Abstract
AIM
To determine the effects of laser photocoagulation on serum levels of angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), soluble angiopoietin receptor Tie-2 (Tie-2), Ang-1/Ang-2 ratio and vascular endothelial growth factor (VEGF) in patients with type 2 diabetes mellitus (T2DM) and proliferative diabetic retinopathy (PDR). We also explored the role of the Ang/Tie system in PDR.
METHODS
160 patients with T2DM, including 50 patients with non-diabetic retinopathy (NDR), 58 patients with non-proliferative diabetic retinopathy (NPDR), and 52 patients with PDR were enrolled in this study. Serum Ang-1, Ang-2, Tie-2 receptor and VEGF levels were measured using enzyme-linked immunosorbent assays for all patients and were repeated in 26 patients who underwent laser photocoagulation two months after the procedure.
RESULTS
The median levels of Ang-2 and VEGF in serum were significantly higher in the NPDR group (4.23 ng/mL and 303.2 pg/mL, respectively) compared to the NDR group (2.67 ng/mL and 159.8 pg/mL, respectively, P<0.01), with the highest level in the PDR group (6.26 ng/mL and 531.2 pg/mL, respectively, P<0.01). The median level of Ang-1 was significantly higher in the NPDR group (10.77 ng/mL) compared to the NDR group (9.31 ng/mL) and the PDR groups (9.54 ng/mL) (P<0.05), while no difference was observed between the PDR and NDR groups. Ang-1/Ang-2 ratio of PDR group was lowest in three groups (1.49 vs 2.69 and 2.90, both P<0.01). The median level of Tie-2 was not significantly different among three groups (P>0.05). Ang-2 was positively correlated with VEGF and Tie-2 in the PDR and NPDR groups (both P<0.05). Among the 26 patients who underwent laser photocoagulation, serum Ang-2 and VEGF levels significantly decreased (both P<0.05), whereas serum Ang-1 level and Ang-1/Ang-2 ratio were weakly increased (P>0.05). The median levels of Ang-2 and VEGF in serum were highest in PDR group, however, Ang-1/Ang-2 ratio of PDR group was lowest in three groups.
CONCLUSION
Laser photocoagulation can reduce serum Ang-2 and VEGF levels. The Ang/Tie system and VEGF play an important role in the development and progression of T2DM patients with PDR.
Keywords: angiopoietin, receptor protein tyrosine kinase, diabetes mellitus, type 2, retinopathy, laser photocoagulation
INTRODUCTION
Diabetic retinopathy (DR) is a common microvascular complication of diabetes. It can lead to blindness and severely impairs the quality of life of diabetic patients. The onset and progression of DR involve various mechanisms including disorders in blood coagulation and hemodynamics, oxidative stress, as well as multiple neovascularization-stimulating cytokines such as vascular endothelial growth factor (VEGF), epidermal growth factor and angiopoietin (Ang)[1]. Of these, VEGF is a key regulatory factor. Microcirculatory disorders result in retinal hypoxia, which ultimately increase VEGF expression via multiple pathways, and thus play a key role in the development of proliferative diabetic retinopathy (PDR). Recent research has shown that Ang is also an important factor that regulates the growth of new blood vessels.
The Ang family consists of a group of angiogenic factors including Ang-1, Ang-2, Ang-3 and Ang-4. All members of this family bind to the tyrosine kinase with immunoglobulin and epidermal growth factor homology domains (Tie-2) on endothelial cells. Ang-1 and Ang-2 have been relatively well studied. Binding of Ang-1 or Ang-2 to the Tie-2 receptor on endothelial cells, induces its phosphorylation, which regulates physiological and pathological vascularization by Ang/Tie pathway[2]–[4]. Joussen et al[5] research showed that, diabetes-associated damage of the blood-retinal barrier was inhibited by Ang-1 in a dose-dependent manner, indicating that Ang-1 has a protective effect against DR[5],[6]. While Ang-2 was found to enhance vascular budding and increase vascular permeability, then causes the pathological changes in retinopathy. They act in an agonistic or antagonistic manner[7]. So, the balance between Ang-1 and Ang-2, together with Ang/Tie signaling pathways, play essential roles in angiogenesis of retina. Recent publication showed that Ang-2 levels were significantly increased in the DR patients, but few involve the relation of Ang-1/Ang-2 ratio with the DR.
The development of new vessels marks the onset of PDR. Recent studies have shown that the expression of Ang-2 and VEGF in the retina are increased in PDR. Ang-2 and VEGF have synergistic effects in terms of promoting vascular permeability and together stimulate retinal neovascularization[8]. According to Peters et al[9], treating pig retinal vascular endothelial cells with Ang-2 or VEGF alone increased the permeability of retinal vascular endothelial cells by 30%-100%. However, when the cells were treated with VEGF and Ang-2 simultaneously, permeability increased by 300%, suggesting that vascular permeability is remarkably increased in the presence of both VEGF and Ang-2.
Laser photocoagulation is currently the most effective way to treat PDR. However, the effects of this procedure on Ang-1, Ang-2 and VEGF remain unclear. Since hypoxia can upregulate the expression of VEGF and Ang-2, we speculated that the improvements in hypoxia following laser photocoagulation may downregulate VEGF and Ang-2 levels. Therefore, in this study, we measured serum levels of Ang-1, Ang-2, Tie-2 receptor, VEGF and calculate the ratio of Ang-1 to Ang-2 before and after laser photocoagulation to investigate the role of the Ang/Tie system in PDR.
SUBJECTS AND METHODS
Patients
A total of 160 inpatients who were treated in our department between February 2011 and January 2012 were enrolled in this study. Type 2 diabetes mellitus (T2DM) was diagnosed based on the 1999 World Health Organization criteria. The staging of retinopathy was based on the DR staging criteria established at the 1985 National Conference on Fundus Disease. DR was divided into non-PDR (NPDR) group and PDR group. Based on these criteria, the patients consisted of 50 patients (30 males and 20 females) with non-DR (NDR) group, 58 patients (32 males and 26 females) with NPDR group, and 52 patients (30 males and 22 females) with PDR group. Twenty-six patients with PDR (14 males and 12 females) underwent laser photocoagulation, and the same parameters were measured 2mo after the procedure (PDR-LP group). DR was confirmed by fundus fluorescein angiography, which was performed by the same ophthalmologist at the Department of Ophthalmology in our hospital. All of the patients were naive to retinal laser photocoagulation. Patients with liver or kidney dysfunction, cancer, cerebrovascular disease (including myocardial infarction, cerebral infarction, and peripheral artery disease), deep vein thrombosis, uncontrolled hypertension (>160/95 mm Hg) or who were using angiotensin-converting enzyme inhibitors were excluded from this study. All patients were fully informed and sign the consent form. The study was approved by the Ethics Committee of Shaoxing People's Hospital.
Methods
Physical examination findings and medical history were recorded for all subjects. After an overnight fast of 10h, the patients were asked to rest for 20min and venous blood samples were taken for biochemical tests, including fasting plasma glucose (FPG), complete blood count, triglyceride (TG), total cholesterol (TC), low density lipoprotein (LDL-C), glycosylated hemoglobin (HbA1c), liver and kidney function. Blood pressure (systolic blood pressure, SBP; diastolic blood pressure, DBP), body weight, and body height were measured, and body mass index (BMI) was calculated. Then 75 g oral glucose tolerance were performed (2h post-load glucose, 2hPG). Ophthalmic examination, fundus fluorescein angiography, electrocardiography, chest radiography, abdominal B-ultrasound scanning and other examinations were also performed.
For measurement of Ang-1, Ang-2, VEGF, and Tie-2, serum samples were separated by centrifuging blood at 2000 r/min for 15min, and stored at -80°C. Enzyme-lined immunosorbent assays (Boster, Wuhan, China) were performed and absorbance measured using a Biochrom Anthos 2010 Microplate Reader (Autobio, Zhengzhou, China). Standard products and quality control products provided in each kit were used to limit measurement error. Other laboratory parameters included TG (glycerol phosphate oxidase method), TC (enzymatic colorimetry), HbA1c (affinity chromatography assay), FPG and 2hPG (glucose oxidase method).
Statistical Analysis
Continuous data are expressed as means±standard deviation. Ang-2 and VEGF levels are represented as medians (IQR) because they were non-normally distributed. Comparisons among three groups were performed using analysis of variance (ANOVA) or Kruskal-Wallis test depending on the distribution of the parameter. Comparisons between two groups were performed using Wilcoxon test. Spearman's correlation test was performed to assess possible association among Ang-1, Ang-2, VEGF and Tie-2 levels. Logistic regression analyses was used to identify factors associated with DR. Data were analyzed using SPSS 13.0 software. Values of P<0.05 were considered statistically significant.
RESULTS
Patient Characteristics
As is shown in Table 1, the age, blood pressure, HbA1c, TC, TG, BMI, FPG and 2hPG were not significantly different among the NDR, NPDR and PDR groups (all P>0.05). However, the duration of diabetes was significantly longer in the PDR group than in the NPDR (P<0.01) and NDR (P<0.05) groups.
Table 1. Patient characteristics.
| Items | NDR (n=50) | NPDR (n=58) | PDR (n=52) | F | P |
| Age (a) | 51.1±12.0 | 53.8±9.9 | 54.2±10.2 | 1.277 | 0.282 |
| Duration of diabetes (a) | 2.52±4.01 | 3.98±3.85 | 6.6±6.71a,c | 8.621 | 0.000 |
| BMI (kg/m2) | 23.6±3.6 | 23.9±3.0 | 23.9±3.5 | 0.151 | 0.860 |
| SBP (mm Hg) | 121±14 | 126±14 | 128±16 | 2.677 | 0.072 |
| DBP (mm Hg) | 73.7±8.1 | 75.3±7.7 | 76.6±8.3 | 1.693 | 0.187 |
| HbA1c (%) | 9.97±2.35 | 9.18±2.37 | 9.01±2.38 | 2.417 | 0.093 |
| FPG (mmol/L) | 11.73±4.21 | 11.45±3.36 | 11.35±4.41 | 0.120 | 0.887 |
| 2hPG (mmol/L) | 22.74±6.14 | 22.53±6.04 | 21.95±5.95 | 0.237 | 0.789 |
| TC (mmol/L) | 5.12±1.25 | 5.05±1.27 | 5.72±2.14 | 2.801 | 0.064 |
| TG (mmol/L) | 1.65±0.90 | 1.69±1.19 | 2.65±3.86 | 2.981 | 0.054 |
| LDL-C (mmol/L) | 2.69±0.78 | 2.66±0.90 | 3.04±1.40 | 2.229 | 0.111 |
aP<0.01 vs NDR; c P<0.05 vs NPDR.
Comparison of Serum Ang-1, Ang-2, VEGF and Tie-2 levels
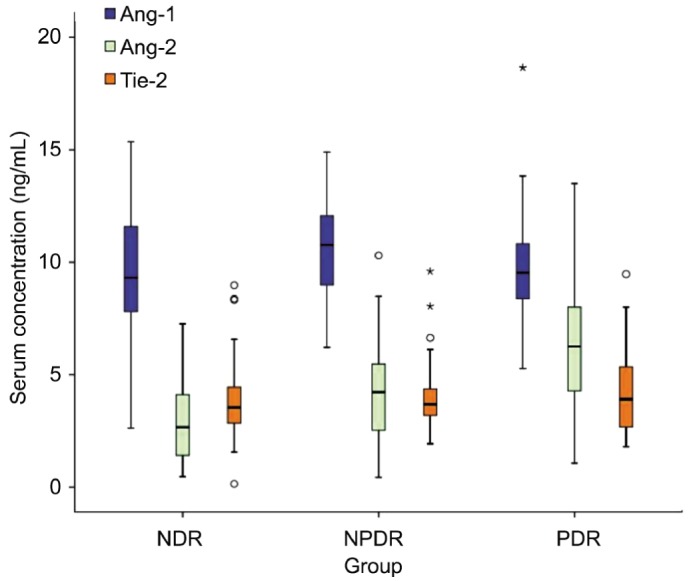
As is shown in Table 2 and Figure 1, Ang-2 and VEGF levels were significantly different among the NDR, NPDR and PDR groups (both P<0.01). The median Ang-2 and VEGF levels in the PDR group (6.26 ng/mL and 531.2 pg/mL, respectively) were significantly higher than those in the NPDR group (4.23 ng/mL and 303.2 pg/mL, respectively) and NDR group (2.67 ng/mL and 159.8 pg/mL, respectively) (all P<0.01). They were also higher in the NPDR group than in the NDR group (both P<0.01). By comparison, the median Ang-1 level was not significantly different between the PDR and NDR groups (9.54 vs 9.31 ng/mL), but was significantly higher in the NPDR group (10.77 ng/mL, P<0.05 vs PDR and NDR). No significant differences were found in Tie-2 levels among the NDR (3.54 ng/mL), NPDR (3.69 ng/mL) and PDR groups (3.91 ng/mL) (P >0.05).
Table 2. Serum Ang-1, Ang-2, VEGF, Tie-2, and Ang-1/Ang-2 ratio levels among the NDR group, NPDR group, and PDR group.
| Items | NDR (n=50) | NPDR (n=58) | PDR (n=52) | Χ2 | P |
| Ang-1 (ng/mL) | 9.31 (7.79-11.64) | 10.77 (9.01-12.07)a | 9.54 (8.64-11.4) | 8.742 | 0.013 |
| Ang-2 (ng/mL) | 2.67 (1.41-4.25) | 4.23 (2.51-5.48)b | 6.26 (4.16-8.01)b,d | 37.033 | 0.000 |
| Ang-1/Ang-2 | 2.90 (1.94-6.40) | 2.69 (1.81-4.45) | 1.49 (1.21-2.20)b,d | 27.955 | 0.000 |
| VEGF (pg/mL) | 159.8 (40.8-376.4) | 303.2 (80.1-527.1)a | 531.2 (299.2-867.6)b,d | 28.541 | 0.000 |
| Tie-2 (ng/mL) | 3.54 (2.84-4.45) | 3.69 (3.18-4.41) | 3.91 (2.65-5.38) | 0.910 | 0.635 |
aP<0.05 and bP<0.01 vs NDR; dP<0.01 vs NPDR.
Figure 1. Serum Ang-2, Ang-1, and Tie-2 levels among the NDR group, NPDR group, and PDR group.
Comparison of the Levels of Ang-1/Ang-2 Ratio
As is shown in Table 2, the ratio of Ang-1/Ang-2 in PDR group (1.49, range 1.21-2.20) were significantly decreased compared to NDR (2.69, range 1.81-4.45) and NPDR group (2.90, range 1.94-6.40), (Z=-4.612, -4.479, respectively, both P<0.01). But was not significantly difference between NPDR and NDR (Z=-0.949, P=0.343). The Ang-1/Ang-2 ratio in PDR group was approximately half that in NDR group.
Correlation Analysis
In PDR group, Spearman correlation analysis showed that Ang-2 was positively correlated with VEGF (r=0.301, P=0.03) and Tie-2 (r=0.278, P=0.046). Similarly, Ang-2 was positively correlated with VEGF (r=0.381, P=0.003) and Tie-2 (r=0.284, P=0.031) in the NPDR group. By comparison, Ang-2 was positively correlated with VEGF (r=0.288, P=0.043) but not with Tie-2 (r=0.084, P=0.573) in the NDR group. Ang-2 was not correlated with age or FPG in any of the three groups.
Effects of Laser Photocoagulation on Ang-1, Ang-2, VEGF, Tie-2 and Ang-1/Ang-2 Ratio Levels
Serum Ang-2 and VEGF levels significantly decreased after laser photocoagulation (both P<0.05; Table 3). In contrast, Ang-1 level and the Ang-1/Ang-2 ratio increased after laser photocoagulation, although this change did not reach statistical significance (P>0.05).
Table 3. Effects of laser photocoagulation on serum Ang-1, Ang-2, VEGF, Tie-2 and Ang-1/Ang-2 ratio levels in patients with PDR.
| Items | Before laser photocoagulation | After laser photocoagulation | Z | P |
| Ang-1 (ng/mL) | 9.57 (8.97-11.56) | 9.93 (8.73-11.18) | -0.826 | 0.409 |
| Ang-2 (ng/mL) | 6.60 (3.13-7.34) | 6.37 (2.55-7.37)a | -2.019 | 0.043 |
| Ang-1/Ang-2 | 1.45 (1.25-2.33) | 1.51 (1.27-2.78) | -1.740 | 0.082 |
| VEGF (pg/mL) | 487.8 (317.9-837.0) | 451.4 (323.4-710.5)a | -2.095 | 0.036 |
| Tie-2 (ng/mL) | 3.05 (2.43-4.03) | 3.04 (2.27-3.66) | -1.689 | 0.091 |
aP<0.05 vs the pre-operative level.
Multivariate Logistic Regression Analysis
Multivariate logistic regression analysis was performed using DR as the dependent variable (yes=1, no=0) and age, duration of diabetes, BMI, SBP, DBP, FPG, 2hPG, HbA1c, TC, TG, Ang-1, Ang-2, Tie-2 and VEGF as independent variables. This analysis revealed that Ang-2 (β=-0.402, P=0.002), VEGF (β=-0.002, P=0.028), duration of diabetes (β=-0.124, P=0.033) and HbA1c (β=-0.106, P=0.043) were independent risk factors for DR.
DISCUSSION
The pathological changes associated with DR include selective loss of retinal capillary pericytes, microaneurysm formation, vascular leakage and neovascularization. The pathogenetic mechanisms are complicated. Recent studies on its pathogenetic mechanisms have mainly focused on growth factors, particularly VEGF and Ang-2. It has consistently been reported that the levels of Ang-2 and VEGF in the retina and serum are increased during DR[10]–[12]. The synergistic actions of Ang-2 and VEGF promote hypoxia-induced retinal neovascularization, a pathological feature of PDR. Although both Ang-2 and VEGF are associated with the severity of retinopathy and their serum levels increase remarkably during PDR, the Ang-1/Ang-2 ratio and the changes of these parameters after laser photocoagulation remain unclear.
In the present study, we found that the serum Ang-2 and VEGF levels were significantly higher in patients with PDR or NPDR than in patients without DR (i.e. NDR group). These levels were also higher in the PDR group than in the NPDR group, suggesting that serum Ang-2 and VEGF levels are associated with the severity of DR. According to Hackett et al[11], the expression of Ang-2 on the surface and in the inner nuclear layer of the retina increased either during embryonic development of the retina and during pathologic neovascularization in adults. Another study revealed that Ang-2 can promote the formation and development of new vessels in retina in response to hypoxia[13]. Hence, Ang-2 is associated with pathologic neovascularization in patients with PDR[14].
Ang-2 and VEGF levels were significantly correlated with Tie-2 level, suggesting that the Ang/Tie signaling system may interact with VEGF during the development of DR. Microcirculatory disorders result in retinal hypoxia, which lead to increased VEGF expression. In addition, VEGF and hypoxia can upregulate the expression of Ang-2[15],[16]. Therefore, VEGF and Ang-2 seem to interact with each other to promote neovascularization. Many studies have demonstrated that the effect of Ang-2 on vessels is dependent on the presence of VEGF[8],[9],[17]. In the absence of VEGF, Ang-2 suppresses the ability of Ang-1 to phosphorylate Tie-2, and thus cause vascular degeneration and endothelial cell apoptosis. In the presence of VEGF, Ang-2 promotes vascularization, which is accompanied by increased vascular permeability. Therefore, the synergistic effects of Ang/Tie and VEGF should be carefully considered when attempting to reduce vascular permeability in DR[18].
We also found that the serum Ang-1 level was significantly higher in patients with NPDR than in those without DR and those with PDR. This suggests that serum Ang-1 levels increase during early DR, which may represent an adaptive compensatory mechanism to promote/support cellular repair, maintain the integrity of endothelial cells, promote the maturation and stabilization of new microvessels, and strengthen the integrity of the vascular structure[5],[19]. Since both of the Ang-1 and Ang-2 compete for the same Tie-2 receptor and cause apposite effects concerning angiogenesis, therefore, it is not sufficient to measure only one angiopoietin[20]. In the study of malignant melanoma, decreased Ang-1/Ang-2 ratio made angiogenesis more easy, and showed progression of the tumor[21]. So we calculated the ratio between the two antagonistic angiooietin, which may be a better parameter to assess the balance of the ongoing angiopoietin process.
According to our measurement, the ratio of Ang-1/Ang-2 began to decline when at NPDR stage, suggesting that the balance between Ang-1 and Ang-2 has already been broken in the early period of DR. With the aggravation of DR, an increased levels of Ang-2 and little changed levels of Ang-1, shift the balance towards angiogenesis. the Ang-1/Ang-2 ratio in PDR group was approximately half that in NDR group. The serum Ang-1 and Ang-1/Ang-2 ratio increased after laser photocoagulation, although postoperative levels were not significantly different from the preoperative levels. As a natural antagonist of Ang-1, Ang-2 can competitively bind to Tie-2, and thus block the vessel-stabilizing effect of Ang-1. In the presence of VEGF, Ang-2 competitively inhibit the effect of Ang-1, disrupting the connection with endothelial cells and increasing vessel permeability and inducing hypoxia-related retinal neovascularization[16]. Increases in the Ang-1/Ang-2 ratio can help vessel stabilization. Therefore, increasing the Ang-1/Ang-2 ratio by increasing Ang-1 or decreasing Ang-2 levels may represent a new therapeutic target for DR. However, the thresholds of the Ang-1/Ang-2 ratio that may benefit vascular stability still need to be established.
Tie-2 levels were not significantly different among the three groups of patients, or between before and after laser photocoagulation. These results indicate that Tie-2 levels may be not associated with the severity of DR.
In our current study, we also found that the serum Ang-2 and VEGF levels significantly decreased after laser photocoagulation in patients with PDR. The change of VEGF is consistent with those reported in other studies[22],[23]. Laser photocoagulation is currently the most effective way to treat PDR. By blocking the retinal neovessels, microangiomas, and diseased capillaries and small vessels, laser photocoagulation can destroy the hypoxic retina, lower its oxygen consumption, and ultimately improve the hypoxic status of the retina. Since hypoxia can upregulate the expression of VEGF and Ang-2, improvements in the hypoxic status after laser photocoagulation may downregulate VEGF and Ang-2 levels. However, we failed to see an increase in Ang-1/Ang-2 raito reached statistic significance after laser photocoagulation, considering that this may relate to the relatively short follow-up time. Furthermore, one study showed that the decrease in serum Ang-2 and VEGF levels after panretinal laser photocoagulation was not statistically significant[24]. One explanation for their finding may be that diabetic patients with retinopathy also have underlying atherosclerosis and/or macroangiopathy, which can affect Ang-2 and VEGF levels. In the current study, we attempted to exclude possible effects of these confounding factors.
Multivariate logistic regression analysis showed that Ang-2, VEGF, duration of diabetes and HbA1c were independent risk factors for DR. Therefore, serum Ang-2 and VEGF levels, duration of diabetes and HbA1c may help clinicians to predict the severity of DR.
In our study, although we tried to avoid possible effects of factors such as hypoxic/ischemic diseases, it is possible that unidentified tumor(s) and underlying ischemic cardiovascular and cerebrovascular diseases may influence the levels of Ang-2 and other markers.
In summary, our study demonstrated that serum Ang-2 and VEGF levels were remarkably increased in T2DM patients with PDR and in those with NPDR compared with patients without DR, and the Ang-1/Ang-2 ratio in PDR group were significantly decreased compared to NPDR group and NDR group. Notably, serum VEGF and Ang-2 levels were downregulated after panretinal laser photocoagulation in diabetic patients with PDR. Therefore, serum Ang-2 and VEGF levels are associated with the severity of PDR in T2DM patients. These results indicate that the Ang/Tie system, together with VEGF, plays an important role in the onset and progression of PDR.
Acknowledgments
Conflicts of Interest: You QY, None; Zhuge FY, None; Zhu QQ, None; Si XW, None.
REFERENCES
- 1.Kinnunen K, Ylä-Herttuala S. Vascular endothelial growth factors in retinal and choroidal neovascular diseases. Ann Med. 2012;44(1):1–17. doi: 10.3109/07853890.2010.532150. [DOI] [PubMed] [Google Scholar]
- 2.Féraud O, Mallet C, Vilgrain I. Expressional regulation of the angiopoietin-1 and -2 and the endothelial-specific receptor tyrosine kinase Tie2 in adrenal atrophy: a study of adrenocorticotropin-induced repair. Endocrinology. 2003;144(10):4607–4615. doi: 10.1210/en.2003-0099. [DOI] [PubMed] [Google Scholar]
- 3.Brkovic A, Pelletier M, Girard D, Sirois MG. Angipoietin chemotactic activities on neutrophils are regulated by PI-3K activation. J Leukoc Biol. 2007;81(4):1093–1101. doi: 10.1189/jlb.0906580. [DOI] [PubMed] [Google Scholar]
- 4.Eklund L, Saharinen P. Angiopoietin signaling in the vasculature. Exp Cell Res. 2013;319(9):1271–1280. doi: 10.1016/j.yexcr.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Joussen AM, Poulaki V, Tsujikawa A, Qin W, Qaum T, Xu Q, Moromizato Y, Bursell SE, Wiegand SJ, Rudge J, Ioffe E, Yancopoulos GD, Adamis AP. Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol. 2002;160(5):1683–1693. doi: 10.1016/S0002-9440(10)61115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nambu H, Nambu R, Oshima Y, Hackett SF, Okoye G, Wiegand S, Yancopoulos G, Zack DJ, Campochiaro PA. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther. 2004;11(10):865–873. doi: 10.1038/sj.gt.3302230. [DOI] [PubMed] [Google Scholar]
- 7.Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, Kutschera S, Bartels S, Appak S, Besemfelder E, Terhardt D, Chavakis E, Wieland T, Klein C, Thomas M, Uemura A, Goerdt S, Augustin HG. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012;122(6):1991–2005. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarlos S, Rizkalla B, Moravski CJ, Cao Z, Cooper ME, Wilkinson-Berka JL. Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. Am J Pathol. 2003;163(3):879–887. doi: 10.1016/S0002-9440(10)63448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters S, Cree IA, Alexander R, Turowski P, Ockrim Z, Patel J, Boyd SR, Joussen AM, Ziemssen F, Hykin PG, Moss SE. Angiopoietin modulation of vascular endothelial growth factor: Effects on retinal endothelial cell permeability. Cytokine. 2007;40(2):144–150. doi: 10.1016/j.cyto.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, Murakami T, Kimura T, Takagi H. Vitreous Levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2005;139(3):476–481. doi: 10.1016/j.ajo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Hackett SF, Ozaki H, Strauss RW, Wahlin K, Suri C, Maisonpierre P, Yancopoulos G, Campochiaro PA. Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184(3):275–284. doi: 10.1002/1097-4652(200009)184:3<275::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Patel JI, Hykin PG, Gregor ZJ, Boulton M, Cree IA. Angiopoietin concentrations in diabetic retinopathy. Br J Ophthalmol. 2005;89(4):480–483. doi: 10.1136/bjo.2004.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai J, Kehoe O, Smith GM, Hykin P, Boulton ME. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49(5):2163–2171. doi: 10.1167/iovs.07-1206. [DOI] [PubMed] [Google Scholar]
- 14.Pfister F, Wang Y, Schreiter K, vom Hagen F, Altvater K, Hoffmann S, Deutsch U, Hammes HP, Feng Y. Retinal overexpression of angiopoietin-2 mimics diabetic retinopathy and enhances vascular damages in hyperglycemia. Acta Diabetol. 2010;47(1):59–64. doi: 10.1007/s00592-009-0099-2. [DOI] [PubMed] [Google Scholar]
- 15.Mandriota SJ, Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res. 1998;83(8):852–859. doi: 10.1161/01.res.83.8.852. [DOI] [PubMed] [Google Scholar]
- 16.Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem. 1999;274(22):15732–15739. doi: 10.1074/jbc.274.22.15732. [DOI] [PubMed] [Google Scholar]
- 17.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 18.Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60(1):9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12(2):235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 20.Hang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10(8):575–585. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 21.Gardizi M, Kurschat C, Riese A, Hahn M, Krieg T, Mauch C, Kurschat P. A decreased ratio between serum levels of the antagonistic angiopoietins 1 and 2 indicates tumour progression of malignant melanoma. Arch Dermatol Res. 2012;304(5):397–400. doi: 10.1007/s00403-012-1228-2. [DOI] [PubMed] [Google Scholar]
- 22.Lip PL, Belgore F, Blann AD, Hope-Ross MW, Gibson JM, Lip GY. Plasma VEGF and soluble VEGF receptor Flt-1 in proliferative retinopathy: relationship to endothelial dysfunction and laser treatment. Invest Ophthalmol Vis Sci. 2000;41(8):2115–2119. [PubMed] [Google Scholar]
- 23.Mahdy RA, Nada WM. Evaluation of the role of vascular endothelial growth factor in diabetic retinopathy. Ophthalmic Res. 2011;45(2):87–91. doi: 10.1159/000317062. [DOI] [PubMed] [Google Scholar]
- 24.Lip P L, Chatterjee S, Caine G J, Hope-Ross M, Gibson J, Blann AD, Lip GY. Plasma vascular endothelial growth factor, angiopoietin-2, and soluble angiopoietin receptor tie-2 in diabetic retinopathy: effects of laser photocoagulation and angiotensin receptor blockade. Br J Ophthalmol. 2004;88(12):1543–1546. doi: 10.1136/bjo.2004.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]


