Abstract
Hemispheric brain injury resulting from a stroke is often accompanied by muscle weakness in limbs contralateral to the lesion. In the present study, we investigated whether weakness in contralesional hand muscle in stroke survivors is partially attributable to alterations in motor unit activation, including alterations in firing rate modulation range. The afterhyperpolarization (AHP) potential of a motoneuron is a primary determinant of motoneuron firing rate. We examined differences in AHP duration in motoneurons innervating paretic and less impaired (contralateral) limb muscles of hemiparetic stroke survivors as well as in control subjects. A novel surface EMG (sEMG) electrode was used to record motor units from the first dorsal interosseous muscle. The sEMG data were subsequently decomposed to derive single-motor unit events, which were then utilized to produce interval (ISI) histograms of the motoneuron discharges. A modified version of interval death rate (IDR) analysis was used to estimate AHP duration. Results from data analyses performed on both arms of 11 stroke subjects and in 7 age-matched control subjects suggest that AHP duration is significantly longer for motor units innervating paretic muscle compared with units in contralateral muscles and in units of intact subjects. These results were supported by a coefficient of variation (CV) analysis showing that paretic motor unit discharges have a lower CV than either contralateral or control units. This study suggests that after stroke biophysical changes occur at the motoneuron level, potentially contributing to lower firing rates and potentially leading to less efficient force production in paretic muscles.
Keywords: motor units, first dorsal interosseous, afterhyperpolarization, paresis, stroke
one of the most common motor impairments after stroke is paresis, or weakness for voluntary movement. While progressive changes in mechanical properties of paretic muscles, such as muscle atrophy, metabolic changes, and connective tissue infiltration, are prevalent and could contribute to paresis, changes at the spinal neuronal level may contribute as well. Indeed, understanding potential changes in the properties and control of motoneurons could offer further insight into mechanisms that may contribute to muscle weakness after stroke. Accordingly, our objective in this study is to assess the potential contributions of changes in motoneuron properties to muscle weakness observed in many stroke survivors.
Earlier electrophysiological studies in stroke survivors have reported reductions of motor unit (MU) number, fibrillation and sharp waves, reduced compound muscle action potential (M wave) amplitude, and lower firing rates in motoneurons of paretic muscles (Brown and Snow 1990; Dattola et al. 1993; Gemperline et al. 1995; McComas et al. 1973; Spaans and Wilts 1982). Gemperline et al. (1995) reported lowered MU discharge rates recorded from paretic biceps muscles of stroke survivors compared with contralateral muscles at the same force level. While reduced firing rates could be a function of diminished descending drive following a cerebral lesion, changes in the electrical properties of the motoneuron could also contribute to the reduced MU firing rates (Gemperline et al. 1995).
The goal of the present study was to determine whether changes in a specific motoneuron property, the postspike afterhyperpolarization (AHP), contribute to the observed reductions in mean firing rates. The AHP is a primary determinant of motoneuron firing rate, and any increase in its duration or amplitude could alter MU firing behaviors (Bakels and Kernell 1993). The relative variability of discharge in motoneurons is dependent upon both the AHP size and time course, as well as the amplitude and frequency content of concurrent synaptic noise. While statistical methods have been used to estimate the AHP duration in neurologically intact human subjects (Gossen et al. 2003; MacDonell et al. 2007; Matthews 1996, 2002), sparse data exist in stroke survivors. As of yet, no study has performed a systematic comparison between the paretic and contralateral sides of stroke survivors in upper limbs (Ivanova et al. 2014).
Currently, two methods of estimating AHP duration in humans exist: interval death rate (IDR) analysis (Matthews 1996) and variability analysis of interspike intervals (ISIs) (Piotrkiewicz 1999). Both methods have been validated through animal models (Powers and Binder 2000). A study in which variability analysis compared average AHP duration of MUs in biceps brachii between poststroke patients and healthy control subjects reported a lengthened AHP duration after cerebral stroke (Liang et al. 2010). However, as the authors state, comparison of ISI variability between groups may have been confounded by a difference in spike generating modes: rhythmic firing mode (short interval range) versus occasional spike mode (long interval range) (Calvin 1974). Rhythmic firing mode is composed of spikes that fire regularly, usually in the faster range of firing rates for a given MU. Occasional spike mode is composed of spikes that fire irregularly, usually in the slowest range of firing rates for a given MU. Results from this study also indicate that interval variability is significantly different in paretic limbs compared with contralateral or control limbs, suggesting that measuring AHP through variability analysis may not be accurate.
Accordingly, the objective of our study was to assess AHP duration systematically, utilizing a modification of the IDR analysis method during similar MU firing modes (Zijdewind and Thomas 2012), and to compare the results obtained from paretic, nonparetic (contralateral), and control muscles. To determine whether distal limb muscles exhibit alterations in motoneuron biophysical properties, we collected EMG data with a novel, noninvasive surface electromyography (sEMG) sensor array (Delsys) from the first dorsal interosseous (FDI). The results of our study suggest that motoneuron/MU recordings obtained from distal paretic muscles display significantly longer AHP durations compared with contralateral muscles of stroke survivors as well as comparable muscles in neurologically intact persons.
METHODS
Subjects
Eleven chronic hemiparetic stroke subjects with moderate to severe weakness of the contralesional extremities were tested (see Table 1). The subjects were recruited from the outpatient clinics of the Rehabilitation Institute of Chicago. Seven age-matched control subjects were recruited as well. All participants gave informed consent via protocols approved by the Institutional Review Board at Northwestern University.
Table 1.
Demographic information for stroke subjects
| ID | Sex | Age, yr | Time Since Stroke, yr | FT | C | Paretic Side |
|---|---|---|---|---|---|---|
| 1 | M | 46 | 13 | 24 | 3 | L |
| 2 | M | 61 | 4 | 63 | 6 | R |
| 3 | F | 53 | 3 | 63 | 7 | R |
| 4 | M | 65 | 17 | 17 | 2 | R |
| 5 | M | 66 | 9 | 16 | 4 | L |
| 6 | F | 63 | 16 | 17 | 2 | L |
| 7 | F | 58 | 5 | 38 | 4 | R |
| 8 | M | 57 | 8 | 63 | 5 | R |
| 9 | F | 58 | 5 | 56 | 4 | R |
| 10 | F | 59 | 23 | 22 | 2 | R |
| 11 | F | 68 | 5 | 66 | 7 | R |
FT, arm motor Fugl-Meyer motor assessment (X/66); C, Chedoke-McMaster stroke assessment (hand).
Stroke participants.
Stroke participants were adults who had sustained a hemiparetic stroke at least 6 mo prior to experimental testing. We evaluated the ability of the participants to complete the specified finger tasks as well as the ability to attain and maintain the experimental sitting posture and arm position, using several clinical assessment scales including the Fugl-Meyer scale and the Chedoke-McMaster hand assessment scale. A research physical therapist performed the clinical evaluation on both sides of our hemiparetic stroke subjects.
Neurologically intact participants.
Our neurologically intact subjects were, on average, age matched to our stroke survivors. A clinical assessment of our intact subjects was performed by the research physical therapist to affirm the lack of neurological impairment and/or motor deficits.
Experimental Setup
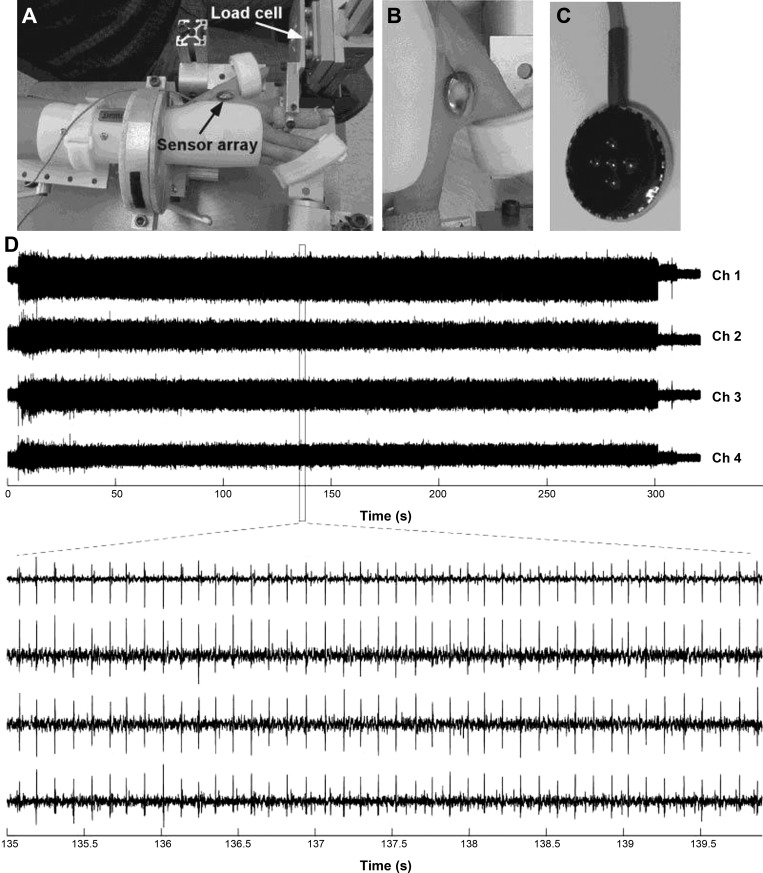
Study participants were seated upright in a Biodex chair with their upper arm comfortably resting on a plastic support. To help standardize hand position as well as to minimize activity of unrecorded muscles, the forearm was casted and placed in a ring-mount interface attached to an elbow rest; this rest was securely mounted with magnetic stands to a heavy steel table. The right shoulder was placed in 45° of abduction and neutral rotation, the elbow in 60° of flexion, and the forearm in full pronation, and the wrist was held neutral with respect to flexion/extension. The third through fifth digits were comfortably splayed and strapped to the support surface. The first digit was secured at a 45° angle to the second digit. The second digit was placed in line with the second metacarpal and the long axis of the forearm, creating a 0° or neutral (abduction/adduction) metacarpophalangeal (MCP) joint angle. The proximal phalanx of the second digit was cast and fixed to a ring-mount interface attached to a six degrees-of-freedom load cell (JR3, ATI) (Fig. 1A). The recorded forces from the x (abduction/adduction) direction were low-pass filtered (cutoff = 200 Hz) and digitized at a sampling frequency of 1 kHz.
Fig. 1.
Experimental setup, surface sensor array, surface EMG (sEMG) signals, and force display. A: brace and cast used to ensure that index finger was aligned with the long axis of the forearm. B: placement of Delsys sensor array on first dorsal interosseous (FDI) muscle. C: 5 cylindrical probes of the sensor array located at the corners and center of a 5 × 5-mm square. D: pairwise differentiation of the 5 electrodes allows for 4 channels of sEMG signals.
MU discharges from the FDI muscle were derived from a sEMG recording using a surface sensor array (Delsys) as shown in Fig. 1B. The sensor consists of five slender cylindrical probes, located at the corners and at the center of a 5 × 5-mm square (Fig. 1C). Pairwise differentiation of the five electrodes yields four channels of sEMG signals (Fig. 1D). The sEMG sensor and a reference electrode were connected to four channels of a Delsys Bagnoli sEMG system. The signals were amplified and filtered with a bandwidth of 20–2,000 Hz. The signals were sampled at 20 kHz.
Procedures
Upon completion of the experimental setup, the subjects were instructed to perform a very small isometric abduction task with their index finger. MU recruitment was monitored both visually on a computer screen and with auditory feedback from an analog speaker by the experimenter. Once a single unit was audibly and visually identified, the participants were requested to maintain a relatively stable firing rate of a single MU at the lowest possible rate in order to ensure that the ISI histogram included intervals longer than the AHP duration (see Data Analysis for further explanation). The subject was given both visual and audio feedback of MU firings. To identify the lowest regular firing frequency for the MU, the subject was asked to increase and decrease the level of effort until a sustained low rate of firing was achieved, and was then requested to maintain that unit firing at that rate for as long as possible. During this period, we also monitored for fatigue, by querying the subject as well as by monitoring the EMG signal. We monitored the EMG signal by ensuring that the isolated unit remained at a relatively stable firing rate and that additional units were not being recruited to maintain the same level of effort. The data for each MU were collected over several trials, allowing the subject to rest between trials.
All four sEMG channels as well as the force signal were monitored online via Spike2 (Cambridge Electronic Design) and subsequently stored for processing. A minimum of four MUs were collected from each of the paretic and contralateral sides. Most stroke subjects were tested on both sides with the same protocol during one session, with the paretic muscle tested first. Two subjects (subjects 1 and 5) were tested on separate days. The same protocol was repeated on a randomly selected side of control subjects.
Data Analysis
Spike2 was used to decompose and classify MUs with a template matching algorithm based on amplitude and shape characteristics. The sEMG trials were selected for further analysis on the basis of the following criteria: 1) >1,000 spikes were generated for a single MU and 2) the instantaneous firing rate variability during the steady-state force had a standard deviation (SD) of <0.5 pps.
Trains of single-MU action potentials were further analyzed in order to compute the AHP duration estimates with a modified IDR method (Matthews 1996) with code written in MATLAB (MathWorks). The modified IDR method is as follows.
As described by Matthews et al., in order to compensate for the length of recordings as well as the high spike variability associated with low firing rates, each spike train was parsed into subpopulations to ensure that ISI histograms represented a specific firing range. Each spike train was divided into subpopulations derived from segments that were spliced together based on a similar running mean firing rate, which was determined by averaging five spikes before and five spikes after a targeted spike (Matthews 1996). The resulting interval histogram of the subpopulation of spikes was then generated based on instantaneous intervals. Interval histograms (5-ms bins) thereby represented a distinct mean firing rate distribution for a given MU. Changing bin size has two opposing effects on estimation accuracy. Increasing bin size reduces noise but also reduces resolution. Bin sizes of 5 ms are conventionally used for IDR analysis. All ISI histograms that consisted of at least 1,000 intervals (mean: 1,583 intervals, SD: 521) were used for further analysis. ISI death rates were then calculated, representing the probability (P) that a MU discharge will terminate a given ISI. The calculation of P is described by the following equation:
| (1) |
N0 is the sum of following bins in the interval histogram, including the bin whose P is being measured, and N1 is the sum of all following bins excluding the bin whose P is being measured. The value P has also been called the interval death rate (IDR; Matthews 1996) because it represents the probability of a spike occurring (i.e., the interval “dying”) as a function of time from the previous spike. The final IDR plot eliminates the last 5% of P values, since the equation diverges quickly when remaining bin numbers are low.
To assess AHP duration, the interval at which the IDR plot reaches a clear “plateau” was determined. The probability of a spike occurring at short intervals is very low, because of the greater membrane hyperpolarization and increased conductance due to the AHP. As the AHP decays, spike probability increases monotonically until it reaches a plateau at the completion of the AHP. Identification of the plateau phase is important because the start of the IDR plot plateau represents the time in the postspike interval after which the probability of spike occurrence is independent of the latency since the previous spike, and thus represents the time after which the AHP has concluded. The death rate plot terminates growth at the completion of the AHP because the membrane potential reaches a “steady state,” where the AHP no longer affects the probability of spike initiation (Matthews 1996; Zijdewind and Thomas 2012). Since IDR plots are constrained by finite interval bins, the “plateau” period is often characterized by erratic behavior resulting from low bin counts, where values substantially deviate from the initial smooth trajectory of the IDR (Fig. 2).
Fig. 2.
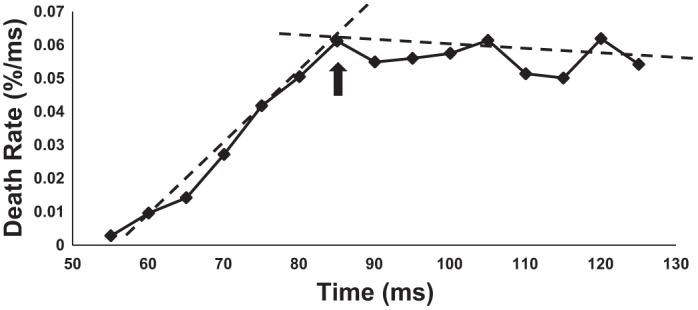
Sample interval death rate plot from subject 3 (contralateral side); arrow indicates beginning of plateau phase and measure of afterhyperpolarization (AHP) duration. The estimate, or “termination interval,” is marked by the intersection of 2 fitted lines that provide the summed least-squares error. An operator indicates the intervals on which the piecewise linear regression is performed due to the fact that the equation diverges quickly when remaining bin numbers are low. Dashed lines show piecewise linear regression results and corresponding intersection of the 2 lines.
As Fig. 2 indicates, this region can be readily identified by the point at which the slope of the plot (qualitatively) reaches a mean of zero. To quantitatively estimate the beginning of the plateau region, the AHP termination interval was determined by performing a piecewise linear regression fit to the trajectory of the IDR.
Control subject AHP estimates were calculated with modified IDR analysis as well as ISI variability analysis. ISI variability analysis utilizes similar firing rate data and determines AHP duration through the relationship between mean interval and SD. AHP duration is quantified by the “turning interval,” which is where the SD substantially increases with mean interval (Piotrkiewicz et al. 2007; Powers and Binder 2000).
The Wilcoxon rank test was used to determine whether control AHP estimate results were significantly different between ISI variability analysis and modified IDR analysis. A previous study confirms that AHP estimate durations derived from modified IDR analysis and ISI variability analysis are significantly correlated with each other (Zijdewind and Thomas 2012).
Additionally, we tested MUs from two control subjects to ensure that AHP estimates did not change significantly with MU firing rate. Subjects were asked to hold steady firing of one MU over three different firing rates. Modified IDR analysis was then performed on these three separate recording trains to ensure that the AHP estimates were independent of firing rate.
Statistical analysis.
Paired t-tests were used to determine whether AHP estimates and firing rates were significantly different between paretic and contralateral sides within subjects. Analysis of variance (ANOVA) with Bonferroni's correction for multiple comparisons was used to compare mean AHP estimate durations as well as mean firing rates among paretic, contralateral, and control MU populations.
MU trials were subsequently parsed into short 20- to 25-s trains and then selected for further coefficient of variation (CV) analysis on the basis of the following criteria: 1) >200 consecutive spikes were generated for a single MU; 2) the ISI train was determined to be normally distributed by the Lillifors test along with visualization of the ISI interval histogram; and 3) firing rates of each MU sample were within the range of 6–9 pps. With these three criteria, the data were assumed to be stationary with sufficient samples to perform higher-order statistics on each MU train. Detailed explanations of this analysis are found in a previous study by Andreassen and Rosenfalck (1980).
Thus only subgroups of MUs between all subjects for both the contralateral and paretic sides were used for CV analysis. This is due to the fact that the CV is a function of MU firing rate and only MUs that demonstrated a firing rate of 6–9 pps (for at least 20–25 s) were used for the calculation of CV. We chose 6–9 pps because this range allowed for the most inclusive representation of both contralateral and paretic MUs. Smaller samples from the original longer spike trains (up to 600 s) were taken since the long spike trains were usually nonstationary and a stationary train of consecutive MU spikes is necessary for a CV analysis.
The CV was calculated, in order to assess normalized variability, with the following equation:
| (2) |
ANOVA with Bonferroni's correction for multiple comparisons was used to determine whether mean CV values were significantly different among paretic, contralateral, and control MUs.
Linear regression analysis was performed on contralateral-to-paretic ratios of AHP estimates and firing rates. Additionally, linear regression analysis was performed on AHP estimates versus CV for contralateral and paretic groups. To determine whether the regression line between paretic and contralateral groups significantly differed, an analysis of covariance (ANCOVA) was performed.
RESULTS
The objective of our study was to investigate whether the estimated duration of the AHP potential, a primary determinant of motoneuronal firing rate, was greater in MUs recorded from contralesional muscle of stroke survivors than in MUs drawn from the equivalent ipsilesional limb muscle. MU data collected from sEMG recordings of the FDI muscles of 11 hemiparetic stroke subjects were analyzed using the modified IDR method. A total of 48 MUs were collected from the contralateral side of 11 stroke survivors and 56 MUs from the paretic side of stroke survivors. Twenty-four MUs in total were collected from seven control subjects. However, because of the requirements of the data analysis method, only 36 contralateral MUs, 39 paretic MUs, and 18 control MUs were further analyzed for AHP duration estimation (all further reporting is on these subsets of units). The limiting factor in including a MU for AHP estimation utilizing the IDR method is that the analysis is only valid on MUs with firing rate characteristics that exhibit a “plateau” in the IDR plot or an exponential decay in the ISI histogram. The rationale is that these features will only emerge for firing rates of MUs for which the interval is largely governed by the AHP, which is generally true only at very low discharge rates.
Subjects attempted to keep a single unit active for as long as possible; MU recordings of <250 s were not used for further analysis because of the low number of ISIs. The range of recording lengths for MUs retained for further analysis was of the order of 250–600 s. An average of 1,583 intervals was collected from each single-unit recording train.
Contralateral and Paretic AHP Estimates Using Modified IDR Analysis
A comparison of AHP duration estimates for each stroke subject was performed with matched pairs, where computed estimates from single MUs were averaged from MUs derived from each side separately. Matched-pairs comparisons were performed between the paretic and contralateral sides within one subject, because of the heterogeneous nature of the stroke population. Our results show that, on average, the estimated AHP duration was significantly longer in MUs recorded on the paretic side compared with the contralateral side (P < 0.05). Shown in Fig. 3 are sample interval histograms as well as IDR plots from one paretic unit and one contralateral unit from a single subject, illustrating the differences in mean AHP duration. The estimated AHP duration was 105 ms for the MU from the contralateral side versus 163 ms for the MU from the paretic side.
Fig. 3.
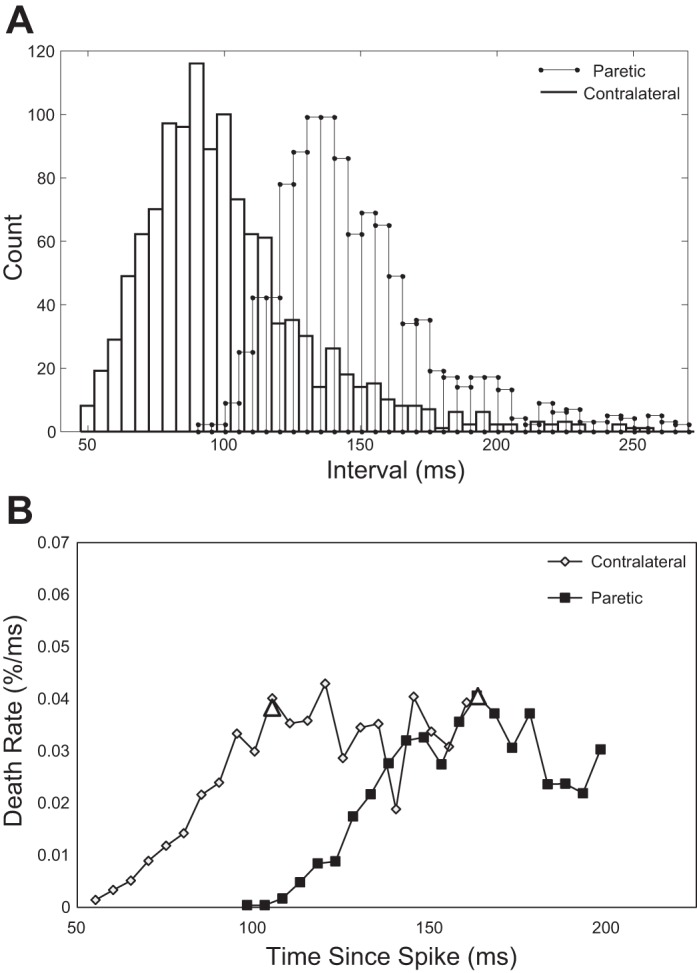
Subject 1. A: interval histogram of spike train from a contralateral single motor unit (MU) and paretic single MU. B: corresponding interval death rate plots, with AHP estimate indicated by white triangle. Contralateral unit: mean firing rate: 9.5 Hz, estimated AHP duration: 105.1 ms. No. of intervals: 1,093. Paretic unit: mean firing rate: 6.7 Hz, estimated AHP duration: 163.3 ms. No. of intervals: 1,044.
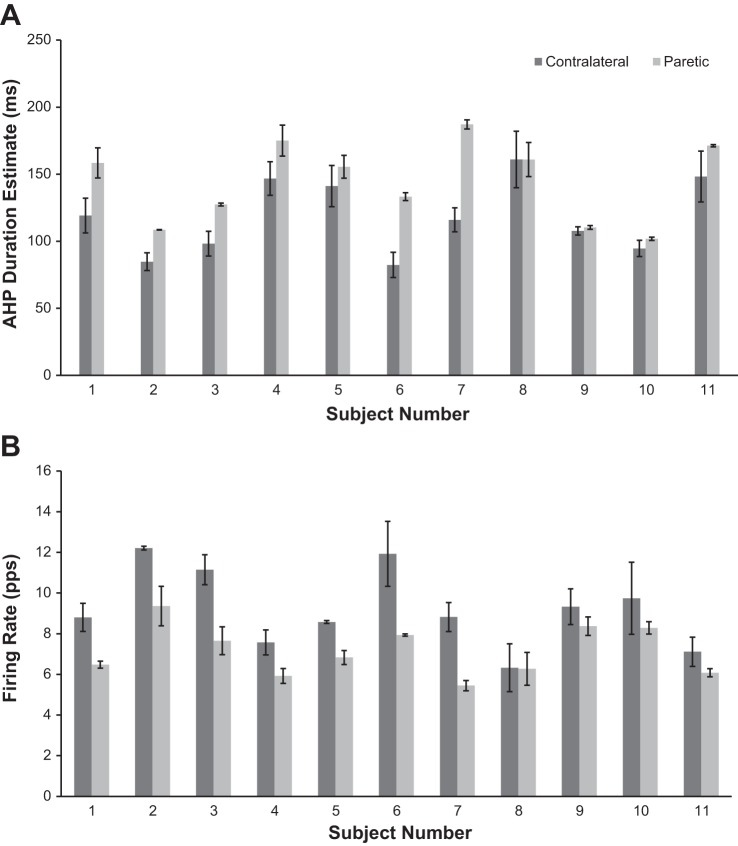
Figure 4 summarizes our results from all tested subjects. AHP duration values are obtained by averaging all the AHP estimates for all MUs (range of 2–6) collected for each subject for each side (paretic or contralateral) separately. We found that for 10 of the 11 tested subjects the average AHP duration estimate derived from the paretic side was longer than the average AHP duration estimate derived from the contralateral side (Fig. 4).
Fig. 4.
Results that illustrate average AHP estimates (A) and firing rates (B) (over 2–6 MUs) on the contralateral and paretic sides for each individual subject. Error bars represent SE for each subject. A matched-pairs statistical comparison suggests that contralateral AHP duration estimates were significantly less than paretic AHP duration estimates (P < 0.05). Contralateral side: subject 1, 4 units; subjects 2–6, 3 units; subject 7, 2 units; subject 8: 5 units; subject 9: 3 units; subject 10, 4 units; subject 11, 3 units. Paretic side: subject 1, 6 units; subject 2, 3 units; subject 3, 5 units; subject 4, 6 units; subject 5, 6 units; subject 6, 3 units; subjects 7–11, 2 units.
To ensure that parametric statistics were valid for these comparisons, the Lilliefors goodness-of-fit test was applied to AHP estimates of both sides. Our analysis shows that the estimates of AHP from both the paretic and contralateral sides follow an approximately normal distribution (P > 0.05). These results indicate that a paired t-test is a valid statistical test for assessment of significant differences in AHP duration estimates between the two sides of a stroke subject.
AHP Estimates and Firing Rate of Control Subjects
All stroke subject data were pooled in order to analyze differences with age-matched control subjects. Comparisons of mean AHP estimates as well as mean firing rates were performed with an ANOVA with Bonferroni's correction for multiple comparisons between control MUs (18), contralateral MUs (36), and paretic MUs (39).
When compared at the α = 0.05 level, our results show that AHP duration estimates were significantly shorter in control MUs (mean: 125 ms; SD: 22 ms) compared with paretic MUs (mean: 147 ms; SD: 29 ms) as well as between contralateral (mean: 116 ms; SD: 27 ms) and contralesional “paretic” MUs. There were no significant differences in AHP duration estimates between control MUs and contralateral MUs. Results for MU mean firing rates also showed the same trend, in that there were significant differences in mean MU firing rates for comparisons made between contralateral (mean: 9.4 pps; SD: 2.0 pps) and paretic (mean: 7.11 pps; SD: 1.2 pps) MUs as well as those between paretic and control MUs (mean: 8.2 pps; SD: 2.1 pps).
In contrast, there were no significant differences in firing rates recorded from contralateral versus control MUs. These values represent averages taken over the entire subject population. Because of the heterogeneous nature of the stroke population, it is difficult to determine limits of high or low firing rates. Although we show average firing rates over the entire population of MUs collected, firing rate values vary considerably between subjects, as demonstrated in the SDs of these measures. As a result, comparisons between the control and paretic sides within each stroke subject better reflect individual poststroke physiological changes.
Validation of Modified IDR Method
To validate our modified IDR method, we compared AHP duration estimates derived from this method with estimates derived with the ISI variability analysis (Piotrkiewicz 1999) for the control subjects only. Using the Wilcoxon paired difference test (P = 0.511) we found that the AHP duration estimates computed with the modified IDR method were not significantly different from estimates computed with the ISI variability analysis (mean: 120 ms; SD 24 ms). Furthermore, both methods have been validated in animal models, as described in a previous study (Powers and Binder 2000). Additionally, a previous study has verified the correlation between the modified IDR method and ISI variability analysis (Zijdewind and Thomas 2012). Modified IDR analysis was chosen as the preferred analysis method for the present study because of the inherent lower MU firing rates exhibited in paretic muscle (Gemperline et al. 1995). For such low firing rates, ISI variability analysis may not offer an accurate comparison of AHP estimates, since the variability of the ISI is strongly influenced by mean interval values (Liang et al. 2010).
Relationship Between AHP Estimates and Clinical Assessments
We also examined the relationship between our experimentally derived parameters in paretic and nonparetic muscle and the experimental and clinical assessments of weakness. For each stroke subject, we computed the ratios between the paretic and contralateral sides for both AHP duration estimates and mean MU firing rates. Maximum voluntary contraction forces (MVCs) were also recorded on the paretic and contralateral side from 8 of 11 subjects, and the force ratio between both sides was also calculated. Table 2 displays these results for each subject. The computed MVC ratios did not significantly correlate with the computed MU firing rate ratio or AHP duration estimate ratios, and there was no apparent trend.
Table 2.
Ratios of paretic to contralateral values for MVC, firing rate, and AHP estimate
| Subject | MVC Paretic, N | MVC Contralateral, N | MVC Ratio | Firing Rate Ratio | AHP Estimate Ratio |
|---|---|---|---|---|---|
| 1 | 14 | 40 | 0.35 | 0.74 | 1.33 |
| 2 | 30 | 39 | 0.77 | 0.77 | 1.28 |
| 3 | 26 | 65 | 0.40 | 0.69 | 1.30 |
| 4 | N/C | N/C | N/C | 0.78 | 1.19 |
| 5 | 6.7 | 22 | 0.30 | 0.80 | 1.10 |
| 6 | 13.2 | 31 | 0.43 | 0.67 | 1.62 |
| 7 | N/C | N/C | N/C | 0.62 | 1.33 |
| 8 | 17.7 | 31 | 0.57 | 0.99 | 1.00 |
| 9 | N/C | N/C | N/C | 0.90 | 1.33 |
| 10 | 8.25 | 37 | 0.22 | 0.85 | 1.08 |
| 11 | 13.6 | 33 | 0.41 | 0.85 | 1.33 |
Ratio data are ratios of paretic to contralateral values for maximum voluntary contraction force (MVC, N), firing rate (pps), and afterhyperpolarization (AHP) estimate (ms). N/C indicates that data were not collected.
We then attempted to find correlations between these ratios derived from experimental measures and the various clinical measures obtained from each subject. Our results show that AHP estimate ratios and firing rate ratios between paretic and contralateral MUs did not correlate with clinical assessment scores (Fugl-Meyer and Chedoke). Overall, the only visible trend was between the ratios of paretic and contralateral AHP duration estimates and the respective MU firing rates. Figure 5 shows that as the ratio between paretic and contralateral firing rate increases, the ratio between paretic and contralateral AHP duration decreases. These data suggest that the longer AHP duration is correlated with lower firing rates in paretic muscle.
Fig. 5.
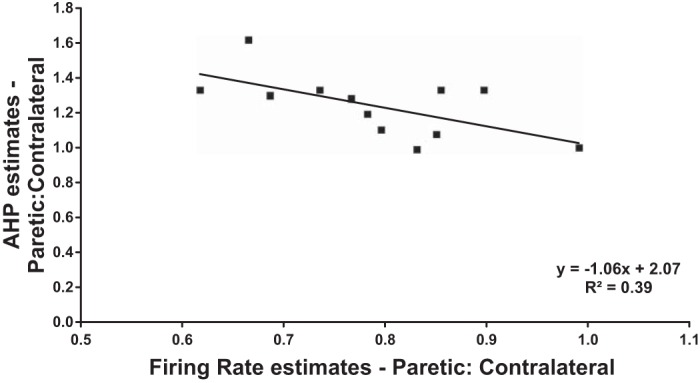
Relationship between paretic-to-contralateral ratio of AHP estimate vs. firing rate estimate for each subject. Plot shows a negative (slope: −1.06) trend, with an R2 value of 0.388. This plot illustrates that as the ratio between paretic and contralateral firing rate increases, the ratio between paretic and contralateral AHP estimates decreases. Therefore, we can infer that longer AHP durations contribute to the lower firing rates exhibited in paretic MUs.
Coefficient of Variation
It was reported in earlier reduced animal studies that the AHP acts to reduce the variability of the motoneuronal firing rate (Brownstone et al. 1992; Manuel et al. 2006; Powers and Binder 2000). To determine whether this relation was also present in our human experiments, the relationship between AHP duration and the CV of the MU firing rate was investigated. With shorter data samples from the long MU trains acquired for AHP estimation, the spike train CV for MUs derived from both the paretic and contralateral sides was computed. Only statistically stationary spike trains can be used for CV analysis, and therefore the record length used for this analysis was necessarily shorter than that utilized for AHP duration estimation. Because of data analysis requirements (see methods), a total of 10 single MUs were analyzed from the contralateral side of eight stroke survivors and 29 single MUs from the paretic side, a subset of the MUs used for AHP analysis. For each single MU, an average of two “trials,” or two separate consecutive intervals of >200 counts were analyzed. Seventeen MUs in total were collected from seven control subjects, with an average of two trials per MU. As described in methods, these data were sampled from the original lengthy spike trains of 250–600 s used for AHP duration estimates—and represent consecutive spike trains of ∼20–30 s.
The CV was calculated for all contralateral, paretic, and control MU samples. Results showed that there was a significant difference between control and paretic units, between contralateral and paretic units, as well as between contralateral and control units. CV distributions illustrate that control units had the highest CV, followed by contralateral and then paretic units. Results also show that for all the data, CV decreases as firing rate (computed over the 25–30 s) increases for a given MU. As described above, the discharge rate for all analyzed units was in the range of 6–9 pps. Figure 6 compares the relation between CV and estimated AHP duration for contralateral and paretic MUs. This plot shows that as the AHP duration increases, the CV for the unit decreases.
Fig. 6.
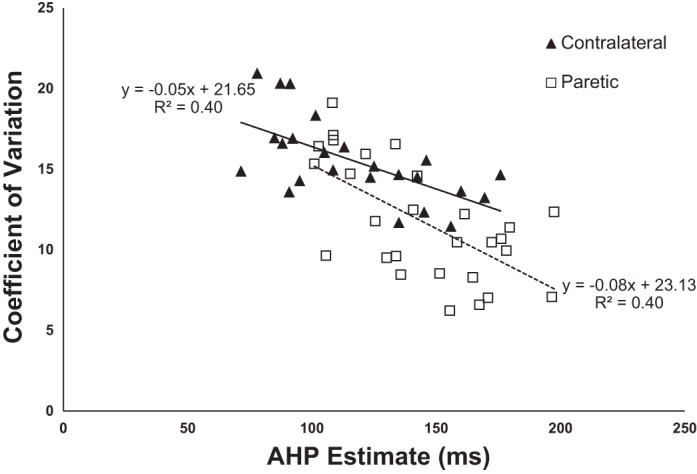
Single MUs: contralateral and paretic. For each unit, the average coefficient of variation (CV) value vs. average AHP estimate is plotted. Both contralateral and paretic units show that as AHP duration increases, CV decreases.
DISCUSSION
The objective of our study was to assess whether the AHP period in human motoneurons, estimated from MU recordings, is longer in MUs recorded from the paretic side of hemiparetic stroke survivors compared with MUs from the nonparetic side of stroke survivors. To achieve this objective, we analyzed data drawn from long-duration recordings derived from the FDI muscles in both paretic and contralateral limbs. Our goal was to understand whether lower MU firing rates, often reported in paretic muscles in stroke, were partially attributable to a longer average motoneuron AHP duration. To derive such AHP duration estimates, we utilized a (novel) modified IDR analysis to estimate AHP duration and a novel, noninvasive surface electrode to record MU activity.
The main result of our study is that in all 11 of our hemiparetic stroke subjects the estimated mean AHP duration was significantly longer in MUs from the affected side compared with the nonparetic (contralateral) side, and this finding was upheld in group data comparisons as well. Additionally, the AHP duration estimates were longer in MUs derived from paretic muscles compared with MUs recorded from neurologically intact controls. No significant differences were observed in comparisons of AHP duration derived from nonparetic (contralateral) MUs with neurologically intact MUs. Our findings also show that CV differs significantly among paretic, contralateral, and control units, with the MUs derived from the paretic side exhibiting the lowest CV. The relationship between firing rate and CV differs among these groups as well.
The longer AHP duration estimates on the paretic side are consistent with findings from an earlier study in which increased AHP durations were reported for MUs recorded from paretic biceps brachii compared with those recorded from neurologically intact individuals (Piotrkiewicz et al. 2007). Thus it appears that MUs derived from both distal and proximal paretic (upper limb) muscle exhibit systematic alterations in AHP duration. A recent study in lower limb muscle in stroke survivors further supports our results (Ivanova et al. 2014).
Our CV analysis also supports this conclusion, albeit indirectly. Presumably because of the presence of a longer AHP, the CV is decreased in paretic units compared with contralateral and control units. A lower CV indicates that spikes are triggered over a more limited range of times around the mean ISI, consistent with a longer AHP. Our CV results support the AHP estimate conclusions in that paretic MUs exhibit longer AHPs with lower CVs, while contralateral/control MUs exhibit shorter AHPs with higher CVs. These CV results are not merely a reflection of the firing rate differences between groups, because we ensured that the range of firing rates was kept similar among groups. Additionally, given that generally the CV of a MU decreases with increased firing rates, the opposite effect would actually be expected with the previously shown firing rate differences between the tested groups. This phenomenon was also reported earlier in cat preparations (Brownstone et al. 1992; Manuel et al. 2006; Powers and Binder 2000). Figure 6 further supports this conclusion, showing that there is a clear negative trend between CV value and AHP for single contralateral as well as paretic MUs. The two regression lines between paretic and contralateral groups are not significantly different, suggesting that the relationship between AHP and CV is similar in both sides and that the lower CV seen in paretic MUs is a function of their longer AHPs.
The AHP also has a significant impact on the slope of the relation between firing rate and injected current (Manuel et al. 2006; Miles et al. 2007; Mottram et al. 2009), i.e., on the input-output gain of the motoneuron. The larger and longer the AHP, the lower the input-output gain. Thus the increased AHP duration seen in this study may account in part for the tendency for there to be lower firing rates in paretic MUs of stroke survivors over a range of contraction levels (Gemperline et al. 1995; Mottram et al. 2009).
Our modified IDR analysis may be better suited to comparisons of AHP durations in stroke survivors than other published methods, as it eliminates the potentially confounding impact of comparing AHP estimates at significantly different firing rates, a point also discussed in a previous study (Piotrkiewicz et al. 2007). ISI variability analysis is governed by the overall variability of the spike train, which is directly influenced by firing rate. Our modified IDR analysis does not rely on the variability of the spike train. Instead, IDR analysis is governed by the “termination probability” of interimpulse intervals. Additionally, in this study we were able to test both the paretic and contralateral sides of the same subject and directly compare how the AHP duration estimate differs within an individual. This is important, given the heterogeneous stroke population and the variation of therapeutic measures experienced by each individual subject.
The use of a novel surface electrode for MU recordings allowed us to record from the same MUs for lengthy periods, up to 7 min in several instances. We can be confident about MU identification because we selected large-amplitude units, whose MU action potential shape remained stable. We were also able to record from the same MU after a brief rest period provided for the subjects. On the negative side, we were limited to recordings of low-threshold MUs.
Sources of Error
A modified IDR analysis was used in order to best accommodate the constraints experienced in our stroke survivor studies. The full IDR analysis requires several lengthy recording trains from one MU (300 s or more) in order to estimate an AHP trajectory. For this study, we were primarily interested in estimating AHP duration, since the IDR analysis of AHP trajectory is based in “noise units,” which cannot be quantified in the human population. Focusing solely on estimating AHP duration allowed for less data collection from each MU, which, in turn, permitted increased inclusion of many stroke subjects who might have been unable to meet the more stringent recording requirements associated with other estimation methods.
Previous studies have illustrated the stability of the IDR analysis-derived AHP estimate within the same unit over a range of MU firing rates, a result that also follows from the theoretical basis of the IDR analysis (Gossen et al. 2003; MacDonell et al. 2007; Matthews 1996; Powers and Binder 2000). The IDR analysis will only converge for intervals (in a MU spike train) that are longer than the AHP, effectively at relatively low MU firing rates.
The reliability of the AHP estimates computed from the IDR method is dependent on whether the firing rate of the MU is in a range in which the IDR plot would plateau, or in other words in a range in which the ISI duration was longer than the actual AHP duration (Piotrkiewicz et al. 2007). Thus our AHP estimates for control, contralateral, and paretic muscle were computed at slightly different firing rates, since firing rates required for a robust estimate (for a convergence of the IDR plot) were generally lower in paretic muscle than in control or contralateral muscle, which in turn would be expected for a longer AHP duration, assuming that the method accurately reflects the actual AHP duration.
Although we tried to make our AHP estimates at comparable MU firing rates, the lower firing rates were not always attainable for our control subjects, or for the contralateral side of our stroke subjects, and consequently almost half of the MU data collected could not be analyzed. In addition, even though we were able to record from MU trains at higher discharge rates on the paretic side, these data were discarded because of nonconvergence of the IDR analysis. Nonetheless, given our results from different firing rate levels in our control subjects, as well as the theoretical framework for the AHP estimates (Matthews 1996) and earlier animal model validations for IDR analysis (Powers and Binder 2000), we believe that we were able to obtain reliable AHP duration estimates for comparisons between control, contralateral, and paretic muscle.
The IDR method utilizes firing rates low enough such that a significant number of the ISIs are longer than the AHP duration. A probability function (illustrated through the IDR plot) can then reliably predict when the interval will most likely be “terminated.” The IDR plot plateaus at the time point of the AHP duration. The AHP is a determinant of firing rate, and it is an intrinsic motoneuron property that is not modulated by feedback control (Lee and Mitchell 2012). Therefore, we expect that an increase in AHP duration is not a result of MU firing rate modulation, and instead contributes to the observed decreases in firing rate (although our arguments are based on correlations only). Animal model validation of modified IDR analysis may offer robust results supporting the hypothesis that a lengthened AHP contributes to lower MU discharge rates in hemispheric stroke.
Mechanisms Contributing to Observed Longer AHP in Paretic MUs
There are a number of possible mechanisms that could cause a significant increase in AHP duration in paretic MUs. One possibility is that increases in AHP duration may result from changes in activity of specific neuromodulator pathways following stroke. The AHP trajectory is controlled by both potassium and calcium conductances, each of which is also influenced by CNS neuromodulators. For example, monoaminergic and cholinergic agents control specific calcium-sensitive channels within the neuron, and therefore any modifications to these neuromodulator pathways can affect AHP-related conductance changes. For example, acetylcholine is able to activate muscarinic receptors, triggering a calcium-sensitive cationic current, which may also impact calcium-sensitive potassium channels. Neuromodulators such as GABA, glutamate, epinephrine, and norepinephrine also have specific effects on L-type calcium conductances and may therefore indirectly affect potassium conductances. Further investigation of these various neuromodulator actions could offer insight into which pathways contribute to changes in AHP duration. This information can potentially be used to pharmacologically target specific channels or conductances and, consequently, the AHP duration.
Functional Implications: Relation Between Motor Unit Firing Rates and Motor Unit Force Production
Although we have demonstrated systematic increases in AHP duration, it is still unclear as to the overall impact of an increase in AHP duration on muscle force generation. Previous studies have shown that muscle force increases both in proportion to MU recruitment and with increases in MU firing rates (Conwit et al. 1999). The AHP is a primary determinant of motoneuron firing rate, and therefore shortening the AHP could lead to higher firing rates and, potentially, to increased and more efficient force production. Ultimately, the effect of changes in firing rate depend on the MU twitch contraction properties, and especially on the relaxation phase, since this will govern the degree of tetanic fusion and overall contractile efficiency. Our present findings show that the ratio of paretic to contralateral MVC does not correlate with the ratio of paretic to contralateral firing rate, or with AHP duration. This is most likely due to the fact that the measured force was maximal voluntary force and the measured firing rate was submaximal, if not the slowest possible firing rates.
As discussed above, a number of other factors may contribute to muscle paresis, and the AHP duration may be just one of many. This may be why we cannot find a consistent correlation between AHP duration changes and MVC changes. Additionally, longer AHP duration may be a compensatory response to various other impairments following stroke, such as changes in muscle properties, disorderly MU recruitment, or decreased descending drive.
Finally, our subject population consisted of chronic stroke survivors, varying from 3 to 23 yr after stroke. MVC ratios between the paretic and contralateral side may be influenced by usage of the paretic limb, by medication, as well as by therapeutic interventions experienced by each individual subject. These variables were not consistent between subjects, especially given the broad range of 3–23 yr after stroke. For future studies, the recruitment of acute stroke subjects may minimize the number of confounding variables.
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke R01 Grant NS-062200.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.K.S. and X.H. performed experiments; A.K.S. analyzed data; A.K.S., R.K.P., C.J.H., N.L.S., and W.Z.R. interpreted results of experiments; A.K.S. and X.H. prepared figures; A.K.S. drafted manuscript; A.K.S., X.H., R.K.P., C.J.H., and W.Z.R. edited and revised manuscript; A.K.S., X.H., R.K.P., C.J.H., N.L.S., and W.Z.R. approved final version of manuscript; R.K.P., C.J.H., N.L.S., and W.Z.R. conception and design of research.
ACKNOWLEDGMENTS
We acknowledge Brian Jeon and April Leung for their technical assistance.
REFERENCES
- Andreassen S, Rosenfalck A. Regulation of the firing pattern of single motor units. J Neurol Neurosurg Psychiatry 43: 897–906, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakels R, Kernell D. Matching between motoneurone and muscle unit properties in rat medial gastrocnemius. J Physiol 463: 307–324, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WF, Snow R. Denervation in hemiplegic muscles. Stroke 21: 1700–1704, 1990 [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Jordan LM, Kriellaars DJ, Noga BR, Shefchyk SJ. On the regulation of repetitive firing in lumbar motoneurones during fictive locomotion in the cat. Exp Brain Res 90: 441–455, 1992 [DOI] [PubMed] [Google Scholar]
- Calvin WH. Three modes of repetitive firing and the role of threshold time course between spikes. Brain Res 69: 341–346, 1974 [DOI] [PubMed] [Google Scholar]
- Conwit RA, Stashuk D, Tracy B, McHugh M, Brown WF, Metter EJ. The relationship of motor unit size, firing rate and force. Clin Neurophysiol 110: 1270–1275, 1999 [DOI] [PubMed] [Google Scholar]
- Dattola R, Girlanda P, Vita G, Santoro M, Roberto ML, Toscano A, Venuto C, Baradello A, Messina C. Muscle rearrangement in patients with hemiparesis after stroke: an electrophysiological and morphological study. Eur Neurol 33: 109–114, 1993 [DOI] [PubMed] [Google Scholar]
- Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve 18: 1101–1114, 1995 [DOI] [PubMed] [Google Scholar]
- Gossen ER, Ivanova TD, Garland SJ. The time course of the motoneurone afterhyperpolarization is related to motor unit twitch speed in human skeletal muscle. J Physiol 552: 657–664, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova TD, Knorr S, Macdonell CW, Pollock CL, Garland SJ. Motoneurone afterhyperpolarisation time-course following stroke. Clin Neurophysiol 125: 544–551, 2014 [DOI] [PubMed] [Google Scholar]
- Lee RH, Mitchell CS. Revisiting the role of spike afterhyperpolarization and spike threshold in motoneuron current-frequency gain. J Neurophysiol 107: 3071–3077, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang LY, Chen JJ, Wang YL, Jakubiec M, Mierzejewska J, Piotrkiewicz M. Changes in spinal motoneuron “fastness” in post-stroke spastic patients. J Med Biol Eng 30: 17–22, 2010 [Google Scholar]
- MacDonell CW, Ivanova TD, Garland SJ. Reliability of the interval death rate analysis for estimating the time course of the motoneurone afterhyperpolarization in humans. J Neurosci Methods 162: 314–319, 2007 [DOI] [PubMed] [Google Scholar]
- Manuel M, Meunier C, Donnet M, Zytnicki D. The afterhyperpolarization conductance exerts the same control over the gain and variability of motoneurone firing in anaesthetized cats. J Physiol 576: 873–886, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol 492: 597–628, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Measurement of excitability of tonically firing neurones tested in a variable-threshold model motoneurone. J Physiol 544: 315–332, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComas AJ, Sica RE, Upton AR, Aguilera N. Functional changes in motoneurones of hemiparetic patients. J Neurol Neurosurg Psychiatry 36: 183–193, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci USA 104: 2448–2453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J Neurophysiol 102: 2026–2038, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrkiewicz M. An influence of afterhyperpolarization on the pattern of motoneuronal rhythmic activity. J Physiol (Paris) 93: 125–133, 1999 [DOI] [PubMed] [Google Scholar]
- Piotrkiewicz M, Kudina L, Mierzejewska J, Jakubiec M, Hausmanowa-Petrusewicz I. Age-related change in duration of afterhyperpolarization of human motoneurones. J Physiol 585: 483–490, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Relationship between the time course of the afterhyperpolarization and discharge variability in cat spinal motoneurones. J Physiol 528: 131–150, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidney S, Rosamond WD, Howard VJ, Luepker RV. The “heart disease and stroke statistics—2013 update” and the need for a national cardiovascular surveillance system. Circulation 127: 21–23, 2013 [DOI] [PubMed] [Google Scholar]
- Spaans F, Wilts G. Denervation due to lesions of the central nervous system. An EMG study in cases of cerebral contusion and cerebrovascular accidents. J Neurol Sci 57: 291–305, 1982 [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Thomas CK. Firing patterns of spontaneously active motor units in spinal cord-injured subjects. J Physiol 590: 1683–1697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]