treatment group: Ctrl (n = 6), 40% oxygen (n = 6), and 70% oxygen (n = 7). Values are expressed as a ratio of ASM surface area/AW surface area. The amount of ASM was significantly increased in the 40% oxygen-exposed mice (0.068 ± 0.015) compared with Ctrl (0.016 ± 0.002) (Fig. 3A). In contrast, the amount of ASM in 70% oxygen-exposed mice (0.039 ± 0.005) was significantly less than in 40% oxygen mice but was not greater than control (Fig. 3A). Representative lung sections illustrating α-smooth muscle actin-positive immunohistochemistry are provided to highlight the presence of ASM from each treatment group (Fig. 3, B–D).
Fig. 3.
Immunohistochemical quantification of airway smooth muscle (ASM)/airway surface area (AW) in mice exposed to neonatal hyperoxia. Note: amount of smooth muscle area expressed as a ratio to the total airway surface area (ASM/AW area) was increased in 40% oxygen-, but not in 70% oxygen-exposed mice compared with control (A). Representative sections illustrate immunostained ASM from each treatment group (B–D). ASM is identified by the green color highlighted by white arrows (Bar = 50 μm). *Significant difference, P < 0.05.
Airway collagen and alveolar morphology.
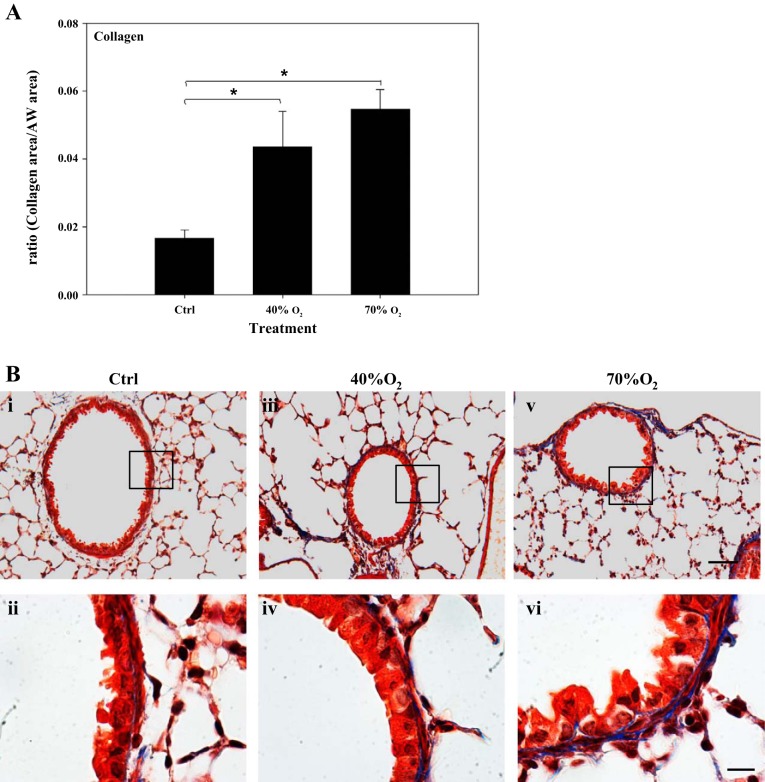
The amount of airway collagen was quantified by determining the surface area of trichrome-positive staining in lung sections from each treatment group: Ctrl (n = 8), 40% oxygen (n = 5), and 70% oxygen (n = 5). Collagen levels were increased in 40% oxygen (0.044 ± 0.011)- and 70% oxygen (0.055 ± 0.006)-exposed mice compared with Ctrl (0.017 ± 0.003) (Fig. 4A). The amount of collagen in 40% oxygen-exposed mice was not significantly different from that following 70% oxygen exposure. Representative lung sections illustrating trichrome staining to highlight presence of airway collagen from each treatment group are also shown under different magnification (Fig. 4B, i–vi).
Fig. 4.
Quantification of airway (AW) collagen in mice exposed to neonatal hyperoxia. Note: collagen was increased (A) in the lungs of 40% oxygen- (iii and iv) and 70% oxygen-exposed mice (v and vi) compared with control (i and ii) mice. Representative sections illustrate collagen staining in lungs from each treatment group (B). Sections are trichrome stained for collagen (blue) and noncollagen tissue (red). Bar = 20 μm in top panels and 100 μm in bottom panels; *significant difference, P < 0.05.
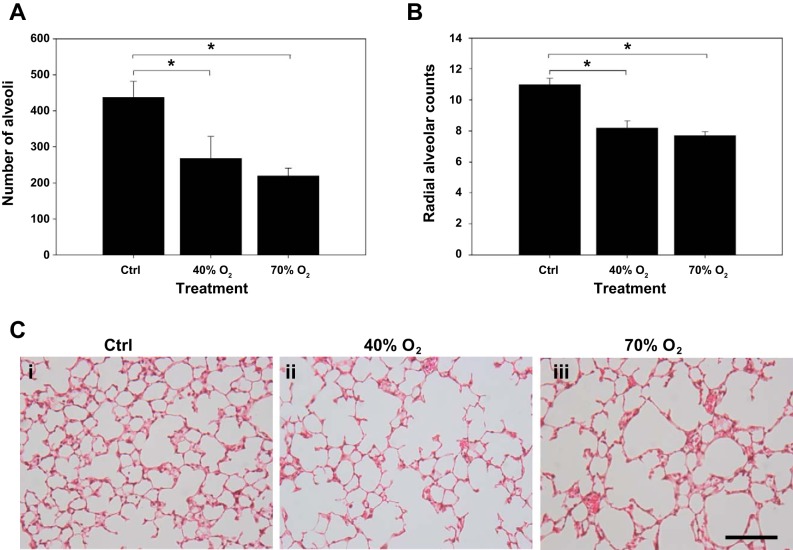
The number of alveoli was quantified following hematoxylin-eosin staining of lung sections from each treatment group (n = 5 animals from each group). The number of alveoli was decreased in both 40% oxygen (266.6 ± 61.3/μm2)- and 70% oxygen (219.2 ± 22.3/μm2)-exposed mice compared with Ctrl (435.9 ± 45.8/μm2, Fig. 5A). Consistent with these data, there was a significant decrease in radial alveolar count in both hyperoxia-exposed groups compared with controls (Ctrl, 10.9 ± 0.4; 40%, 8.1 ± 0.5; and 70%, 7.7 ± 0.3 alveoli; P < 0.01). Radial alveolar counts did not differ between 40% and 70%-exposed pups (Fig. 5B). The number of alveoli in 40% oxygen-exposed mice was not significantly different to 70%-exposed pups. Representative lung sections demonstrate lung parenchyma and alveolar morphology for each treatment group (Fig. 5C, i–iii).
Fig. 5.
Quantification of numbers of alveoli and radial alveolar counts (RAC) in control and hyperoxia-exposed mice. Note: the numbers of alveoli (A) and RAC were (B) decreased in 40% oxygen and 70% oxygen-exposed mice compared with control mice. There was no difference in the number of alveoli and RAC between the lungs of 40% and 70% oxygen-exposed mice. Representative sections (C) of paraffin-embedded lungs from each treatment group stained with hematoxylin and eosin are also shown (i, ii, and iii represent control, 40%, and 70% oxygen, respectively; bar = 20 μm). *Significant difference, P < 0.05.
DISCUSSION
This study was driven by the consistent observation that increased airway reactivity is a prominent longer term consequence of preterm birth, especially as a sequelae of neonatal lung injury (8, 9). We deliberately chose to study our model at 3 wk postnatal age due to the high incidence of childhood wheezing disorders in former preterm infants. Recent anatomic data indicate that neonatal hyperoxic exposure results in enhanced bronchiolar smooth muscle in adult mice, consistent with our present data (16). To our knowledge this is the first study in which a neonatal rodent model was exposed to modest hyperoxia and airway reactivity was assessed beyond the period of hyperoxic exposure. Our major finding is that methacholine-induced increase in airway reactivity was only clearly observed after exposure to 40% oxygen compared with either normoxia or 70% exposure. This was associated with a significant increase in ASM after 40% oxygen exposure, in contrast to 70%. These findings may have implications for the sustained increase in airway reactivity observed in preterm infants, many of whom may have only modest and/or transient hyperoxic exposure.
In response to either 40% or 70% hyperoxia, there was an increase in baseline respiratory Rrs and decrease baseline Crs compared with normoxia-exposed mice. A prior study in which mouse pups were exposed to 85% oxygen for 14 days demonstrated an increase in resistance and decrease in compliance at completion of hyperoxic exposure, but these changes were absent after a subsequent 14-day recovery in room air (19). In another recent study newborn mice were exposed to 40–100% oxygen for 4 days, followed by recovery in room air until 8 wk of age (22). Exposure to less than 60% oxygen did not significantly affect lung function and this differs from our own findings. Our data do demonstrate a modest but significant change in baseline respiratory function after recovery from hyperoxic exposure. The discrepancies in these findings may be related to duration (4 vs. 7 days) of exposure or time of study after recovery from hyperoxia. Neither of the prior studies measured the effect of a bronchoconstrictor challenge on airway function.
There are limited available data on the longer lasting effects of hyperoxic exposure on airway structure and function in different neonatal animal models. They have demonstrated both recovery of hyperoxia-induced airway remodeling 2 wk after hyperoxia exposure in rat pups and persistence of airway hyperreactivity 9 days after cessation of hyperoxic exposure in neonatal guinea pigs (7, 17). Both studies employed exposure to high supplemental oxygen (>95% and 70%, respectively) and neither study used neonatal mice, confounding comparison to our own data. We have demonstrated a profound increase in respiratory system resistance that was manifest after 2-wk recovery in room air.
The greater increase in airway reactivity with modest hyperoxia (vs. normoxia or severe hyperoxia) may appear counterintuitive, and therefore we sought to explore potential underlying mechanisms that reflect functional or structural change in the airway after modest hyperoxia exposure. Newtonian resistance (Rn) was not different between groups at any dose of methacholine (data not shown), suggesting an effect of hyperoxia on smaller airway structures that probably do not contribute to Rn measures. Although we cannot exclude changes in ASM function, or its innervation, we have observed a selective increase in the amount of ASM associated with modest hyperoxia. This was documented by quantification of α-smooth muscle actin protein and confirmed by immunofluorescence. Although these changes in α-smooth muscle actin are suggestive of increased ASM mass, they do not provide direct evidence for increased contractility of that mass per se. Accordingly, future studies will need to explore other proteins including calcium regulatory proteins and myosin light chains to determine the functional effect of increased ASM mass. Although we have not focused on changes in airway epithelium in this study, it is apparent that it was affected by oxygen exposure and therefore it is conceivable that a potential increase in epithelial thickness after 70% oxygen exposure may impair methacholine delivery to ASM and attenuate a contractile response. Since body weights between treatment groups were not significantly different, this is unlikely to account for the observed effects of hyperoxia on ASM. We do not anticipate that the differences in methacholine responses would be explained by sex differences since there was not a predominance of male pups in the 40% oxygen-exposed group. However, we acknowledge that sex differences should be an important contributing factor to airway reactivity, as shown previously (12).
Prior studies have reported on the role of 95–100% hyperoxic exposure in promoting apoptosis in the mesenchyme of the developing lung (4). In contrast, more recent data have proposed that 60% oxygen exposure for 14 days may inhibit apoptosis and contribute to parenchymal thickening in neonatal rat lungs (23). In a prior study we sought to determine the role of various oxygen concentrations in inducing proliferation of human fetal ASM cells under in vitro conditions (6). We documented enhanced proliferation at <60% oxygen exposure but increased apoptosis at >60% was accompanied by corresponding changes in proliferative vs. apoptotic markers (6). These prior data lead us to speculate that the enhanced airway reactivity observed in our neonatal mouse model at 40% vs. 70% oxygen may result from a shift in the balance from enhanced ASM proliferation toward greater apoptosis with excessive oxygen exposure.
Over the last 15 years hyperoxia-exposed neonatal mice have been increasingly employed to characterize acute and longer term neonatal lung injury (15, 21, 22). Although the etiology of BPD is clearly multifactorial, this model does exhibit many features of BPD exhibited by human infants (18). We therefore sought to confirm that our recovering oxygen-exposed model does exhibit some of these features. Both 40% and 70%-exposed pups exhibited decreased alveolar numbers compared with normoxia controls, and histological evidence for enlarged air spaces was confirmed by the decreased radial alveolar counts after both 40% and 70% oxygen exposure. These findings support the concept that different mechanisms may contribute to the longer-lasting airway reactivity, which is enhanced by modest hyperoxic exposure, and to the process of alveolar simplification, which appears to be aggravated with greater hyperoxic exposure. Interestingly, a recent study of school-age former preterm infants with BPD has demonstrated recovery of alveolarization, but persistent airway obstruction (13).
We also sought to characterize the effects of hyperoxic exposures on airway-related collagen content. We anticipated an increase in collagen based on our prior data that a 7-day exposure of rat pups to 50% oxygen increased arginase 1 expression in airway epithelium compared with normoxic controls (1). This would be expected to decrease the bioavailability of l-arginase for NO-cGMP signaling and airway relaxation and, at the same time, increase downstream collagen production. We have now demonstrated a significant increase in airway related collagen content after hyperoxic recovery. This was comparable after both 40% and 70% oxygen exposure and consistent with the decrease in Crs observed in both oxygen-exposed groups. One might speculate that greater airway related collagen content after 70% vs. 40% oxygen exposure might restrict airway contractility after more severe hyperoxic exposure and explain our findings of greater airway reactivity after modest hyperoxic exposure. However, it is important to note that the increase in collagen content did not differ significantly between the two hyperoxic groups, and therefore this explanation of a differential effect of oxygen exposure on airway reactivity is unlikely. Nonetheless, these data point to a novel mechanism by which hyperoxia may alter airway structure. It is also possible that hyperoxia-induced upregulation of arginase enhances ASM proliferation and hyperresponsiveness via an increase in ornithine-derived polyamines as reported in a mature mouse model of asthma (14).
In summary, we have documented enhanced airway reactivity in neonatal mice that have recovered from modest hyperoxic exposure. We speculate that the attenuated airway reactivity after recovery from more severe hyperoxic exposure may be the result of increased ASM apoptosis as concentration of oxygen increases. These findings contrast to the increase in airway-related collagen content and decrease in alveolar number, which appeared comparable after both 40% and 70% oxygen exposure, suggesting a unique and selective response of immature ASM to such exposures. We speculate that these findings will contribute to our greater understanding of the later predisposition of both early and moderately preterm infants to airway hyperreactivity (20).
GRANTS
This study was supported by the National Heart, Lung and Blood Institute, Grant R01HL056470 (R. J. Martin and Y. S. Prakash) and R01HL088029 (Y. S. Prakash), grant NSFC81070521 (H. Wang), and the Julia Yu Chao Memorial Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.W., R.J.M., Y.S.P., and P.M.M. conception and design of research; H.W., A.J., J.N., and P.M.M. performed experiments; H.W., A.J., C.F., Y.S.P., and P.M.M. analyzed data; H.W., R.J.M., C.F., Y.S.P., and P.M.M. interpreted results of experiments; H.W., A.J., R.J.M., C.F., and P.M.M. prepared figures; H.W., A.J., R.J.M., J.N., C.F., Y.S.P., and P.M.M. edited and revised manuscript; H.W., A.J., R.J.M., J.N., C.F., Y.S.P., and P.M.M. approved final version of manuscript; R.J.M. and P.M.M. drafted manuscript.
ACKNOWLEDGMENTS
Sincere appreciation is extended to Catherine Mayer (Case) and Michael Thompson (Mayo) for excellent technical assistance throughout this study.
Present address for H. Wang: West China Second University Hospital Sichuan, Chengdu, Sichuan, China.
REFERENCES
- 1.Ali NK, Jafri A, Sopi RB, Prakash YS, Martin RJ, Zaidi SI. Role of arginase in impairing relaxation of lung parenchyma of hyperoxia-exposed neonatal rats. Neonatology 101: 106–115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belik J, Jankov RP, Pan J, Tanswell AK. Chronic O2 exposure enhances vascular and airway smooth muscle contraction in the newborn but not adult rat. J Appl Physiol 94: 2303–2312, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Denis D, Fayon MJ, Berger P, Molimard M, de Lara MT, Roux E, Marthan R. Prolonged moderate hyperoxia induces hyperresponsiveness and airway inflammation in newborn rats. Pediatr Res 50: 515–519, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Dieperink HI, Blackwell TS, Prince LS. Hyperoxia and apoptosis in developing mouse lung mesenchyme. Pediatr Res 59: 185–190, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, Thomas S, Stocks J. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med 182: 237–245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartman WR, Smelter DF, Sathish V, Karass M, Kim S, Aravamudan B, Thompson MA, Amrani Y, Pandya HC, Martin RJ, Prakash YS, Pabelick CM. Oxygenxy dose responsiveness of human fetal airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 303: L711–L719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hershenson MB, Abe MK, Kelleher MD, Naureckas ET, Garland A, Zimmermann A, Rubinstein VJ, Solway J. Recovery of airway structure and function after hyperoxic exposure in immature rats. Am J Respir Crit Care Med 149: 1663–1669, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, Jaakkola MS. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol 118: 823–830, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Joshi S, Powell T, Watkins WJ, Drayton M, Williams EM, Kotecha S. Exercise-induced bronchoconstriction in school-aged children who had chronic lung disease in infancy. J Pediatr 162: 813–818.e1, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Laughon M, Allred EN, Bose C, O'Shea TM, Van Marter LJ, Ehrenkranz RA, Leviton A. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics 123: 1124–1131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin RJ, Prakash YS, Hibbs AM. Why do former preterm infants wheeze? J Pediatr 162: 443–444, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenzie R, Burton MD, Royze SG, Tang ML. Age and sex influences on airway hyperresponsiveness. J Asthma 47: 651–654, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Narayanan M, Beardsmore CS, Owers-Bradley J, Dogaru CM, Mada M, Ball I, Garipov RR, Kuehni CE, Spycher BD, Silverman M. Catch-up alveolarization in ex-preterm children. Am J Respir Crit Care Med 187: 1104–1109, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North ML, Grasemann H, Khanna N, Inman MD, Gauvreau GM, Scott JA. Increased ornithine-derived polyamines cause airway hyperresponsiveness in a mouse model of asthma. Am J Respir Cell Mol Biol 48: 694–702, 2013 [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly MA, Marr SH, Yee M, McGrath-Morrow SA, Lawrence BP. Neonatal hyperoxia enhance the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med 177: 1103–1110, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Reilly M, Harding R, Sozo F. Altered small airways in aged mice following neonatal exposure to hyperoxic gas. Neonatology 105: 39–45, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Schulman SR, Canada AT, Fryer AD, Winsett DW, Costa DL. Airway hyperreactivity produced by short-term exposure to hyperoxia in neonatal guinea pigs. Am J Physiol Lung Cell Mol Physiol 272: L1211–L1216, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Sosenko IRS, Bancalari E. New developments in the presentation, pathogenesis, epidemiology and prevention of bronchopulmonary dysplasia. In: The Newborn Lung: Neonatology Questions and Controversies, edited by Bancalari E. Philadelphia, PA: Saunders, p. 187–207, 2010 [Google Scholar]
- 19.Velten M, Heyob KM, Rogers LK, Welty SE. Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. J Appl Physiol 108: 1347–1356, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrijlandt EJ, Kerstjens JM, Duiverman EJ, Bos AF, Reijneveld SA. Moderately preterm children have more respiratory problems during their first 5 years of life than children born full term. Am J Respir Crit Care Med 187: 1234–1240, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Warner BB, Stuart LA, Papes RA, Wispé JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol Lung Cell Mol Physiol 275: L110–L117, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Yee M, Chess PR, McGrath-Morrow SA, Wang Z, Gelein R, Zhou R, Dean DA, Notter RH, O'Reilly MA. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am J Physiol Lung Cell Mol Physiol 297: L641–L649, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi M, Masood A, Ziino A, Johnson BH, Belcastro R, Li J, Shek S, Kantores C, Jankov RP, Tanswell AK. Inhibition of apoptosis by 60% oxygen: a novel pathway contributing to lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol 300: L319–L329, 2011 [DOI] [PubMed] [Google Scholar]