Abstract
Idiopathic pulmonary fibrosis (IPF) is characterized by the relentless spread of fibroblasts from scarred alveoli into adjacent alveolar units, resulting in progressive hypoxia and death by asphyxiation. Although hypoxia is a prominent clinical feature of IPF, the role of hypoxia as a driver of the progressive fibrotic nature of the disease has not been explored. Here, we demonstrate that hypoxia robustly stimulates the proliferation of IPF fibroblasts. We found that miR-210 expression markedly increases in IPF fibroblasts in response to hypoxia and that knockdown of miR-210 decreases hypoxia-induced IPF fibroblast proliferation. Silencing hypoxia-inducible factor (HIF)-2α inhibits the hypoxia-mediated increase in miR-210 expression and blocks IPF fibroblast proliferation, indicating that HIF-2α is upstream of miR-210. We demonstrate that the miR-210 downstream target MNT is repressed in hypoxic IPF fibroblasts and that knockdown of miR-210 increases MNT expression. Overexpression of MNT inhibits hypoxia-induced IPF fibroblast proliferation. Together, these data indicate that hypoxia potently stimulates miR-210 expression via HIF-2α, and high miR-210 expression drives fibroblast proliferation by repressing the c-myc inhibitor, MNT. In situ analysis of IPF lung tissue demonstrates miR-210 expression in a similar distribution with HIF-2α and the hypoxic marker carbonic anhydrase-IX in cells within the IPF fibrotic reticulum. Our results raise the possibility that a pathological feed-forward loop exists in the IPF lung, in which hypoxia promotes IPF fibroblast proliferation via stimulation of miR-210 expression, which in turn worsens hypoxia.
Keywords: idiopathic pulmonary fibrosis, hypoxia, miR-210, fibroblast proliferation
idiopathic pulmonary fibrosis (IPF) is a prevalent and progressive fibrotic lung disease that does not respond to therapy (10, 37, 42, 47). It is characterized by unrelenting proliferation of fibroblasts with deposition of type I collagen within alveolar airspaces, resulting in scarred nonfunctional airspaces and hypoxia (1, 2, 18, 31, 32, 43, 48). This progressive fibrosis clinically correlates with worsening hypoxia, and increasing desaturation during exercise has been found to be a significant predictor of subsequent mortality (17). Inevitably, death occurs by asphyxiation.
Our prior studies indicate that the majority of the fibroblast population derived from IPF lung manifests a distinct pathological phenotype (51–53). Low oxygen tension has variable effects on cellular proliferation depending on cell type. While arresting alveolar epithelial cell proliferation, low oxygen tension has been shown to promote normal fibroblast proliferation, suggesting the possibility that hypoxia may promote IPF fibroblast proliferation (4, 15, 16, 28, 30). Although hypoxia is a prominent clinical feature of IPF, few mechanistic studies have explored the role of hypoxia as a driver of the progressive fibrotic nature of the disease. However, experimental evidence indicates that tissue hypoxia leads to fibrosis (21, 23, 34, 38, 40, 49), and hypoxic lung tissue is associated with lung fibrosis (46). A recent study found that lactic acid levels are high in IPF lung tissue. Lactic acid is generated by cells in response to hypoxia, supporting the concept that IPF fibroblasts may exist in a hypoxic microenvironment (29).
Mechanistically, hypoxia stimulates the proliferation of various cell types, including cancer cells via the hypoxia-inducible transcription factors HIF-1α and HIF-2α (together denoted HIF-α) (22). Increased HIF-α expression correlates with poor patient survival in cancer, and importantly HIF-2α has been found to be preferentially expressed in tumor cells and facilitates maintenance of cancer stem cell characteristics (22, 35, 45). HIF-α regulates the transcription of a variety of genes controlling the hypoxic response, including genes that control proliferation and growth (25). Recent studies indicate that hypoxia can also upregulate the expression of a specific set of microRNAs via HIF-α, which have been termed hypoxamirs. miR-210 is a unique hypoxamir in that it is expressed in a variety of cell types in response to hypoxia and it regulates many vital cell functions including proliferation (3, 6, 12, 13, 23, 40). Although miR-210 is thought to be regulated by HIF-1α via a hypoxia-responsive element, several studies suggest that miR-210 expression may also be regulated by HIF-2α (6, 25, 54). Interestingly, miR-210 has been found to be both a positive and negative regulator of cell proliferation (7). Because miR-210 modulates the expression of multiple genes controlling cell proliferation, the mechanism(s) by which miR-210 controls proliferation may vary depending on cell type as well as contextual cues. Importantly, miR-210 expression has been found to be increased in patients with rapidly progressive IPF, a group of patients who typically have severe hypoxia (44). Furthermore, a preliminary study found a significant correlation between miR-210 expression levels and severity of lung function in patients with idiopathic interstitial pneumonias (54).
We have discovered that hypoxia robustly stimulates the proliferation of IPF fibroblasts. The mechanism for the hypoxia-induced proproliferative response involves HIF-2α, which upregulates the expression of miR-210. Prior studies have found that miR-210 regulates cell proliferation by modulating the translational efficiency/stability of target mRNAs that control the cell cycle, including the c-myc inhibitor MNT (11, 55). Here, we demonstrate that increased miR-210 expression in IPF fibroblasts correlates with decreased MNT expression. Knockdown of miR-210 increases MNT and inhibits IPF fibroblast proliferation in response to hypoxia, whereas overexpression of MNT blocks hypoxia-induced IPF fibroblast proliferation. Our results indicate that hypoxia robustly promotes the proliferation of IPF fibroblasts via a HIF-2α/miR-210/MNT pathway and raise the possibility that a pathological feed-forward circuit exists in the IPF lung, in which hypoxia promotes fibroblast proliferation, which in turn worsens hypoxia.
MATERIALS AND METHODS
Study approval.
Deidentified patient samples were obtained under a waiver of informed consent, and all protocols were approved by the University of Minnesota Institutional Review Board.
Primary cell lines.
Seven primary mesenchymal cell lines were established from patients with IPF. All patients fulfilled the criteria for the diagnosis of IPF as established by the American Thoracic Society and European Respiratory Society (1). In all cases, the diagnosis of IPF was confirmed by microscopic analysis of lung tissue, which demonstrated the characteristic morphological findings of usual interstitial pneumonia. Cell lines were derived from lungs removed at the time of transplantation or death as previously described (8, 33). Patient controls were selected to be similar in age to patients with IPF with nonfibrotic lung disorders. Based on these criteria, six nonfibrotic primary control adult human lung fibroblast lines were established and utilized. These lines were established from lung tissue uninvolved by the primary disease process: adenocarcinoma (n = 4), bronchoalveolar carcinoma (n = 1), or inflammation (n = 1). Primary lung mesenchymal cell lines were generated by explant outgrowth culture and/or mechanical/chemical dispersion and maintained in high-glucose DMEM containing 10% FCS. Cells were used from passage 0 (the initial isolate before subcultivation) through passage 5.
Proliferation assay.
Equal numbers of IPF or control fibroblasts were seeded on tissue culture dishes and cultured in various amounts of oxygen (3% through 21%), as specified for up to 6 days. Cells were stained with Trypan blue, and live cell numbers were quantified using an automated cell counter (Countess; Invitrogen, Grand Island, NY). Alternatively, cell numbers were quantified using the water-soluble tetrazolium salt (WST-1) proliferation assay kit according to manufacturer's instructions (Millipore, Billerica, MA). Briefly, 2,000 cells were plated in each well of a 96-well plate in a final volume of 100 μl. The cells were then cultured in normoxia or hypoxic conditions for the specified time. WST-1 reagent (10 μl/well) was then added to each well and incubated (30 min; 37°C). Cell numbers were quantified by measuring absorbance at 440/600 nm.
Antibodies.
HIF-1α antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). HIF-2α antibodies were obtained from Novus Biologicals (Littleton, CO) and Abcam (Cambridge, MA). MNT antibodies were from Sigma-Aldrich (St. Louis, MO) and Novus Biologicals. Carbonic anhydrase-IX (CA-IX) rabbit polyclonal antibody was obtained from Abcam.
Western blot analysis.
IPF fibroblasts were isolated at the indicated times and lysed using cell lysis buffer containing 150 mM NaCl, 1 mM EGTA, 50 mM Tris, pH 7.4, 1% Triton X-100, 1% Nonidet P-40, 1% sodium deoxycholate, with protease inhibitors (complete protease inhibitor mixture tablets; Roche Applied Science, Indianapolis, IN). Western analysis was performed on the resulting lysates as previously described (51–53).
qRT-PCR.
Cell lysates were prepared by adding 1 ml Trizol (Invitrogen) to each dish to solubilize the sample and stored at −80°C. Total RNA was isolated by chloroform extraction, treated with DNase, and cleaned using an RNeasy kit (Qiagen, Valencia, CA). cDNA was reverse transcribed using TaqMan reverse transcriptase kit (Roche), and real-time PCR was performed using the Roche Light-Cycler with SYBR Green dye (Roche). The primer sequences were as follows: HIF-1α forward: 5′ ATCGCGGGGACCGATT 3′; HIF-1α reverse: 5′ TGTGGACTTGGGAGAGGACT 3′; HIF-2α forward: 5′ TTGATGTGGAAACGGATGAA 3′; HIF-2α reverse: 5′ GGAACCTGCTCTTGCTGTTC 3′. miScript PCR Control (RNU6) and miR-210 primers were purchased from Qiagen.
Lentiviral vectors, HIF-1α, HIF-2α, miR-210, and MNT knockdown, and/or overexpression.
siRNA for HIF-1α and HIF-2α and scrambled siRNA were purchased from Novus Biologicals. Lentiviral particles for shRNA knockdown of MNT were purchased from Santa Cruz Biotechnology. The plasmid-expressing MNT (CMV-MNTcmyc tag SV40-eGFPIRES puro) was obtained from System Biosciences (Mountain View, CA). Lentivirus plasmids expressing either hsa-miR210 (PMIRH210-PA1) or a hsa-miR210 antagomir (PMZIP210PA-1) were obtained from System Biosciences. HEK293T/c17 cells were purchased from American Type Culture Collection (Manassas, VA) and cultured in DMEM-10% FBS with antibiotics (penicillin 100 IU/ml, streptomycin 100 IU/ml, and amphotericin B 25 μg/ml). Third-generation lentivirus-packaging plasmids, pMDLg/RRE, and pRSV-REV were obtained from Addgene (Cambridge, MA). For generation of VSV-G-pseudotyped lentivirus, either pMIRH210 or pMZIP210 (9.3 μg) along with pMD2.G (2.9 μg), pMDLg/RRE (5.4 μg), and pRSV-REV (2.3 μg) were cotransfected into 10-cm plates of subconfluent HEK293T/c17 cells using Fugene 6 (60 μl). Cells were incubated (4 h, 37°C, 5% CO2) with the DNA complexes, and then medium was removed and replaced with 5 ml DMEM-10%FBS-antibiotic medium containing 2 mM caffeine. The transfected cells were incubated (24 h, 37°C, 5% CO2), and then the media was replaced with another 5 ml DMEM-10%FBS-antibiotic medium containing 2 mM caffeine. Viral supernatants were harvested 48 h posttransfection, filtered through a 0.45-μm-pore-size PVDF filter, and then aliquoted and frozen at −80°C. IPF fibroblasts were plated in 12-well dishes and incubated under hypoxic (3% O2) conditions overnight. Virus was added to cells in DMEM-10% FBS with polybrene (final concentration 8 μg/ml), and the plate was centrifuged (1,200 g; 25°C) for 1 h and then incubated for 16 h (37°C, 5% CO2). The virus was then removed, 2 ml fresh medium per well added, and incubation continued for a total of 48 h.
Immunohistochemistry and in situ hybridization.
Immunohistochemistry and in situ hybridization were performed on 4-μm serial-sectioned paraffin-embedded IPF lung tissue obtained at the time of lung transplantation. Control lung tissue specimens were obtained from patients undergoing lung nodule resection and represents uninvolved lung tissue distant from the lung nodule. A strict protocol for handling the lung tissue was followed and was identical for IPF and control patient tissue specimens. At the time of transplantation or resection, the lung tissue was immediately placed on ice and brought to the laboratory for processing. Upon arrival to the laboratory, the lung tissue was immediately placed in 10% formalin and fixed for 48 h. The total time from removal of the lung tissue to arrival in the laboratory for fixation was <10 min. Immunohistochemical analysis to detect hypoxic tissue regions was performed using antibodies to CA-IX and HIF-2α. Immunohistochemical analysis was also performed to analyze MNT immunoreactivity in the IPF fibrotic reticulum. In situ hybridization was performed to detect miR-210 expression using a double-digoxigenin (DIG)-labeled miRCURY locked nucleic acid detection probe system per the manufacturer's instructions (miRNA ISH Optimization Kit 2; Exiqon, Woburn, MA). The DIG-labeled probe was detected using alkaline phosphatase-conjugated anti-DIG and nitro-blue tetrazolium/BCIP substrate. For the ISH experiments, eosin was used as a counterstain. For the immunohistochemical experiments, the sections were deparaffinized in xylene, rehydrated through a graded Methanol series, quenched with 0.3% hydrogen peroxide in methanol to inhibit endogenous peroxidases, and immersed in a 98°C water bath for 30 min in citrate buffer (pH 6.0) for antigen retrieval. Sections were blocked with 5% goat or horse serum (depending on primary antibody) to block nonspecific binding of secondary antibodies. Endogenous avidin and biotin binding sites were blocked by sequential incubation for 15 min, each with an avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA), and incubated overnight (16 h, 4°C) with the primary monoclonal antibodies; CA-IX (1:1,000) (Abcam); HIF-2α (1:100) (Santa Cruz Biotechnology) or HIF-2α (1:1,000) (Abcam); and MNT (1:100) (Novus Biologicals). Sections were rinsed with PBS, placed in biotinylated goat anti-rabbit or horse anti-mouse secondary antibodies (30 min, RT); followed by RTU horseradish peroxidase-streptavidin complex (Vector Laboratories). Controls consisted of secondary antibody only. Specific antibody binding was detected by 3,3′ diaminobenzidine (Vector Laboratories), and RTU hematoxylin was used as a counterstain. Sections were viewed and analyzed using a Leica Leitz DMRB microscope, and images were produced using LASV4.3 software.
Statistical analysis.
Comparisons of data among experiments were performed with the unipolar unpaired or paired Student's t-test. All experiments were independently replicated a minimum of three times. Data are expressed as means ± SD. P < 0.05 was considered significant.
RESULTS
Hypoxia robustly promotes the proliferation of IPF fibroblasts.
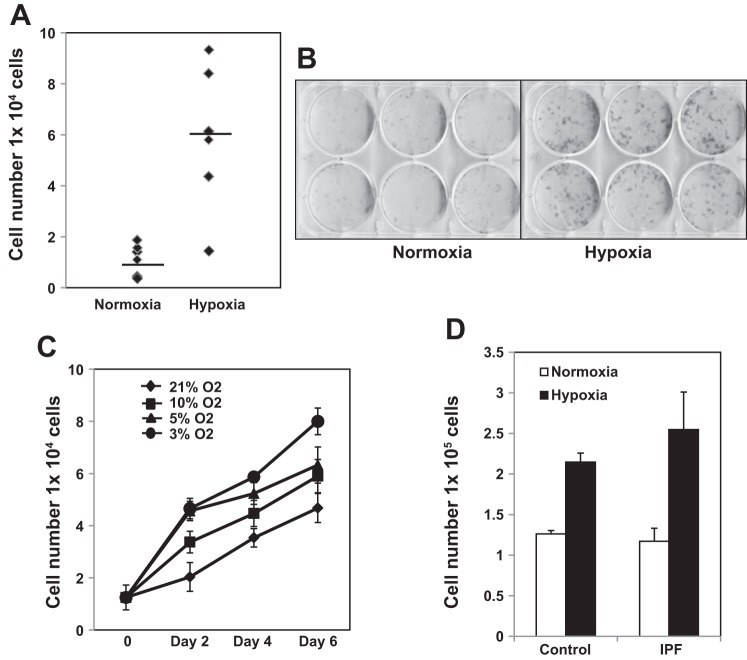
Hypoxia is a prominent clinical feature of IPF, and worsening hypoxia correlates with fibrotic disease progression (17). Although low oxygen tension has variable effects on cellular proliferation depending on cell type, studies from the 1960s and 1970s demonstrated that hypoxia promotes the proliferation of normal fibroblasts (4, 15, 16, 28, 30). Therefore, the objective of this study was to determine whether hypoxia also stimulates IPF fibroblast proliferation and, if so, by what mechanism. Primary human IPF fibroblasts were cultured in either a normoxic or a hypoxic (3% oxygen concentration) environment for 6 days. We found that hypoxia robustly stimulated IPF lung fibroblast proliferation (Fig. 1, A and B).
Fig. 1.
Hypoxia robustly promotes the proliferation of idiopathic pulmonary fibrosis (IPF) fibroblasts. A and B: IPF fibroblasts (n = 6 primary IPF fibroblast lines) were seeded on tissue culture dishes and exposed to normoxia (21% O2) or hypoxic (3% O2) conditions for 6 days. Cell numbers were quantified by automated cell counter (A). Alternatively, the cells were fixed and stained with crystal violet (B). Top and bottom rows represent 2 different primary IPF fibroblast lines. C: IPF fibroblast proliferation on tissue culture dishes was defined as a function of oxygen concentration (3, 5, 10, and 21% oxygen) and time (2, 4, and 6 days). Cell numbers were quantified by automated cell counter. D: IPF and control fibroblasts were seeded on tissue culture dishes and exposed to normoxic or hypoxic (3% O2) conditions for 6 days. Data shown are from 12 independent primary human lung fibroblast cell lines [IPF (n = 6), control (n = 6)]. Cell numbers were quantified by automated cell counter as a function of time.
To our knowledge, there have been no studies that have directly measured the partial pressure of O2 within IPF lung tissue (e.g., O2 concentration within the IPF fibrotic reticulum). Most data on measuring lung Po2 levels in IPF concern air oxygen levels or blood oxygen levels, not the Po2 inside the tissue. However, the level of oxygen in the normal lung parenchyma has been measured to be 14% (27). Interestingly, although oxygen concentrations can vary widely in tissues, at the cellular level physiological O2 concentrations are generally in the 1–11% range (6). With regard to pathological O2 concentrations, moderate tissue hypoxia has been defined as ∼2.5% or less (12, 20, 27). Oxygen concentrations in severely hypoxic tissue range from <0.1–0.5% (12). Because there is an absence of data concerning O2 concentrations in IPF lung tissue during the course of the disease process, we chose to examine the effect of a range of O2 concentrations on IPF fibroblast proliferation. We next performed experiments to define IPF fibroblast proliferation as a function of oxygen concentration (3, 5, 10, and 21% oxygen) and time (2, 4, and 6 days). There was an inverse correlation between oxygen concentration and fibroblast numbers. IPF fibroblast proliferation increased with decreasing oxygen concentration. IPF fibroblast proliferation increased 126%, 135%, and 171% compared with normoxia at 10%, 5%, and 3% oxygen concentration, respectively (Fig. 1C). This indicates that more severe decreases in oxygen concentration can more robustly stimulate IPF fibroblast proliferation. Fibroblast proliferation continued for the duration of the experiment (6 days) at each of the different oxygen concentrations.
Prior studies have shown that hypoxia stimulates normal fibroblasts to proliferate. Because we have found that hypoxia also stimulates IPF fibroblast proliferation, we next examined whether there were differences in the ability of IPF vs. control lung fibroblasts to proliferate in response to hypoxia. Primary IPF (n = 6 primary IPF fibroblast lines) and control (n = 6 primary control fibroblast lines) fibroblasts were cultured in 3% oxygen concentration for 2 days. Hypoxia increased the proliferation of IPF and control fibroblast proliferation by 2.2-fold and 1.7-fold, respectively (Fig. 1D). These data indicate that hypoxia is a potent stimulus driving both IPF and control fibroblast proliferation.
miR-210 expression is upregulated in IPF fibroblasts in response to hypoxia and knockdown to miR-210 decreases the hypoxia-induced proliferation of IPF fibroblasts.
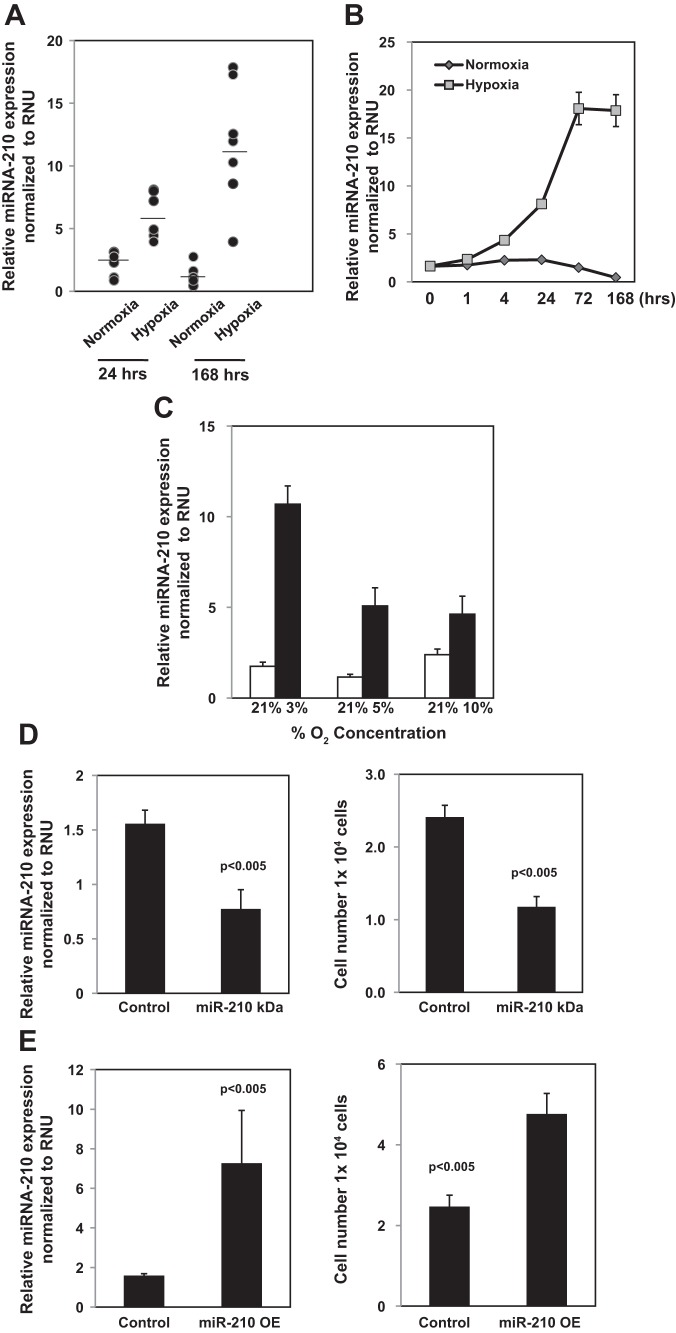
Recent studies have demonstrated that a specific set of microRNA molecules are upregulated by hypoxia and have been termed hypoxamirs. In particular, the expression of the hypoxamir, miR-210, is robustly upregulated by hypoxia (7). Studies indicate that miR-210 can either promote or inhibit cell proliferation depending on cell type and culture conditions (7). To begin to examine the role of miR-210 in regulating the proliferative response of IPF fibroblasts to hypoxia, we first examined the effect of exposure to normoxic or hypoxic conditions on miR-210 expression in IPF fibroblasts. After 24-h exposure to 3% oxygen, miR-210 levels were increased approximately fivefold compared with normoxia. By day 7, miR-210 levels were increased >10 fold compared with cells exposed to normoxic conditions (Fig. 2A). Of note, induction of miR-210 expression in response to hypoxia was slightly less robust in control fibroblasts, increasing ∼2.5-fold in response to exposure to 3% oxygen for 24 h (data not shown). We next analyzed the effect of culturing IPF fibroblasts on miR-210 expression as a function of time and oxygen concentration. After 4-h exposure to hypoxia (3% O2), miR-210 levels had increased roughly 2.5-fold. By 24 h, miR-210 expression had increased approximately fivefold, and, at 72 h, miR-210 levels were elevated >10-fold. By day 7, miR-210 levels had plateaued and were roughly equivalent to miR-210 levels at day 3 (Fig. 2B). Furthermore, miR-210 levels inversely correlated with the oxygen concentration; as the oxygen concentration decreased, miR-210 levels increased. miR-210 expression in IPF fibroblasts increased ∼6-fold, ∼4.4-fold, and 2-fold in IPF fibroblasts exposed to 3%, 5%, and 10% oxygen concentration for 24 h, respectively, compared with normoxia (Fig. 2C). Because 3% oxygen concentration robustly increased miR-210 levels and IPF fibroblast proliferation and this level of oxygen tension corresponds to moderate levels of tissue hypoxia, 3% oxygen concentration was used for the remainder of the experiments unless specified.
Fig. 2.
miR-210 expression is upregulated in IPF fibroblasts in response to hypoxia, and knockdown to miR-210 decreases the hypoxia-induced proliferation of IPF fibroblasts. A: IPF fibroblasts (n = 7 primary IPF fibroblast lines) were seeded on tissue culture dishes and cultured under normoxia or hypoxia (3% O2). miR-210 expression was quantified by qRT-PCR at day 1 and day 7. B: IPF fibroblasts were seeded on tissue culture dishes and cultured under normoxic or hypoxic (3% O2) conditions as a function of time. miR-210 expression was quantified by qRT-PCR. C: IPF fibroblasts were seeded on tissue culture dishes and cultured under normoxia or hypoxia (3%, 5%, and 10% O2) conditions for 24 h. miR-210 expression was quantified by qRT-PCR. D: miR-210 was knocked down (miR-210 KD) in IPF fibroblasts by infecting the cells with a lentiviral vector containing hsa-miR210 antagomir (pMZIP210). Cells infected with empty vector (pMZIP) served as control. The cells were seeded on tissue culture dishes and cultured under hypoxic conditions for 3 days. Left: miR-210 expression was quantified by qRT-PCR. Right: cell number was quantified by automated cell counter. E: miR-210 was overexpressed (miR-210 OE) in IPF fibroblasts by infecting the cells with a lentiviral vector expressing hsa-miR210 (PMIRH210-PA1). Controls consisted of IPF fibroblasts infected with empty vector. The cells were seeded on tissue culture dishes and cultured under normoxia conditions for 3 days. Left: miR-210 expression was quantified by qRT-PCR. Right: cell number was quantified by automated cell counter.
Because both miR-210 and IPF fibroblast proliferation robustly increase in response to hypoxia, we sought to determine the role of miR-210 in regulating this proproliferative response. Because both miR-210 expression and IPF fibroblast proliferation were robustly elevated at 72 h, we analyzed the effect of knockdown of miR-210 on the proliferation of IPF fibroblasts in response to hypoxia at the 72-h time point. The miR-210 antagomir knocked down miR-210 levels by 49% (Fig. 2D, left). Knockdown of miR-210 decreased the proliferative response of IPF fibroblasts to hypoxia by 48% (Fig. 2D, right). We also analyzed the effect of overexpression of miR-210 on IPF fibroblast proliferation exposed to normoxic conditions. Overexpression of miR-210 elevated miR-210 expression to levels comparable to that induced by hypoxia and stimulated IPF fibroblast proliferation by approximately twofold (Fig. 2E). These data indicate that miR-210 levels markedly increase in response to hypoxia and that silencing of miR-210 attenuates hypoxia-induced IPF fibroblast proliferation.
HIF-2α regulates miR-210 expression and miR-210-mediated proliferation of IPF fibroblasts in response to hypoxia.
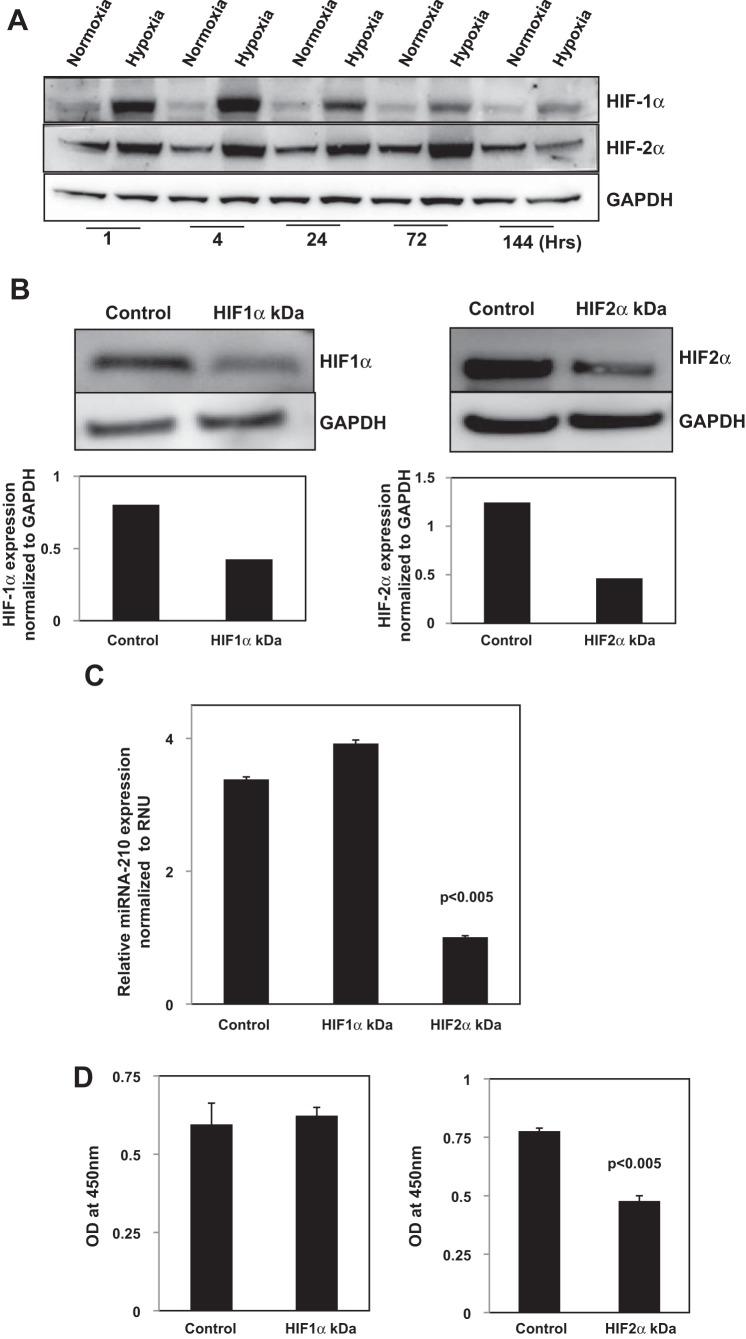
The expression of HIF-1α and HIF-2α transcription factors rapidly increases in response to low oxygen tension and regulates miR-210 expression (7, 22, 25, 35, 45). Under normoxic conditions, the family of HIF-α are degraded by the proteasome (22, 25). In response to hypoxia, HIF-α is not ubiquinated and HIF-α protein levels are stabilized. To begin to investigate the role of HIF-α in regulating the miR-210-mediated proliferative response of IPF fibroblasts to hypoxia, we examined the effect of culturing the cells under normoxic and hypoxic conditions on the expression of HIF-1α and HIF-2α at the protein level as a function of time. A 3% oxygen concentration stabilized both HIF-1α and HIF-2α protein levels in IPF fibroblasts, resulting in increased expression of HIF-1α and HIF-2α protein compared with normoxia (Fig. 3A). Interestingly, in response to hypoxia, HIF-1α protein levels were increased at 1 and 4 h but decreased progressively for the duration of the experiment up to day 6. In contrast, HIF-2α protein levels increased progressively over the first 3 days of exposure to hypoxia, closely mirroring the increase in miR-210 expression. By day 6, HIF-2α levels had returned to baseline levels. This indicates that there is differential expression of HIF-1α and HIF-2α protein levels in IPF fibroblasts in response to hypoxia, with HIF-2α demonstrating a more durable increase in protein expression.
Fig. 3.
Hypoxia-inducible factor (HIF)-2α regulates miR-210 expression and miR-210 mediated proliferation of IPF fibroblasts in response to hypoxia. A: IPF fibroblasts were seeded on tissue culture dishes and exposed to normoxic or hypoxic (3% O2) conditions as a function of time. HIF-1α and HIF-2α protein levels were quantified by Western analysis. B–D: HIF-1α and HIF-2α were knocked down in IPF fibroblasts using HIF-1α or HIF-2α siRNA. Scrambled siRNA was used as a control. The cells were seeded on tissue culture dishes and cultured under hypoxic conditions (3% O2) for 3 days in DMEM + 10% FCS. HIF-1α and HIF-2α protein levels were quantified by Western analysis to confirm knockdown (B). miR-210 expression was quantified by qRT-PCR (C). Cell numbers were quantified using the water-soluble tetrazolium salt proliferation assay (D).
Although several studies indicate that miR-210 expression is regulated by HIF-1α binding to a hypoxic response element in the miR-210 promoter, other studies have shown that HIF-2α can also regulate miR-210 expression (7, 41). We next analyzed the role of HIF-α in regulating miR-210 expression in IPF fibroblasts by knocking down HIF-1α and HIF-2α by siRNA and examining the effect on miR-210 expression in response to hypoxia. HIF-1α siRNA knocked down HIF-1α expression by 47% (Fig. 3B, left). Knockdown of HIF-2α siRNA decreased HIF-2α levels by 63% (Fig. 3B, right). HIF-1α knockdown had no significant effect on HIF-2α expression, and knockdown of HIF-2α did not significantly alter HIF-1α expression (data not shown). We found that knockdown of HIF-2α by siRNA decreased hypoxia-induced miR-210 expression in IPF fibroblasts by 70% (Fig. 3C). Knockdown of HIF-1α did not significantly change miR-210 expression in IPF fibroblasts in response to hypoxia. This indicates that HIF-2α regulates miR-210 expression in IPF fibroblasts exposed to hypoxia. Consistent with this, we found that knockdown of HIF-2α by HIF-2α siRNA decreased the proliferation of IPF fibroblasts in response to hypoxia by 39%, whereas knockdown of HIF-1α had no significant effect on the proliferation of IPF fibroblasts (Fig. 3D). These data indicate that HIF-2α regulates miR-210 expression and IPF fibroblast proliferation in response to hypoxia.
miR-210 promotes IPF fibroblast proliferation via repression of its downstream target MNT.
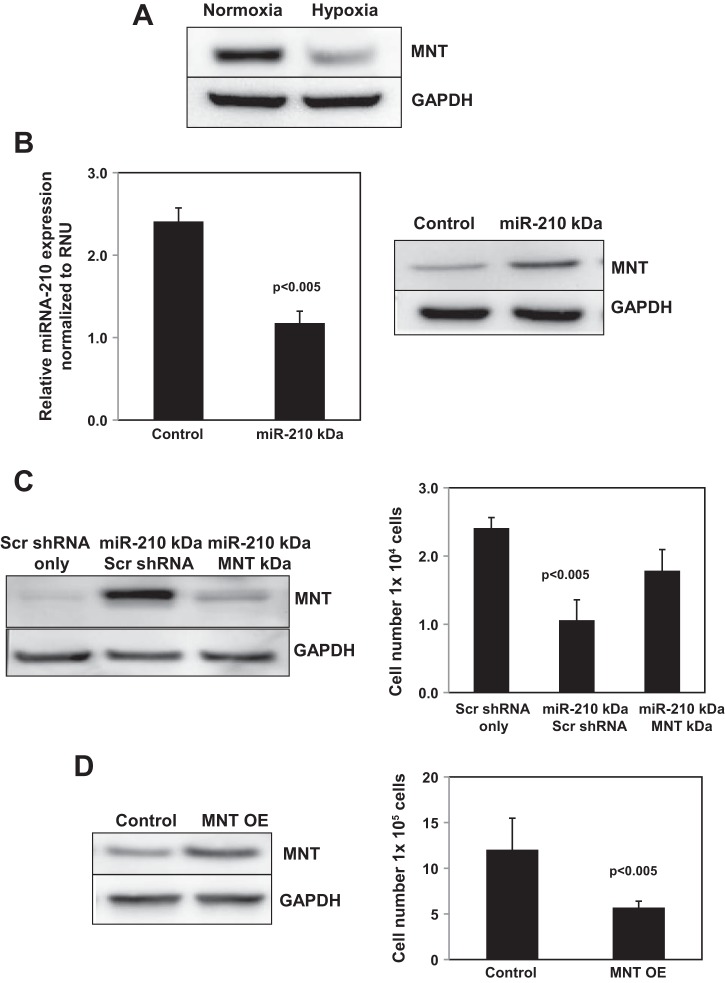
miR-210 regulates cell proliferation by modulating the translational efficiency/stability of target mRNAs that control the cell cycle, including MNT, a negative regulator of c-myc (7, 11, 13, 14, 55). The MNT 3′UTR contains multiple miR-210 binding sites, indicating that MNT is a bona fide miR-210 target (55). To begin to determine the role of MNT in regulating the miR-210-mediated hypoxic proliferative response of IPF fibroblasts, we first analyzed the effect of hypoxia on MNT expression in IPF fibroblasts. Exposure of IPF fibroblasts to hypoxia decreased MNT expression compared with normoxia (Fig. 4A). This suggested that miR-210 represses MNT expression in response to hypoxia. Therefore, we next examined the effect of knockdown of miR-210 on MNT expression in IPF fibroblasts cultured under hypoxic conditions. Knockdown of miR-210 increased MNT expression in hypoxic IPF fibroblasts (Fig. 4B). These data strongly suggest that miR-210 drives IPF fibroblast proliferation in response to hypoxia via repressing the c-myc inhibitor MNT. To examine this possibility, we knocked down MNT in IPF fibroblasts in which miR-210 had been silenced and examined the effect on IPF fibroblast proliferation. We found that, in IPF fibroblasts in which only miR-210 had been knocked down, MNT expression was increased (Fig. 4C, left, lane 2), and knockdown of miR-210 suppressed IPF fibroblast proliferation (Fig. 4C, right, middle bar). In contrast, knockdown of MNT decreased MNT expression in IPF fibroblasts in which miR-210 had been knocked down (Fig. 4C, left, lane 3) and partially restored their proliferation in response to hypoxia (Fig. 4C, right, right bar). To confirm the ability of MNT in suppressing IPF fibroblast proliferation, we overexpressed MNT and examined the effect on IPF fibroblast proliferation. We found that overexpression of MNT significantly increased MNT levels and decreased the IPF fibroblast hypoxic proliferative response (Fig. 4D). These data demonstrate that hypoxia stimulates IPF fibroblast proliferation via potently inducing miR-210 expression, which represses the c-myc inhibitor MNT.
Fig. 4.
miR-210 promotes IPF fibroblast proliferation via repression of its downstream target MNT. A: IPF fibroblasts were seeded on tissue culture dishes and exposed to normoxia or hypoxia (3% O2) for 3 days. MNT expression was quantified by Western analysis. B: miR-210 was knocked down in IPF fibroblasts by infecting the cells with a lentiviral vector containing hsa-miR210 antagomir (pMZIP210). Cells infected with empty vector (pMZIP) served as control. The cells were seeded on tissue culture dishes and exposed to hypoxic (3% O2) conditions for 3 days. MNT expression was quantified by Western analysis. C: IPF fibroblasts in which miR-210 had been knocked down were infected with a lentiviral vector containing MNT shRNAs to silence MNT (miR-210 KD/MNT KD). Controls consisted of IPF fibroblasts in which miR-210 had been knocked down and then treated with scrambled shRNA (miR-210 KD/Scr shRNA) and IPF fibroblasts treated with scrambled shRNA only (Scr shRNA only, no miR-210 knock down). Left: Western analysis was performed to confirm MNT knockdown. The cells were seeded on tissue culture dishes and exposed to hypoxic (3% O2) conditions for 3 days. Right: cell numbers were quantified by automated cell counter. D: MNT was overexpressed in IPF fibroblasts using a lentiviral vector containing a MNT construct. Control consisted of empty vector. The cells were seeded on tissue culture dishes and exposed to hypoxic (3% O2) conditions for 3 days. Cell numbers were quantified by automated cell counter.
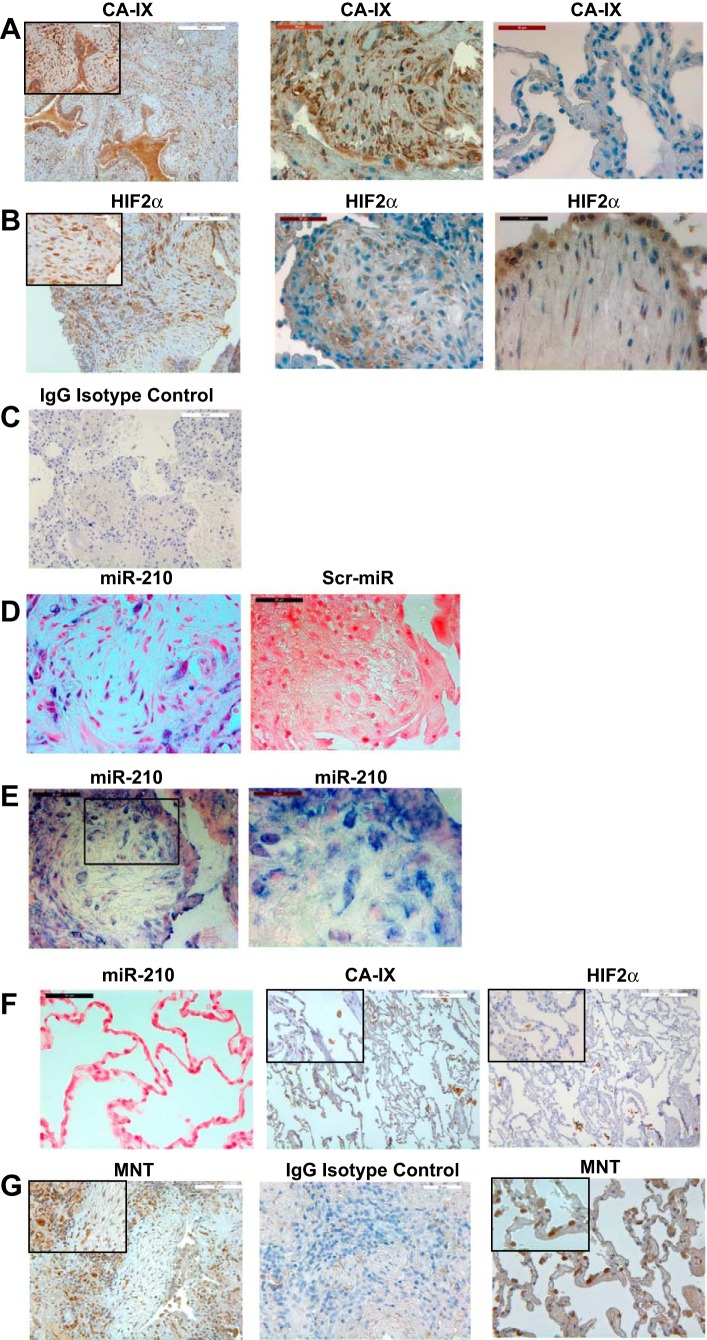
miR-210 is expressed in the fibrotic reticulum of IPF lung tissue in a similar distribution with the tissue hypoxia marker CA-IX and HIF-2α.
IPF disease progression clinically correlates with worsening hypoxia, and eventually patients die from asphyxiation. To determine whether cells within fibrotic regions of IPF lung tissue are exposed to hypoxic conditions, we performed immunohistochemistry on IPF lung tissue using antibodies to CA-IX and HIF-2α. CA-IX is a cell-intrinsic marker of hypoxia and has been used to detect regions of hypoxic tissue in pathological tissue specimens, including radiation-induced lung injury/fibrosis and lung cancer (36, 46, 50). We found strong CA-IX immunoreactivity in fibrotic regions of IPF lung tissue (Fig. 5A, left and middle). In contrast, weak CA-IX immunoreactivity was present in relatively normal-appearing alveolar structures in nonfibrotic regions of IPF lung tissue (Fig. 5A, right). Consistent with the staining pattern of CA-IX, HIF-2α immunoreactivity was also present in cells within fibrotic regions and in epithelial cells (Fig. 5B). As a control, very weak or no immunoreactivity was present in IPF lung tissue stained with isotype-matched IgG control antibody plus secondary antibody (Fig. 5C). Of note, macrophages in the airspaces of IPF lung tissue specimens were immunoreactive for both CA-IX and HIF-2α. When we analyzed IPF lung tissue for miR-210 expression by in situ hybridization, miR-210-expressing cells could be detected interspersed throughout the fibrotic reticulum (Fig. 5D, left). Immunoreactive cells were absent in IPF lung tissue stained with scrambled LNA-DIG probe (Fig. 5D, right). Analysis of IPF fibrotic tissue demonstrated that miR-210-expressing cells could be detected within IPF fibrotic foci. Shown is a fibroblastic focus containing relatively few cells embedded within loose connective tissue (Fig. 5E, left). The cells within the focus strongly expressed miR-210 (Fig. 5E, high-magnification image, right). Interestingly, in addition to the presence of miR-210-expressing cells within the IPF fibrotic reticulum, miR-210 expression was also detected in epithelial cells overlying fibrotic regions. In contrast, miR-210 expression was not detectable in normal-appearing alveolar structures in control lung tissue specimens (Fig. 5F, left). In addition, weak CA-IX and HIF-2α staining was present in normal alveolar structures in control lung tissue (Fig. 5F, middle and right, respectively) although macrophages in airspaces displayed immunoreactivity to CA-IX and HIF-2α. Our in vitro studies indicate that miR-210 represses MNT expression in response to hypoxia. Consistent with this, the majority of cells within IPF fibrotic foci displayed relatively weak MNT immunoreactivity (Fig. 5G, left), compared with cells in fibrotic regions adjacent to fibrotic foci, which displayed stronger MNT immunoreactivity. Furthermore, numerous cells with very strong MNT staining were present in anatomically normal alveolar structures in control lung tissue (Fig. 5G, right). Taken together, these data support the concept that fibroblasts within the IPF fibrotic reticulum express miR-210 as a consequence to exposure to hypoxic conditions.
Fig. 5.
miR-210 is expressed in the fibrotic reticulum of IPF lung tissue in a similar distribution with the hypoxia marker carbonic anhydrase IX (CA-IX) and HIF-2α. IPF and control human lung tissue specimens (n = 6 each) were analyzed for (CA-IX) and HIF-2α immunoreactivity by immunohistochemistry and miR-210 expression by in situ hybridization. Please note that the CA-IX, HIF-2α, and miR-210 images shown are representative of all 6 IPF patient tissue samples. A, left: representative low-power image of CA-IX staining pattern in IPF lung tissue. Inset: higher-power image showing CA-IX immunoreactive cells in the IPF fibrotic reticulum. Middle and right: representative images displaying CA-IX staining in fibrotic and nonfibrotic regions of IPF lung tissue from the same IPF patient tissue specimen. B, left: representative low-power image of HIF-2α immune reactive cells in the IPF fibrotic reticulum. Inset: higher-power image displaying cells with nuclear and cytoplasmic HIF-2α immunoreactivity. Middle: HIF-2α immune reactive cells in fibrotic regions of IPF lung tissue. Right: shown is a representative image of IPF lung tissue stained for HIF-2α demonstrating cells in the IPF fibrotic focus and overlying epithelium with a nuclear and cytoplasmic staining pattern. C: IPF (right) lung tissue stained with an IgG isotype-matched control antibody (control for CA-IX and HIF-2α antibodies) showed a lack of immunoreactivity. D: in situ hybridization of IPF lung tissue demonstrating the presence of miR-210 expressing cells in the IPF fibrotic reticulum (left). miR-210 is shown in blue (NBT/BCIP, nitro-blue tetrazolium), and the tissue was counterstained with eosin (red). Control: IPF lung tissue stained using a scrambled LNA-digoxigenin-labeled probe (right). E, left: shown is representative image of IPF lung tissue displaying miR-210 expression in cells within an IPF fibrotic focus. Right: high-magnification image of the boxed region in left displaying miR-210-expressing cells within the fibroblastic focus. Please note that the ISH images shown in D and E were obtained from 2 separate IPF patient tissue specimens. F: miR-210 expressing cells could not be detected in anatomically normal alveolar structures from a control lung tissue specimen (left). In addition, there was weak CA-IX and HIF-2α immunoreactivity in normal-appearing alveolar structures of control lung tissues (middle and right). Insets: high-power images of CA-IX and HIF-2α immunostaining of control lung tissue specimens. Of note, macrophages within airspaces displayed relatively strong immunoreactivity for both CA-IX and HIF-2α. The miR-210, CA-IX, HIF-2α, and miR-210 images shown are representative of all 6 control patient tissue samples. G: IPF and control human lung tissue specimens (n = 3 each) were analyzed for MNT immunoreactivity by immunohistochemistry. Left: shown are representative images of IPF lung tissue displaying weak MNT staining in cells within the IPF fibrotic focus. Inset: higher-power image showing weak MNT immunoreactive cells in the IPF fibrotic reticulum. Of note, relatively strong MNT immunoreactivity was detected in cells in fibrotic regions adjacent to the fibrotic foci. Middle: IPF lung tissue stained with IgG isotype-matched control antibody and secondary antibody. Right: strong MNT immunoreactivity was present in numerous cells in anatomically normal alveolar structures from control lung tissues. Inset: higher-power image showing strong MNT immunoreactive cells in anatomically normal alveolar structures in control lung tissue.
DISCUSSION
In IPF, fibrosis spreads from scarred alveolar units into adjacent alveolar structures, creating an expanding reticular network of fibrotic tissue that obliterates the gas-exchange surface, resulting in hypoxia (9). As the disease progresses, hypoxia worsens and inevitably death occurs by asphyxiation. Although hypoxia is a prominent clinical feature of IPF, the role of hypoxia as a driver of the progressive fibrotic nature of the disease has not been explored. Studies from the 1960s and 1970s demonstrated that hypoxia promotes the proliferation of normal fibroblasts (4, 15, 16). This suggested to us that hypoxia may not just be a consequence of progressive lung fibrosis but may drive IPF fibrotic disease progression. Therefore, the objective of this study was to determine whether hypoxia also stimulates IPF fibroblast proliferation and, if so, to examine the mechanism. Here, we report that hypoxia robustly promotes the proliferation of IPF fibroblasts. We demonstrate that the mechanism involves HIF-2α-mediated increase in miR-210 expression. Knockdown HIF-2α reduced miR-210 expression and largely abrogated the hypoxia-induced proliferation of IPF fibroblasts. Likewise, silencing miR-210 reduced the proliferative capacity of the IPF fibroblasts in response to hypoxia. Our data raise the possibility that, in the IPF lung, a pathological feed-forward loop exists in which hypoxia drives IPF fibroblast proliferation, which in turn worsens hypoxia.
There is considerable experimental evidence that tissue hypoxia leads to fibrosis (21, 23, 34, 38, 40, 49) and that hypoxic lung tissue is associated with lung fibrosis (46). Interestingly, a recent study found that lactic acid levels are high in IPF lung tissue. Lactic acid is generated by cells in response to hypoxia, further supporting the concept that cells comprising the fibrotic reticulum in IPF may exist in a hypoxic microenvironment (29). Our immunohistochemical analysis of IPF lung tissue using probes for hypoxic tissue provides evidence for tissue hypoxia in IPF. We demonstrate CA-IX and HIF-2α immunoreactivity within the IPF fibrotic reticulum. This supports the concept that cells within the IPF fibrotic reticulum are exposed to hypoxic conditions.
The hypoxia-inducible transcription factors are sensitive physiological sensors of oxygen tension. By regulating the expression of a variety of genes, they help cells adapt to a hypoxic environment. Mechanistically, hypoxia regulates the proliferation of various cell types, as well as pathological cancer cells via increasing HIF-1α and HIF-2α (reviewed in Refs. 22 and 25). Recent work has demonstrated that a specific set of microRNA molecules is upregulated by hypoxia via HIF-α and that miR-210 is the most consistently and robustly induced miR in response to hypoxia (7). miR-210 expression can be upregulated by either HIF-1α or HIF-2α depending on cell type (5, 19, 39). miR-210 is a direct transcriptional target of HIF-α, and five HIF-α binding sites have been identified upstream of the miR-210 transcriptional start site, indicating a key role for HIF-α in regulating miR-210 expression (19). We have found that miR-210 expression rises up to 10-fold in IPF fibroblasts in response to hypoxia. Somewhat surprisingly, our data indicate a role for HIF-2α but not HIF-1α in regulating this robust increase in miR-210 expression and hypoxia-induced proliferation of IPF fibroblasts. We have found a more durable increase in HIF-2α protein levels in IPF fibroblasts compared with HIF-1α. HIF-2α protein levels progressively increased over 72 h, whereas HIF-1α expression peaked within 24 h of hypoxia exposure. This more prolonged duration of HIF-2α protein expression paralleled the increase in miR-210, which, like HIF-2α, progressively increased over several days and peaked at 72 h. Importantly, knockdown of HIF-2α both decreased miR-210 levels and decreased the hypoxia-induced proliferative response of IPF fibroblasts, whereas knockdown of HIF-1α did not affect IPF fibroblast proliferation in response to hypoxia.
Consistent with our in vitro findings demonstrating a role for HIF-2α in regulating the hypoxia-mediated increase in miR-210 and IPF fibroblast proliferation, our in situ hybridization and immunohistochemistry analysis revealed miR-210 and HIF-2α immunoreactive cells within fibrotic foci of IPF lung tissue. HIF-1α immunoreactive cells were not observed within the fibrotic foci, but epithelial cells overlying the fibrotic foci were immunoreactive for HIF-1α. As discussed above, the presence of both CA-IX and HIF-2α immunoreactive cells in fibrotic regions suggests the presence of a hypoxic environment in the IPF fibrotic reticulum. Taken together, these data suggest that cells within the IPF fibrotic reticulum exist in a hypoxic environment and as a result express HIF-2α and miR-210, which stimulate IPF fibroblast proliferation, which in turn drives fibrotic progression. Interestingly, miR-210 expression and HIF-1α and HIF-2α staining were apparent in epithelial cells overlying the fibrotic foci. Although not the focus of this study, the presence of HIF-α and miR-210 expression in epithelial cells overlying the fibrotic foci suggests a possible role for miR-210 in regulating epithelial cell function in IPF.
miR-210 is unique in that it is expressed in a variety of cell and tissue types in response to hypoxia, and it regulates many vital cell functions including proliferation, thus conferring it with the title of “master hypoxamir” (3, 7, 24). miR-210 has been found to be both a positive and negative regulator of cell proliferation. Because miR-210 modulates the expression of multiple genes controlling cell proliferation, the mechanism(s) by which miR-210 controls cell proliferation is thought to vary depending on cell type as well as contextual cues. Our studies indicate that miR-210 is a positive regulator of IPF fibroblast proliferation in response to hypoxia. Knockdown of miR-210 decreases hypoxia-induced proliferation of IPF fibroblasts.
miR-210 silences gene expression by binding to the 3′UTR of target transcripts inhibiting their translation or degrading their target mRNA. miR-210 regulates cell proliferation by modulating the translational efficiency/stability of target mRNAs that control the cell cycle (7). Gain-of-function studies demonstrate that miR-210 can override hypoxia-induced cell cycle arrest by decreasing the proportion of cells in G1 and increasing the fraction of cells in S phase of the cell cycle. Prior work indicates that the mechanism involves the ability of miR-210 to target a variety of mRNAs of proteins that regulate the cell cycle, including MNT, a negative regulator of c-myc (55). The MNT 3′UTR contains multiple miR-210 binding sites, indicating that MNT is a bona fide miR-210 target (55). MNT is a transcription factor that functions as a transcriptional repressor of MAX. MAX forms complexes with c-myc, and MAX/Myc complexes enhance c-myc activity. By repressing MAX expression, MNT antagonizes c-myc activity. Knockdown of MNT has been shown to phenocopy miR-210 gain of function by promoting cell cycle progression under hypoxic conditions. Our work demonstrates that MNT expression is suppressed in IPF fibroblasts exposed to hypoxic conditions. We have found that knockdown of miR-210 decreases IPF fibroblast proliferation in response to hypoxia, and this is associated with increases in MNT expression. Importantly, we found that knockdown of MNT in IPF fibroblasts in which miR-210 had been knocked down partially restored the proliferative response to hypoxia. In addition, we found that overexpression of MNT attenuates hypoxia-induced IPF fibroblast proliferation. This indicates that the ability of miR-210 to increase IPF fibroblast proliferation involves repression of MNT. However, we recognize that the mechanism by which miR-210 stimulates IPF fibroblast proliferation is likely complex, and additional work will need to be performed to comprehensively identify miR-210 targets in IPF fibroblasts.
Worsening hypoxia is a prominent clinical feature of IPF progression. Our data support the concept that a pathological feed-forward circuit is operational in the IPF lung, in which hypoxia drives fibrotic progression by stimulating miR-210 expression and promoting fibroblast proliferation, which in turn worsens hypoxia. We suggest that interdicting this feed-forward loop could provide a means to halt the relentless fibroproliferative process. Our data indicate that this hypoxia-mediated feed-forward circuit operates through a HIF-2α/miR-210/MNT pathway. In support of this concept, a preliminary report from Schwartz and colleagues (54) found that miR-210 was the most significantly associated miRNA with severity of lung function in patients with idiopathic interstitial pneumonias. Our work can be immediately translated into a clinical trial of oxygen early in the course of IPF to determine whether treatment of hypoxia would slow IPF disease progression. In addition, it would support development of pharmacological treatments targeting the hypoxia-mediated miR-210 proliferation pathway as a new therapeutic strategy for slowing the progression of IPF.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) grants (R01 HL074882 and P01 HL91775) to C. Henke, and R01 HL089249 to P. Bitterman.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: V.B., P.B.B., and C.A.H. conception and design of research; V.B., P.H., K.S., J.H., M.P., and J.K. performed experiments; V.B., W.K., J.K., P.B.B., and C.A.H. analyzed data; V.B., H.X., W.K., P.B.B., and C.A.H. interpreted results of experiments; V.B., H.X., and C.A.H. prepared figures; V.B., P.B.B., and C.A.H. drafted manuscript; V.B., P.B.B., and C.A.H. edited and revised manuscript; V.B., P.B.B., and C.A.H. approved final version of manuscript.
REFERENCES
- 1.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 161: 646–664, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Basset F, Ferrans VJ, Soler P, Takemura T, Fukuda Y, Crystal RG. Intraluminal fibrosis in interstitial lung disorders. Am J Pathol 122: 443–461, 1986 [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas S, Roy S, Banerjee J, Hussain S, Khanna S, Meenakshisundarm G, Kuppusamy P, Friedman A, Sen CK. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc Natl Acad Sci USA 107: 6976–6981, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley TR, Hodgson GS, Rosendaal M. The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J Cell Physiol 97: 517–522, 1978 [DOI] [PubMed] [Google Scholar]
- 5.Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Raqoussis J. has-miR-210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res 14: 1340–1348, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15: 1239–1253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan SY, Loscalzo J. MicroRNA210. A unique and pleiotropic hypoxamir. Cell Cycle 9: 1072–1083, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen B, Polunovsky V, White J, Blazar B, Nakhleh R, Jessurun J, Peterson M, Bitterman P. Mesenchymal cells isolated after acute lung injury manifest an enhanced proliferative phenotype. J Clin Invest 90: 1778–1785, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cool CD, Groshong SD, Rai PR, Henson PM, Stewart JS, Brown KK. Fibroblast foci are not discrete sites of lung injury or repair: the fibroblast reticulum. Am J Respir Crit Care Med 174: 654–658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 150: 967–972, 1994 [DOI] [PubMed] [Google Scholar]
- 11.DeGregori LG, Yan Z, Jakoi L, Ishida S, Williams RS, Nevins JR. E2F3 activity is regulated during the cell cycle and is required for the induction of S phase. Genes Dev 12: 2120–2130, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans SM, Koch CJ. Prognostic significance of tumor oxygenation in humans. Cancer Lett 195: 1–16, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Fasanaro P, Alessandra YD, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand ephrin-A3. J Biol Chem 283: 15878–15883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fasanaro P, Greco S, Lorenzi M, Pescatori M, Brioschi M, Kulshreshtha R, Banfi C, Stubbs A, Calin GA, Ivan M, Capogrossi MC, Martelli F. An integrated approach for experimental target identification of hypoxia-induced miR-210. J Biol Chem 284: 35134–35143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferencz N, Nardone RM. A non-continuous flow gas chamber for the cultivation of mammalian cells grown in petri dishes. Exp Cell Res 53: 139–144, 1968 [DOI] [PubMed] [Google Scholar]
- 16.Fisher AR. Some effects of different oxygen tensions of oxygen on the respiration and growth of L-strain fibroblasts. Nature 186: 315–216, 1960 [DOI] [PubMed] [Google Scholar]
- 17.Flaherty KR, Andrei AC, Murray S, Fraley C, Colby TV, Travis WD, Lama V, Kazerooni EA, Gross BH, Toews GB, Martinez FJ. Idiopathic pulmonary fibrosi s. Prognositc value of changes in physiology and six-minute walk test. Am J Respir Crit Care Med 174: 803–809, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda Y, Ishizaki M, Masuda Y, Kimura G, Kawanami O, Masugi Y. The role of intraalveolar fibrosis in the process of pulmonary structural remodeling in patients with diffuse alveolar damage. Am J Pathol 126: 171–182, 1987 [PMC free article] [PubMed] [Google Scholar]
- 19.Giannakakis A, Huang J, Greshock J, Liang S, Dionyssios K, Weber BL, Sandaltzopoulos R, Simon C, Coukos G, Zhang L. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther 7: 255–264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyton AC, Hall JE. Physical principles of gas exchange; diffusion of oxygen and carbon dioxide through the respiratory membrane. In: Textbook of Medical Physiology, 10th edition Philadelphia, PA: W. B. Saunders, 2000, p. 454 [Google Scholar]
- 21.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang U, Brekken RA, Scherer PE. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29: 4467–4483, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris AL. Hypoxia-a key regulatory factor in tumour growth. Nat Rev Cancer 2: 38–47, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, Fasanaro P, Sun N, Wang X, Martelli F, Robbins RC, Wu JC. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 122: S124–S131, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang LE, Bunn HF. Hypoxia-inducible factor and its biomedical relevance. J Biol Chem 278: 19575–19578, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le QT, Giaccia AJ. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell 35: 856–867, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanovic Z. Hypoxia or in situ normoxia: the stem cell paradigm. J Cell Physiol 219: 271–275, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Jain M, Sznajder JI. Effects of hypoxia on the alveolar epithelium. Proc Am Thorac Soc 2: 202–205, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Kottmann RM, Kulkami AA, Smolnycki KA, Lyda E, Dahanayake T, Salibi R, Honnons S, Jones C, Isern NG, Hu JZ, Nathan SD, Grant G, Phipps RP, Sime PJ. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiationvia pH-dependent activation of transforming growth factor-β. Am J Respir Crit Care Med 186: 740–751, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krick S, Eul BG, Hanze J, Savai R, Grimminger F, Seeger W, Rose F. Role of hypoxia-inducible factor-1 alpha in hypoxia-induced apoptosis of primary alveolar epithelial type II cells. Am J Respir Cell Mol Biol 32: 395–403, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Kuhn C, 3rd, Boldt J, King TE, Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis 140: 1693–1703, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol 138: 1257–1265, 1991 [PMC free article] [PubMed] [Google Scholar]
- 33.Larsson O, Diebold D, Fan D, Peterson M, Nho RS, Bitterman PB, Henke CA. Fibrotic myofibroblasts manifest genome-wide derangements of translational control. PLoS One 3: e3220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leungwattanakij S, Bivalacqua TJ, Mustafa FU, Yang DY, Hyun JS, Champion HC, Abdel-Mageed AB, Hellstrom WJG. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Androl 24: 239–245, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15: 501–513, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loncaster JA, Harris AL, Davidson SE. Carbonic anhydrase (CAIX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements, and prognosis in locally advanced carcinoma of the cervix. Cancer Res 61: 6394–6399, 2001 [PubMed] [Google Scholar]
- 37.Mannino DM, Etzel RA, Parrish RG. Pulmonary fibrosis deaths in the United States, 1979–1991. An analysis of multiple-cause mortality data. Am J Respir Crit Care Med 153: 1548–1552, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol 295: G709–G717, 2008 [DOI] [PubMed] [Google Scholar]
- 39.McCormick RI, Blick C, Ragoussis J, Schoedel J, Mole DR, Young AC, Selby PJ, Banks RE, Harris AL. miR-210 is a target of hypoxia-inducible factors 1 and 2 in renal cancer, regulates ISCU and correlates with good prognosis. Br J Cancer 108: 1133–1142, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michailidou Z, Turban S, Miller E, Zou X, Schrader J, Ratcliffe PJ, Hadoke PWF, Walker BR, Iredale JP, Morton NM, Seckl JR. Increased angiogenesis protects against hypoxia and fibrosis in metabolic disease-resistant 11β-hydroxysteroid dehydrogenase type 1 (HSD1)-deficient mice. J Biol Chem 287: 4188–4197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutharasan RK, Nagpal V, Ichikawa Y, Ardehali H. microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exert cytoprotective effects. Am J Physiol Heart Circ Physiol 301: H1519–H1530, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noble PW. Idiopathic pulmonary fibrosis: natural history and prognosis. Clin Chest Med 27: S11–S16, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Noble PW, Homer RJ. Idiopathic pulmonary fibrosis: new insights into pathogenesis. Clin Chest Med 25: 749–758, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Oak SR, Murray L, Herath AS, Sleeman M, Anderson I, Joshi AD, Coelho AL, Flaherty KR, Toews GB, Knight D, Martinez FJ, Hogaboam CM. A micro RNA processing defect in rapidly progressing idiopathic pulmonary fibrosis. PLoS One 6: e21253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietras A, Hansford LM, Johnsson AS, Bridges E, Sjolund J, Gisselsson D, Rehn M, Beckman S, Noguera R, Navarro S, Cammenga J, Fredlund E, Kaplan DR, Pahlman S. HIF2α maintains an undifferentiated state in neural crest-like human neuroblastoma tumor-initiating cells. Proc Natl Acad Sci USA 106: 16805–16810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabbani ZN, Mi J, Zhang Y, Delong M, Jackson IL, Flecenstein K, Salahuddin FK, Zhang X, Clary B, Anscher MS, Vujaskovic Z. Hypoxia inducible factor 1α signaling in fractionated radiation induced lung injury: role of oxidative stress and tissue hypoxia. Radiat Res 173: 165–174, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 174: 810–816, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 134: 136–151, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Sun S, Ning X, Zhang Y, Lu Y, Nie Y, Han S, Liu L, Du R, Xia L, He L, Fan D. Hypoxia-inducible factor-1α induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int 75: 1278–1287, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Swinson DEB, Lones JL, Richardson D, Wykoff C, Turley H, Pastorek J, Taub N, Harris AL, O'Byrne KJ. Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small cell lung cancer. J Clin Oncol 21: 473–382, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med 205: 1659–1672, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia H, Khalil W, Kahm J, Jessurun J, Kleidon J, Henke CA. Pathologic caveolin-1 regulation of PTEN in idiopathic pulmonary fibrosis. Am J Pathol 176: 2626–2637, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia H, Seeman J, Hong J, Hergert P, Bodem V, Jessurun J, Smith K, Nho R, Kahm J, Gaillard P, Henke C. Low α2β1 integrin function enhances the proliferation of fibroblasts from patients with idiopathic pulmonary fibrosis by activation of the β-catenin pathway. Am J Pathol 181: 222–233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang IV, Luna L, Coldren CD, Fingerlin T, Leach S, Murphy E, Lin J, Cosgrove GP, Lynch DA, Groshong SD, Brown KK, Schwarz MI, Schwartz DA. Genes and miRNAs associated with severity of lung function impairment in idiopathic interstitial pneumonias. Am J Respir Crit Care Med 183: A3559, 2011 [Google Scholar]
- 55.Zhang Z, Sun H, Dai H, Walsh RM, Imakura M, Schelter J, Buchard J, Dai X, Chang AN, Diaz RL, Marszalek JR, Bartz SR, Carleton M, Cleary MA, Linsley PS, Grandori C. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle 8: 2756–2768, 2009 [DOI] [PubMed] [Google Scholar]