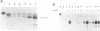Abstract
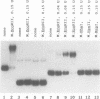
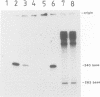
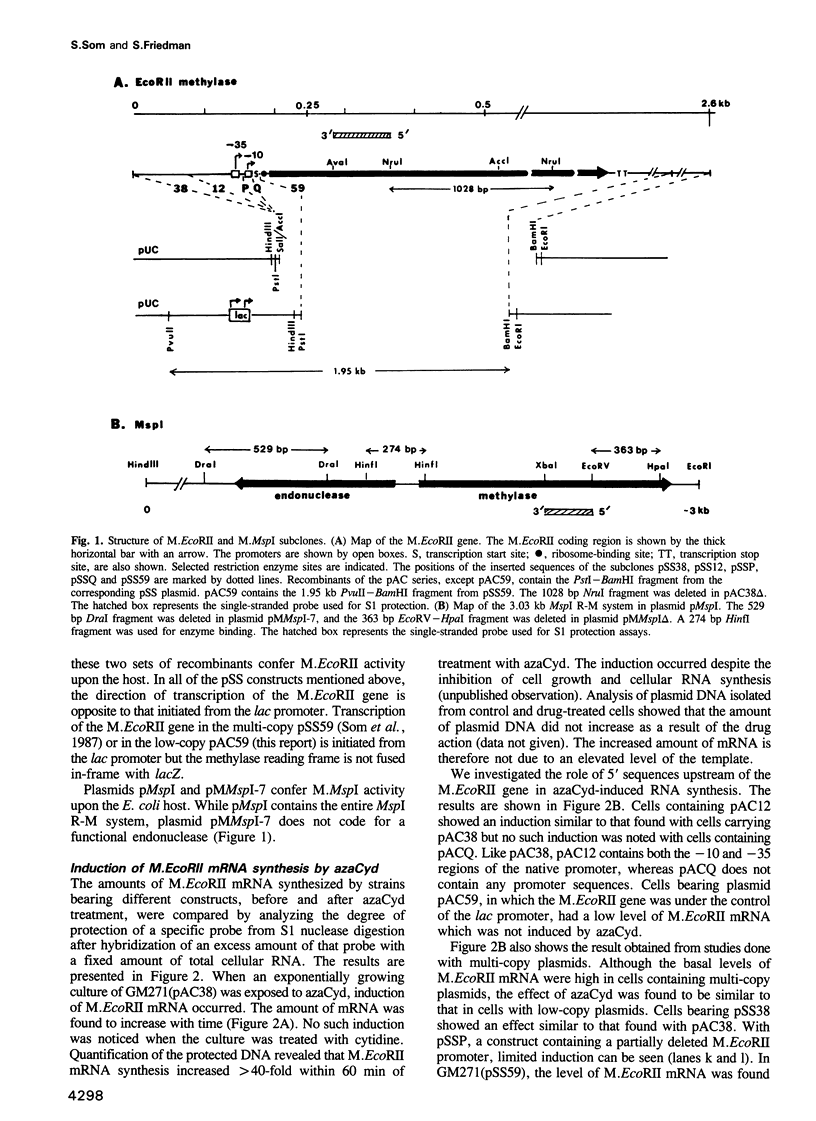
mRNA of the EcoRII methylase (M.EcoRII), a type II modification enzyme, was induced when Escherichia coli carrying a cloned M.EcoRII gene was exposed to the bacteriocidal drug 5-azacytidine. Induction occurred only when transcription was initiated from its own promoter. When the 5' promoter sequences were deleted or replaced with the lac promoter sequences, no induction occurred. The induction was independent of the template DNA level, but the presence of an intact M.EcoRII protein was a requirement. The drug is incorporated into DNA which then inhibits M.EcoRII by binding tightly to the enzyme. A deletion within the M.EcoRII coding region caused a marked increase in the basal level of mRNA transcribed from the M.EcoRII promoter, but no induction occurred upon 5-azacytidine treatment. The level could be reduced to normal by M.EcoRII in trans. In vitro, the enzyme bound to the sequences upstream of the transcription start sites and inhibited the initiation of transcription. These experiments indicate that expression of the M.EcoRII gene was autogenously regulated at the transcriptional level. Similar regulation is also noted in another DNA (cytosine-5) methylase, M.MspI.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister D., Glover S. W. Restriction and modification of bacteriophages by R+ strains of Escherichia coli K12. Biochem Biophys Res Commun. 1968 Mar 27;30(6):735–738. doi: 10.1016/0006-291x(68)90575-5. [DOI] [PubMed] [Google Scholar]
- Bhagwat A. S., Johnson B., Weule K., Roberts R. J. Primary sequence of the EcoRII endonuclease and properties of its fusions with beta-galactosidase. J Biol Chem. 1990 Jan 15;265(2):767–773. [PubMed] [Google Scholar]
- Bhagwat A. S., Roberts R. J. Genetic analysis of the 5-azacytidine sensitivity of Escherichia coli K-12. J Bacteriol. 1987 Apr;169(4):1537–1546. doi: 10.1128/jb.169.4.1537-1546.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. Bactericidal effect of 5-azacytidine on Escherichia coli carrying EcoRII restriction-modification enzymes. J Bacteriol. 1982 Jul;151(1):262–268. doi: 10.1128/jb.151.1.262-268.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. Binding of the EcoRII methylase to azacytosine-containing DNA. Nucleic Acids Res. 1986 Jun 11;14(11):4543–4556. doi: 10.1093/nar/14.11.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. The inhibition of DNA(cytosine-5)methylases by 5-azacytidine. The effect of azacytosine-containing DNA. Mol Pharmacol. 1981 Mar;19(2):314–320. [PubMed] [Google Scholar]
- Friedman S. The irreversible binding of azacytosine-containing DNA fragments to bacterial DNA(cytosine-5)methyltransferases. J Biol Chem. 1985 May 10;260(9):5698–5705. [PubMed] [Google Scholar]
- Gilman M. Z., Chamberlin M. J. Developmental and genetic regulation of Bacillus subtilis genes transcribed by sigma 28-RNA polymerase. Cell. 1983 Nov;35(1):285–293. doi: 10.1016/0092-8674(83)90231-3. [DOI] [PubMed] [Google Scholar]
- Hanck T., Gerwin N., Fritz H. J. Nucleotide sequence of the dcm locus of Escherichia coli K12. Nucleic Acids Res. 1989 Jul 25;17(14):5844–5844. doi: 10.1093/nar/17.14.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. C., Friedman S. Inhibition of recA-mediated strand exchange by adducts of azacytosine-containing DNA and the EcoRII methylase. J Biol Chem. 1991 Sep 15;266(26):17424–17429. [PubMed] [Google Scholar]
- Ives C. L., Nathan P. D., Brooks J. E. Regulation of the BamHI restriction-modification system by a small intergenic open reading frame, bamHIC, in both Escherichia coli and Bacillus subtilis. J Bacteriol. 1992 Nov;174(22):7194–7201. doi: 10.1128/jb.174.22.7194-7201.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosykh V. G., Repik A. V., Kaliman A. V., Bur'ianov Ia I., Baev A. A. Pervichnaia struktura gena restriktsionnoi éndonukleazy EcoRII. Dokl Akad Nauk SSSR. 1989;308(6):1497–1499. [PubMed] [Google Scholar]
- Lal D., Som S., Friedman S. Survival and mutagenic effects of 5-azacytidine in Escherichia coli. Mutat Res. 1988 May;193(3):229–236. doi: 10.1016/0167-8817(88)90033-8. [DOI] [PubMed] [Google Scholar]
- Lauster R., Trautner T. A., Noyer-Weidner M. Cytosine-specific type II DNA methyltransferases. A conserved enzyme core with variable target-recognizing domains. J Mol Biol. 1989 Mar 20;206(2):305–312. doi: 10.1016/0022-2836(89)90480-4. [DOI] [PubMed] [Google Scholar]
- Lin P. M., Lee C. H., Roberts R. J. Cloning and characterization of the genes encoding the MspI restriction modification system. Nucleic Acids Res. 1989 Apr 25;17(8):3001–3011. doi: 10.1093/nar/17.8.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S., Stewart V. Autogenous regulation of gene expression. J Bacteriol. 1993 Jan;175(2):307–316. doi: 10.1128/jb.175.2.307-316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pósfai J., Bhagwat A. S., Pósfai G., Roberts R. J. Predictive motifs derived from cytosine methyltransferases. Nucleic Acids Res. 1989 Apr 11;17(7):2421–2435. doi: 10.1093/nar/17.7.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Som S., Bhagwat A. S., Friedman S. Nucleotide sequence and expression of the gene encoding the EcoRII modification enzyme. Nucleic Acids Res. 1987 Jan 12;15(1):313–332. doi: 10.1093/nar/15.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Som S., Friedman S. Direct photolabeling of the EcoRII methyltransferase with S-adenosyl-L-methionine. J Biol Chem. 1990 Mar 15;265(8):4278–4283. [PubMed] [Google Scholar]
- Tao T., Blumenthal R. M. Sequence and characterization of pvuIIR, the PvuII endonuclease gene, and of pvuIIC, its regulatory gene. J Bacteriol. 1992 May;174(10):3395–3398. doi: 10.1128/jb.174.10.3395-3398.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T., Bourne J. C., Blumenthal R. M. A family of regulatory genes associated with type II restriction-modification systems. J Bacteriol. 1991 Feb;173(4):1367–1375. doi: 10.1128/jb.173.4.1367-1375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. G. Organization of restriction-modification systems. Nucleic Acids Res. 1991 May 25;19(10):2539–2566. doi: 10.1093/nar/19.10.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M. W., Gabbara S., Bhagwat A. S. Substitutions of a cysteine conserved among DNA cytosine methylases result in a variety of phenotypes. Nucleic Acids Res. 1992 Jan 25;20(2):319–326. doi: 10.1093/nar/20.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoshimori R., Roulland-Dussoix D., Boyer H. W. R factor-controlled restriction and modification of deoxyribonucleic acid: restriction mutants. J Bacteriol. 1972 Dec;112(3):1275–1279. doi: 10.1128/jb.112.3.1275-1279.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gemen B., Twisk J., van Knippenberg P. H. Autogenous regulation of the Escherichia coli ksgA gene at the level of translation. J Bacteriol. 1989 Jul;171(7):4002–4008. doi: 10.1128/jb.171.7.4002-4008.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]