Abstract
Advanced age is associated with increases in muscle passive stiffness, but the contributors to the changes remain unclear. Our purpose was to determine the relative contributions of muscle fibers and extracellular matrix (ECM) to muscle passive stiffness in both adult and old animals. Passive mechanical properties were determined for isolated individual muscle fibers and bundles of muscle fibers that included their associated ECM, obtained from tibialis anterior muscles of adult (8–12 mo old) and old (28–30 mo old) mice. Maximum tangent moduli of individual muscle fibers from adult and old muscles were not different at any sarcomere length tested. In contrast, the moduli of bundles of fibers from old mice was more than twofold greater than that of fiber bundles from adult muscles at sarcomere lengths >2.5 μm. Because ECM mechanical behavior is determined by the composition and arrangement of its molecular constituents, we also examined the effect of aging on ECM collagen characteristics. With aging, muscle ECM hydroxyproline content increased twofold and advanced glycation end-product protein adducts increased threefold, whereas collagen fibril orientation and total ECM area were not different between muscles from adult and old mice. Taken together, these findings indicate that the ECM of tibialis anterior muscles from old mice has a higher modulus than the ECM of adult muscles, likely driven by an accumulation of densely packed extensively crosslinked collagen.
Keywords: muscle mechanics, passive tension, collagen, age crosslinking, tangent modulus
the extracellular matrix (ECM) of muscle plays a vital role in the transmission of force produced by muscle fibers to the tendon (32, 36). To perform this function effectively, the ECM must remain intact during large strains associated with lengthening and shortening of muscle (31), and proper functioning of the ECM is largely determined by the mechanical properties of the tissue. Therefore, changes in ECM mechanical properties have the potential to influence both force transmission to the skeleton and mobility of the organism. Aging is associated with declines in skeletal muscle force generation (7) and increases in muscle passive stiffness (1). The decreased muscle force is due to a combination of reduced fiber size and number along with qualitative changes within the remaining fibers that reduce force generating capacity (15), but the precise contributors to increased muscle stiffness with age remain unclear.
Whole muscle stiffness is determined by the properties of both muscle fibers and ECM, with ECM hypothesized to be the major contributor (30, 34). We showed previously that in mice, tibialis anterior (TA) tendon stiffness increases dramatically with age, in particular in the region near the muscle (40). Because muscle ECM represents a functional extension of the tendon (26), a reasonable hypothesis is that similar age-related changes in the mechanical properties of muscle ECM would be observed. ECM mechanical properties are determined by the underlying composition, which in tendon is primarily fibrillar collagen. With aging, a dramatic slowing of the turnover of type I collagen (19, 38) allows for the accumulation of posttranslational modifications of collagen molecules including advanced glycation end products (AGEs). AGEs can form permanent molecular crosslinks that stiffen collagen fibrils (33), and age-related tendon stiffening is associated with increased concentration of AGE crosslinking (3). Similar changes may also play an important role in altering the mechanical characteristics of muscle ECM with age.
Given the practical difficulty in isolating and mechanically testing muscle ECM in mammals, the effects of aging on muscle ECM properties have not been thoroughly investigated. Previous estimates of ECM stiffness for muscles of young adult mice have compared mechanical properties of single muscle fibers with those of muscle fiber bundles that include fibers and their surrounding ECM (22). We aimed to expand upon those results by determining the relative contributions of fibers and ECM to the passive stiffness of muscle from both adult and old mice and by assessing the underlying collagen characteristics of the ECM. We tested the specific hypotheses that muscle ECM stiffens with age and that the increased stiffness correlates with greater collagen and AGE protein adduct content.
MATERIALS AND METHODS
Animals.
Male C57BL/6 mice 8–12 (adult, n = 21) and 28–30 (old, n = 12) mo of age were obtained from Charles River Laboratories (Wilmington, MA) and housed under specific-pathogen-free conditions with food and water provided ad libitum. To obtain tissues for experimentation, mice were anesthetized with an intraperitoneal injection of tribromoethanol (250 mg/kg), and the tibialis anterior (TA) muscles were removed. Muscles were divided with some prepped for mechanical experiments and others snap frozen at −80°C for biochemical analysis or frozen in Tissue Tek for histology. Inasmuch as extraction of the TA muscle with the epimysium intact cannot be assured, the epimysium was removed completely from the muscles to eliminate it as a source of variability. Therefore, our histological and biochemical data are from ECM that is within the muscle (i.e., the perimysium and endomysium). Immediately following removal of the muscles, mice were killed with an overdose of anesthetic followed by administration of a bilateral thoracotomy. All experimental procedures were approved by the University of Michigan Committee for the Use and Care of Animals.
Muscle fiber/bundle isolation and storage.
The solutions used in the fiber and bundle experiments have been described previously (9). TA muscles used for mechanical experiments were immediately placed in cold permeabilizing solution and grossly divided into bundles ∼4–6 mm in length and 0.5 mm in diameter. After dissection, bundles were incubated for 30 min in permeabilizing solution to which the nonionic detergent Brij 58 (0.5% wt/vol) had been added. Bundles were then placed in storage solution and maintained for 16 h at 4°C followed by storage at −80°C. On the day of an experiment, fiber bundles were removed from storage solution and placed in relaxing solution on ice. For single fiber experiments, individual fibers were gently pulled from a bundle with fine forceps and transferred to an experimental chamber containing relaxing solution maintained at 15°C. For experiments on fiber bundles, stored bundles were further trimmed to a diameter of ∼300 μm using microdissecting spring scissors, taking care to preserve the integrity of all fibers remaining in the bundle. The trimmed bundles were then transferred to an experimental chamber containing relaxing solution maintained at 15°C.
Single fiber experiments.
Twenty-one adult fibers from three animals and twelve old fibers from two animals were tested. Single fiber contractility experiments were performed using techniques modified from Claflin et al. (9). Briefly, one end of the fiber was secured to a force transducer (Aurora Scientific, Model 403A) using two ties of 10–0 monofilament nylon suture. The other end of the fiber was attached in a similar manner to the lever arm of a servomotor (Aurora Scientific, model 322C). The solution-changing system (Aurora Scientific, model 802A) consisted of six separate glass-bottom chambers machined into a moveable, temperature-controlled stainless-steel plate. The length of the fiber was adjusted to obtain a sarcomere length of 2.5 μm, determined by projecting a laser diffraction pattern produced by the fiber onto a calibrated target screen. A sarcomere length of 2.5 μm was chosen as the reference length because it corresponds to the length at which isometric force is maximum in rodent skeletal muscle (12, 27). Fiber length (Lf) was determined with sarcomere length maintained at 2.5 μm. Subsequently, all sarcomere lengths were set by changing the length of the fiber to Lf × (new sarcomere length)/2.5. This approach results in sarcomere lengths that are accurate to within 5% of the target value in relaxed fibers (25). Fiber cross-sectional area (CSA) was estimated with fiber length at Lf using fiber width and depth measurements from high-magnification digital images of both top and side views of the fiber. Side views were obtained using a prism embedded in the side of the chamber. Five width-depth measurement pairs were obtained at 100-μm intervals along the midsection of the fiber. Fiber CSA was calculated for each width-depth pair assuming an elliptical cross section, and overall CSA was estimated by averaging the five individual areas.
Relaxed single fibers were activated by first immersing them in a chamber containing a low-[Ca2+] preactivating solution for 3 min and then immersing them in a separate chamber containing high-[Ca2+] activating solution (pCa ∼4.5) to elicit maximum isometric force (Fo). The preactivating solution was weakly buffered for Ca2+, which results in rapid activation and force development upon introduction of the activating solution (23). Specific force was calculated as Fo/CSA.
After maximum calcium activation, fibers were returned to relaxing solution and adjusted to a length corresponding to a mean sarcomere length of 2.1 μm. The relaxed fiber was then conditioned with a series of five constant-velocity (“ramp”) lengthening movements applied at a velocity of 1 Lf/s and sufficient in magnitude to increase mean sarcomere length from 2.1 to 3.2 μm. A 2-min refractory period was maintained between conditioning ramps. The conditioning was performed to ensure that all fibers had a common strain history before assessment of passive tension characteristics because passive tension responses are affected by strain history (25). Passive tension responses were then recorded during a series of five ramp stretches (1 Lf/s) designed to increase sarcomere length by 0.2, 0.4, 0.6, 0.8, and 1.0 μm. All stretches were initiated from a sarcomere length of 2.1 μm. After each stretch, force was monitored while the fiber was held at the new length for 2 min and the force level reached at the end of the 2-min hold was recorded (Fig. 1).
Fig. 1.
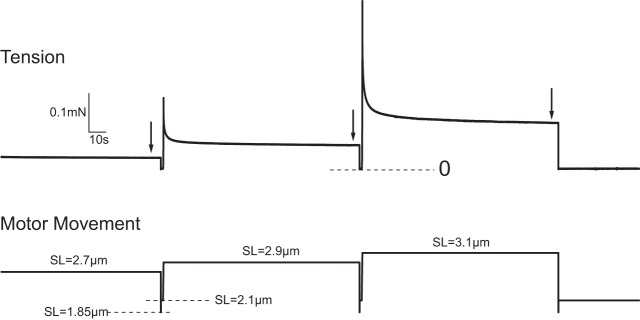
Representative passive tension responses of mouse tibialis anterior fiber to step length increases. Five stretches were applied at a velocity of 1 fiber length (Lf/s), all from a resting sarcomere length (SL) of 2.1 μm and increasing in 0.2 μm/sarcomere increments, resulting in a mean sarcomere length of 3.1 μm after the final stretch. Detailed records for the final 2 stretches are shown. Tension was measured 2 min after each stretch as indicated by the arrows. Fibers were forced to slacken for 50 ms immediately after each tension measurement to indicate the location of zero on the tension record. Fibers were then returned to a sarcomere length of 2.1 μm and held there for 1.3 s before the next stretch.
Bundle experiments.
Eight adult fiber bundles from two animals and eight old bundles from five animals were tested. The experiments on bundles of fibers were conducted as described for single fibers and illustrated in Fig. 1, except that there was no activation of the bundles at the beginning of the experiment. To accommodate the higher passive forces associated with the fiber bundle experiments, a force transducer with a higher maximum force limit was used (Aurora Scientific, model 400A).
Immunohistochemical analysis.
Five TA muscles from each age group were sectioned at a thickness of 10 μm in a cryostat. Sections were permeabilized with 0.2% Triton X-100 and incubated with wheat germ agglutinin conjugated to AlexaFluor 488 (WGA, Invitrogen) to identify the ECM and DAPI to visualize cell nuclei. Slides were mounted in ProLong Gold (Invitrogen) and imaged using a Zeiss Apotome fluorescence microscope with an 8 megapixel camera. Five random images were taken at ×20 for each sample, and the total number of green pixels (i.e., WGA-stained ECM tissue) was determined with a custom MATLAB program. Total ECM area was determined by dividing the number of green pixels by the total number of pixels in the image.
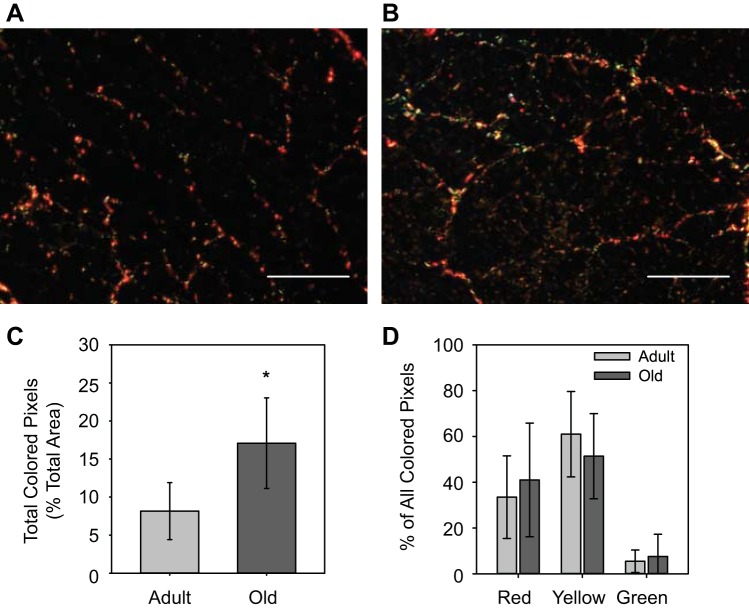
Picrosirius red staining was performed on 10-μm-thick frozen muscle sections by incubating in 0.2% phosphomolybdic acid for 2 min, 0.1% picrosirius red for 90 min, and 0.01 N HCl for 2 min followed by drying and mounting in Permount. All samples were imaged using an Olympus BX51 microscope with an 8-megapixel camera under linearly polarized light. In muscle cross sections, picrosirius red-stained collagen appears red if the fibrils are oriented at large angles to the muscle fibers and yellow and green if the fibrils are oriented more parallel with the muscle fibers (2). A custom MATLAB program was used to determine the number of pixels of each color present in each image. The distribution of red, yellow, and green pixels was determined by dividing the number of pixels of each individual color by the total number of colored pixels in each image.
AGE protein adducts.
Five TA muscles from five adult mice and five TA muscles from five old mice were finely homogenized and digested into peptides using a solution of 40 mg/ml proteinase K in PBS at 55°C. The concentration of AGE protein adducts was determined as previously described (17).
Hydroxyproline content.
A hydroxyproline assay was performed as described previously (21). Briefly, five flash-frozen TA muscles from each age group (five animals per group) were thawed, dried, and then digested using 6.0 N hydrochloric acid overnight at 110°C. Hydroxyproline content was determined using a colorimetric assay (39).
Mechanics data analysis.
Fiber and bundle passive tension values were divided by corresponding CSA measurements to generate passive length-stress curves. Length-stress curves were then converted to strain-stress curves using a sarcomere length 2.5 μm as the reference length. Sarcomere lengths of 2.1, 2.3, 2.5, 2.7, 2.9, and 3.1 μm thus corresponded to strains of −0.16, −0.08, 0.00, 0.08, 0.16, and 0.24, respectively. A third-order polynomial was fitted to the strain-stress responses of each fiber or bundle (R2 ≥ 0.985 for all calculations) and the slope of the fitted curve (“tangent modulus,” referred to hereafter simply as “modulus”) was calculated at strains of 0, 0.08, 0.16, and 0.24 by evaluating the first derivative of the fitted curve at those strain levels.
Statistical analysis.
Results are presented as mean ± SD. Statistical analysis was performed using JMP software (SAS Institute). Differences in mechanical properties for the four experimental groups (fibers and bundles, adult and old mice) were determined at each strain level using analysis of variance (ANOVA). In cases where the ANOVA indicated significance, individual differences were determined using a Tukey's honestly significant difference post hoc test. Differences in mean values for ECM structural and compositional characteristics were determined with a Student's t-test. Significance was set at P < 0.05.
RESULTS
Active and passive mechanics of single fibers.
Maximum calcium-activated specific force for permeabilized TA muscle fibers was unchanged with aging (adult: 109 ± 14 kPa, n = 21; old: 98 ± 15 kPa, n = 12). The passive stress-strain relationships for permeabilized fibers were nonlinear, exhibiting a slope that increased with increasing sarcomere length (Fig. 2A). No differences were observed in single fiber passive stresses between the two age groups at any of the sarcomeres lengths tested.
Fig. 2.
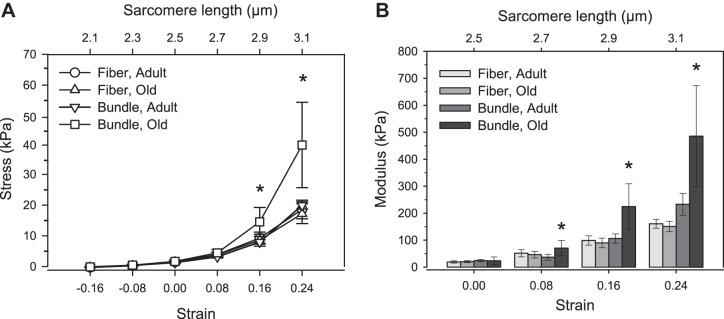
Stress-strain responses (A) and tangent moduli (B) for single fibers and fiber bundles from adult and old TA muscles. At long sarcomere lengths, old muscle bundles exhibited larger stresses than old single fibers, indicating increased bundle modulus at sarcomere lengths at 2.7, 2.9, and 3.1 μm. Moduli of adult fibers and bundles were similar, and single fiber modulus was unchanged with age. *Significantly different from all other groups at a given strain (P < 0.05). Data presented as means ± SD.
Passive mechanics of fiber bundles.
The stress responses of bundles from adult mice were not different from those of individual fibers from either adult or old mice at any of the tested sarcomere lengths (Fig. 2A). In contrast, old fiber bundles exhibited significantly higher stress levels than bundles from adult mice for sarcomere lengths greater than 2.7 μm. At sarcomere lengths of 3.1 μm, corresponding to the highest strain applied in these experiments, the average stress level in bundles from old mice was 96% greater than the average stress level in bundles from adult mice.
Modulus of single fibers and bundles.
Moduli of single fibers of adult and old mice as well as bundles of fibers from adult mice were all similar at all sarcomere lengths (Fig. 2B). In contrast to single fibers, the moduli of bundles of fibers from old mice were higher than those from adult mice at all sarcomere lengths >2.5 μm (Fig. 2B).
Morphology of muscle ECM.
Representative images from adult and old TA muscle cross sections immunostained with WGA and DAPI are shown in Fig. 3. WGA stains for general ECM components, including collagens and proteoglycans, and DAPI marks cell nuclei. Total ECM area revealed with WGA remained unchanged with age (adult: 18.4 ± 1.7%, old: 18.8 ± 1.8%), indicating no overall age-associated hypertrophy of ECM components. Figure 4, A and B, show representative cross-sections of muscles from adult and old mice, respectively, stained with picrosirus red and imaged under polarized light. The total number of colored pixels in each image increased with aging from 8.2 ± 3.7% area colored to 17.1 ± 6.0% area colored (Fig. 4C), suggesting an age-associated increase in total collagen content in the muscle cross sections. When analyzed as a percent of all colored pixels, the distribution of red, yellow, and green pixels remained constant with aging, indicating no age-associated change in collagen orientation (Fig. 4D).
Fig. 3.
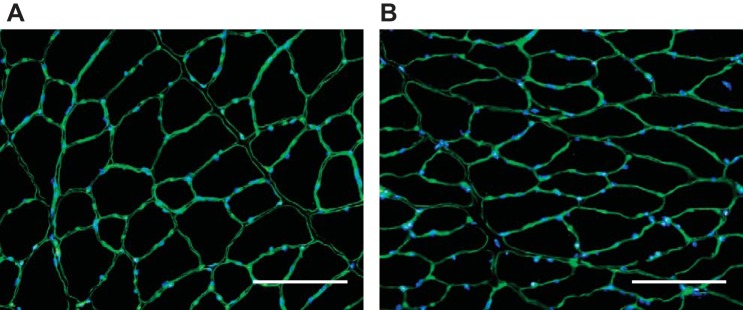
Representative adult (A) and old (B) tibialis anterior muscle cross sections immunostained with wheat germ agglutinin and DAPI for visualization of extracellular matrix (ECM) and cell nuclei, respectively. Total ECM area remained unchanged with age (P = 0.79). Scale bars = 75 μm.
Fig. 4.
Representative cross sections of tibialis anterior (TA) muscles of adult (A) and old (B) mice stained with picrosirius red and viewed under polarized light. Red staining indicates collagen fibrils that are oriented at a large angle with respect to the muscle fiber, and yellow and green staining indicates collagen fibrils that are more parallel to the fibers. Scale bars = 200 μm. Total number of colored pixels increased with aging, suggesting an age-associated increase in total collagen content in the muscle cross sections (C). When analyzed as a percent of all colored pixels, the distribution of red, yellow, and green pixels remained constant with aging, indicating no age-associated change in collagen orientation (D). *Significantly different from adult group (P < 0.05). Data presented as means ± SD.
Collagen content and crosslinking.
Hydroxyproline content in muscles from old mice was significantly elevated compared with muscles from adult mice (1.13 ± 0.38 vs. 0.49 ± 0.30 μm/mg wet muscle mass; Fig. 5A). The concentration of AGE protein adducts in TA muscles also increased with aging from 690 ± 251 to 1,490 ± 620 pg/mg muscle (Fig. 5B).
Fig. 5.
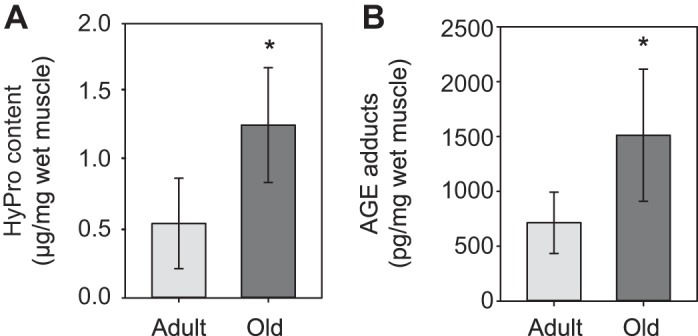
Hydroxyproline (A) and advanced glycation end product (AGE) protein adduct (B) concentration in whole TA muscles. Old muscles had increased hydroxyproline content and AGE adduct concentration compared with muscles from adult animals. *Significantly different from adult group (P < 0.05). Data presented as means ± SD.
DISCUSSION
The main findings of the present study are that 1) the intrinsic stiffness, or modulus (stiffness/CSA), of individual muscle fibers from adult and old muscles are not different; 2) the modulus of fiber bundles from old muscles is more than twofold greater than that of fiber bundles from adult muscles; and 3) collagen content and AGE protein adduct concentration are increased with aging in muscles independent of a change in collagen fibril orientation or significant hypertrophy of total muscle ECM components. Taken together, these findings support the conclusions that, in tibialis anterior muscles of adult mice, the moduli of ECM and muscle fibers are similar, but an accumulation of densely packed extensively crosslinked collagen with aging results in a significant increase in the modulus of the ECM. The similarity in modulus for fibers and ECM of muscles from adult animals indicates that the relative contribution of the two components to stiffness during passive stretch is simply proportional to the fractional cross-sectional areas of each component, i.e., 18% ECM and 82% fibers. Thus a large majority of the passive stiffness in muscles of adult animals reflects the properties of the muscle fibers. The higher modulus of the ECM in muscles from old mice coupled with no changes with aging in fiber modulus indicates that the contribution of ECM to stiffness is much greater than that of fibers on a “per cross-sectional area” basis. The threefold greater modulus (at the highest strain tested, sarcomere length 3.1 μm) of fiber bundles from muscles of old compared with isolated fibers and the observation that ECM occupies ∼25% as much area as the fibers indicates that the modulus of the ECM actually increased by ∼12-fold. Using the rule of mixtures (22), the corresponding contributions of ECM and muscle fibers to the passive stiffness of muscles of old mice is 73% ECM and 27% fibers. The consequences of this shift with aging in which structures bear the load during passive stretch are not known, but the magnitude of the change suggests the potential for important functional effects.
Muscle stiffness has been shown to increase with aging in both humans (6) and rodents (16, 28), although the basis for the increase is unclear. Considering a simple mechanical model of muscle as muscle fibers in parallel with ECM, the increase in muscle stiffness must be caused by an increase in fiber stiffness, ECM stiffness, or both. Muscle ECM in mammals is extremely difficult to isolate and mechanically test. As such, little data exist regarding mechanical properties of muscle ECM and how those properties are altered in aging. Estimates have been made in cardiac muscle by determining the mechanical properties of the tissue before and after enzymatic degradation of collagen (e.g., Ref. 20). These methods, however, may produce incomplete digestion that is not completely specific for ECM (for review, see Ref. 13). In skeletal muscle, Gao and colleagues (14) successfully isolated epimysium of rat TA muscles and observed an age-associated increase in stiffness of the tissue via uniaxial tensile testing. The present study expands upon those results by demonstrating an age-related increase in stiffness in fiber bundles containing intact ECM, with no increase in the stiffness of isolated fibers. Our data are in contrast to previous reports of a sixfold greater modulus of muscle fiber bundles compared with single fibers from adult mice as well as a linear stress-strain response of single fibers (22). The basis for these apparent discrepancies is unclear, but numerous methodological differences exist between the studies. In the present study, we report the tangent modulus at four different strains, with a maximum strain of 24%, whereas the previous study (22) reports quadratic modulus with maximum strains of 100%. Additional differences between the previous and present studies include the muscle that was analyzed, the strains of mice that were used, and the ages of the mice, all of which may contribute to the observed discrepancies.
Our results indicate that the modulus of muscle ECM increases with age. ECM mechanical properties are determined by the composition of the underlying components, the most abundant of which is fibrillar collagen. Collagen has an exceptionally long half-life, which makes it highly susceptible to the accumulation of glucose-mediated permanent intermolecular AGE crosslinks (38). AGE crosslinks stiffen the collagen fibrils (29), and AGE crosslink concentration increases significantly with age in muscle (18, 36). Collagen concentration also increases in muscle with aging (1, 16). Consistent with these previous reports, the present data show that the observed increase in ECM modulus with age is accompanied by increased collagen and AGE protein adduct concentration exclusive of significant hypertrophy of the ECM. We also examined muscle cross sections stained with picrosirius red, which gives an indication of the anisotropy of collagen fibrils when viewed under polarized light. Collagen fibrils that are aligned parallel with the muscle fiber axis appear green, whereas fibrils that are at a greater angle relative to the muscle fiber appear yellow and red (2). Because the load applied to a collagen fibril is determined by the cosine of the angle between the muscle axis and the fibril axis, fibrils that are more aligned with the muscle bear a greater proportion of the applied load, effectively increasing ECM stiffness (2). The present data show that collagen fibril orientation is unchanged with aging in TA muscles of mice, suggesting that age-related changes in ECM mechanical properties result from changes in the intrinsic material properties of the ECM. Given that WGA is a general stain for all ECM components and picrosirius red predominantly stains type 1 collagen, the data imply that with age, muscle ECM is becoming more densely packed with highly crosslinked collagen, likely contributing to the increased ECM modulus and muscle stiffness seen in aging.
Muscle ECM is directly continuous with tendon tissue. Consequently, the similarity between the age-associated increase in stiffness observed in the present study for TA muscle ECM and the increase in stiffness we reported previously for mouse TA tendons with aging (40) may not be surprising. Given that the muscle ECM and tendon form a functional link that transfers force from muscle fibers to the skeleton, any change in the mechanical properties of either tissue has the opportunity to influence the functional performance of the muscle-tendon unit, leading to altered functional outcomes. The precise implications of the age-related changes in stiffness reported in the present and our previous studies, whether protective or harmful, are still unclear. Increased stiffness of the muscle-tendon unit may indeed facilitate more effective force transmission from muscle contractile elements to the bone (5); however, it may also impair balance stability in the elderly (4) and decrease range of motion. During lengthening contractions, sarcomere lengths are stabilized by the lateral transmission of force from the muscle fibers to the ECM (31), and changes in ECM mechanical properties may contribute to the impaired lateral transmission of force seen in old age (32). Increased stiffness of muscle ECM may also serve the protective role of limiting the amount of stretch a fiber undergoes during a lengthening muscle contraction. As such, future investigations are warranted to determine the precise influence of age-associated increases in ECM stiffness on functional performance of the muscle-tendon unit.
Our study is not without limitations. Ideally, ECM mechanics would be determined by directly measuring the properties of isolated ECM tissue. Isolated ECM is very delicate, however, and isolation of ECM without tissue damage is extremely difficult. As such, the present study estimates ECM properties by comparing the properties of single fibers and fiber bundles. Given that the modulus of groups of fibers with their associated ECM removed is similar to that of single fibers (22), we are confident that this approach to determining the material properties of ECM is valid. Although great care was taken during isolation of fibers and fiber bundles, the possibility of some tissue damage cannot be discounted. Finally, the extent to which the present findings generalize to all muscles in all species is not known. For example, in contrast to the increase in stiffness we observed for mouse TA tendons with aging (40), an age-associated decrease in the stiffness of human Achilles tendon has been reported (11, 24, 35) and the stiffness of human patellar tendons is unchanged with aging (8, 10). Thus the effect of age on the mechanical properties of tendons as well as muscle ECM is likely dependent on anatomy, loading, and patterns of use, which may vary across species even for a particular muscle. Additionally, ECM properties are dependent on muscle fiber type (29). Thus the conclusions from this study may not generalize to muscles that do not contain primarily fast fibers.
In summary, we demonstrated that aging results in increased modulus of fiber bundles but no increase in the modulus of single fibers. We also demonstrated that increased modulus is accompanied by an increase in hydroxyproline content and AGE protein adducts. Coupled with the findings that the ECM occupies the same fractional area in adult and old muscle, we conclude that the ECM of old muscles is more densely packed with highly crosslinked collagen and has a higher modulus than the ECM of adult muscles. Changes in ECM mechanical properties have the potential to influence force transmission to the skeleton, susceptibility to contraction-induced muscle damage, and the ability of the muscle to respond to alterations in external loading.
GRANTS
Financial support for the work was provided by National Institute of Arthritis and Musculoskeletal Skin Diseases Grant AR055624 to SVB and National Institute on Aging Grant AG000114 that provided fellowship support to LKW.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.K.W. and E.K. performed experiments; L.K.W., E.K., D.R.C., and S.V.B. analyzed data; L.K.W., E.K., J.P.G., C.L.M., D.R.C., and S.V.B. interpreted results of experiments; L.K.W. and D.R.C. prepared figures; L.K.W. and D.R.C. drafted manuscript; L.K.W., E.K., J.P.G., C.L.M., D.R.C., and S.V.B. edited and revised manuscript; L.K.W., E.K., J.P.G., C.L.M., D.R.C., and S.V.B. approved final version of manuscript; D.R.C. and S.V.B. conception and design of research.
REFERENCES
- 1.Alnaqeeb MA, Alzaid NS, Goldspink G. Connective tissue changes and physical properties of developing and aging skeletal muscle. J Anat 139: 677–689, 1984 [PMC free article] [PubMed] [Google Scholar]
- 2.Arruda EM, Mundy K, Calve S, Baar K. Denervation does not change the ratio of collagen I and collagen III mRNA in the extracellular matrix of muscle. Am J Physiol Regul Integr Comp Physiol 292: R983–R987, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev 106: 1–56, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Baudry S, Lecoeuvre G, Duchateau J. Age-related changes in the behavior of the muscle-tendon unit of the gastrocenemius medialis during upright stance. J Appl Physiol 112: 296–304, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Bojsen-Moller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol 99: 986–994, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Blanpied P, Smidt GL. The difference in stiffness of the active plantarflexors between young and elderly human females. J Gerontol 48: M58-M63, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol 105: 1907–1915, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claflin DR, Larkin LM, Cederna PS, Horowitz JF, Alexander NB, Cole NM, Galecki AT, Chen S, Nyquist LV, Carlson BM, Faulkner JA, Ashton-Miller JA. Effects of high- and low-velocity resistance training on the contractile properties of skeletal muscle fibers from young and older humans. J Appl Physiol 111: 1021–1030, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couppe C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol 107: 880–886, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Csapo R, Malis V, Hodgson J, Sinha S. Age-related greater Achilles tendon compliance is not associated with larger plantar flexor muscle fascicle strains in senior women. J Appl Physiol 116: 961–969, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edman KA. Contractile properties of mouse single muscle fibers, a comparison with amphibian muscle fibers. J Exp Biol 208: 1905–1913 [DOI] [PubMed] [Google Scholar]
- 13.Fomovsky GM, Thomopoulos S, Holmes JW. Contribution of extracellular matrix to the mechanical properties of the heart. J Mol Cell Cardiol 48: 490–496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao YX, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech 41: 465–469, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61: 1059–1064, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Gosselin LE, Adams C, Cotter TA, McCormick RJ, Thomas DP. Effect of exercise training on passive stiffness in locomotor skeletal muscle: role of extracellular matrix. J Appl Physiol 85: 1011–1016, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Gumucio JP, Korn MA, Saripalli AL, Flood MD, Phan AC, Roche SM, Lynch EB, Claflin DR, Bedi A, Mendias CL. Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg 23: 99–108, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 103: 2068–2076, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kostrominova TY, Brooks SV. Age-related changes in structure and extracellular matrix protein expression levels in rat tendon. Age (Dordr) 35: 2203–2214, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacKenna DA, Omens JH, McCulloch AD, Covell JW. Contribution of collagen matrix to passive left ventricular mechanics in isolated rat hearts. Am J Physiol Heart Circ Physiol 266: H1007–H1018, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Mendias CL, Marcin JE, Calerdon DR, Faulkner JA. Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol 101: 898–905, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer GA, Lieber RL. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. J Biomech 44: 771–773, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moisescu DG, Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in external bathing solution. J Physiol 275: 241–262, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol 100: 2048–2056, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Palmer ML, Claflin DR, Faulkner JA, Panchangam A. Non-uniform distribution of strain during stretch of relaxed skeletal muscle fibers from rat soleus muscle. J Muscle Res Cell Motil 32: 39–48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passerieux E, Rossignol R, Letellier T, Delage JP. Physical continuity of the perimysium from myofibers to tendons: involvement in lateral force transmission in skeletal muscle. J Struct Biol 159: 19–28, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Philips SK, Woledge RC. A comparison of isometric force, maximum power, and isometric heat rate as a function of sarcomere length in mouse skeletal muscle. Pflügers Arch 420: 578–583, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Plate JF, Wiggins WF, Haubruck P, Scott AT, Smith TL, Saul KR, Mannava S. Normal aging alters in vivo passive biomechanical response of the rat gastrocenemius-Achilles muscle-tendon unit. J Biomech 46: 450–455, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Prado LG, Makarenko I, Andresen C, Kruger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol 126: 461–480, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purslow PP. Strain-induced reorientation of an intramuscular connective tissue network—implications for passive muscle elasticity. J Biomech 22: 21–31, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Purslow PP. The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol A Physiol 133: 947–966, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, Faulkner JA. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol 589: 1195–1208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit Achilles tendon. Exp Diabesity Res 5: 143–153, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowe RWD. Morphology of perimysial and endomysial connective tissue in skeletal muscle. Tissue Cell 13: 681–690, 1981 [DOI] [PubMed] [Google Scholar]
- 35.Stenroth L, Peltonen J, Cronin NH, et al. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol 113: 1537–1544, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Street SF. Lateral transmission of tension in frog myofibers - a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol 114: 346–364, 1983 [DOI] [PubMed] [Google Scholar]
- 37.Takahashi M, Hoshino H, Kushida K, Inoue T. Direct measurement of crosslinks, pyridinoline, deoxypyridinoline, and pentosidine, in the hydrolysate of tissues using high-performance liquid chromatography. Anal Biochem 232: 158–162, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Thorpe CT, Streeter I, Pinchbeck GL, Goodship AE, Clegg PD, Birch HL. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J Biol Chem 285: 15674–15681, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woessner JF. Determination of hydroxyproline in tissue and protein samples containing small properties of this imino acid. Arch Biochem Biophys 93: 440–447, 1961 [DOI] [PubMed] [Google Scholar]
- 40.Wood LK, Arruda EM, Brooks SV. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol 111: 999–1006, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]