Abstract
In normal animals, operant conditioning of the spinal stretch reflex or the H-reflex has lesser effects on synergist muscle reflexes. In rats and people with incomplete spinal cord injury (SCI), soleus H-reflex operant conditioning can improve locomotion. We studied in normal humans the impact of soleus H-reflex down-conditioning on medial (MG) and lateral gastrocnemius (LG) H-reflexes and on locomotion. Subjects completed 6 baseline and 30 conditioning sessions. During conditioning trials, the subject was encouraged to decrease soleus H-reflex size with the aid of visual feedback. Every sixth session, MG and LG H-reflexes were measured. Locomotion was assessed before and after conditioning. In successfully conditioned subjects, the soleus H-reflex decreased 27.2%. This was the sum of within-session (task dependent) adaptation (13.2%) and across-session (long term) change (14%). The MG H-reflex decreased 14.5%, due mainly to task-dependent adaptation (13.4%). The LG H-reflex showed no task-dependent adaptation or long-term change. No consistent changes were detected across subjects in locomotor H-reflexes, EMG activity, joint angles, or step symmetry. Thus, in normal humans, soleus H-reflex down-conditioning does not induce long-term changes in MG/LG H-reflexes and does not change locomotion. In these subjects, task-dependent adaptation of the soleus H-reflex is greater than it is in people with SCI, whereas long-term change is less. This difference from results in people with SCI is consistent with the fact that long-term change is beneficial in people with SCI, since it improves locomotion. In contrast, in normal subjects, long-term change is not beneficial and may necessitate compensatory plasticity to preserve satisfactory locomotion.
Keywords: spinal cord, synergists, plasticity, learning, rehabilitation
an operant conditioning protocol can markedly increase or decrease the H-reflex or the spinal stretch reflex (SSR) in monkeys, rats, mice, and humans (Carp et al. 2006b; Chen and Wolpaw 1995; Thompson et al. 2009; Wolpaw 1987). In monkeys, rats, and humans, this conditioning has similar but lesser effects on the reflexes of synergist muscles (Chen et al. 2005; Wolf and Segal 1996; Wolpaw et al. 1983). Thompson et al. (2009) showed in humans that conditioned H-reflex change is the sum of rapid within-session (i.e., task dependent) adaptation and gradual across-session (i.e., long term) change. The former is thought to reflect task-dependent change in cortical influence (e.g., on presynaptic inhibition) (Baudry et al. 2010; Brooke et al. 1997; Meunier and Pierrot-Deseilligny 1998; Stein 1995; Stein and Capaday 1988) that affects the activity of H-reflex pathway only during the conditioning protocol. The latter appears to reflect spinal cord plasticity (Thompson and Wolpaw, in press; Thompson and Wolpaw 2014; Wolpaw 2010 for review) that affects the activity of the pathway even outside of the conditioning protocol (Thompson et al. 2013a) and thereby affects other behaviors. Indeed, in rats and humans with incomplete spinal cord injury (SCI), appropriate soleus H-reflex conditioning can improve locomotion (Y Chen et al. 2006; Thompson et al. 2013b). Comparison of conditioning results from normal humans and humans with incomplete SCI suggested that the relative magnitudes of task-dependent adaptation and long-term change depend on the functional impact of long-term change on other behaviors such as locomotion (i.e., on whether it improves them or disturbs them) (Thompson et al. 2013b; Wolpaw 2010).
On the basis of these findings, the present study asked two questions about soleus H-reflex conditioning in normal humans. First, is soleus H-reflex conditioning accompanied by task-dependent adaptation and/or long-term change in the H-reflexes of the synergist muscles, medial (MG) and lateral gastrocnemii (LG)? A recent study (Makihara et al. 2012), showing that human soleus, MG, and LG H-reflexes are similarly modulated during standing and walking, suggested that soleus H-reflex conditioning would affect MG and LG H-reflexes.
Second, does soleus H-reflex conditioning in normal humans affect locomotion? In normal rats, it does not disturb major features of locomotion (e.g., right/left symmetry), but it does affect locomotor electromyography (EMG) and kinematics (Chen et al. 2005, 2011). Analysis indicated that compensatory plasticity in other reflex pathways prevents the conditioned change in the soleus H-reflex pathway from disturbing locomotion (Chen et al. 2011). These results in normal rats suggested that soleus H-reflex conditioning might have similar effects on locomotion in normal humans. Soleus H-reflex modulation over the step-cycle, locomotor kinematics (i.e., joint angles), locomotor EMG activity in proximal and distal muscles of both legs, and step symmetry were assessed before and after down-conditioning of the soleus H-reflex in one leg.
MATERIALS AND METHODS
Study overview.
The operant conditioning protocol for the human soleus H-reflex reflex has been described in detail previously (Thompson et al. 2009). It is summarized below, with several minor modifications noted. The protocol comprised 6 baseline sessions and 30 conditioning sessions spread over 12 wk (i.e., 3/wk). Each subject's sessions always occurred at the same time of day to control for diurnal variations in H-reflex size (Carp et al. 2006a; Chen and Wolpaw 1994; Lagerquist et al. 2006; Wolpaw and Seegal 1982). The H-reflexes of the MG and LG were concurrently measured with the soleus H-reflex in every sixth session over the course of study. Before beginning and after finishing the block of 30 conditioning sessions, each subject walked on a treadmill at a self-selected speed while EMG activity, H-reflexes, and joint angle data were measured. The study protocol was approved by the Institutional Review Board of Helen Hayes Hospital, New York State Department of Health, and all subjects gave written consent before participating. Eight subjects (2 women and 6 men, age 21–54 yr) with no known history of neurological disease or injury participated in the study.
Operant conditioning sessions.
In the preliminary session preceding the baseline sessions, the maximum voluntary contraction (MVC) values for the soleus, MG, and LG muscles of the conditioned leg were concurrently measured as the rectified EMG levels for maximum isometric contraction during standing. Then, for each subject, target background EMG levels and target soleus M-wave size (i.e., rectified, equivalent to absolute value) for H-reflex trials were then determined. The target background EMG levels were set at about the subject's natural standing level [i.e., typically 10–15% MVC for the soleus and <8 μV (resting level) for the tibialis anterior (TA)]. Tibial nerve stimulation just above the soleus M-wave threshold was used to elicit the H-reflex. For each subject, rectified soleus M-wave size and soleus and TA background EMG amplitudes were kept constant for all the H-reflex trials of all the baseline and conditioning sessions. Small adjustments of the stimulus level were occasionally needed to maintain the target soleus M-wave size. In the sessions in which MG and LG EMG activity was also recorded, MG and LG M-waves were monitored throughout the H-reflex trials. MG and LG M-wave sizes are typically highly correlated with soleus M-wave size (Makihara et al. 2012). In the present study, the level of tibial nerve stimulation that produced a soleus M-wave just above threshold generally produced MG and LG M-waves that were also just above threshold.
Each baseline and conditioning session began with an H-reflex/M-wave recruitment curve measurement during standing while the subject maintained the soleus background EMG activity within the preset level (i.e., 10–15% MVC). Then, in each of the baseline sessions, 225 control H-reflexes were elicited in three 75-trial blocks. In each of the conditioning sessions, 20 control H-reflexes were elicited as in the baseline sessions, and then 225 conditioned H-reflexes were elicited in three 75-trial blocks. The difference between the control and conditioning trials was that in the conditioning trials, the subject was asked to decrease the soleus H-reflex and was given visual feedback of the reflex size immediately after each stimulus. The visual feedback showed whether the resulting H-reflex was smaller than a criterion value (i.e., successful or not). In contrast, in the control trials, the H-reflex was simply elicited without any feedback on H-reflex size. The MG and LG H reflexes were measured every sixth session (i.e., in baseline session 6, conditioning sessions 6, 12, 18, 24, and 30) concurrently with the soleus H-reflex. The subject received no feedback regarding the MG or LG background EMG activity or H-reflex size.
EMG recording and electrical stimulation.
In all the sessions, EMG activity was recorded from soleus and TA. Soleus EMG activity was recorded with a pair of self-adhesive surface Ag-AgCl electrodes (2.2 × 3.5 cm; Vermed, Bellows Falls, VT) placed longitudinally just below the gastrocnemii with the centers of electrodes ∼3 cm apart, but consistent within each subject. To evaluate antagonist activity during the conditioning, another pair of EMG recording electrodes was placed on the skin over the belly of the TA. Every sixth session, EMG recording electrodes were also placed over the center of the MG and LG muscle bellies (see Operant conditioning sessions). The MG and LG EMG electrodes were placed so as to minimize the H-reflex threshold and to maximize the maximum H-reflex (Hmax) and the maximum M-wave (Mmax). The soleus, MG, and LG electrode pairs were separated by at least 7 cm apart to minimize cross talk (i.e., overlap among the motor unit populations contributing to the activity recorded by each pair).
To elicit the H-reflex and M-wave, the tibial nerve was stimulated in the popliteal fossa by a Grass S48 stimulator (with CCU1 constant current unit and SIU5 stimulus isolation unit; Astro-Med, West Warwick, RI) through self-adhesive surface Ag-AgCl electrodes (2.2 × 2.2 cm for the cathode and 2.2 × 3.5 cm for the anode; Vermed). The stimulating electrode pair was placed so as to minimize the soleus H-reflex threshold, maximize the soleus Hmax and Mmax sizes, and avoid stimulation of other nerves. To prevent session-to-session variability in EMG or stimulating electrode placement, the locations of all electrodes were mapped in relation to permanent skin marks (e.g., scars and moles) in the preliminary session, and the electrodes were placed on the basis of this mapping in all subsequent sessions.
During the baseline and conditioning sessions, EMG signals were amplified, bandpass filtered at 10–1,000 Hz, and sampled at 5,000 Hz. During the period of soleus EMG maintenance prior to stimulus delivery, soleus EMG activity was rectified and averaged every 100 ms, and the result was immediately provided as visual feedback on a computer monitor to help the subject maintain soleus background EMG activity within the specified window (see Thompson et al. 2009 for details). If soleus background EMG remained in the target window (typically 10–15% MVC) for 2 s, and if 5 s had passed since the last stimulus, a 1-ms square-wave stimulus pulse was delivered to elicit the H-reflex and M-wave. The nerve stimulus pulse and EMG activity from 50 ms before to 150 ms after the pulse were recorded.
Analysis of conditioned and control H-reflexes.
For control and conditioning trials, soleus, MG, and LG H-reflex and M-wave sizes were defined as the mean rectified size in the reflex window (i.e., for the soleus, typically 33–47 ms poststimulus for the H-reflex and 6–23 ms poststimulus for the M-wave) minus average background EMG. For each session, we calculated average H-reflex sizes for the 225 H-reflex trials (i.e., three 75-trial blocks together) and for the first 20 within-session control trials (i.e., the first 20 trials of the first 75-trial block of a baseline session, or the 20 control H-reflex trials of a conditioning session). Control and conditioned soleus H-reflex sizes across sessions were quantified as a percentage of their average values for the six baseline sessions. That is, the control H-reflex size at each conditioning session was expressed as a percentage of the average of the first 20 trials of the 6 baseline sessions (i.e., 100% indicates no change), and the conditioned H-reflex size was expressed as a percentage of the average of the entire 225 trials of the 6 baseline sessions.
As noted above, MG and LG reflexes were simply recorded in every sixth session; the subject received no feedback as to their size. MG and LG H-reflexes recorded during soleus H-reflex control trials are referred to here as control MG and LG H-reflexes; and those recorded during soleus H-reflex conditioning trials are referred to as conditioned MG and LG H-reflexes. Control and conditioned MG and LG H-reflex sizes across sessions were quantified as a percentage of their average values in baseline session 6. For soleus, MG, and LG H-reflexes, we also calculated within-session (i.e., task dependent) change as average conditioned H-reflex size minus average control H-reflex size.
To determine for each subject whether down-conditioning was successful, the soleus H-reflex sizes during the six baseline sessions were compared with the conditioned reflex sizes in the last six conditioning sessions by using a one-sided unpaired t-test (Thompson et al. 2009). If the average for the six final sessions was less than that for the six baseline sessions with P < 0.05, conditioning was considered to be successful. For the subjects in whom conditioning was successful, the final conditioned soleus H-reflex size was calculated as the average conditioned soleus H-reflex for the final three sessions (i.e., sessions 28–30) and expressed as a percentage of the average soleus H-reflex for the 225 trials of the three 75-trial blocks of the 6 baseline sessions. Similarly, the final control soleus H-reflex size was calculated as the average control H-reflex for the final three sessions and expressed as a percentage of the average soleus H-reflex for the first 20 trials of the 6 baseline sessions. For MG and LG, the conditioned and control H-reflexes in the last conditioning session were used as the final conditioned and control H-reflexes.
Locomotor evaluation: EMG, H-reflex modulation, and kinematics.
Before and after the block of 30 conditioning sessions, locomotor EMG activity, H-reflex modulation, and joint motion were measured during treadmill walking. The postconditioning locomotor session occurred within 2 days of the last conditioning session. At the beginning of each locomotor session, the H-reflex and M-wave recruitment curves were simultaneously obtained from soleus, MG, and LG of the conditioned leg.
In each locomotor session, the subject walked on a treadmill at his/her comfortable speed (average 3.9 km/h; range 3.0–5.1 km/h) three times for 1) bilateral locomotor EMG measurement, 2) locomotor H-reflex measurement, and 3) joint motion measurement. For each subject, the treadmill speed was kept the same for all three measurements and for both locomotor sessions. Locomotor EMG activity was measured bilaterally from the soleus, TA, MG, LG, vastus lateralis (VL), and biceps femoris (BF). Locomotor H-reflexes were measured for soleus, MG, and LG of the conditioned side. For locomotor EMG and locomotor H-reflex measurements from soleus, MG, LG, and TA, the locations of the EMG and stimulating electrodes were the same ones used during the baseline and conditioning sessions. The locations of the VL and BF electrodes were the same for the two locomotor sessions.
During bilateral locomotor EMG measurement, we also assessed step-cycle symmetry [i.e., the ratio of the time between the nonconditioned leg's foot contact (nFC) and the conditioned leg's foot contact (cFC) to the time between cFC and nFC]. A ratio of 1 indicates a symmetrical gait (Thompson et al. 2013b). To detect foot contact, footswitch cells (Bortec Biomedical, Calgary, AB, Canada) were inserted between the subject's shoe and heel. For the entire locomotor evaluation, the step cycle for each leg went from its foot contact (FC) to its next FC (Thompson et al. 2013b).
For evaluation of locomotor EMG activity, the subject walked on the treadmill for about 4 min (i.e., >200 steps). To determine EMG modulation over the step cycle for each muscle of each subject, the step cycles were normalized to a standard length of time, that time was divided into 12 bins of equal duration, and the average EMG absolute values for each bin were averaged across the step cycles (Kido et al. 2004b; Makihara et al. 2012). For each muscle, the average rectified EMG amplitude for each of the 12 bins was expressed as a percentage of the amplitude in the bin with the highest amplitude. To determine the degree to which each muscle's activity was modulated during locomotion, the modulation index (MI) was calculated in percent as follows: 100 × [(highest bin amplitude − lowest bin amplitude)/highest bin amplitude] (Zehr and Kido 2001; Zehr and Loadman 2012). Finally, the subjects' average EMG levels for each bin for the two locomotor sessions were compared using a paired t-test with Ŝidák correction [1 − (1 − α)1/n] (Ŝidák 1967). We chose Ŝidák correction because the individual tests are statistically independent. The MIs for the two locomotor sessions were compared using a paired t-test.
To measure locomotor H-reflexes in the soleus, MG, and LG of the conditioned leg, EMG and nerve stimulation signals were continuously recorded while the H-reflexes of the soleus, MG, and LG were elicited by a 1-ms square-wave pulse at pseudo-random intervals (i.e., interstimulus interval of 2.5–4.5 s). This ensured that H-reflexes were obtained throughout the entire step cycle and that there was at least one full unstimulated step cycle between successive stimuli (Kido et al. 2004a, 2004b; Yang and Stein 1990). To compare the H-reflexes elicited at the same stimulus efficacy (i.e., the same M-wave size) across different phases of the step cycle, various stimulus intensities were used (Capaday and Stein 1986; Edamura et al. 1991; Llewellyn et al. 1990), and only the H-reflexes with comparable M-wave sizes were included in the further analysis. The M-wave size for locomotor H-reflex measurement was consistent for the two locomotor sessions. H-reflex and M-wave sizes during locomotion were measured as mean rectified values in each reflex window (i.e., as in the conditioning/control trials, typically 33–47 ms poststimulus for the H-reflex and 6–23 ms poststimulus for the M-wave for the soleus). Soleus, MG, and LG H-reflex sizes during locomotion were expressed in units of Mmax for the muscle. As described above for locomotor EMG analysis, the step cycle was divided into 12 bins of equal duration, and average H-reflex size for each bin was determined. The average of these 12 values defined the average locomotor H-reflex. Typically >10 responses were averaged in each bin. H-reflex sizes in each bin for the two locomotor sessions were compared using a paired t-test with Ŝidák correction; the significance level was corrected to P = 0.004 for 12 potential comparisons. In addition, the H-reflex modulation during locomotion was assessed using MIs as described above.
Following the locomotor EMG and H-reflex measurements, the ankle, knee, and hip joint motions during locomotion were recorded. Active infrared markers were placed on five landmarks (i.e., acromion, greater trochanter, lateral epicondyle of the femur, lateral malleolus, and tips of the toes) of the conditioned leg, and their two-dimensional (2-D) trajectories in the sagittal plane were recorded using an infrared motion capture system (http://wiimotion.sourceforge.net/) at a rate of 66 frames/s. The 2-D joint angle data in the sagittal plane (i.e., dorsiflexion/plantarflexion for the ankle, flexion/extension for the knee and hip) were calculated as an angle between adjacent segments. As in the locomotor EMG and H-reflex analyses, ankle, knee, and hip joint angles were analyzed across 12 equal bins of the step cycle. The joint angles in each bin for the two locomotor sessions were compared using a paired t-test with Ŝidák correction.
RESULTS
Down-conditioning of the soleus H-reflex was successful (as defined above) in seven of the eight subjects. (In the remaining subject, the soleus did not change significantly.) Since the purpose of this study was to examine the effects of successful soleus H-reflex down-conditioning on gastrocnemius H-reflexes and on locomotion, the data from the seven successfully down-conditioned subjects are presented in this article.
Stability of EMG recording and nerve stimulation.
To verify that EMG recording quality and effective stimulus strength in each subject were constant throughout study, Mmax and Hmax sizes, background (i.e., prestimulus) EMG level, and M-wave size were compared across sessions and between control reflexes and conditioned reflexes. Neither the Mmax nor the Hmax changed significantly throughout the entire study period in soleus, MG, or LG (i.e., soleus: Mmax P = 0.69, Hmax P = 0.88; MG: Mmax P = 0.45, Hmax P = 0.46; LG: Mmax P = 0.61, Hmax P = 0.40; 1-way repeated-measures ANOVA). Background EMG level remained within a narrow window for each muscle [typically 10–13% MVC for soleus and 3–8% MVC for both MG and LG, depending on the subject (e.g., 15–20 μV, 4–11 μV, and 3–9 μV for soleus, MG, and LG, respectively)] throughout study (P = 0.23, 0.75, and 0.91 for soleus, MG, and LG, respectively; 1-way repeated-measures ANOVA). M-wave size for each muscle stayed in a narrow window (typically 3–5% Mmax for soleus, 15–20% Mmax for MG, and 17–22% Mmax for LG, depending on the subject) throughout study (P = 0.22, 0.70, and 0.61 for soleus, MG, and LG, respectively; 1-way repeated-measures ANOVA).
Background EMG level and M-wave size did not differ significantly between the initial 20 trials and the entire 225 trials of the individual sessions (P = 0.09, 0.42, and 0.95, for soleus, MG, and LG background EMG level, respectively; P = 0.84, 0.67, and 0.92, for soleus, MG, and LG M-wave size, respectively; paired t-test). Furthermore, TA (i.e., antagonist) background activity remained very low (i.e., <8 μV) throughout study (P = 0.11; 1-way repeated-measures ANOVA).
Similarly, to verify that EMG recording quality and stimulus strength were constant for the two locomotor sessions, Mmax and Hmax sizes and M-wave size were compared. Mmax size was not significantly different between the two sessions in soleus, MG, or LG (P = 0.24, 0.46, and 0.32 for soleus, MG, and LG, respectively; paired t-test). Hmax size did not differ between the two locomotor sessions in any of the three muscles (P = 0.72, 0.92, and 0.56 for soleus, MG, and LG, respectively; paired t-test). Finally, M-wave size during locomotor H-reflex measurement did not differ between the two locomotor sessions (P = 0.65, 0.54, and 0.65 for soleus, MG, and LG, respectively; paired t-test).
Changes in conditioned and control H-reflexes.
Table 1 summarizes the final conditioned and control H-reflex results for soleus, MG, and LG. It also shows average final task-dependent adaptation (i.e., final conditioned H-reflex minus final control H-reflex). Conditioned and control soleus reflexes are significantly reduced, and the magnitudes of their reductions are comparable to those in previous human and animal studies (Carp et al. 2006b; Chen and Wolpaw 1995; Thompson et al. 2009; Wolpaw 1987). The soleus H-reflex also shows significant task-dependent adaptation comparable to that in the previous study of normal humans (Thompson et al. 2009). The conditioned MG H-reflex is significantly reduced by about half as much as the soleus H-reflex. This result is similar to that found in synergist muscles with down-conditioning of the monkey biceps spinal stretch reflex (SSR) (Wolpaw et al. 1983). The control MG H-reflex shows no significant change; MG task-dependent adaptation is significant and comparable to that in soleus. Thus essentially all the change in the conditioned MG H-reflex is task-dependent adaptation. In contrast, the conditioned and control LG H-reflexes do not change.
Table 1.
H-reflex sizes in the last conditioning session in percent of baseline values
| Conditioned H-Reflex | Control H-Reflex | Within-Session Change | |
|---|---|---|---|
| Soleus | 72.8 ± 3.6† | 86.0 ± 3.1† | −13.2 ± 2.6† |
| MG | 85.5 ± 5.1* | 98.9 ± 6.8 | −13.4 ± 5.1* |
| LG | 93.1 ± 12.0 | 99.1 ± 11.7 | −6.0 ± 4.2 |
P < 0.05,
P < 0.01, significant differences from baseline for the H-reflexes and from 0 for within-session change.
MG, medial gastrocnemius; LG, lateral gastrocnemius.
Figure 1 illustrates these results with data from a representative subject for the sixth baseline session and the last conditioning session. For soleus, the final conditioned and control H-reflexes are both smaller than baseline; for MG, only the final conditioned H-reflex is smaller; and for LG, neither is smaller.
Fig. 1.
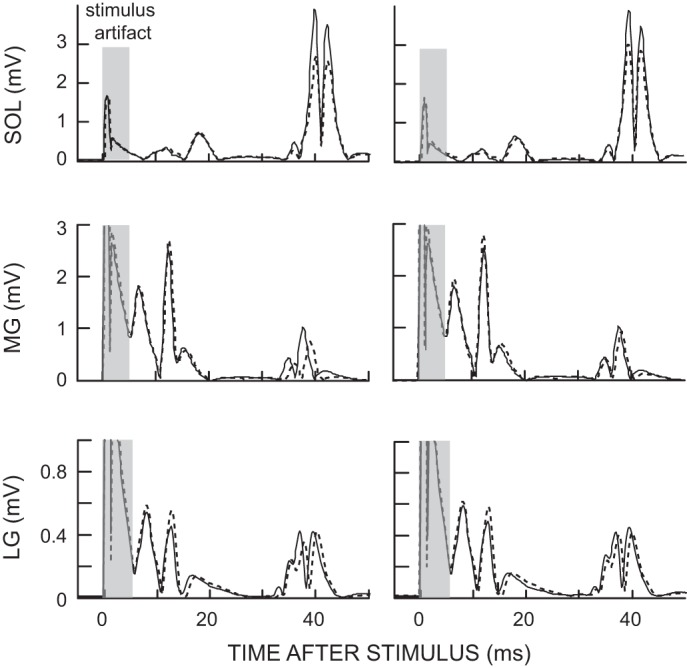
Average conditioned H-reflexes (left; 225 trials) and control H-reflexes (right; 20 trials) in soleus, medial gastrocnemius (MG), and lateral gastrocnemius (LG) in the sixth baseline session (solid line) and the last conditioning session (dotted line) from a subject whose H-reflex decreased significantly. The conditioned H-reflex size decreased after soleus H-reflex down-conditioning in soleus and MG, but not in LG. The control H-reflex decreased after conditioning only in soleus. Background electromyography (EMG) and M-wave size did not change between the baseline and last conditioning sessions. Sol, soleus.
Locomotor symmetry, EMG activity, H-reflex modulation, and kinematics.
H-reflex conditioning did not affect locomotor symmetry. Right/left step symmetry in the seven subjects averaged 1.0 (±0.03 SE) before and 1.0 (±0.04) after conditioning (P = 0.95; paired t-test).
EMG activity during locomotion was recorded as described above in six muscles of each leg while the subject walked on the treadmill at a comfortable speed. For each of the 12 muscles, the MI was high (>0.85) and did not differ significantly for the two locomotor sessions (i.e., before and after the 30 conditioning sessions) (P > 0.07 in each muscle; paired t-test). For each muscle, the amplitudes (absolute value in mV) of the highest of the 12 step-cycle time bins did not differ between the two sessions (P > 0.12 in all; paired t-test). Furthermore, in none of the 12 muscles did normalized EMG amplitude for any of the 12 bins differ significantly for the two locomotor sessions (P > 0.01 in each; paired t-test; nonsignificant after Ŝidák correction). Figure 2 shows the average normalized results from each of the 12 muscles before and after down-conditioning. As evident in Fig. 2, each muscle's average EMG pattern across the step cycle was essentially the same before and after the 30 conditioning sessions.
Fig. 2.

Average locomotor EMG activity for all 7 successfully conditioned subjects from soleus, tibialis anterior (TA), MG, LG, vastus lateralis (VL), and biceps femoris (BF) of both legs before (solid line) and after (dotted line) the 30 conditioning sessions. For each muscle, EMG amplitude in each of the 12 equal bins was normalized by the amplitude in the bin with the highest amplitude. There were no significant differences between pre- and postconditioning in any of the 12 bins in any of the 12 muscles.
In accord with previous locomotor studies (Makihara et al. 2012), the locomotor H-reflex was smaller in MG and LG than in soleus. H-reflex modulation over the step cycle was similar in magnitude and pattern in the three muscles. The MI was high (>0.89) in all three and did not differ for the two locomotor sessions (P = 0.73, 0.67, and 0.48 for soleus, MG, and LG, respectively; paired t-test). In all three muscles, the H-reflex was low during swing, increased to its maximum value during stance, and then fell rapidly at the beginning of swing. For all three muscles, the H-reflexes for each of the 12 bins of the step cycle did not differ between the two locomotor sessions (P > 0.10, 0.06, and 0.23 for soleus, MG, and LG, respectively; paired t-test). The average locomotor H-reflexes calculated by averaging values in 12 bins (Thompson et al. 2013a) did not change after conditioning in all three muscles (P > 0.36; paired t-test). Figure 3 shows average H-reflex modulation over the step-cycle for soleus, MG, and LG (in percent of Mmax) before and after the 30 conditioning sessions. In all three muscles, modulation is high, and the magnitude and pattern of modulation are comparable before and after soleus H-reflex down-conditioning.
Fig. 3.
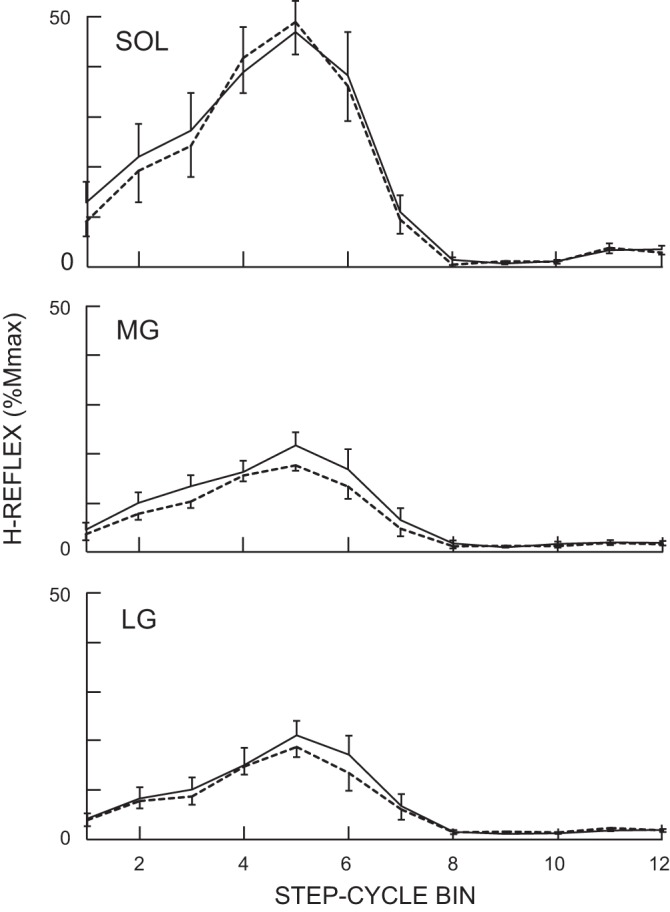
Average locomotor H-reflexes for all 7 successfully conditioned subjects from soleus, MG, and LG in the conditioned leg before (solid line) and after (dotted line) the 30 conditioning sessions. H-reflex size is expressed as a percentage of the maximum M-wave (%Mmax). As for locomotor EMG activity in Fig. 2, the step cycle was divided into 12 equal bins and an average reflex size for each bin was calculated. There were no significant differences between pre- and postconditioning in any of the 12 bins in any of the 3 muscles.
Ankle, knee, and hip joint angles in each of the 12 step-cycle bins were calculated and compared between the two locomotor sessions. No significant differences were found in any bin of any joint angle (P > 0.13; paired t-test). Furthermore, the maximum dorsiflexion and plantar flexion angles of the ankle, the maximum extension and flexion angles of the knee and hip, and the timings of these maxima in the step cycle did not differ between the two sessions before and after the conditioning (P > 0.05 for all). Figure 4 displays the average results for all subjects before and after conditioning.
Fig. 4.
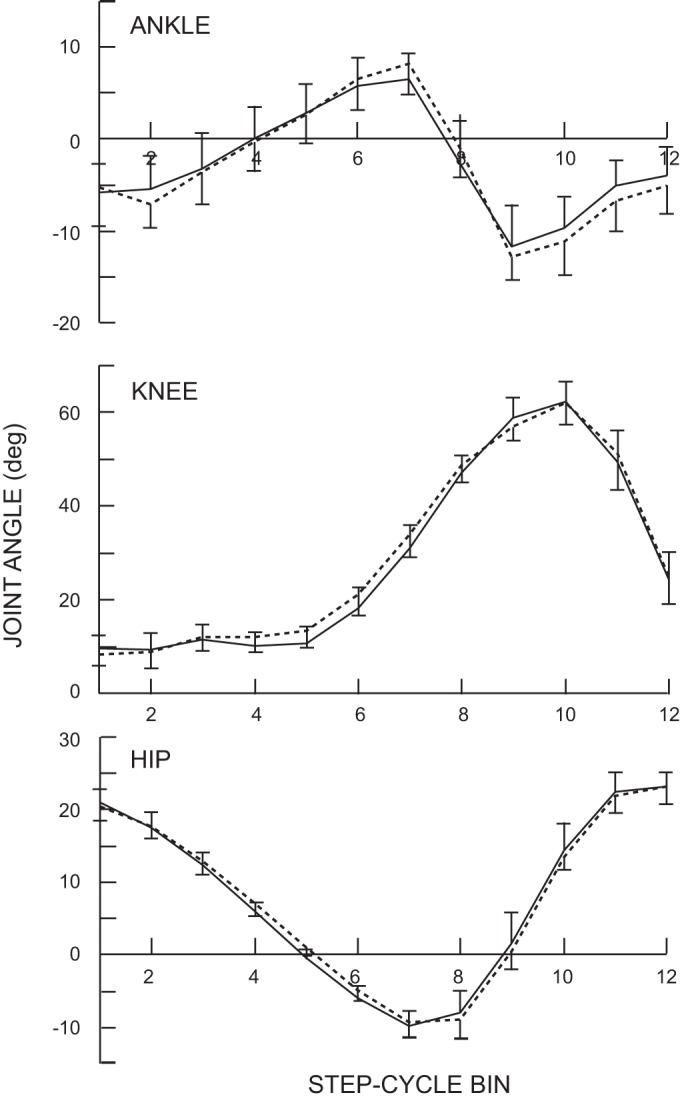
Average ankle, knee, and hip joint angles for all 7 successfully conditioned subjects in the conditioned leg over the 12 bins of the step cycle before (solid line) and after (dotted line) the 30 conditioning sessions. There were no significant differences between pre- and postconditioning in any of the 12 bins in any of the three joints. Ankle: +, dorsiflexion; −, plantarflexion; knee: +, flexion; −, extension; Hip: +, flexion; −, extension.
DISCUSSION
This study examined two different aspects of the impact of soleus H-reflex down-conditioning in neurologically normal humans: its impact on MG and LG H-reflexes, with particular focus on the extent of task-dependent adaptation (i.e., within-session change) and/or long-term change (i.e., across-session change), and its impact on locomotor EMG activity and kinematics. We found task-dependent adaptation in the MG H-reflex comparable to that in the soleus H-reflex, no task-dependent adaptation in the LG H-reflex, and no long-term change in the MG or LG H-reflex. Thus the change in the conditioned H-reflex (i.e., the sum of task-dependent adaptation and long-term change) was less in MG than in soleus, a result comparable to previous results in the upper limb (Wolf and Segal 1996; Wolpaw et al. 1983). We also found that soleus H-reflex down-conditioning produced no changes in locomotor EMG activity or kinematics that were consistent across subjects.
Task-dependent adaptation in gastrocnemius H-reflexes with down-conditioning of soleus H-reflex.
As discussed in Thompson et al. (2009), task-dependent adaptation in the H-reflex is likely to reflect task-dependent change in corticospinal tract (CST)-mediated presynaptic inhibition at the primary afferent synapse on the motoneuron (Baudry et al. 2010; Brooke et al. 1997; Meunier and Pierrot-Deseilligny 1998; Stein 1995; Stein and Capaday 1988). The fact that soleus H-reflex down-conditioning produced task-dependent adaptation in MG equal to that in soleus is consistent with the strong correlations between soleus and MG H-reflexes noted in previous studies (Makihara et al. 2012). More surprising is the lack of detectable task-dependent adaptation in the LG H-reflex. Although the mechanisms of this striking MG/LG difference are not clear, the three muscles do differ in other respects [e.g., in activation patterns during voluntary plantar flexion (Giordano and Segal 2006) and heel raise exercise (Segal and Song 2005) and in autogenic (homonymous) and heterogenic (heteronymous) excitation and inhibition (Nichols 1989)], and presynaptic inhibition can even differ between collaterals from the same muscle afferent (Rudman et al. 1998).
Lack of long-term change in the gastrocnemius H-reflexes.
Whereas both components of the final change in the conditioned reflex, task-dependent adaptation and long-term change, appear to depend on activity in sensorimotor cortex that reaches the spinal cord through the CST (Chen and Wolpaw 2002; Chen et al. 2002; BY Chen et al. 2006), they almost certainly differ in their spinal mechanisms. As noted above, task-dependent adaptation probably reflects change in presynaptic inhibition at the primary afferent synapse. In contrast, long-term change probably reflects plasticity in motoneuron properties, other synaptic terminals on the motoneuron, and/or spinal interneurons (see Wolpaw 2010 for review). It is not at present clear to what extent these two components are linked. Although both depend on the CST, they may depend on different aspects of CST activity. Furthermore, evidence indicates that their relative magnitudes vary across subject populations. In previous studies, long-term change was significantly greater in people with spinal cord injury than in neurologically normal people (−24% vs. −16% from Thompson et al. 2009), whereas task-dependent adaptation was significantly less (−7% vs. −15% from Thompson et al. 2009). The magnitudes of task-dependent adaptation and long-term change in the present study of normal subjects (Table 1) are similar to those of Thompson et al. (2009): more task-dependent adaptation (P = 0.05 by unpaired t-test) and significantly less long-term change (P < 0.05) than in the people with spinal cord injury of Thompson et al. (2013b).
The impact of task-dependent adaptation is specific to the conditioning protocol. That is, task-dependent adaptation affects H-reflex size only during the conditioning protocol; it does not affect the contribution of the reflex pathway to other behaviors, such as locomotion. The lesser task-dependent adaptation in people with spinal cord injury is probably due to deficits in descending control caused by the spinal cord damage (Thompson et al. 2013b). Long-term change lacks the specificity of task-dependent adaptation: it does affect the pathway's contribution to other behaviors. In rats or people with spinal cord injuries, long-term change in the soleus H-reflex is doubly adaptive; it increases reward probability in the conditioning protocol and it improves locomotion (Y Chen et al. 2006; Thompson et al. 2013b). On the other hand, in people who are neurologically normal, long-term change is both adaptive and maladaptive; it increases reward probability but can impair locomotion and/or existing motor skills. For example, it can cause locomotor asymmetries, unless its effect is counteracted by additional changes that offset the asymmetries (Chen et al. 2005, 2011, 2013). Thus the difference in specificity between task-dependent adaptation and long-term change is consistent with their relative magnitudes in the two subject groups (i.e., greater task-dependent adaptation in normal subjects, greater long-term change in subjects with spinal cord injuries). Task-dependent adaptation is preferable in normal subjects because it does not affect locomotion and other existing motor skills, whereas long-term change is preferable in subjects with spinal cord injuries because it does affect locomotion.
The present finding that soleus H-reflex conditioning produces comparable task-dependent adaption in soleus and MG, but long-term change only in soleus, is consistent with this difference in specificity. The long-term change is modest in the soleus H-reflex because it is adaptive for the H-reflex conditioning protocol but maladaptive for existing motor skills; and long-term change does not occur in the MG H-reflex because it is not adaptive for the conditioning protocol and is maladaptive for existing motor skills. This outcome is explicable in terms of the negotiated equilibrium hypothesis (Wolpaw 2010). According to the hypothesis, the state of the spinal cord (including the pathway of the soleus H-reflex) is the product of concurrent adaptive processes that acquire a new behavior (e.g., a smaller H-reflex) and at the same time preserve previously acquired behaviors (e.g., locomotion) (Chen et al. 2014; Thompson et al. 2013b; Thompson and Wolpaw, in press; Thompson and Wolpaw 2014; Wolpaw 2010 for discussion).
Lack of detectable impact on normal locomotion.
The present results indicate that soleus H-reflex conditioning in humans does not affect locomotion; there were no detectable consistent (i.e., across subjects) effects on step symmetry, locomotor EMG activity, H-reflex modulation, or joint motion. In normal rats, soleus H-reflex conditioning does not affect step symmetry, but it does alter locomotor EMG activity, H-reflexes, and kinematics (Chen et al. 2005, 2011). Thus the mechanisms ensuring preservation of locomotion appear to differ between rats and humans. In rats, in which the long-term change in the soleus H-reflex was still evident during locomotion, the key features of locomotion (e.g., step-cycle symmetry) appeared to be preserved by compensatory changes in other locomotor EMG activity and in kinematics (Chen et al. 2011). In humans, the long-term change in the soleus H-reflex change was not evident during locomotion, and no consistent changes in locomotor EMG or kinematics were detected. Several factors are likely to account for this difference.
First, the long-term soleus H-reflex change is considerably smaller in normal humans than it is in normal rats (i.e., −14% in the present human study vs. about −40% in rats; Chen et al. 2001 and subsequent data). Thus it would be expected to have much less effect on locomotion.
Second, the fact that there is no consistent effect on the human locomotor H-reflex suggests that compensatory changes prevented the long-term change in the soleus H-reflex from being apparent during locomotion. In humans, the H-reflex decreases from standing to walking (Capaday and Stein 1986; Stein and Capaday 1988). This task-dependent decrease probably reflects increased presynaptic inhibition produced by supraspinal descending pathways (Stein and Capaday 1988; Stein 1995). In the present subjects, the normal standing-to-walking decrease in presynaptic inhibition may have been smaller after soleus H-reflex down-conditioning so that the reflex pathway's locomotor contribution was maintained. Group I afferent pathways, including the soleus H-reflex pathway, are important in locomotion (Bennett et al. 1996; Stein et al. 2000; Yang et al. 1991). Thus preserving their activity during locomotion may be the compensation that maintains normal locomotor EMG activity and kinematics after soleus H-reflex down-conditioning in normal humans. The difference between rats and humans in the compensation that maintains normal locomotion may reflect interspecies differences in the task-dependent regulation of presynaptic inhibition and/or differences between quadrupedal and bipedal locomotion (Courtine et al. 2007; Knout et al. 2012).
Third, it is important to note that the changes in locomotor EMG and kinematics with H-reflex conditioning in rats were detected only because they were consistently present across animals. If different rats had compensated in different ways, the fact that compensatory EMG and kinematic changes occurred would have been much more difficult to document. For humans, who are likely to differ more widely than laboratory rats in previous motor experience, inter-subject differences in compensatory mechanisms might be considerably greater, and such variation could prevent statistical detection of the occurrence of compensatory changes in locomotor EMG or kinematics. Studies of populations with more uniform past motor experiences (e.g., specific groups of athletes) might clarify this issue.
GRANTS
This work was supported in part by the New York State Spinal Cord Injury Research Trust Fund C023685 (A. K. Thompson) and C020932 (X. Y. Chen and R. L. Segal) and National Institute of Neurological Disorders and Stroke Grants NS069551 (A. K. Thompson), NS22189 (J. R. Wolpaw), and NS061823 (J. R. Wolpaw and X. Y. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.M., R.L.S., J.R.W., and A.K.T. conception and design of research; Y.M. and A.K.T. performed experiments; Y.M. analyzed data; Y.M., R.L.S., J.R.W., and A.K.T. interpreted results of experiments; Y.M. and A.K.T. prepared figures; Y.M. drafted manuscript; Y.M., R.L.S., J.R.W., and A.K.T. edited and revised manuscript; Y.M., R.L.S., J.R.W., and A.K.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Jonathan Carp and Dennis McFarland for helpful comments on the manuscript and Briana Favale for assistance in data collection.
REFERENCES
- Baudry S, Maerz AH, Enoka RM. Presynaptic modulation of Ia afferents in young and old adults when performing force and position control. J Neurophysiol 103: 623–631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, De Serres SJ, Stein RB. Gain of the triceps sure stretch reflex in decerebrate and spinal cats during postural and locomotor activities. J Physiol 496: 837–850, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke JD, Cheng J, Collins DF, McElroy WE, Misiaszek JE, Staines WR. Sensori-sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Prog Neurobiol 51: 393–421, 1997 [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci 6: 1308–1313, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp JS, Tennissen AM, Chen XY, Wolpaw JR. Diurnal H-reflex variation in mice. Exp Brain Res 168: 517–528, 2006a [DOI] [PubMed] [Google Scholar]
- Carp JS, Tennissen AM, Chen XY, Wolpaw JR. H-reflex operant conditioning in mice. J Neurophysiol 96: 1718–1727, 2006b [DOI] [PubMed] [Google Scholar]
- Chen XY, Carp JS, Chen L, Wolpaw JR. Corticospinal tract transection prevents operantly conditioned H-reflex increase in rats. Exp Brain Res 144: 88–94, 2002 [DOI] [PubMed] [Google Scholar]
- Chen XY, Carp JS, Chen L, Wolpaw JR. Sensorimotor cortex ablation prevents H-reflex up-conditioning and causes a paradoxical response to down-conditioning in rats. J Neurophysiol 96: 119–127, 2006 [DOI] [PubMed] [Google Scholar]
- Chen XY, Chen L, Wolpaw JR. Time course of H-reflex conditioning in the rat. Neurosci Lett 302: 85–88, 2001 [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Circadian rhythm in rat H-reflex. Brain Res 648: 167–170, 1994 [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol 73: 411–415, 1995 [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Probable corticospinal tract control of spinal cord plasticity in the rat. J Neurophysiol 87: 645–652, 2002 [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen L, Liu R, Wang Y, Chen XY, Wolpaw JR. Locomotor impact of beneficial or nonbeneficial H-reflex conditioning after spinal cord injury. J Neurophysiol 111: 1249–1258, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen L, Liu RL, Wang Y, Wolpaw JR, Chen XY. Locomotor effects of H-reflex conditioning in rats with transection of the dorsal column ascending tract. Program No 64504. 2013 Neuroscience Meeting Planner San Diego, CA: Society for Neuroscience, 2013. Online. [Google Scholar]
- Chen Y, Chen L, Wang Y, Wolpaw JR, Chen XY. Operant conditioning of rat soleus H-reflex oppositely affects another H-reflex and changes locomotor kinematics. J Neurosci 31: 11370–11375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci 26: 12537–12543, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Schalk G, Stokes BT, Wolpaw JR. The interaction of a new motor skill and an old one: H-reflex conditioning and locomotion in rats. J Neurosci 25: 6898–6906, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, Rouiller EM, Schnell L, Wannier T, Schwab ME, Edgerton VR. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med 13: 561–566, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edamura M, Yang JF, Stein RB. Factors that determine the magnitude and time course of human H-reflexes in locomotion. J Neurosci 11: 420–427, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano SB, Segal RL. Leg muscles differ in spatial activation patterns with differing levels of voluntary plantarflexion activity in humans. Cells Tissues Organs 184: 42–51, 2006 [DOI] [PubMed] [Google Scholar]
- Kido A, Tanaka N, Stein RB. Spinal reciprocal inhibition in human locomotion. J Appl Physiol 96: 1969–1977, 2004a [DOI] [PubMed] [Google Scholar]
- Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol 82: 238–248, 2004b [DOI] [PubMed] [Google Scholar]
- Lagerquist O, Zehr EP, Baldwin ER, Klakowicz PM, Collins DF. Diurnal changes in the amplitude of the Hoffmann reflex in the human soleus but not in the flexor carpi radialis muscle. Exp Brain Res 170: 1–6, 2006 [DOI] [PubMed] [Google Scholar]
- Llewellyn M, Yang JF, Prochazka A. Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res 83: 22–28, 1990 [DOI] [PubMed] [Google Scholar]
- Makihara Y, Segal RL, Wolpaw JR, Thompson AK. H-reflex modulation in the human medial and lateral gastrocnemii during standing and walking. Muscle Nerve 45: 116–125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Cortical control of presynaptic inhibition of Ia afferents in humans. Exp Brain Res 119: 415–426, 1998 [DOI] [PubMed] [Google Scholar]
- Nichols TR. The organization of heterogenic reflexes among muscles crossing the ankle joint in the decerebrate cat. J Physiol 410: 463–477, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nout YS, Rosenzweig ES, Brock JH, Strand SC, Moseanko R, Hawbecker S, Zdunowski S, Nielson JL, Roy RR, Courtine G, Ferguson AR, Edgerton VR, Beattie MS, Bresnahan JC, Tuszynski MH. Animal models of neurologic disorders: a nonhuman primate model of spinal cord injury. Neurotherapeutics 9: 380–392, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Jimenez I, Quevedo J. Selectivity of the presynaptic control of synaptic effectiveness of group I afferents in the mammalian spinal cord. In: Presynaptic Inhibition and Neural Control, edited by Rudomin P, Romo R, Mendell LM. New York: Oxford University Press, 1998, p. 282–302 [Google Scholar]
- Segal RL, Song AW. Nonuniform activity of human calf muscles during an exercise task. Arch Phys Med Rehabil 86: 2013–2017, 2005 [DOI] [PubMed] [Google Scholar]
- Ŝidák Z. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc 62: 626–633, 1967 [Google Scholar]
- Stein RB. Presynaptic inhibition in humans. Prog Neurobiol 47: 533–544, 1995 [DOI] [PubMed] [Google Scholar]
- Stein RB, Capaday C. The modulation of human reflexes during functional motor tasks. Trends Neurosci 11: 328–332, 1988 [DOI] [PubMed] [Google Scholar]
- Stein RB, Misiaszek JE, Pearson KG. Functional role of muscle reflexes for force generation in the decerebrate walking cat. J Physiol 525: 781–791, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci 29: 5784–5792, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Chen XY, Wolpaw JR. Soleus H-reflex operant conditioning changes the H-reflex recruitment curve. Muscle Nerve 47: 539–544, 2013a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Pomerantz FR, Wolpaw JR. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci 33: 2365–2375, 2013b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Wolpaw JR. Restoring walking after SCI: operant conditioning of spinal reflexes can help. Neuroscientist. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Wolpaw JR. Operant conditioning of spinal reflexes: From basic science to clinical therapy. Front Integr Neurosci 8: 25, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SL, Segal RL. Reducing human biceps brachii spinal stretch reflex magnitude. J Neurophysiol 75: 1637–1646, [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Operant conditioning of primate spinal reflexes: the H-reflex. J Neurophysiol 57: 443–459, 1987 [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. What can the spinal cord teach us about learning and memory? Neuroscientist 16: 532–549, 2010 [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Seegal RF. Diurnal rhythm in the spinal stretch reflex. Brain Res 244: 365–369, 1982 [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Seegal RF, O'Keefe JA. Adaptive plasticity in primate spinal stretch reflex: behavior of synergist and antagonist muscles. J Neurophysiol 50: 1312–1319, 1983 [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB. Phase-dependent reflex reversal in human leg muscles during walking. J Neurophysiol 63: 1109–1117, 1990 [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Exp Brain Res 87: 679–687, 1991 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Kido A. Neural control of rhythmic, cyclical human arm movement: task dependency, nerve specificity and phase modulation of cutaneous reflexes. J Physiol 537: 1033–1045, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Loadman PM. Persistence of locomotor-related interlimb reflex networks during walking after stroke. Clin Neurophysiol 123: 796–807, 2012 [DOI] [PubMed] [Google Scholar]


