Abstract
The exercise pressor reflex (EPR) is generated by group III and IV muscle afferents during exercise to increase cardiovascular function. Muscle contraction is triggered by ACh, which is metabolized into choline that could serve as a signal of exercise-induced activity. We demonstrate that ACh can induce current in muscle afferents neurons isolated from male Sprague-Dawley rats. The nicotinic ACh receptors (nAChRs) appear to be expressed by some group III-IV neurons since capsaicin (TRPV1) and/or ATP (P2X) induced current in 56% of ACh-responsive neurons. α7- And α4β2-nAChRs have been shown to be expressed in sensory neurons. An α7-nAChR antibody stained 83% of muscle afferent neurons. Functional expression was demonstrated by using the specific α7-nAChR blockers α-conotoxin ImI (IMI) and methyllycaconitine (MLA). MLA inhibited ACh responses in 100% of muscle afferent neurons, whereas IMI inhibited ACh responses in 54% of neurons. Dihydro-β-erythroidine, an α4β2-nAChR blocker, inhibited ACh responses in 50% of muscle afferent neurons, but recovery from block was not observed. Choline, an α7-nAChR agonist, elicited a response in 60% of ACh-responsive neurons. Finally, we demonstrated the expression of α7-nAChR by peripherin labeled (group IV) afferent fibers within gastrocnemius muscles. Some of these α7-nAChR-positive fibers were also positive for P2X3 receptors. Thus choline could serve as an activator of the EPR by opening α7-nAChR expressed by group IV (and possible group III) afferents. nAChRs could become pharmacological targets for suppressing the excessive EPR activation in patients with peripheral vascular disease.
Keywords: exercise pressor reflex, α4β2, choline, TRPV1, P2X
the exercise pressor reflex (EPR) is a neural mechanism that regulates cardiovascular adjustments to exercise (Kaufman and Hayes 2002). Certain disease states such as peripheral vascular disease (claudication) can activate this reflex to drive the cardiovascular system inappropriately (Lorentsen 1972). A better understanding of the ion channels and receptors that activate this reflex could lead to the development of drugs that would suppress the reflex to normalize cardiovascular function and reduce myocardial infarction risk in patients with peripheral vascular disease. Prior investigations have shown functional expression of transient receptor potential channels (TRPV1, TRPA1; Koba et al. 2011; Smith et al. 2010) and ionotropic purinergic receptors (P2X3) by group III and/or IV muscle afferent neurons (Hanna and Kaufman 2003; Hayes et al. 2008).
ACh is released from efferent terminals to initiate muscle contraction and is rapidly metabolized into acetic acid and choline (Katz and Miledi 1973). Thus high choline levels could serve as a signal for intense muscle activity to initiate the EPR. Nicotinic ACh receptors (nAChRs) have been shown to be expressed in dorsal root ganglia (DRG) neurons (Genzen et al. 2001) but have not been investigated in muscle afferent neurons. Interestingly, one of the nAChRs (α7) expressed by DRG neurons (Genzen et al. 2001) is activated by choline (Alkondon and Albuquerque 2006). We demonstrate that muscle afferent neurons express functional nAChRs and that the α7-nAChR is a dominant receptor type. In addition, we show that α7-nAChRs are expressed by group IV afferent fibers within gastrocnemius muscle. Thus ACh and/or choline could serve as chemical activator(s) of the EPR.
MATERIALS AND METHODS
All animal protocols were approved by the Kirksville College of Osteopathic Medicine Institutional Animal Care and Use Committee and were consistent with the National Research Council Guide for the Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats (Hilltop Lab Animals, Scottsdale, PA) weighing between 150 and 400 g were used in these experiments. Rats were housed in a U.S. Department of Agriculture-approved, Association for Assessment and Accreditation of Laboratory Animal Care-certified animal care facility at a constant temperature of 24 ± 1°C, under controlled 12:12-h light-dark cycles, and with access to commercial rat chow and water ad libitum.
Labeling and isolation of DRG neurons.
To label fluorescently the muscle afferent neurons within the DRG, male Sprague-Dawley rats were anesthetized by intraperitoneal injection of sterile 50 mg/kg ketamine, 5 mg/kg xylazine, and 1 mg/kg acepromazine. Once anesthetized, the hair over the calf area of both legs was shaved, and 100 μl of a 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Life Technologies, Grand Island, NY) solution (1.5% in DMSO; Ramachandra et al. 2012) was injected into both the left and right gastrocnemius muscles (Smith et al. 2006).
Four to five days were allowed for transport of DiI from the muscle to the somata of muscle afferents in the DRG. Rats were killed using CO2 followed by decapitation using a laboratory guillotine (Kent Scientific, Torrington, CT; Ramachandra et al. 2012). The lumbar 4 (L4) and L5 DRG were isolated from both sides and dissociated in Earle's balanced salt solution containing 7 mg of collagenase (Worthington Biochemical, Lakewood, NJ), 10 mg of trypsin, and 1 mg of DNase at 35°C for 60 min (Ramachandra et al. 2012). Dissociated neurons were plated in MEM supplemented with 10% fetal bovine serum, 1% glutamine, and 1% penicillin-streptomycin solution (all from Life Technologies) and stored in a humidified atmosphere of 5% CO2 in air at 37°C until use (typically 12–24 h later; Ramachandra et al. 2012).
Solutions.
The extracellular solution contained, in mM, 45 NaCl, 100 N-methyl-d-glucamine (NMG)-Cl, 4 MnCl2, 10 Na-HEPES, and 10 glucose, with pH 7.4 and osmolarity of 320 mosM. The lower extracellular Na+ (50 vs. 145 mM) allows for better voltage-clamp control of the neuron when recording voltage-dependent sodium (NaV) currents. Mn2+ was used to replace Ca2+ as it does not readily permeate calcium channels (Ramachandra et al. 2012) and still maintains membrane surface charge screening (Zhou and Jones 1995). The intracellular solution contained, in mM, 104 NMG-Cl, 14 creatine-PO4, 6 MgCl2, 10 NMG-HEPES, 5 Tris-ATP, 10 NMG2-EGTA, and 0.3 Tris2-GTP with pH 7.4 and osmolarity of 300 mosM.
Depending on hydrophobicity, agonist and antagonist solutions were made up as stock solutions in either water or DMSO. Stock solutions were then diluted into the external solution to make the working concentrations used in experiments. Solutions were delivered via a gravity-fed perfusion system to provide a rapid exchange of solutions around the neuron (1–2 s). To achieve the fastest solution exchange, the neuron from which we recorded was positioned directly under the 200-μm-diameter tip of the flow pipe. Methyllycaconitine (MLA) was obtained from Abcam (Cambridge, MA), whereas α-conotoxin ImI (IMI) and dihydro-β-erythroidine (DHβE) were from Tocris Bioscience (Minneapolis, MN). Unless otherwise stated, all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Patch-clamp system.
Recorded currents were measured using an Axopatch 200A amplifier (Molecular Devices, Sunnyvale, CA) and digitized using an ITC-18 analog-to-digital converter (InstruTECH, Port Washington, NY) after analog filtering with the four-pole low-pass Bessel filter of the amplifier. Glass microelectrodes were fabricated from 8250 glass (inside diameter 0.90 mm, outside diameter 1.5 mm; King Precision Glass, Claremont, CA) using a Flaming/Brown P-97 electrode puller (Sutter Instrument, Novato, CA), and electrodes were polished using a custom microforge. The polished electrodes typically had a resistance of 1–3 MΩ. The series resistance was compensated by at least 80% using the electronic circuits of the Axopatch 200A amplifier. Bright fluorescently labeled, isolated neurons with no obvious neuronal outgrowths were chosen for the experiments (Ramachandra et al. 2012).
A Power Macintosh computer (Apple Computer, Cupertino, CA) running S5 data acquisition software written by Dr. Stephen R. Ikeda (National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD) was used to control the experiments. Traces used to identify NaV channels expressed by muscle afferent neurons were “leak subtracted” using averaged and scaled hyperpolarizing steps of 1/4 amplitude. All experiments were conducted at room temperature and with a holding potential of −80 mV.
Data analysis.
Patch-clamp data were analyzed using custom routines written with IGOR Pro (WaveMetrics, Lake Oswego, OR). Cell capacitance, measured by the Axopatch circuitry, was used to calculate the somal diameter, assuming a specific capacitance of 1 μF/cm2 and that the neuron was spherical (Ramachandra et al. 2012). Statistical significance between two groups was determined using either Student's t-test or an ANOVA plus Tukey honestly significant difference post hoc test. Significance was defined as P < 0.05.
Immunostaining.
For immunocytochemistry, neurons were fixed with 4% formaldehyde and permeabilized with 2% Tween 20 as previously described (Ramachandra et al. 2012). Neurons were incubated overnight with primary antibodies for α7-nAChR (rabbit, 1:500; Alomone Labs, Jerusalem, Israel) and visualized using secondary antibodies Alexa Fluor 488 IgG goat anti-rabbit (Life Technologies; Ramachandra et al. 2012). Images were captured using a Nikon Eclipse 80i epifluorescence microscope, and neurons were measured using ImageJ (http://rsbweb.nih.gov/ij/index.html). Cell size was calculated, and positive fluorescent labeling was determined as described previously (Ramachandra et al. 2012).
For immunohistochemistry, rats were killed as described above, and both gastrocnemius muscles were dissected out along with the tendons. The muscles were washed in ice-cold PBS solution and flash-frozen in dry ice-cooled isopentane. The muscles were kept frozen at −80°C until use. Frozen muscles were cut longitudinally in 25-μm sections using a Leica CM1900 cryostat (Leica Microsystems, Buffalo Grove, IL). The sections were mounted on polylysine-coated slides, allowed to dry, postfixed with 4% formaldehyde, and permeabilized with 2% Triton X-100. The slides were incubated with blocking solution for 1 h followed by overnight incubation with the primary antibodies chicken polyclonal anti-peripherin (1:1,000; Aves Labs, Tigard, OR), rabbit polyclonal anti-α7-nAChR (1:100; Abcam), and guinea pig polyclonal anti-P2X3R (1:100; EMD Millipore, Billerica, MA). The sections were washed with PBS and incubated for 1 h in secondary antibodies anti-chicken FITC (1:200; Aves Labs), anti-rabbit Alexa Fluor 633 (1:250; Life Technologies), and anti-guinea pig Alexa Fluor 546 (1:500; Life Technologies). The sections were visualized and images captured using the Nikon epifluorescence microscope.
RESULTS
Ionotropic receptors in muscle afferent neurons.
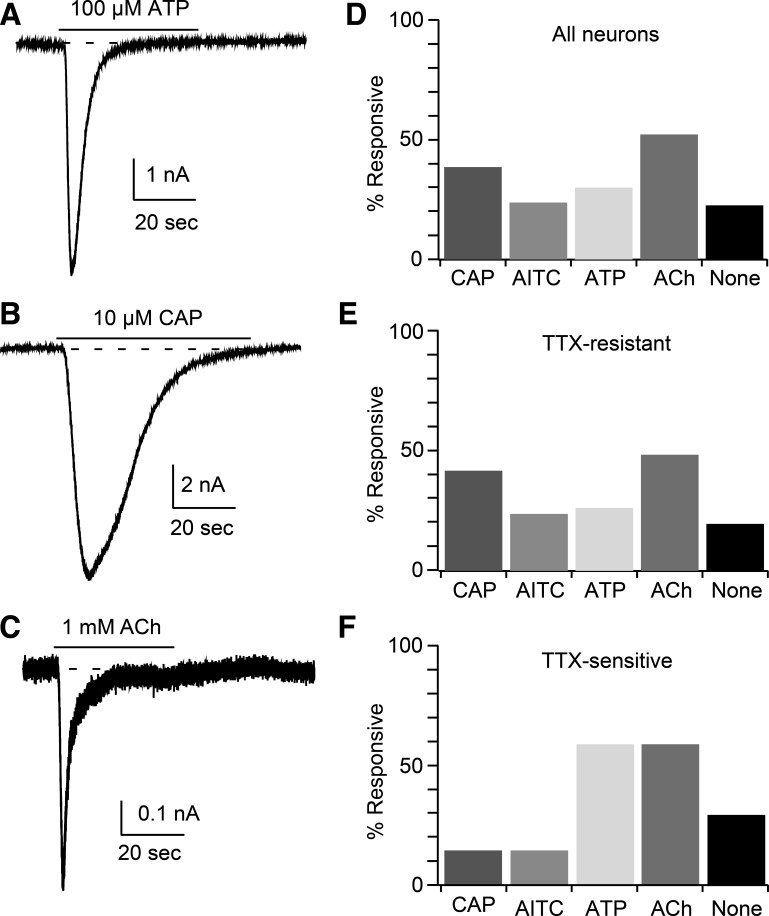
To determine whether muscle afferent neurons express nAChR, we applied 1 mM ACh to DiI-positive sensory neurons and found ACh-induced current in 52% (27/52; Figs. 1 and 2A) of these neurons. We wanted to determine whether the ACh-sensitive neurons included group III and/or IV muscle afferents, which mediate the EPR. These neurons have previously been demonstrated to express TRPV1, TRPA1, or P2X receptors (Gao et al. 2007; Hayes et al. 2008; Koba et al. 2011; Ro et al. 2009; Smith et al. 2010). In the 52 neurons, we measured responses to application of selective activators 10 μM capsaicin (CAP; TRPV1; Smith et al. 2010) and 100 μM ATP (P2X; Hayes et al. 2008). In 32 of the ACh-tested neurons, we also applied 100 μM allyl isothiocyanate (AITC; TRPA1; Koba et al. 2011; Figs. 1 and 2C). Of the total population (ACh-responsive and nonresponsive) examined, 38% of muscle afferent neurons responded to CAP, 29% to ATP, and 25% to AITC. Twenty-one percent of the tested neurons failed to respond to any of the applied agonists, including ACh (Fig. 1D). Of ACh-responsive muscle afferent neurons, 59% responded to one or more of the other agonists, with 33% (9/27) responding to CAP, 41% (11/27) responding to ATP, and 35% (7/20) to AITC (Fig. 1 and Table 1). Some ACh-sensitive muscle afferent neurons responded to multiple agonists, with 20% (4/20) sensitive to all 3 agonists (Table 1). The coexpression of TRPV1, TRPA1, and/or P2X receptors with nAChRs suggests that group III and IV neurons can express nAChRs.
Fig. 1.
Muscle afferent neurons express nicotinic ACh receptors (nAChRs). A–C: example currents recorded from muscle afferent neurons in response to 100 μM ATP (A), 10 μM capsaicin (CAP; B), and 1 mM ACh (C). The application of each agonist is indicated by the horizontal bar above each trace. D–F: the percentage of responsive neurons is shown for 3 groups, which are all muscle afferent neurons tested (D; n = 52), neurons expressing TTX-resistant voltage-dependent sodium (NaV) current (E; n = 45), and neurons expressing TTX-sensitive NaV current (F; n = 7). “None” indicates muscle afferent neurons that failed to respond to any of the 4 applied activators. The agonist concentrations are listed above. AITC, allyl isothiocyanate.
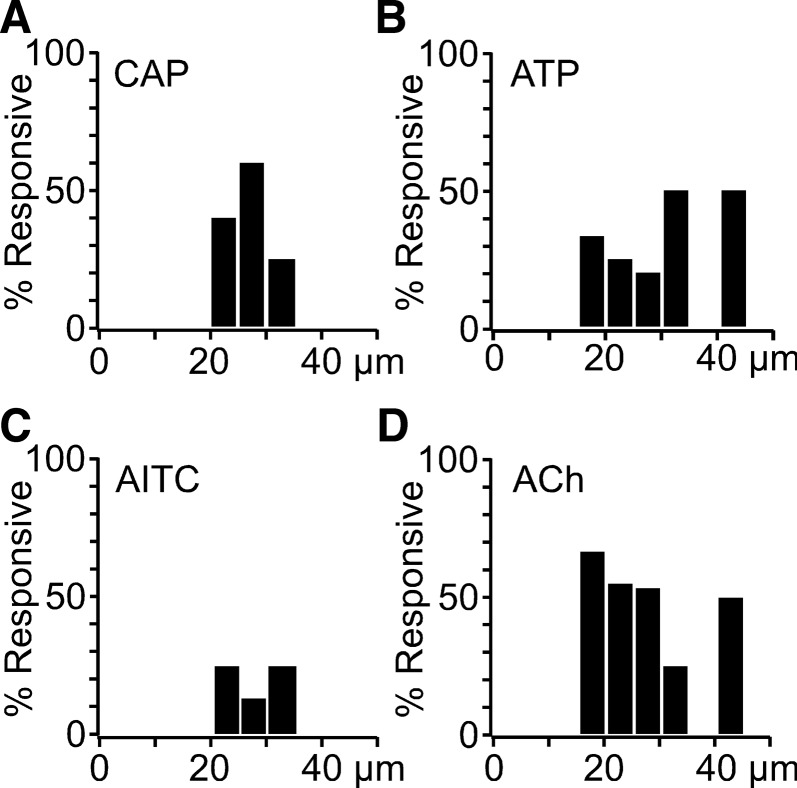
Fig. 2.
The majority of agonist-responsive neurons have diameters <40 μm. These data are from TTX-resistant neurons (n = 45). The histograms show the percentage of neurons responding to CAP (A), ATP (B), AITC (C), and ACh (D) vs. neuronal diameter binned into 5-μm bins. One CAP-sensitive neuron was found to have a diameter >50 μm but is not shown since only bins with 2 or more neurons were included. The agonist concentrations are listed in Fig. 1 legend.
Table 1.
The responsiveness of ACh-sensitive muscle afferent neurons to CAP, ATP, and AITC
| Agonist | +CAP | +ATP | +AITC | All 3 Agonists | %Agonist Sensitive | ACh-Sensitive Neurons Tested |
|---|---|---|---|---|---|---|
| CAP | 4* | 1 | 0 | 4 | 33.3% | 27 |
| ATP | 1 | 4* | 2 | 4 | 40.7% | 27 |
| AITC | 0 | 2 | 1* | 4 | 35.0% | 20 |
The number of neurons sensitive to the 1 agonist in addition to ACh.
CAP, capsaicin; AITC, allyl isothiocyanate.
Another classification scheme that we used was based on TTX sensitivity of NaV current in muscle afferent neurons (Ramachandra et al. 2012). We have previously reported that the NaV current (holding potential −80 mV) in 86% of these neurons was blocked <30% by 300 nM TTX (TTX-resistant), whereas the current in the remaining 14% of muscle afferent neurons was blocked by >90% (TTX-sensitive; Ramachandra et al. 2012). We wondered whether the neurons within these 2 groups were differentially responsive to the agonists described above. Of the 52 neurons examined, 45 (87%) were TTX-resistant, and 7 (13%) were TTX-sensitive, which matches our previous results (Ramachandra et al. 2012). Of the TTX-resistant neurons recorded, 23/45 (51%) responded to ACh, 19/45 (42%) responded to CAP, 11/45 (24%) respond to ATP, and 7/28 (25%) responded to AITC (Fig. 1B). Although our sample size of TTX-sensitive neurons was small, this population appeared to be less responsive to the TRP channel activators CAP (1/7, 14%) and AITC (1/7, 14%) compared with TTX-resistant muscle afferent neurons (Fig. 1C). However, responses to both ACh (4/7, 57%) and ATP (4/7, 57%) were similar to those of TTX-sensitive neurons (Fig. 1C). It appears that the TTX-resistant muscle afferent neurons preferentially express TRPV1 and TRPA1 channels.
Neuronal cell size.
A third method that can be used to distinguish sensory neurons is by somal diameter. We and others have found that muscle afferent neurons have larger diameters (Hu and McLachlan 2003; Ramachandra et al. 2012) compared with cutaneous afferents (Djouhri et al. 2003). We have defined group IV neurons as those with diameters <30 μm and group III neurons as those with diameters from 30 to 40 μm (Ramachandra et al. 2012). Of the seven TTX-sensitive neurons, one had a diameter of 51 μm (ACh- and ATP-sensitive), one had a diameter of 32 μm (ACh-, ATP-, and AITC-sensitive), and the remaining five neurons had diameters between 20 and 30 μm (group IV range). Of these five neurons, two failed to respond to any of the four agonists tested, one responded to both ACh and CAP, one to both ACh and ATP, and one responded only to ATP.
For the TTX-resistant neurons (n = 45), diameters ranged from 17 to 51 μm. Three neurons had diameters >40 μm with two between 40 and 45 μm and one at 52 μm. The majority (n = 42) of these muscle afferent neurons had diameters between 20 and 35 μm. To compare the somal size distribution for each response type, we generated histograms (5-μm bin width) of neuronal diameters (Fig. 2). The diameters of most CAP-responsive neurons ranged from 20 to 35 μm (Fig. 2A) with one neuron having a diameter of 51 μm (data not shown). Neurons responsive to ATP ranged from 17 to 42 μm in diameter (Fig. 2B) with 50% in the 30- to 35-μm range. One of the two neurons in the 40- to 45-μm range was ATP-sensitive, whereas the other was ACh-sensitive. The majority of ACh-responsive neurons ranged from 17 to 35 μm in diameter with most <30 μm (Fig. 2D). Twenty-eight TTX-resistant neurons were tested with 100 μM AITC, and responsive neurons had diameters that ranged from 22 to 33 μm (Fig. 2C). The size range for ACh-responsive neurons suggests that the majority were group IV with fewer in the range for group III afferents.
nAChR identification.
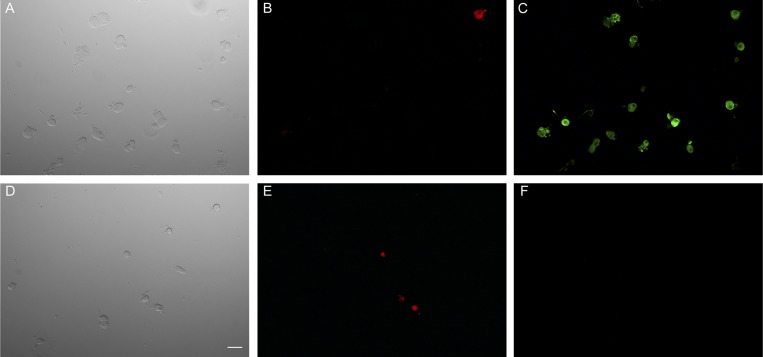
Multiple genes generate nAChR that are differentially expressed in the nervous systems, and these nAChRs can have distinctive response properties. Sensory neurons have been shown to express several nAChR subunits including α3, α4, α6, α7, β2, β3, and β4 (Genzen et al. 2001). α7-nAChR are widely expressed in sensory neurons (Genzen et al. 2001; Haberberger et al. 2004; Rau et al. 2005). As a first step, we used immunocytochemistry to determine expression of α7-nAChR. An α7-nAChR antibody labeled 11 of 13 muscle afferent neurons (Fig. 3), which ranged in diameter from 27 to 90 μm. Of the 7 neurons with diameters <40 μm (group III or IV size range), 6 (86%) were positively labeled by the antibody. Given the prominent expression of these receptors, we tested α7-nAChR antagonists and an agonist on ACh-sensitive muscle afferent neurons.
Fig. 3.
Immunocytochemistry shows α7-nAChRs expressed by muscle afferent neurons. A–C: the top 3 images show “test” sensory neurons that were stained with the antibody. The bright-field image (A) shows the neurons within the field, and the 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) panel (B) shows 1 labeled muscle afferent neuron. The majority of neurons were positive for α7-nAChR (C). D–F: the bottom 3 images show the controls for antibody staining (F) along with the bright-field image (D) and the DiI image (E) showing 3 labeled muscle afferent neurons. The white bar in D indicates 50 μm.
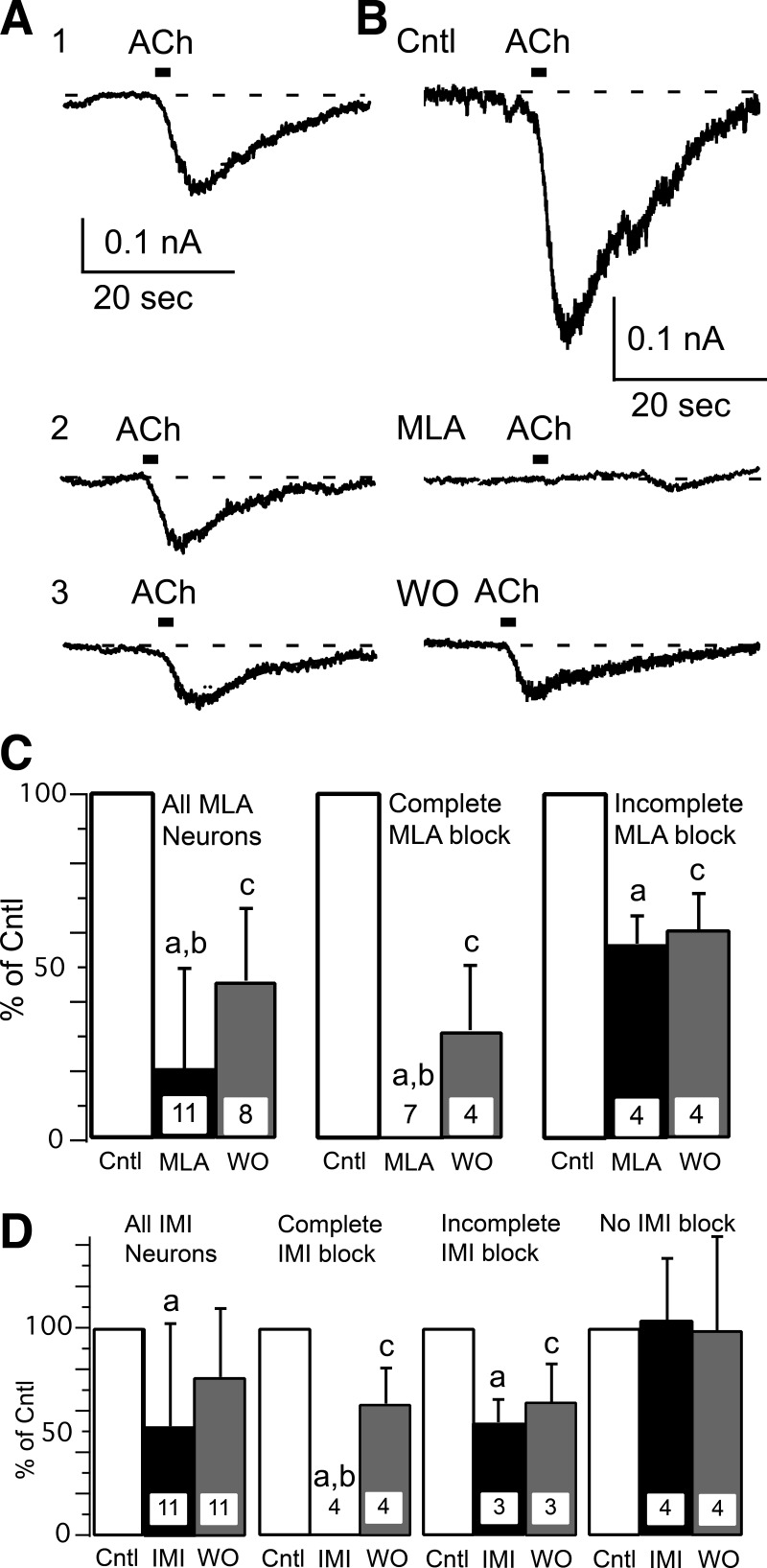
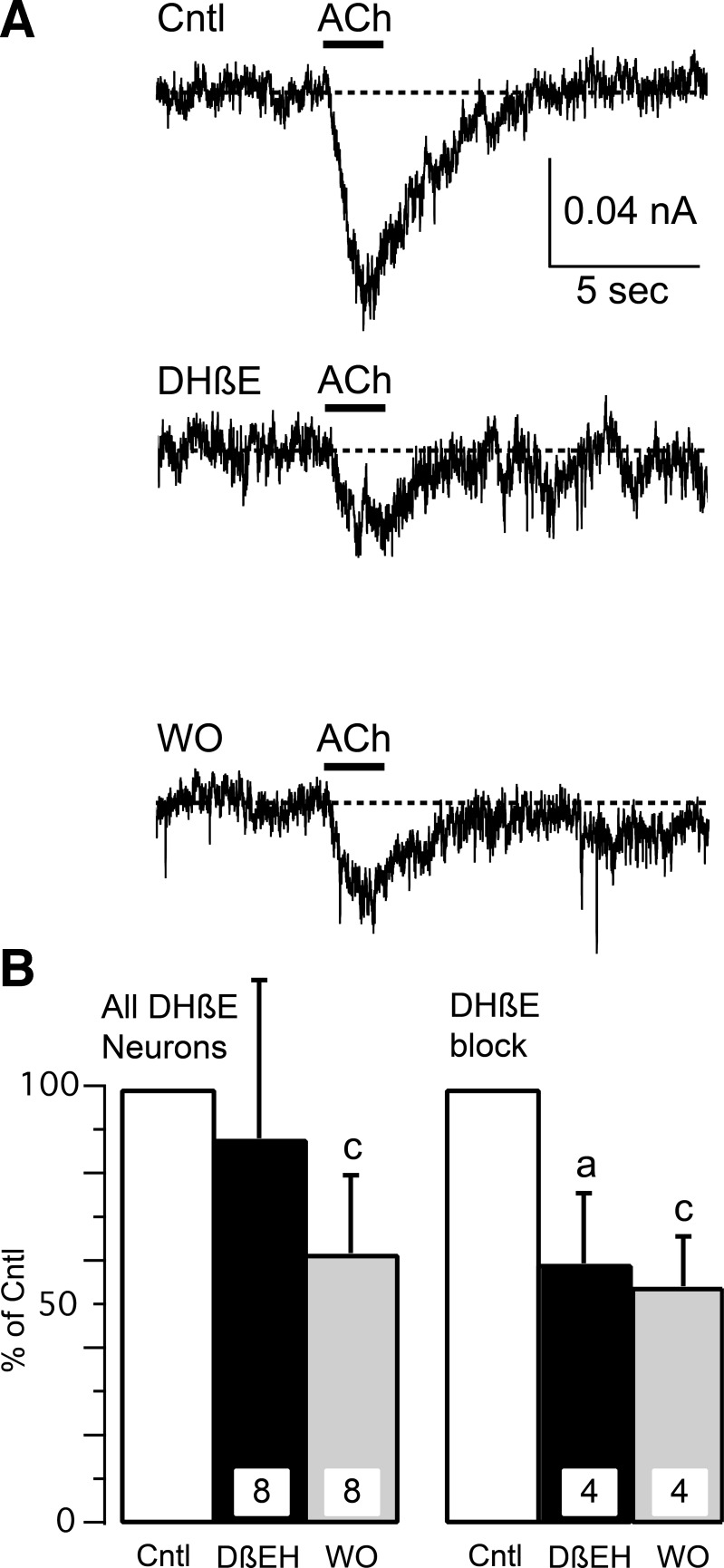
Two α7-nAChR antagonists were used with very different chemical structures. MLA is small organic molecule that completely blocks α7-nAChR at concentrations ≥10 nM (Zappettini et al. 2011). IMI is a small peptide that completely blocks α7-nAChR at concentrations ≥100 μM (Johnson et al. 1995). ACh responses were tested on the same muscle afferent neuron before, during, and on washout of the antagonist (MLA or IMI). This presented a potential problem since ACh responses desensitize. We used two strategies to enhance our ability to record multiple ACh responses within the same neuron. The first was that ACh was applied as a 2-s pulse (vs. 20-s applications that were used for the earlier results), and, second, the ACh pulses were applied at 3-min intervals. Using this protocol, we were able to obtain stable ACh responses from single neurons (Fig. 4A). MLA (10 nM) significantly blocked ACh responses in all 11 muscle afferent neurons tested (Fig. 4, B and C), which is consistent with at least part of the ACh response being mediated by α7-nAChR. Closer examination of the records showed that these 11 neurons could be separated into 2 groups by the magnitude of MLA block. MLA completely blocked the current in 7 neurons but only partially blocked current in the remaining 4 neurons (Fig. 4C). This suggests that the majority (64%) of these neurons exclusively expressed α7-nAChR, whereas the other neurons expressed α7- along with at least one other nAChR type. Partial recovery from MLA block was recorded in 8 neurons, but the other 3 neurons were lost before recovery could be measured. The neuronal diameters of all 11 neurons were <40 μm, with 5 neurons having diameters <30 μm, which suggests that group III and IV neurons can express α7-nAChR. Similarly to MLA, ACh responses were significantly blocked by IMI (1 μM; Fig. 4D). However, the effect of IMI on ACh responses was more diverse than that observed by MLA. Of the 11 muscle afferent neurons tested, ACh responses in 4 neurons were unaffected by IMI, whereas the ACh responses in 4 of 11 neurons were completely blocked and 3 of 11 neurons were partially blocked (Fig. 4D). Thus 7 of the 11 neurons tested appeared to express α7-nAChR, with 4 muscle afferent neurons exclusively expressing α7-nAChR. A different nAChR type was expressed by 7 of 11 neurons tested by IMI, with 4 neurons apparently expressing no α7-nAChR. All 7 of the IMI-sensitive neurons had diameters between 24 and 29 μm, as did 2 of the 4 insensitive neurons. The 2 other IMI-resistant neurons had diameters of 33 and 39 μm.
Fig. 4.
ACh responses can be blocked by specific α7-nAChR antagonists. A: multiple ACh responses can be measured with limited desensitization, which was minimized by applying ACh (1 mM) for 2 s once every 3 min. An example is shown from 1 control neuron for 3 (1–3) ACh responses recorded at 3-min intervals. B: the specific α7-nAChR antagonist methyllycaconitine (MLA; 10 nM, 3-min application) completely blocked ACh-induced current in this muscle afferent neuron. A partial recovery was recorded after 3 min of MLA washout (WO). C: these bar graphs show percentage block and WO for 10 nM MLA. The left set of bar graphs show mean (+ SD) for all neurons tested with MLA, whereas the middle graph shows the 7 neurons in which MLA completely blocked the ACh response, and the right graph shows the remaining 4 neurons in which the block was partial. Note that 3 neurons were lost before recovery from MLA block could be recorded. D: bar graphs show the percentage block of ACh responses and WO for 100 μM α-conotoxin ImI (IMI). The left set of bar graphs show mean block (+ SD) for all neurons tested with IMI, whereas the middle graphs show the 4 neurons in which IMI completely blocked (middle left) and 3 neurons in which IMI partially blocked (middle right) the ACh response. The right graph shows the remaining 4 neurons in which IMI did not affect the ACh response. The ACh concentration was 1 mM. The lowercase letters above the bars indicate significant differences with “a” indicating significant difference between test (MLA or IMI) and control (Cntl), “b” indicating significant difference between test and WO, and “c” indicating significant difference between WO and Cntl.
The incomplete block of the ACh response by MLA and IMI suggests that non-α7-nAChRs are expressed by some muscle afferent neurons, and previous publications have shown expression of α4β2-nAChR by sensory neurons (Genzen et al. 2001; Haberberger et al. 2004). We investigated α4β2-nAChR using the specific blocker DHβE at a concentration of 1 μM (Fig. 5), which is 5–10 times the IC50 for block of α4β2-nAChR (Iturriaga-Vásquez et al. 2010; Karadsheh et al. 2004). When averaged from all 8 neurons tested, DHβE did not significantly block ACh-induced currents (Fig. 5B). However, the ACh response was significantly reduced in 4 of the 8 neurons (Fig. 5). One problem was that ACh responses failed to recover during washout of DHβE (Fig. 5B) even though ACh responses were recorded up to 12 min after washout of DHβE had commenced. Previous reports have shown full recovery from DHβE block following 2 min of washout (Dourado and Sargent 2002). Although the lack of recovery complicates interpretation, it is likely that part of the ACh response in some muscle afferent neurons is mediated by α4β2-nAChR.
Fig. 5.
ACh responses can be blocked by a specific α4β2-nAChR antagonist. A: the specific α4β2-nAChR antagonist dihydro-β-erythroidine (DHβE; 1 μM, 3-min application) partially blocked ACh-induced current in this muscle afferent neuron. A slight recovery was recorded after 3 min of DHβE WO. B: the bar graphs show percentage block and WO for 1 μM DHβE. The left bar graphs show mean (+ SD) for all neurons tested with DHβE, whereas the right graph shows the 4 neurons in which DHβE blocked at least part of the ACh response. The ACh concentration was 0.3 mM. The lowercase letters above the bars indicate significant differences with “a” indicating significant difference between DHβE and Cntl and “c” indicating significant difference between WO and Cntl.
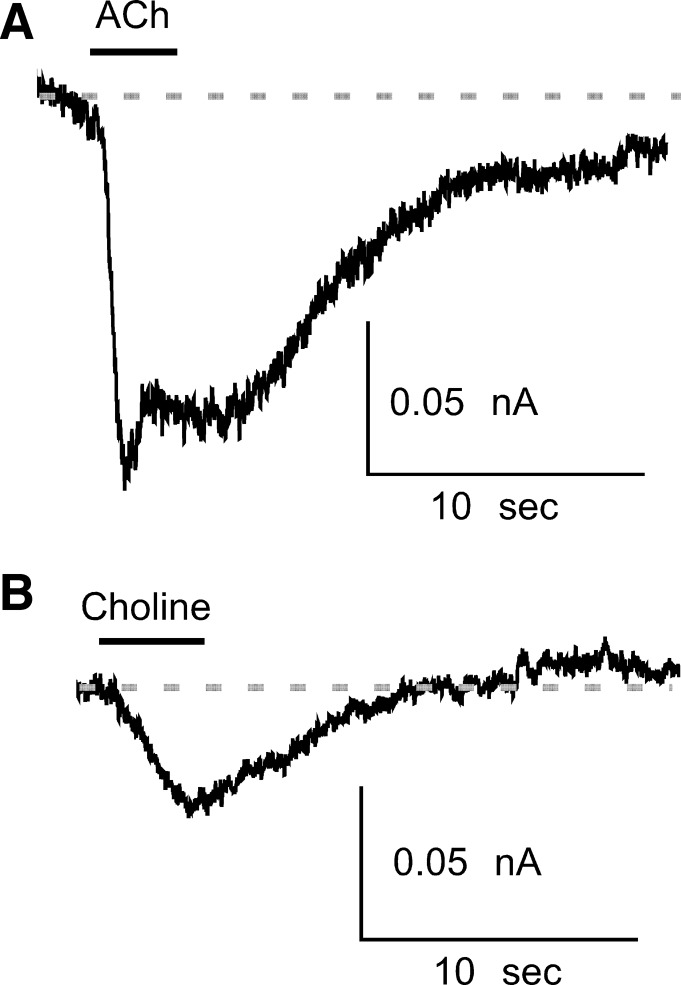
Choline is a major metabolite of ACh resulting from breakdown by acetylcholinesterase (Katz and Miledi 1973) and is an agonist for α7-nAChRs (Alkondon et al. 1997). Choline (10 mM) elicited current in three of five (60%) muscle afferent neurons (Fig. 6) that were responsive to ACh. The combination of antagonist and choline data demonstrate the expression of α7-nAChR by a majority of ACh-responsive muscle afferent neurons.
Fig. 6.
The specific α7-nAChR agonist choline generates current in ACh-responsive muscle afferent neurons. Example currents are from 1 muscle afferent neuron in response to 1 mM ACh (A) and 10 mM choline (B). The application of each agonist is indicated by the horizontal bar above each trace.
Muscle expression of α7-nAChR.
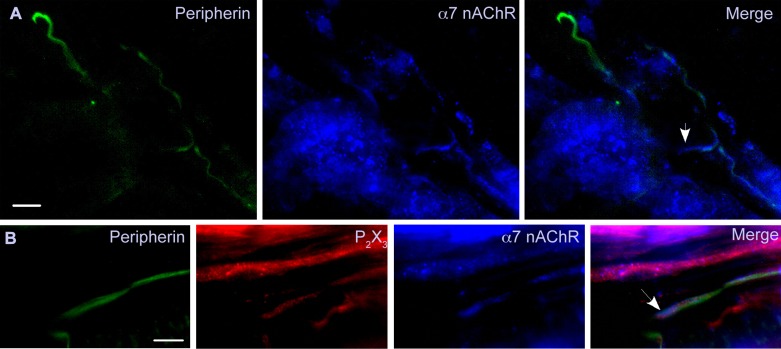
Although the generation of choline during intense muscle activity could be a source for α7-nAChR activation, the receptors must be present in the muscle to be activated. We used immunohistochemistry to visualize α7-nAChR on muscle afferent axons (Fig. 7A). Peripherin is a neurofilament protein that is selectively expressed in unmyelinated axons (Black et al. 2012; Goldstein et al. 1991), and we stained muscle sections with an antibody directed against peripherin to reveal the group IV afferents. These sections were also exposed to antibodies against α7-nACh and P2X3 receptors. P2X3 receptors are involved in generating the EPR (Hayes et al. 2008), and our electrophysiology recordings demonstrated that 41% ACh-sensitive muscle afferent neurons also respond to ATP (Table 1). Thus we reasoned that the chance of capturing afferents colabeled by both the α7-nAChR and P2X3R antibodies should be higher than either TRPV1 or TRPA1. We found peripherin-positive afferents that were also positive for α7-nAChR (Fig. 7A) and both α7-nAChR and P2X3R (Fig. 7B). One interesting finding is that the α7-nAChR labeling appears to extend beyond that of the peripherin (Fig. 7, A and B, arrows). In both examples, the extended labeling appears to correspond with the end of the axon as expected for the terminal sensory transduction zone. The examples shown in Fig. 7 are representative of the labeling observed in gastrocnemius muscle sections from the three rats for both α7-nAChR and P2X3R.
Fig. 7.
Group IV fibers within the muscle express α7-nAChR. Sections are shown from 2 gastrocnemius muscles (different animals) that were labeled with antibodies directed against peripherin (green), α7-nAChR (blue), and P2X3R (red). A: several peripherin-positive fibers (group IV) are shown, but only those in the lower right corner were also positive for α7-nAChR. In the merged image (right), the arrow shows α7-nAChR labeling extending beyond that of peripherin into an apparent axon terminal region. B: a peripherin-positive fiber is also stained with the α7-nAChR and P2X3R antibodies. The α7-nAChR and P2X3R labeling appears to extend beyond that of peripherin to the terminal end of the fiber (marked by arrow). There appears to be a parallel fiber that is only positive for P2X3R, which could be a group III fiber since it was not positive for peripherin. The bar in the lower corner of each peripherin panel indicates 12.5 μm. The examples shown here are representative of labeling experiments done on gastrocnemius muscle tissue from the 3 animals for both α7-nAChR and P2X3R.
In addition to sensory afferents, the α7-nAChR antibody also labeled oval or round cells that could be muscle satellite cells since these receptors are highly expressed during skeletal muscle development or following denervation (Corriveau et al. 1995; Fischer et al. 1999). The labeling of cells was clearly distinguishable from that of muscle afferent fibers, which were also colabeled by the peripherin antibody (Fig. 7).
DISCUSSION
Muscle afferent neurons express a number of receptors that can affect group III and IV afferent activity and thus the EPR. These include P2X receptors, TRPV1 channels, and TRPA1 channels (Hayes et al. 2008; Koba et al. 2011; Smith et al. 2010). We have demonstrated most muscle afferent neurons also express nAChR. We used antibody labeling, specific antagonists (MLA and IMI), and an agonist (choline) to show that α7-nAChR were expressed in the majority of ACh-responsive neurons. ACh responses were also blocked by DHβE to support expression of α4β2 by smaller fraction of muscle afferent neurons. nAChRs are expressed by neurons in the size range of group III (<40-μm diameter) and group IV (<30-μm) afferents. In addition, neurons expressing TRPV1, TRPA1, and/or P2XR can express nAChR. To our knowledge, this is the first study to demonstrate that muscle afferent neurons express nAChRs. Since α7-nAChR are expressed on group IV (and perhaps group III) afferent fibers, it is possible that choline released during intense muscle activity could be a physiological activator of the EPR.
Identification of muscle afferents as groups III and IV.
We used a number of methods to separate different classes of sensory afferent neurons. One method was to correlate muscle afferent responses between ACh (nAChR), CAP (TRPV1), AITC (TRPA1), and ATP (P2X). TRPV1, TRPA1, and P2X receptors have been shown to be involved in generating the EPR and to be expressed by group III and/or IV neurons (Hayes et al. 2008; Koba et al. 2011; Smith et al. 2010). We found that 59% of ACh-sensitive neurons also responded to one or more of the other activators (CAP, AITC, ATP), including 20% that were sensitive to all three agonists. By this classification method, group III and/or IV neurons can express nAChRs.
We also used somal diameter for classification of sensory neuron classes. Group IV muscle afferent neurons have diameters <30 μm, whereas group III neuronal diameters are <40 μm (Hu and McLachlan 2003; Ramachandra et al. 2012). The majority of neurons recorded for this study had diameters <35 μm. Within the size range for group III and IV neurons, there were no clear differences in responsiveness to the four agonists tested in this study. However, of the few neurons recorded with diameters >40 μm, nearly half were responsive to either ACh or ATP, but only one responded to CAP and none to AITC. The TRP channels appear to have a more limited expression pattern than either nAChR or P2XR, perhaps being expressed only in group III and IV neurons (Connor et al. 2005). Muscle afferent neurons that respond to ACh can also respond to activators of TRPV1, TRPA1, and P2X channels, and most have diameters <35 μm. Together, these results support the expression of nAChR by group III and IV neurons.
We also tested the TTX sensitivity of the NaV current in the recorded neurons. Some investigations have supported selective expression of NaV1.8 by nociceptive sensory neurons (C- and Aδ-type; Djouhri et al. 2003). However, we and others have demonstrated that NaV1.8 expression is not restricted to a particular sensory neuron class (Coward et al. 2000; Persson et al. 2010; Ramachandra et al. 2012, 2013; Shields et al. 2012). We have previously shown that a small fraction (∼14%) of muscle afferent neurons primarily express TTX-sensitive NaV channels (Ramachandra et al. 2012). Thus we were interested to determine whether there was differential responsiveness to ACh, ATP, CAP, and AITC between TTX-sensitive vs. TTX-resistant neurons. Although the TTX-sensitive muscle afferent neurons comprised only ∼14% of the total population (7/52), some differences compared with TTX-resistant muscle afferents were noticed. One finding was that TRPV1 and TRPA1 channels did not appear to be as widely expressed by the TTX-sensitive neurons. Only one out of seven (14%) TTX-sensitive neurons responded to CAP (TRPV1), and only one other (14%) responded to AITC (TRPA1), whereas 42% and 25% of TTX-resistant muscle afferent neurons responded to CAP or AITC, respectively. The expression of nACh and P2X receptors was similar between TTX-sensitive and -resistant neurons. Although the number of TTX-sensitive neurons is small, it appears that TRPV1 and TRPA1 channel expression may be less prominent in these neurons.
nAChR in muscle afferent neurons.
Our electrophysiological testing of muscle afferent neurons showed that 52% were ACh-responsive, which was the highest percentage amount of all agonists tested. However, our antibody staining showed that 86% of muscle afferent neurons were labeled by α7-nAChR antibody. Two possible explanations for the differences are nAChR desensitization and ACh responses below our detection limits (this includes the lack of functional expression). nAChR can desensitize rapidly with α7-nAChR showing the fastest desensitization (Rau et al. 2005). The exchange rate for our flow system ∼1 s, which could be too slow to reveal all ACh-responsive neurons. It is also possible that some ACh responses were so small that they could not be detected against the background noise. We did not test these possibilities since we were most interested in determining whether group III and IV muscle afferent neurons can express nAChR.
There are many genes that generate nAChR subunits, but only a subset of these are expressed in sensory neurons, including α3-, α4-, α5-, α7-, α10-, β2-, and β4-subunits (Haberberger et al. 2004; Rau et al. 2005). We used immunocytochemistry to show expression of α7-nAChR and pharmacological tests to demonstrate functional expression of α7- and, perhaps, α4β2-nAChR by muscle afferent neurons. The involvement of α7-nAChR was supported by using specific antagonists MLA and IMI (Albuquerque et al. 2009; Freitas et al. 2013; Olivera et al. 2008; Zappettini et al. 2011) and the α7-nAChR agonist choline (Alkondon et al. 1997). MLA blocked ACh-induced currents in 100% of muscle afferent neurons with 64% of these neurons showing a complete block of the ACh response. The effectiveness of IMI was somewhat lower relative to that of MLA. IMI blocked the ACh response in 64% of muscle afferent neurons. Of those neurons, 57% showed complete block by IMI. One advantage of using these two antagonists is that they are chemically very different. MLA is a small organic molecule, whereas IMI is a peptide toxin that was originally isolated from cone snail venom. They both block α7-nAChRs, but their different chemical structures suggest nonoverlapping off-target effects. The similar effect of these two blockers greatly strengthens the conclusion that α7-nAChRs are expressed by muscle afferent neurons.
When testing with the α7-nAChR agonist, we found that 60% of the ACh-sensitive neurons responded to choline, which suggests that α7-nAChR are expressed by the majority of these neurons. This conclusion is supported by the MLA and IMI experiments, which block ACh responses in 100% and 64% of muscle afferent neurons, respectively. The choline and IMI results demonstrate α7-nAChR expression by more than half of the ACh-sensitive neurons. The MLA results suggest that a much larger fraction (100%) of muscle afferents express α7-nAChR. It is likely that the difference results from selection bias in that only α7-nAChR-expressing neurons happened to be selected for the MLA experiments. One interesting finding was that MLA and IMI completely blocked the ACh response in ∼50% of the ACh-sensitive muscle afferents, which suggests that α7-nAChRs were exclusively expressed in those neurons. Although the different experiments provide somewhat different expression levels, the results clearly demonstrate that the majority of ACh-sensitive muscle afferent neurons express α7-nACh. The small to medium diameter (<40 μm) of these neurons suggests that they are group III or IV neurons.
The partial block of ACh responses by MLA and IMI along with the inability of choline to activate nAChR in some neurons support expression of non-α7-nAChR. α4β2-nAChRs have been shown to be expressed by sensory neurons (Genzen et al. 2001; Haberberger et al. 2004), and we used the specific blocker DHβE to test for this receptor type (Iturriaga-Vásquez et al. 2010; Karadsheh et al. 2004). We found significant block of the ACh response in 50% of neurons tested, which supports α4β2-nAChR expression. However, this result was complicated by the absence of recovery from DHβE block. One mitigating factor is that ACh responses measured from all eight neurons tested showed an overall reduction in responsiveness from control to test to washout (Fig. 5B). Thus it appears that AChR desensitization was more severe for this set of experiments than for those using either IMI or MLA. It is clear that non-α7-nAChRs are expressed by muscle afferent neurons, and it is likely that the α4β2-nAChR is one of those expressed. The failure of DHβE to block completely ACh responses suggests that α4β2-nAChRs are expressed with other nAChRs by muscle afferent neurons. Further work is required to determine which other nAChR types are also expressed.
Expression of α7-nAChR by muscle afferent terminals.
Our results clearly demonstrate the expression of α7-nAChR in muscle afferent somata, but we also wanted to know whether these channels were expressed along the afferent fibers within the muscle where they could sense ACh and/or choline released during intense muscle activity. Peripherin is a cytoskeletal marker for small-diameter unmyelinated sensory axons (Black et al. 2012; Goldstein et al. 1991; Oblinger et al. 1989), and we used an antibody raised against peripherin to visualize group IV afferent axons within the muscle. P2X3Rs have been demonstrated to mediate the ATP-induced activity in group III and IV muscle afferents (Light et al. 2008), and these receptors participate in generating the EPR (Hayes et al. 2008). In addition, 41% of ACh-sensitive muscle afferent neurons also responded to ATP (Table 1). Thus we used P2X3R as an additional marker for afferent fibers that were potentially involved in the EPR. We found fibers colabeled with peripherin and α7-nAChR, and, in some cases, the fibers were also positive for P2X3R. This demonstrates that α7-nAChR are expressed on group IV afferents and could serve as a sensor for intense muscle activity.
We note that the α7-nAChR labeling can be found in fibers beyond the end of the peripherin labeling. In these cases, the α7-nAChR labeling appears to extend to the nerve terminal as expected if this were a sensory signal transduction zone. The absent or reduced peripherin labeling suggests that this neurofilament protein does not extend into the signal transduction zones at the end of muscle afferents.
Role for nAChRs in the EPR.
The expression of nAChRs by small to medium muscle afferent neurons that also express TRPV1, TRPA1, and/or P2XR suggests a role for nAChR in activation of the EPR. ACh is released by motor efferent neurons to initiate muscle contraction. During intense exercise, large amounts of ACh are released, and some may diffuse out of the neuromuscular junction to dilate muscle arterioles by activation of muscarinic AChR (Welsh and Segal 1997). However, ACh is rapidly metabolized in the synaptic cleft by acetylcholinesterase into choline and acetate (Katz and Miledi 1973), and it has been suggested that the enzyme activity is sufficiently rapid to destroy all ACh molecules before they could diffuse from the neuromuscular junction (Clifford 2007). The spillover of ACh (10 μM), if it exists, may be sufficient to activate muscarinic AChR (Welsh and Segal 1997) but would be insufficient (100–300 μM) to activate nAChRs on afferent nerve endings. However, α7-nAChRs are activated by choline (Alkondon et al. 1997), and we demonstrate that choline is an activator of nAChRs expressed by muscle afferent neurons. The EC50 for choline activation of α7-nAChRs is 1.6 mM (Alkondon et al. 1997), but we could not find literature that determined choline levels in muscle during exercise. Resting choline levels have been measured to be 12 μM (Korth et al. 2000). There is evidence of both high- and low-affinity choline transporters that would reduce levels of extracellular choline during exercise, but the high-affinity choline transporter (CHT) that is expressed in nerve terminals saturates at choline concentrations >5 μM (Ferguson and Blakely 2004). Another high-affinity transporter, the choline-like transporter (CLT), is highly expressed in skeletal muscle (Yuan et al. 2004), but this transporter also saturates at choline concentrations >10 μM. Thus both of these transporters should be saturated at resting levels of extracellular choline (12 μM). A candidate for the low-affinity choline transporter is the organic cation transporter type 2 (OCT2), which has a choline Km >100 μM (Ferguson and Blakely 2004). This transporter could reduce choline levels in active muscle, but it is not clear by how much. Thus it seems possible that choline levels in a working muscle could increase sufficiently to activate the α7-nAChRs that are expressed on group IV (and perhaps group III) afferent nerve terminals.
The role of α7-nAChRs in the EPR can be tested using MLA or IMI since these blockers are specific for α7-nAChRs and should not affect the α1β1ϵδ-nAChR type expressed at the neuromuscular junction (Fagerlund and Eriksson 2009). Therefore, introduction of these blockers to the muscle blood supply (Kaufman and Rybicki 1987) will inhibit α7-nAChRs expressed on the afferent nerve terminals but not muscle nAChRs required for muscle contraction.
Disease states such as peripheral vascular disease and heart failure can reduce muscle blood flow to produce ischemia, which can result in claudication (muscle pain) as well as activation of the EPR (Hayes et al. 2007; Lorentsen 1972). This pressor reflex activation leads to inappropriate cardiovascular responses that can increase risk for myocardial infarction. Recent studies have shown a variety of stimuli can activate muscle afferent neurons and contribute to the EPR, including hydrogen ions, ATP, and bradykinin (Hayes et al. 2008; Koba et al. 2011; Smith et al. 2010). Our findings support the possibility that choline could be added to the growing list of compounds that stimulate the EPR and suggest that α7-nAChR blockers could be useful in suppressing inappropriate EPR activity in patients suffering from cardiovascular diseases such as peripheral vascular disease and heart failure.
GRANTS
This work was funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-059397 (K. S. Elmslie) and the Graduate Program Committee of the Kirksville College of Osteopathic Medicine, A.T. Still University of Health Sciences (J. C. Baxter).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.C.B., R.R., D.R.M., and K.S.E. conception and design of research; J.C.B., R.R., and D.R.M. performed experiments; J.C.B., R.R., D.R.M., and K.S.E. analyzed data; J.C.B., R.R., and K.S.E. interpreted results of experiments; J.C.B., R.R., and K.S.E. prepared figures; J.C.B., R.R., and K.S.E. drafted manuscript; J.C.B., R.R., D.R.M., and K.S.E. edited and revised manuscript; J.C.B., R.R., D.R.M., and K.S.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Stephanie McGrew for technical support and assistance during these experiments.
REFERENCES
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89: 73–120, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Subtype-specific inhibition of nicotinic acetylcholine receptors by choline: a regulatory pathway. J Pharmacol Exp Ther 318: 268–275, 2006 [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 9: 2734–2742, 1997 [DOI] [PubMed] [Google Scholar]
- Black J, Frézel N, Dib-Hajj SD, Waxman SG. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol Pain 8: 82, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS. Skeletal muscle vasodilatation at the onset of exercise. J Physiol 583: 825–833, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Naves LA, McCleskey EW. Contrasting phenotypes of putative proprioceptive and nociceptive trigeminal neurons innervating jaw muscle in rat. Mol Pain 1: 31–31, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau R, Romano S, Conroy W, Oliva L, Berg D. Expression of neuronal acetylcholine receptor genes in vertebrate skeletal muscle during development. J Neurosci 15: 1372–1383, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward K, Plumpton C, Facer P, Birch R, Carlstedt T, Tate S, Bountra C, Anand P. Immunolocalization of SNS/PN3 and NaN/SNS2 sodium channels in human pain states. Pain 85: 41–50, 2000 [DOI] [PubMed] [Google Scholar]
- Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol 550: 739–752, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourado M, Sargent PB. Properties of nicotinic receptors underlying Renshaw cell excitation by α-motor neurons in neonatal rat spinal cord. J Neurophysiol 87: 3117–3125, 2002 [DOI] [PubMed] [Google Scholar]
- Fagerlund MJ, Eriksson LI. Current concepts in neuromuscular transmission. Br J Anaesth 103: 108–114, 2009 [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Blakely RD. The choline transporter resurfaces: new roles for synaptic vesicles? Mol Interv 4: 22–37, 2004 [DOI] [PubMed] [Google Scholar]
- Fischer U, Reinhardt S, Albuquerque EX, Maelicke A. Expression of functional α7 nicotinic acetylcholine receptor during mammalian muscle development and denervation. Eur J Neurosci 11: 2856–2864, 1999 [DOI] [PubMed] [Google Scholar]
- Freitas K, Ghosh S, Ivy Carroll F, Lichtman AH, Imad Damaj M. Effects of α7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology 65: 156–164, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Li JD, Sinoway LI, Li J. Effect of muscle interstitial pH on P2X and TRPV1 receptor-mediated pressor response. J Appl Physiol 102: 2288–2293, 2007 [DOI] [PubMed] [Google Scholar]
- Genzen JR, Van Cleve W, McGehee DS. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol 86: 1773–1782, 2001 [DOI] [PubMed] [Google Scholar]
- Goldstein ME, House SB, Gainer H. NF-L and peripherin immunoreactivities define distinct classes of rat sensory ganglion cells. J Neurosci Res 30: 92–104, 1991 [DOI] [PubMed] [Google Scholar]
- Haberberger RV, Bernardini N, Kress M, Hartmann P, Lips KS, Kummer W. Nicotinic acetylcholine receptor subtypes in nociceptive dorsal root ganglion neurons of the adult rat. Auton Neurosci 113: 32–42, 2004 [DOI] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol 94: 1437–1445, 2003 [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol 581: 1271–1282, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SG, McCord JL, Kaufman MP. Role played by P2X and P2Y receptors in evoking the muscle chemoreflex. J Appl Physiol 104: 538–541, 2008 [DOI] [PubMed] [Google Scholar]
- Hu P, McLachlan EM. Selective reactions of cutaneous and muscle afferent neurons to peripheral nerve transection in rats. J Neurosci 23: 10559–10567, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga-Vásquez P, Carbone A, García-Beltrán O, Livingstone PD, Biggin PC, Cassels BK, Wonnacott S, Zapata-Torres G, Bermudez I. Molecular determinants for competitive inhibition of α4β2 nicotinic acetylcholine receptors. Mol Pharmacol 78: 366–375, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DS, Martinez J, Elgoyhen AB, Heinemann SF, McIntosh JM. α-Conotoxin Imi exhibits subtype-specific nicotinic acetylcholine receptor blockade: preferential inhibition of homomeric α7 and α9 receptors. Mol Pharmacol 48: 194–199, 1995 [PubMed] [Google Scholar]
- Karadsheh MS, Shah MS, Tang X, Macdonald RL, Stitzel JA. Functional characterization of mouse α4β2 nicotinic acetylcholine receptors stably expressed in HEK293T cells. J Neurochem 91: 1138–1150, 2004 [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol 231: 549–574, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002 [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res 61: I60–I65, 1987 [PubMed] [Google Scholar]
- Koba S, Hayes SG, Sinoway LI. Transient receptor potential A1 channel contributes to activation of the muscle reflex. Am J Physiol Heart Circ Physiol 300: H201–H213, 2011 [DOI] [PubMed] [Google Scholar]
- Korth U, Merkel G, Fernandez FF, Jandewerth O, Dogan G, Koch T, van Ackern K, Weichel O, Klein J. Tourniquet-induced changes of energy metabolism in human skeletal muscle monitored by microdialysis. Anesthesiology 93: 1407–1412, 2000 [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentsen E. Systemic arterial blood pressure during exercise in patients with atherosclerosis obliterans of the lower limbs. Circulation 46: 257–263, 1972 [DOI] [PubMed] [Google Scholar]
- Oblinger M, Wong J, Parysek L. Axotomy-induced changes in the expression of a type III neuronal intermediate filament gene. J Neurosci 9: 3766–3775, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera BM, Quik M, Vincler M, McIntosh JM. Subtype-selective conopeptides targeted to nicotinic receptors: concerted discovery and biomedical applications. Channels (Austin) 2: 143–152, 2008 [DOI] [PubMed] [Google Scholar]
- Persson AK, Black JA, Gasser A, Cheng X, Fischer TZ, Waxman SG. Sodium-calcium exchanger and multiple sodium channel isoforms in intra-epidermal nerve terminals. Mol Pain 6: 84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra R, McGrew SY, Baxter JC, Howard JR, Elmslie KS. NaV1.8 channels are expressed in large, as well as small, diameter sensory afferent neurons. Channels (Austin) 7: 34–37, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra R, McGrew SY, Baxter JC, Kiveric E, Elmslie KS. Tetrodotoxin-resistant voltage-dependent sodium (NaV) channels in identified muscle afferent neurons. J Neurophysiol 108: 2230–2241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau KK, Johnson RD, Cooper BY. Nicotinic AChR in subclassified capsaicin-sensitive and -insensitive nociceptors of the rat DRG. J Neurophysiol 93: 1358–1371, 2005 [DOI] [PubMed] [Google Scholar]
- Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain 144: 270–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Ahn HS, Yang Y, Han C, Seal RP, Wood JN, Waxman SG, Dib-Hajj SD. Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain 153: 2017–2030, 2012 [DOI] [PubMed] [Google Scholar]
- Smith SA, Leal AK, Williams MA, Murphy MN, Mitchell JH, Garry MG. The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J Physiol 588: 1179–1189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006 [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Coactivation of resistance vessels and muscle fibers with acetylcholine release from motor nerves. Am J Physiol Heart Circ Physiol 273: H156–H163, 1997 [DOI] [PubMed] [Google Scholar]
- Yuan Z, Wagner L, Poloumienko A, Bakovic M. Identification and expression of a mouse muscle-specific CTL1 gene. Gene 341: 305–312, 2004 [DOI] [PubMed] [Google Scholar]
- Zappettini S, Grilli M, Lagomarsino F, Cavallero A, Fedele E, Marchi M. Presynaptic nicotinic α7 and non-α7 receptors stimulate endogenous GABA release from rat hippocampal synaptosomes through two mechanisms of action. PLoS One 6: e16911–e16911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Jones SW. Surface charge and calcium channel saturation in bullfrog sympathetic neurons. J Gen Physiol 105: 441–462, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]