Abstract
AIM
To study the feasibility of using the discoloration to evaluate the biomechanical properties after treating with genipin.
METHODS
Porcine cadaver eyes were treated for 30min with 1.0% (by w/v) genipin. Untreated samples were used as controls. After treatment, scleral strips of 4.0×10.0-mm2 were cut. The denaturation temperature (Td) measurement and stress-strain test were performed after taking photograph to analyze the color.
RESULTS
Within 24h after treating with genipin, the sclera exhibited a bluish color which became deeper with time. And the denaturation temperature also was increased gradually. Compared with untreated groups, at 1, 6, 12, 24 and 36h after treatment, the ultimate stress were increased by 56%, 153%, 173%, 225% and 211% respectively. The Young's modulus at 10% strain also increased by 170%, 246%, 264%, 389% and 288% respectively. There were strong correlation between the discoloration and the biomechanical properties (ΔE-Ultimate stress: R2=0.892, P=0.00; ΔE-Young's modulus: R2=0.602, P=0.00).
CONCLUSION
Genipin could be used to strengthen collagen gradually in a relatively short time span. And the biomechanical properties could be reliably evaluated via simple visible discoloration.
Keywords: genipin, crosslinking, sclera, biomechanics, discoloration
INTRODUCTION
Myopia is a very common refractive error around the world. It affects about 20%-30% general population in USA and Europe, and up to 80%-90% in school-leavers in east Asia[1]–[4]. Now the pathogenesis of myopia is still unclear, however most of myopia (>95%) have strong correlation to the axial extension[5]. The axial elongation is closely related to the sclera biomechanics. In high myopia the sclera extension was associated with a reduction in the size of individual collagen fiber and as well the number of collagen fiber[6]–[9].
Corneal crosslinking with riboflavin/UVA has been successfully used to prevent the progress of keratoconus and keratectasia after laser in situ keratomileusis[10]–[12]. Based on these studies, Wollensak and Spoerl[11]suggested that collagen crosslinking may be a potential treatment to prevent the progression of high myopia. At present, there are some methods that could improve the sclera biomechanical properties, including riboflavin/UVA, glyceraldehydes, Aliphatic β-nitro alcohols and genipin[13]–[16].
As a natural crosslinking reagent, Genipin has been suggested as an alternative reagent for improving the mechanics of bioartificial tissues[17]–[19]. Meanwhile the tissue treated with genipin exhibited blue discoloration and emitted the fluorescence[20]. There was strong correlation of the fluorescence intensity to the biomechanical properties[21]. However there has not report about the correlation of the visible discoloration to the biomechanics. In our previous study showed that genipin could improve the sclera biomechanics and it may be the potential method to prevent the progression of high myopia; meanwhile during the preliminary experiment we found that the discoloration of sclera strips did not occur immediately after treating with genipin for 30min[16]. So in this paper we represented the change of color, denaturation temperature and biomechanical properties at 1, 6, 12, 24 and 36h after treat with 1.0% genipin for 30min and investigated the feasibility of using the discoloration to evaluate the biomechanical properties.
MATERIALS AND METHODS
Porcine Eye Preparation
Forty-eight enucleated porcine eyes were retrieved from the local abattoir within 5h postmortem. Adherent tissue was trimmed carefully from the scleral surface. These eyes were equally divided into 6 groups including control and experimental groups treating with 1.0% genipin for 30min. After treatment, 5 eyes were used to take the stress-strain test and another 3 eyes for the denaturation temperature measurement. Our study were approved by the Institutional Animal Care and Use Committee of the Sun Yet-sen University (2012-087).
Genipin Crosslinking
10×15-mm2 filter paper was laid onto the target sclera located at the 12 o'clock position between 4 mm after corneal limbus and the posterior sclera. 1.0% Genipin (Wako, Japan) in normal saline (NS) was dropped on the filter at 3-5min intervals, which the sclera could keep to contact genipin during the treatment. The cross-linking reaction was performed at 25±1°C for 30min. After treatment, the eyes were rinsed extensively with NS to remove all free genipin.
Scleral Strips
After the treatment, a 4×10-mm2 strip was cut from 4 mm after corneal limbus in direction to the optic nerve at 12 o'clock with a self-constructed double-blade scalpel. To preserve the sclera hydration, the strips were covered with hypromellose eye drops. And then the strips were preserved at 25±1°C in a moist chamber about 1, 6, 12, 24 and 36h before measurement.
Digital Photography and Analysis the Discoloration
We used a Nikon Coolpix S220 (Nikon CORP., Japan) to take the photographs before measuring the denature temperature and stress-strain test. All the photograph were performed at the same conditions and every strip were taken photography three times.
We employed Lab system of Adobe Photoshop CS6 software (Adobe Systems, San Jose, CA, USA) for image analysis. The L1, a1, and b1 values were measured on the surface of sclera strips. L1 (from black to white) is the lightness component which ranges from 0 to 100; a1 (from green to red) and b1 (from blue if negative to yellow if positive) are two color components which range from -120 to +120[22]. The kinetics of color formation of sclera at different time after treatment with genipin was followed by the parameter total color change (ΔE) which was calculate the following way ΔE=[(L1-L0)2+(a1-a0)2+(b1-b0)2]1/2. The L1a1b1 values correspond to the values of sclera at different times and the values of L0a0b0 corresponds to the color of the sclera before treatment.
Denaturation Temperature Measurement
Differential scanning calorimeter (DSC) is a well developed analytic tool used for measurement of denaturation temperature in collagenous tissues[23],[24]. In our experiment, the denaturation temperature of sclera was determined using TA DSC instrument (TA Q20, USA). One aluminum pan contains about 5.0 mg sclera sample and using an empty pan as reference. The tissues were heated at 10°C per minute from 30°C to an appropriate specified temperature.
Stress-strain Test
The scleral thickness was determined using a digimatic caliper [Sanling group (H.K.) Ltd.]. The stress-strain test was performed using an Instron 3343 microtester (Illinois Tool Works Inc., Glenview, IL, USA). The strips were clamped vertically with 6 mm between the jaws. They were subjected to a stress level of 0.02 mPa and 5 cycles in total of deformation-load testing. And then, strain was increased linearly at a velocity of 1.5 mm/min up to tissue rupture. The parameters ultimate stress, ultimate strain and Young's modulus at 10% strain were used for analysis.
Statistical Analysis
The ultimate stress, Young's modulus and ΔE were analyzed using SPSS for Windows (SPSS, Inc, Chicago, IL, USA. version 17.0). A value of P<0.05 was considered statistically significant.
RESULTS
The mean thickness of the porcine sclera was 815±20 µm (607-1085 µm). There were no statistically significant differences among all groups (all P>0.05).
Figure 1 and Table 1 display the coloration of sclera at different time after treating with genipin and the L1a1b1 value respectively. The total color change (ΔE) refers to the difference of sclera color untreated and post-treated with genipin. All untreated sclera show white. At one hour after treating, the strips turn into yellowish, and then sclera exhibited blue gradually with time with 24h (Table 1). At 24h and 36h, the strips become dark bluish and had very closed ΔE value.
Figure 1. The scleral color after treating with 1.0% genipin/30min (Control, 1, 6, 12, 24, 36h).

Table 1. Analysis of the discoloration after treatment with genipin.
| Time (h) | Lab |
ΔE | ||
| L | a | b | ||
| Control | 66.60±2.91 | -0.20±0.20 | 2.00±0.32 | 0 |
| 1 | 65.40±1.99 | -3.20±2.49 | 9.60±1.81 | 10.98±1.89 |
| 6 | 40.20±4.53 | -9.20±2.06 | -5.60±1.86 | 32.10±3.69 |
| 12 | 28.00±1.58 | -13.4±1.94 | -6.00±1.30 | 44.15±1.31 |
| 24 | 20.00±2.55 | -5.60±1.99 | -13.40±0.93 | 51.91±2.23 |
| 36 | 20.00±3.85 | -6.80±1.16 | -12.00±0.55 | 51.36±3.59 |
The stability of triple-helical structure of collagen depends on the weak hydrogen bonds. Genipin crosslinking could strength the bonds by forming intramolecular and intermolecular crosslinks of the amino residues in collagen molecules[25]. When the collagen is heat-denatured, these bonds are broken and the triple-helical structure collapse into random coils[26],[27]. In the crosslinking of collagenous tissue, the denaturation temperature is often regarded as an indicator of degree of crosslinking. Higher Td means higher degree of collagen crosslinking[28]. The Td of sclera strips were measured using differential scanning calorimeter (Table 2). At one hour after treatment, the Td (about 70°C) was higher than that of fresh sclera (about 64°C). Subsequently, the Td was increased gradually with time up to 24h after treatment. These results suggested that within 24h after treatment the degree of collagen crosslinking-induced by genipin could be enhanced gradually.
Table 2. Denaturation temperature and biomechanical properties of porcine sclera after treatment.
| Time (h) | Td (°C) | Ultimate stress (mPa) | Ultimate strain (%) | Modulus (mPa) |
| Control | 64.33±0.33 | 5.12±0.60 | 30.85±2.58 | 11.71±4.10 |
| 1 | 70.50±1.50 | 8.02±0.56 | 24.31±0.82 | 31.63±3.37 |
| 6 | 74.00±2.00 | 12.96±0.64 | 30.45±3.08 | 40.52±3.69 |
| 12 | 77.50±1.24 | 13.96±1.46 | 28.20±1.46 | 42.57±6.45 |
| 24 | 83.00±3.51 | 16.63±1.00 | 28.18±0.89 | 51.38±8.20 |
| 36 | 81.00±4.04 | 15.94±1.12 | 28.91±3.25 | 45.43±10.96 |
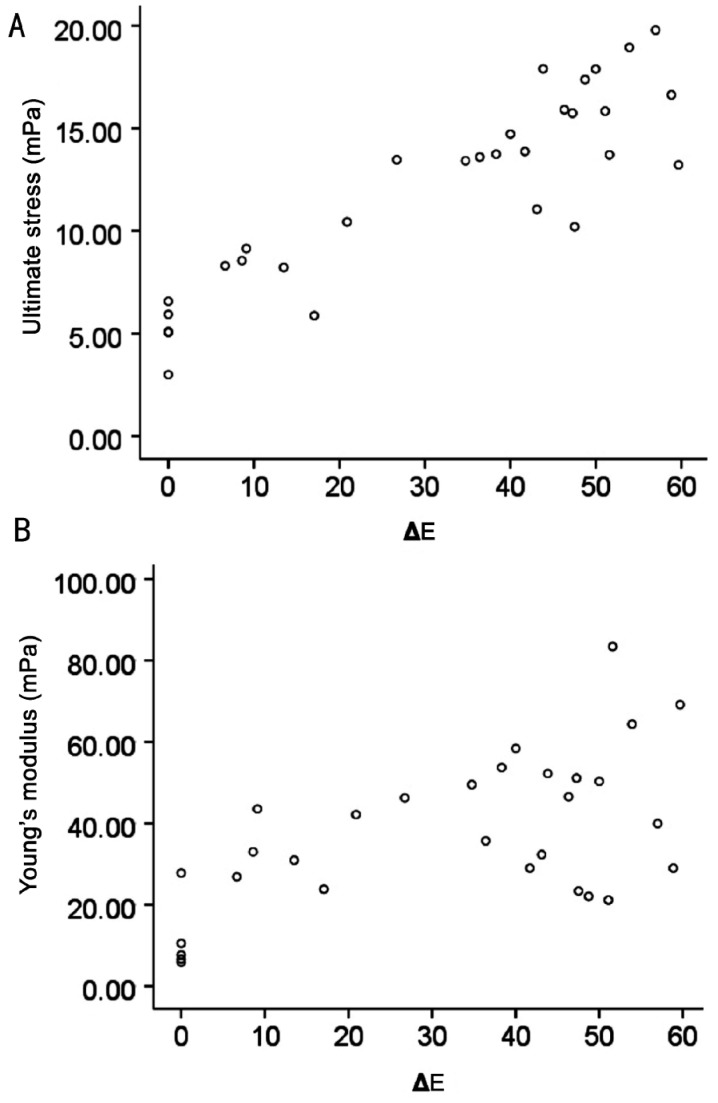
After treatment, the stress-strain curve of strip is nonlinear curve (Figure 2). The curves became steeper with time after treatment. Meanwhile 24h after treating had similar stress-strain curve with 36h. At 1, 6, 12, 24 and 36h, compared to the untreated group, the ultimate stress increased by 56%, 153%, 173%, 225% and 211% respectively (all P<0.05, Table 2). The modulus at 10% strain also increased by 170%, 246%, 264%, 389% and 288% respectively (all P<0.05, Table 2). However, the ultimate strain had no significant difference between untreated and treated groups. The peak of the change of ultimate stress and Young's modulus occurred at 24h after treatment. And there had strong correlation of the total color difference (ΔE) to biomechanical properties (ΔE-ultimate stress: R2=0.892, P=0.00; ΔE-Young's modulus: R2=0.602, P=0.00. Figure 3).
Figure 2. The stress-strain curves of porcine sclera.
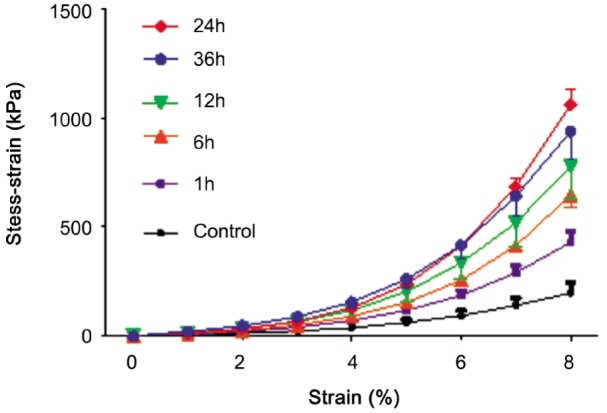
Figure 3. The scatter diagram.
A: ΔE-Ultimate stress, R2=0.874, P=0.00; B: ΔE-Young's modulus, R2=0.437, P=0.02.
DISCUSSION
In this paper we analyzed the changes of color, thermal stability and biomechanical properties in porcine sclera treated by 1.0% genipin for 30min. Our results showed that at room temperature, the discoloration, biomechanical properties and denaturation temperature were enhanced gradually. There was strong correlation of discoloration to mechanical properties.
Collagen crosslinking is a common phenomenon in biological tissues. There are various methods to induce crosslinking, including physical, chemical reagent and enzyme. Crosslinking reagent could strengthen the collagen biomechanics by inducing the crosslinking covalent bonds of intramolecular and intermolecular in collagen fiber[29]. As a natural collagen crosslinking reagent, Genipin has been used as an alternative reagent to improve the biomechanical properties of collagenous tissues[17]–[19].
In the crosslinking of collagenous tissue, the denaturation temperature is often regarded as an indicator of degree of crosslinking. Td means that the triple-helical structure of collagen collapse into random coil[26],[27]. Higher Td shows higher degree of collagen crosslinking[28]. In the present experiment, the Td of sclera genipin-induced was higher than that of the fresh tissue. And the denaturation temperature increased with time within 24h after treatment. This suggested that the crosslinking in collagen molecular was continued to occur up to the finish of the reaction process of genipin with amino acid residues at 24h after treatment.
Genipin could strengthen the biomechanics of collagenous tissues, while the tissues appeared bluish discoloration and emitted the fluorescence[20]. And there had strong correlation of fluorescence intensity to the biomechanical properties[21]. Due to its sensitivity to the bluish discoloration of biological tissues, genipin was used to detect the fingerprint[20]. The reaction mechanism is still not well clear at present. It could form blue pigment upon spontaneous reaction with amino acid residues[30]. In our study at one hour after treatment, the sclera strips appeared yellowish, and then exhibited bluish discoloration with time, up to dark blue at 24h and 36h. We speculated that the sclera appeared yellowish because of the small amount of crosslinking genipin-induced at the early stage. Subsequently, more and more crosslinking were occurred; and the sclera discoloration depended on the degree of collagen crosslinking. However, in our previous and present investigation, the color of the specimens were not uniform across the surface[16]. Even at the same time or concentration, there was still a certain range of color variation. It may be due to the thickness and proportion of collagen of sclera. Meanwhile, the stress and Young's modulus measurements also were increasingly improved over time. Compared to the untreated group, the ultimate stress increased by 56%, 153%, 173%, 225% and 211% respectively at 1, 6, 12, 24 and 36h after treatment. The modulus also increased by 170%, 246%, 264%, 389% and 288%. And these changes were strong correlation to the sclera discoloration. These showed that the visible discoloration may be a feasible method to evaluate the biomechanical properties after genipin-treatment.
In conclusion, the preliminary study has shown that the effect genipin-induced could be improved gradually within 24h at the room temperature. The degree of collagen crosslinking and the biomechanical properties could be indirectly reflected by the discoloration of sclera. And this ability also may help to directly observe the area of crosslinking in biological tissues. It is a potential method using the simple visible discoloration to evaluate the biomechanical properties of genipin-induced collagen cross-linking. However, in the long run, due to the metabolism the color and biomechanical properties of collagenous tissues after treatment with genipin may change with time. The further study should be performed about the correlation of discoloration to biomechanical properties in vivo and we also need more accurate and reliable method to monitor the discoloration. Of course the safety of genipin will be examined in the future. Once the proper concentration which has no side effect is decided, genipin might be a potential reagent for strengthening scleral tissue to prevent the myopic progression.
Acknowledgments
Foundations: Supported by Science and Technology Projects of Guangdong Province, China (No.2007B031002001, No.2008B030301086)
Conflicts of Interest: Liu TX, None; Luo X, None; Gu YW, None; Yang B, None; Wang Z, None.
REFERENCES
- 1.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthalmol. 2009;127(12):1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 2.Sherwin JC, Khawaja AP, Broadway D, Luben R, Hayat S, Dalzell N, Wareham NJ, Khaw KT, Foster PJ. Uncorrected refractive error in older British adults: the EPIC-Norfolk Eye Study. Br J Ophthalmol. 2012;96(7):991–996. doi: 10.1136/bjophthalmol-2011-301430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attebo K, Ivers RQ, Mitchell P. Refractive errors in an older population: the Blue Mountains Eye Study. Ophthalmology. 1999;106(6):1066–1072. doi: 10.1016/S0161-6420(99)90251-8. [DOI] [PubMed] [Google Scholar]
- 4.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379(9827):1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 5.Zadnik K. The Glenn A. Fry Award Lecture (1995). Myopia development in childhood. Optom Vis Sci. 1997;74(8):603–608. [PubMed] [Google Scholar]
- 6.Curtin BJ, Teng CC. Scleral changes in pathological myopia. Trans Am Acad Ophthalmol Otolaryngol. 1958;62(6):777–790. [PubMed] [Google Scholar]
- 7.Curtin BJ, Iwamoto T, Renaldo DP. Normal and staphylomatous sclera of high myopia. An electron microscopic study. Arch Ophthalmol. 1979;97(5):912–915. doi: 10.1001/archopht.1979.01020010470017. [DOI] [PubMed] [Google Scholar]
- 8.Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995;35(9):1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- 9.Guggenheim JA, Mcbrien NA. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci. 1996;37(7):1380–1395. [PubMed] [Google Scholar]
- 10.Henriquez MA, Izquierdo L, Jr, Bernilla C, Zakrzewski PA, Mannis M. Riboflavin/Ultraviolet A corneal collagen cross-linking for the treatment of keratoconus: visual outcomes and Scheimpflug analysis. Cornea. 2011;30(3):281–286. doi: 10.1097/ICO.0b013e3181eeaea1. [DOI] [PubMed] [Google Scholar]
- 11.Wollensak G, Spoerl E. Collagen crosslinking of human and porcine sclera. J Cataract Refract Surg. 2004;30(3):689–695. doi: 10.1016/j.jcrs.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Kymionis GD, Diakonis VF, Kalyvianaki M, Portaliou D, Siganos C, Kozobolis VP, Pallikaris AI. One-year follow-up of corneal confocal microscopy after corneal cross-linking in patients with post laser in situ keratosmileusis ectasia and keratoconus. Am J Ophthalmol. 2009;147(5):774–778. doi: 10.1016/j.ajo.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Wollensak G, Iomdina E, Dittert DD, Salamatina O, Stoltenburg G. Cross-linking of scleral collagen in the rabbit using riboflavin and UVA. Acta Ophthalmol Scand. 2005;83(4):477–482. doi: 10.1111/j.1600-0420.2005.00447.x. [DOI] [PubMed] [Google Scholar]
- 14.Wollensak G, Iomdina E. Crosslinking of scleral collagen in the rabbit using glyceraldehyde. J Cataract Refract Surg. 2008;34(4):651–656. doi: 10.1016/j.jcrs.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Paik DC, Wen Q, Airiani S, Braunstein RE, Trokel SL. Aliphatic beta-nitro alcohols for non-enzymatic collagen cross-linking of scleral tissue. Exp Eye Res. 2008;87(3):279–285. doi: 10.1016/j.exer.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Liu TX, Wang Z. Collagen crosslinking of porcine sclera using genipin. Acta Ophthalmol. 2013;91(4):e253–257. doi: 10.1111/aos.12172. [DOI] [PubMed] [Google Scholar]
- 17.Liang HC, Chang WH, Lin KJ, Sung HW. Genipin-crosslinked gelatin microspheres as a drug carrier for intramuscular administration: in vitro and in vivo studies. J Biomed Mater Res A. 2003;65(2):271–282. doi: 10.1002/jbm.a.10476. [DOI] [PubMed] [Google Scholar]
- 18.Chen SC, Wu YC, Mi FL, Lin YH, Yu LC, Sung HW. A novel pH-sensitive hydrogel composed of N, O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J Control Release. 2004;96(2):285–300. doi: 10.1016/j.jconrel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Moffat KL, Marra KG. Biodegradable poly (ethylene glycol) hydrogels crosslinked with genipin for tissue engineering applications. J Biomed Mater Res B Appl Biomater. 2004;71(1):181–187. doi: 10.1002/jbm.b.30070. [DOI] [PubMed] [Google Scholar]
- 20.Almog J, Cohen Y, Azoury M, Hahn TR. Genipin--a novel fingerprint reagent with colorimetric and fluorogenic activity. J Forensic Sci. 2004;49(2):255–257. [PubMed] [Google Scholar]
- 21.Sundararaghavan HG, Monteiro GA, Lapin NA, Chabal YJ, Miksan JR, Shreiber DI. Genipin-induced changes in collagen gels: correlation of mechanical properties to fluorescence. J Biomed Mater Res A. 2008;87(2):308–320. doi: 10.1002/jbm.a.31715. [DOI] [PubMed] [Google Scholar]
- 22.Yamanel K, Caglar A, Özcan M, Gulsah K, Bagis B. Assessment of color parameters of composite resin shade guides using digital imaging versus colorimeter. J Esthet Restor Dent. 2010;22(6):379–388. doi: 10.1111/j.1708-8240.2010.00370.x. [DOI] [PubMed] [Google Scholar]
- 23.Duan X, Sheardown H. Crosslinking of collagen with dendrimers. J Biomed Mater Res A. 2005;75(3):510–518. doi: 10.1002/jbm.a.30475. [DOI] [PubMed] [Google Scholar]
- 24.Tonda-Turo C, Gentile P, Saracino S, Chiono V, Nandagiri VK, Muzio G, Canuto RA, Ciardelli G. Comparative analysis of gelatin scaffolds crosslinked by genipin and silane coupling agent. Int J Biol Macromol. 2011;49(4):700–706. doi: 10.1016/j.ijbiomac.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Lima EG, Tan AR, Tai T, Marra KG, DeFail A, Ateshian GA, Hung CT. Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement. J Biomed Mater Res A. 2009;3(91):692–700. doi: 10.1002/jbm.a.32305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser RD, MacRae TP, Suzuki E. Chain conformation in the collagen molecule. J Mol Biol. 1979;129(3):463–481. doi: 10.1016/0022-2836(79)90507-2. [DOI] [PubMed] [Google Scholar]
- 27.Bella J, Eaton M, Brodsky B, Berman HM. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science. 1994;266(5182):75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- 28.Tu R, Shen SH, Lin D, Hata C, Thyagarajan K, Noishiki Y, Quijano RC. Fixation of bioprosthetic tissues with monofunctional and multifunctional polyepoxy compounds. J Biomed Mater Res. 1994;28(6):677–684. doi: 10.1002/jbm.820280604. [DOI] [PubMed] [Google Scholar]
- 29.Bailey AJ. Structure, function and ageing of the collagens of the eye. Eye. 1987;1(Pt2):175–183. doi: 10.1038/eye.1987.34. [DOI] [PubMed] [Google Scholar]
- 30.Zhu K, Slusarewicz P, Hedman T. Thermal analysis reveals differential effects of various crosslinkers on bovine annulus fibrosis. J Orthop Res. 2011;29(1):8–13. doi: 10.1002/jor.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]