Abstract
AIM
To describe the initial outcomes and safety of femtosecond laser-assisted deep anterior lamellar keratoplasty (DALK) for keratoconus and post-LASIK keratectasia.
METHODS
In this non-comparative case series, 10 eyes of 9 patients underwent DALK procedures with a femtosecond laser (Carl Zeiss Meditec AG, Jena, Germany). Of the 9 patients, 7 had keratoconus and 2 had post-LASIK keratectasia. A 500 kHz VisuMax femtosecond laser was used to perform corneal cuts on both donor and recipient corneas. The outcome measures were the uncorrected visual acuity (UCVA), best-corrected visual acuity (BCVA), corneal thickness, astigmatism, endothelial density count (EDC), and corneal power.
RESULTS
All eyes were successfully treated. Early postoperative evaluation showed a clear graft in all cases. Intraoperative complications included one case of a small Descemet's membrane perforation. Postoperatively, there was one case of stromal rejection, one of loosened sutures, and one of wound dehiscence. A normal corneal pattern topography and transparency were restored, UCVA and BCVA improved significantly, and astigmatism improved slightly. There was no statistically significant decrease in EDC.
CONCLUSION
Our early results indicate that femtosecond laser-assisted deep anterior lamellar keratoplasty could improve UCVA and BCVA in patients with anterior corneal pathology. This approach shows promise as a safe and effective surgical choice in the treatment of keratoconus and post-LASIK keratectasia.
Keywords: femtosecond laser, deep anterior lamellar keratoplasty, keratoconus, post-LASIK keratectasia
INTRODUCTION
Penetrating keratoplasty (PK), a procedure that involves the full-thickness replacement of the cornea, has been the definitive surgical treatment for keratoconus for many years[1]–[3]. Although the procedure is effective, resulting in excellent best-corrected visual acuity (BCVA), it is not without limitations. However, PK can lead to significantly high or irregular astigmatism and the loss of endothelial cells, with the patient's vision rehabilitating very slowly. Since the wound never heals to the original corneal strength, it is highly susceptible to trauma, leading to severe vision loss in some cases. Furthermore, it has been associated with a high risk of graft rejection, secondary glaucoma, complicated cataracts, and the constant loss of endothelial cells [4].
In an attempt to overcome these limitations, researchers have developed more conservative keratoplasty techniques which selectively replace only the diseased layers of the cornea, including deep anterior lamellar keratoplasty (DALK), microkeratome-assisted lamellar keratoplasty (LK), excimer laser assisted LK (ELLK), and peripheral tectonic lamellar keratoplasty [5],[6]. Through lamellar keratoplasty, a partial-thickness donor cornea can be transplanted onto a complementary recipient bed in which only the abnormal stroma has been removed. In deep anterior DALK, the donor lamella is positioned directly on Descemet's membrane, preserving the recipient's endothelium and decreasing the risk of immunologic rejection[7],[8]. The procedure may be descemetic, involving the removal of the entire stroma, or pre-descemetic whereby at least 75% but not all of the stroma is removed[3].
Comparative studies have shown the incidence of graft rejection episodes after anterior lamellar keratoplasty (ALK) to be 50% lower, and the life expectancy of the graft to be longer than that observed with PK[9],[10].
When performed manually, the DALK procedure has resulted in clinically significant topographical irregularities to the lamellar interface in some patients, leading to a reduction in BCVA. Advancements have been made in this field that have reduced the surgery time, increased the safety of the procedure, and improved visual outcomes, making the DALK procedure more attractive than PK[7],[9].
Recently, a femtosecond laser-assisted DALK (FDALK) procedure that uses a laser programmed to produce bladeless, precise lamellar cuts, allowing both anterior and posterior lamellar transplantation, has been developed[5].
Here we report the outcomes of patients that were treated for keratoconus and post-laser in situ keratomileusis (LASIK) keratectasia with FDALK at our center.
SUBJECTS AND METHODS
Subjects
This study adhered to the tenets of Declaration of Helsinki and ethical approval was given by the medical ethics committee of Jinling hospital, and informed consent was obtained from all participants. Data were collected from procedures performed between April 2012 and December 2013 by a single surgeon (Huang ZP) at the Department of Ophthalmology, Jinling Hospital, Nanjing, China.
A complete ophthalmologic examination performed pre and postoperatively included Snellen uncorrected visual acuity (UCVA) and BCVA, anterior segment slitlamp examination, anterior segment optical coherence tomography (Carl Zeiss Meditec Atlas 995, Jena, Germany), computerized corneal topography (CAS; EyeSys, Houston, TX, USA), 50-MHz ultrasound corneal pachymetry (Pachmate DGH Technology, Exton, PA), endothelium cell density (ECD) was calculated with a specular microscope Topcon SP2111p (Topcon Corp, Tokyo, Japan), and dilated fundus examination.
The inclusion criteria were a total corneal pachymetry of more than 300 µm, evidence of ongoing keratoconus with follow-up and intolerance to rigid gas permeable (RGP) contact lenses, or poor results with the use of RGP. Exclusion criteria were glaucoma, retinal disorders, deep amblyopia, total corneal pachymetry less than 300 µm, and any other coexisting ocular disease that could affect the patients visual acuity as well a pregnancy, evidence of ongoing bacterial infection, or corneal perforation.
Methods
Surgical technique
The surgical technique was performed in two steps. The first step was carried out in the laser room, where a 500 kHz VisuMax femtosecond laser (Carl Zeiss Meditec AG) was used to cut the donor and recipient corneas. The second step, in which the donor lamella was sutured into the receiving stromal bed, was performed in the operating room. All procedures were performed by a single surgeon. All donor tissue was obtained from fresh human globes from voluntary donor eyeballs of healthy young adults with accidental death. Donors were between the age of 20-40y and the globes were evaluated for thinnest corneal thickness. The donors were evaluated within 24h of the death of the donor and stored in a container filled with ice. To create the donor graft, the entire donor globe was fixed in an eyeball retainer (Figure 1A, 1B) and placed directly under the laser. A 500 kHz VisuMax femtosecond laser with preprogrammed parameters set by the surgeon was used for cutting the donor graft. The parameters were set to a lenticule thickness of 300 to 510 µm; a lenticule diameter of 7.3 to 8.0 mm, spiral method; 230 to 240 nanojoules spiral energy; 230 to 240 nanojoules side cut energy; 90° side cut angle. The track distance and spot distance was 1.9 µm in the graft and 1.5 µm on the graft side. Depending on the donor tissue quality and edema, up to 20% additional thickness was added to the donor lenticule to adjust for donor tissue swelling. The mean donor diameter was 7.73±0.21 mm (range, 7.3-8.0 mm) and mean thickness was 399±55.27µm (range, 300-510 µm). Before the graft was separated and lifted from the stromal bed the eyeball was wrapped with wet sterile gauze and stored in a container.
Figure 1. The eyeball retainer.
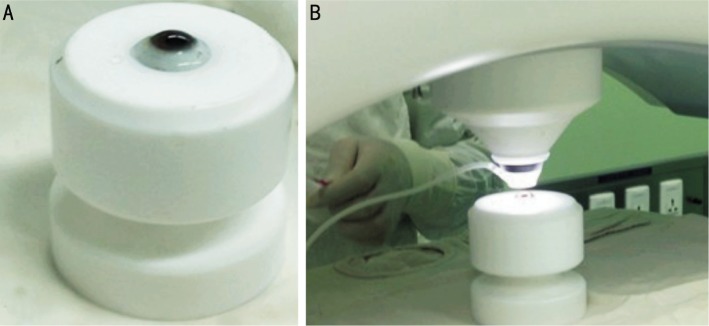
A: Preoperative, the entire donor globe was fixed in the eyeball retainer; B: Introperative, the donor cornea was cut with femtosecond laser.
Under topical anesthesia (oxybuprocaine drops given 3 times), the recipient eye was stabilized with a disposable suction cone positioned at the sclerolimbal margin.
After the docking, the femtosecond laser cut on receiving corneal stroma was performed with settings for the recipient corneal lenticule at a 0.2 mm smaller diameter than the donor graft diameter. Targets were a mean stromal cut deepness of 309.5±53.87 µm, a mean diameter of 7.54±0.16 mm, (range, 7.3-7.8 mm), and a mean residual stromal bed of 84.4±8.67 µm.
In the second phase, the patient was transferred to the surgery room, and the remaining surgery was performed under retrobulbar block anesthesia with light sedation using lidocaine and bupivacaine. The donor corneal button (Figure 2A) was lifted with a blunt spatula and forceps and placed on the recipient residual corneal stromal bed (Figure 2B). It was sutured on using a continuous or 16 interrupted suture technique with 10-0 nylon sutures. Eventually the sutures had to be removed, the time to removal depended upon the wound healing process and if any sutures loosened they were removed. Topical antibiotics (netilmicin) and steroids (dexamethasone 0.18%) were applied several times a day and tapered off and titrated, on the basis of the corneal transparency and scarring of the surgical wound. Patients were followed up every two weeks or once a month and UCVA, BCVA, anterior segment slit lamp examination, anterior segment optical coherence tomography, endothelial cell density measurements and computerized corneal topography measurements were used at follow up.
Figure 2. Femtosecond laser-assisted deep anterior lamellar keratoplasty.
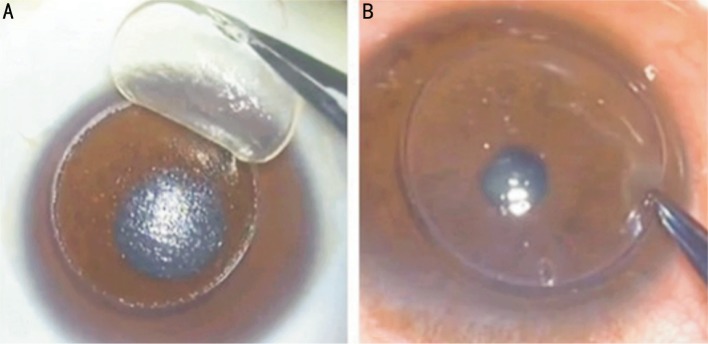
A: Corneal recipient specimen lifting after femtosecond separation showing smooth stromal surface; B: Donor graft placement.
Statistical Analysis
Preoperative data versus postoperative data were analyzed using the paired Student's t-test. If not otherwise indicated, statistical measures are the mean±standard deviation and significant P values were less than 0.05. Statistical analysis was performed using SPSS software (Version 17, SPSS, Inc.).
RESULTS
The preoperative and postoperative findings are listed in Table 1.
Table 1. Patient's characteristics and postoperative-outcome with deep anterior lamellar keratoplasty assisted by a femtosecond laser.
| Patient | Sex/Age | Diagnosis | Corneal pachy- metry (µm) | Preoperative UCVA/BCVA | Postoperative UCVA/BCVA | Stromal bed residual thickness (µm) | Follow up (mo) | Preoperative keratometry | Postoperative keratometry |
| 1 | M /42 | KC | 454 | 0.1/0.4 | 0.4/0.6 | 74 | 21 | 60.21 ×92° 49.7×172° |
50.20×79° 48.71×147° |
| 2 | M /40 | Bilateral KC with scaring | 355 | 0.01/0.2 | 0.12/0.4 | 105 | 18 | 73.93 ×51° 67.26×141° |
43.11 ×139° 40.51×49° |
| 3 | F /23 | KC with scaring | 319 | 0.1/0.6 | 0.3/0.8 | 89 | 17.5 | 66.79 ×105° 64.13×15° |
47.11 ×32° 45.13×122° |
| 4 | M /20 | KC | 398 | 0.15/1.0 | 0.6/1.0 | 88 | 16.5 | 50.79 ×46° 47.00×130° |
48.12 ×113° 38.43×23° |
| 5 | M /20 | KC | 400 | 0.01/0.12 | 0.4/0.5 | 80 | 16.5 | 75.33 ×85° 56.87×178° |
47.75 ×61° 40.67×160° |
| 6 | M /40 | Bilateral KC with scaring | 380 | 0.01/0.12 | 0.12/0.2 | 80 | 13 | 72.05×120° 64.49×30° |
53.46×113° 44.75×23° |
| 7 | M/32 | Ectasia post-LASIK | 435 | 0.15/0.8 | 0.2/0.6 | 80 | 19 | 42.30×120° 38.94×30° |
36.49×9° 34.51×99° |
| 8 | M/26 | Ectasia post-LASIK | 320 | 0.12/0.25 | 0.06/0.4 | 80 | 20 | 50.82×124° 50.45×34° |
41.88×63° 39.96×153° |
| 9 | M/22 | KC | 448 | 0.04/0.3 | 0.1/0.3 | 88 | 9.5 | 43.16×110° 41.24×20° |
47.95×93° 45.62×3° |
| 10 | M/29 | KC | 430 | 0.2/0.2 | 0.4/0.4 | 80 | 10 | 55.73×103° 50.16×13° |
48.82×89° 42.62×179° |
KC: Keratoconus; BCVA: Best-corrected visual acuity; UCVA: Uncorrected visual acuity.
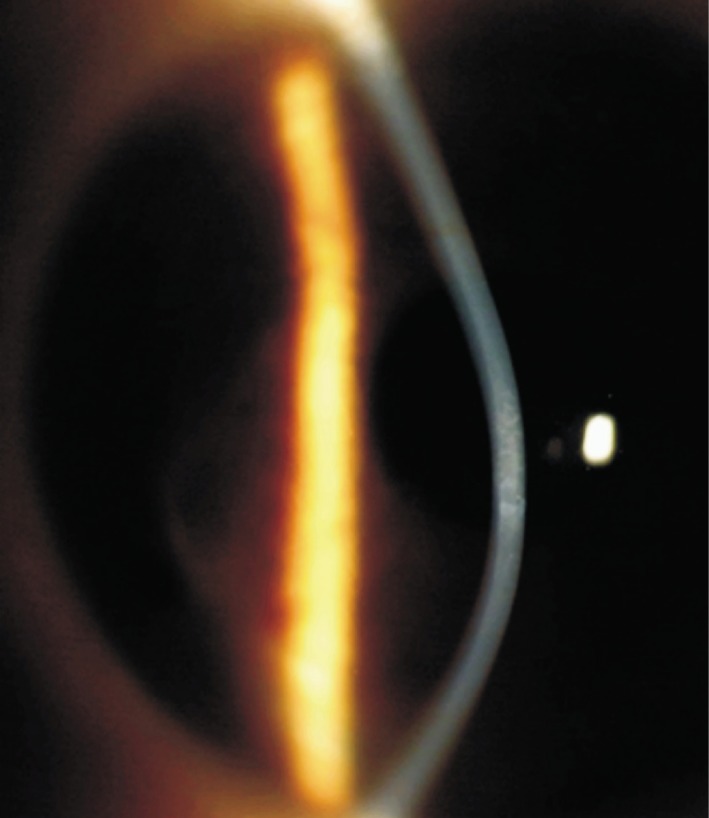
Ten eyes (9 patients; 1 female and 8 males) with keratoconus or post-LASIK keratectasia were enrolled in the study. The mean patient age was 29.4±8.76y (range, 20-42y). Of the 9 patients, 7 had keratoconus (Figure 3), and 2 had post-LASIK keratectasia.
Figure 3. Slit-lamp photograph of advanced keratoconus with scarring.
Maximum follow-up was 13mo (mean, 16.10±4.00mo, range 21-9.5mo). Data of the last follow-up visit of each patient were recorded and selected for analysis.
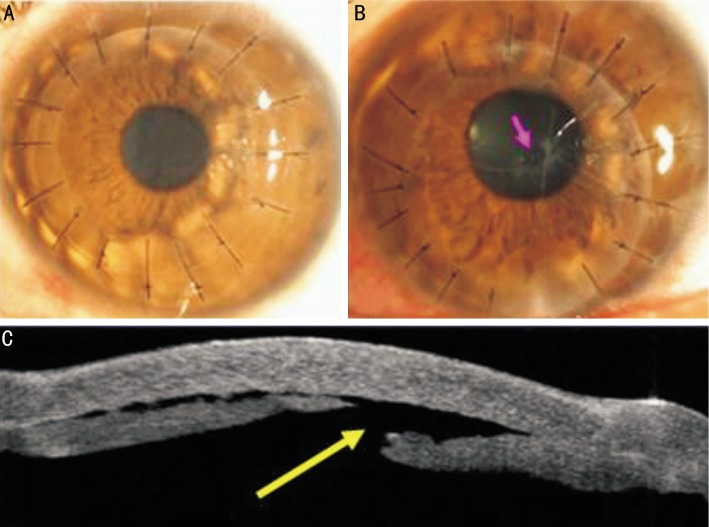
All FDALK procedures were uneventful except one case of a small Descemet's membrane perforation intraoperatively, in the early postoperative period, a corneal examination showed a clear graft (Figure 4A). Intraoperative complications included one small perforation of Descemet's membrane in a patient with post-LASIK keratectasia (Figure 4B, 4C). Topography measurements taken pre- and post-operatively showed a physiologic corneal pattern after 1wk. (Figure 5A, 5B).
Figure 4. Slit-lamp photograph of FDALK for keratoconus showed a clear graft at postoperative day 5.
A:Slit-lamp photograph of small perforation of Descemet's membrane (red arrow) at postoperative 4.5wk; B: Anterior segment optical coherence tomography of small perforation of Descemet's membrane (yellow arrow) at postoperative 2wk.
Figure 5. Corneal topography of a keratoconus case before surgery (A) and 1wk after FDALK (B).
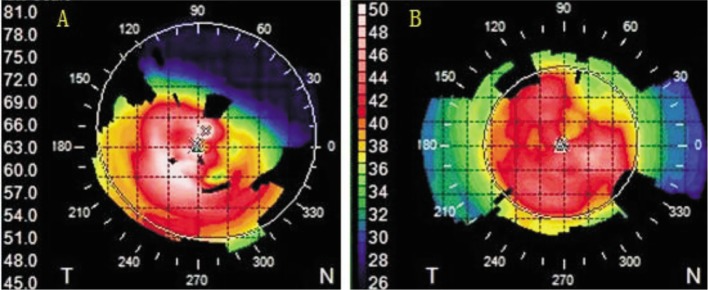
Note the changes in corneal shape from 67 D ectasia (A) to a physiologic topographic pattern with a maximum curvature of 43 D.
Postoperative complications included one patient with wound dehiscence that was resolved with wound resuturing. One eye experienced loosened sutures that had to be removed. Stromal rejection in one eye was managed with the application of topical steroids.
Following keratoplasty, the mean UCVA increased from 0.09±0.07 (SD) to 0.25±0.17, and the mean BCVA increased from 0.40±0.30 (SD) to 0.49±0.25, both of which were statistically significant improvements (P<0.05). The preoperative mean thinnest corneal thickness, evaluated with ultrasound corneal pachymtry and with anterior segment optical coherence tomography, was 393.90±49.85 µm (range, 319-454 µm). The postoperative corneal mean thickness was 483.40±53.70 µm. The mean corneal power decreased from 57.13±12.09 (SD) to 44.53±4.53 (SD), a statistically significant reduction (P<0.05). A significant improvement in mean corneal refractive astigmatism from 6.87±5.50 D to 3.65±2.68 D (P<0.05) was recorded. The mean endothelial cell density (ECD) was 2711.58±300.9 cell/mm2 (range, 2196.9–3294.5 cell/mm2) preoperatively. Although this decreased to 2587.08±398.34 cell/mm2 with a mean cell loss after surgery of 4.6%, the difference was not statistically significant (P=0.149).
DISCUSSION
Keratoconus is the most common primary ectasia encountered in ophthalmology. Both keratoconus and keratectasia following LASIK can progressively lead to the loss of BCVA as well as the reduction in visual acuity that cannot be corrected with the use of spectacles[11]–[13]. It is therefore important to manage the condition with safe and effective procedures.
Lamellar keratoplasty is an alternative to PK that works to remove and replace most of the diseased stroma with a graft, leaving intact the patient's own Descemet's membrane and endothelial cells, avoiding allograft rejection[14]. Although this was a novel concept theoretically, it was associated with its own difficulties, limiting its popularity in the management of keratoconus or keractasia[15],[16]. An uneven or irregular dissection plane left interface opacities that impaired visual acuity with manual dissection techniques[15]. The development of techniques that present the possibility of removing the diseased stroma layer with greater efficacy has led to a renewed interest in these lamellar keratoplasty.
The automated DALK with microkeratome showed critical limits because of corneal curvature, thickness, and shape, leading to unpredictable postoperative results[17]. DALK assisted by excimer laser requires long surgical times, high levels of laser energy with possible risk of endothelium damage, and presents significant challenges especially for corneal thickness and curvature[18],[19]. The femtosecond laser is yet another innovation that can create precise computer guided corneal lamellar cuts at any depth together with trephination cuts of the required diameter in both anterior and posterior lamellar keratoplasty. The focusable, near-infrared (1053 nm) laser generated ultrashort pulses in the femtosecond (10-15s) range[5].
The laser creates an increased surface area and interlocking surfaces, which provide more stable and faster healing graft-host interfaces. In addition, the precision of computer-guided cuts allows for accurate and reproducible placement of incisions at desired depths in the corneal stroma[20].
There are several reports in the literature demonstrating improvements in visual acuity of patients with keratoconus that have been treated using femtosecond lasers. The approaches used include femtosecond laser-assisted LK, femtosecond laser-assisted mushroom configuration DALK, femtosecond laser and big-bubble DALK as well as femtosecond laser intracorneal ring implantation[21]–[25]. Femtosecond laser keratoplasty has presented a more predictable and simpler technique that is not limited to corneal shape, thickness, and curvature abnormalities that are commonly found in other LK techniques.
The present study assesses the initial outcomes and safety of DALK assisted by a femtosecond laser for keratoconus and post- LASIK keratectasia. It was impressive to note that all grafts were successfully created in both donor and recipient corneas. Furthermore, an overall improvement in UCVA and BCVA (P<0.05) was recorded following FDALK. FDALK also led to an improvement of corneal transparency, thickness, and shape, restoring the corneal optical integrity and structure. The ability to create a vertical side cut with the femtosecond laser allowed for a better fit at the graft-host junction. Although astigmatism was relatively high postoperatively, we expect that the true potential reduction of astigmatism, as well as overall vision, will become apparent only once the sutures have been removed. Corneal thickness was restored in all cases, with no significant improvement in EDC.
The results are limited by a small sample size (10 eyes), the retrospective nature of the study, as well as the lack of a comparison group of patients treated with automated mechanical microkeratome or PK. Future prospective comparative randomized studies including more patients are needed to address these limitations.
In conclusion, our results suggest that FDALK is a precise, safe, and reproducible alternative to other LK techniques. FDALK in patients with keratoconus seems to be an alternative to conventional anterior LK and PK without significant short-term complications.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No. 81270979); Natural Science Foundation of Jiangsu Province, China (No.BK2012777)
Conflicts of Interest: Lu Y, None; Shi YH, None; Yang LP, None; Ge YR, None; Chen XF, None; Wu Y, None; Huang ZP, None
REFERENCES
- 1.Troutman RC, Lawless MA. Penetrating keratoplasty for keratoconus. Cornea. 1987;6(4):298–305. doi: 10.1097/00003226-198706040-00013. [DOI] [PubMed] [Google Scholar]
- 2.Lawless MA, Troutman RC. The role of penetrating keratoplasty and epikeratoplasty in the surgical management of keratoconus. Aust N Z J Ophthalmol. 1989;17(4):387–393. doi: 10.1111/j.1442-9071.1989.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 3.Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379(9827):1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YM, Wu SQ, Yao YF. Long-term comparison of full-bed deep anterior lamellar keratoplasty and penetrating keratoplasty in treating keratoconus. J Zhejiang Univ Sci B. 2013;14(5):438–450. doi: 10.1631/jzus.B1200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soong HK, Malta JB, Mian SI, Juhasz T. Femtosecond laser-assisted lamellar keratoplasty. Arq Bras Oftalmol. 2008;71(4):601–606. doi: 10.1590/s0004-27492008000400028. [DOI] [PubMed] [Google Scholar]
- 6.Tan DT, Por YM. Current treatment options for corneal ectasia. Curr Opin Ophthalmol. 2007;18(4):284–289. doi: 10.1097/ICU.0b013e3281a7ecaa. [DOI] [PubMed] [Google Scholar]
- 7.Shimmura S, Tsubota K. Deep anterior lamellar keratoplasty. Curr Opin Ophthalmol. 2006;17(4):349–355. doi: 10.1097/01.icu.0000233953.09595.91. [DOI] [PubMed] [Google Scholar]
- 8.Vajpayee RB, Tyagi J, Sharma N, Kumar N, Jhanji V, Titiyal JS. Deep anterior lamellar keratoplasty by big-bubble technique for treatment corneal stromal opacities. Am J Ophthalmol. 2007;143(6):954–957. doi: 10.1016/j.ajo.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 9.Tan DT, Anshu A. Anterior lamellar keratoplasty: ‘Back to the Future’- a review. Clin Experiment Ophthalmol. 2010;38(2):118–127. doi: 10.1111/j.1442-9071.2009.02180.x. [DOI] [PubMed] [Google Scholar]
- 10.Borderie VM, Guilbert E, Touzeau O, Laroche L. Graft rejection and graft failure after anterior lamellar versus penetrating keratoplasty. Am J Ophthalmol. 2011;151(6):1024–1029. doi: 10.1016/j.ajo.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Binder PS, Lindstrom RL, Stulting RD, Donnefeld E, Wu H, McDonnell P, Rabinowitz Y. Keratoconus and corneal ectasia after LASIK. J Refract Surg. 2005;21(6):749–752. doi: 10.3928/1081-597X-20051101-15. [DOI] [PubMed] [Google Scholar]
- 12.Seiler T, Quurke AW. Iatrogenic keratectasia after LASIK in a case of forme fruste keratoconus. J Cataract Refract Surg. 1998;24(7):1007–1009. doi: 10.1016/s0886-3350(98)80057-6. [DOI] [PubMed] [Google Scholar]
- 13.Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010;33(4):157–166. doi: 10.1016/j.clae.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Cassidy D, Beltz J, Jhanji V, Loughnan MS. Recent advances in corneal transplantation for keratoconus. Clin Exp Optom. 2013;96(2):165–172. doi: 10.1111/cxo.12047. [DOI] [PubMed] [Google Scholar]
- 15.Buzzonetti L, Petrocelli G, Valente P. Big-bubble deep anterior lamellar keratoplasty assisted by femtosecond laser in children. Cornea September. 2012;31(9):1083–1086. doi: 10.1097/ICO.0b013e31823f8efc. [DOI] [PubMed] [Google Scholar]
- 16.Richard J, Paton D, Gasset A. A comparison of PK and lamellar keratoplasty in the surgical management of keratoconus. Am J Ophthalmol. 1978;86(6):807–811. doi: 10.1016/0002-9394(78)90126-5. [DOI] [PubMed] [Google Scholar]
- 17.Coullet J, Fournié P, Malecaze F, Arné JL. Inadequate results for microkeratome-assisted additive stromal keratoplasty for management of keratoconus. J Refract Surg. 2008;24(2):166–172. doi: 10.3928/1081597X-20080201-07. [DOI] [PubMed] [Google Scholar]
- 18.Buratto L, Belloni S, Valeri R. Excimer laser lamellar keratoplasty of augmented thickness for keratoconus. J Refract Surg. 1998;14(5):517–525. doi: 10.3928/1081-597X-19980901-09. [DOI] [PubMed] [Google Scholar]
- 19.Eckhardt HB, Hutz WW, Heinrich AW, Kaiser WE. Lamellar keratoplasty with the excimer laser. Initial clinical results. Ophthalmologe. 1996;93(3):242–246. [PubMed] [Google Scholar]
- 20.Chamberlain W, Cabezas M. Femtosecond-assisted deep anterior lamellar keratoplasty using big-bubble technique in a cornea with 16 radial keratotomy incisions. Cornea. 2011;30(2):233–236. doi: 10.1097/ICO.0b013e3181e16db0. [DOI] [PubMed] [Google Scholar]
- 21.Mosca L, Fasciani R, Tamburelli C, Buzzonetti L, Guccione L, Mandarà E, Balestrazzi E. Femtosecond laser-assisted lamellar keratoplasty: early results. Cornea. 2008;27(6):668–672. doi: 10.1097/ICO.0b013e31816736b1. [DOI] [PubMed] [Google Scholar]
- 22.Yoo SH, Al-Ageel S. Femtosecond laser (WaveLight FS200) customized keratoplasty for keratoconus: case report. J Refract Surg. 2012;28(11 Suppl):S826–828. doi: 10.3928/1081597X-20121005-03. [DOI] [PubMed] [Google Scholar]
- 23.Chan CC, Ritenour RJ, Kumar NL, Sansanayudh W, Rootman DS. Femtosecond laser-assisted mushroom configuration deep anterior lamellar keratoplasty. Cornea. 2010;29(3):290–295. doi: 10.1097/ICO.0b013e3181b77873. [DOI] [PubMed] [Google Scholar]
- 24.Buzzonetti L, Laborante A, Petrocelli G. Refractive outcome of keratoconus treated by combined femtosecond laser and big-bubble deep anterior lamellar keratoplasty. J Refract Surg. 2011;27:189–194. doi: 10.3928/1081597X-20100520-01. [DOI] [PubMed] [Google Scholar]
- 25.Jabbarvand M, Salamatrad A, Hashemian H, Mazloumi M, Khodaparast M. Continuous intracorneal ring implantation for keratoconus using a femtosecond laser. J Cataract Refract Surg. 2013;39(7):1081–1087. doi: 10.1016/j.jcrs.2013.02.054. [DOI] [PubMed] [Google Scholar]