Abstract
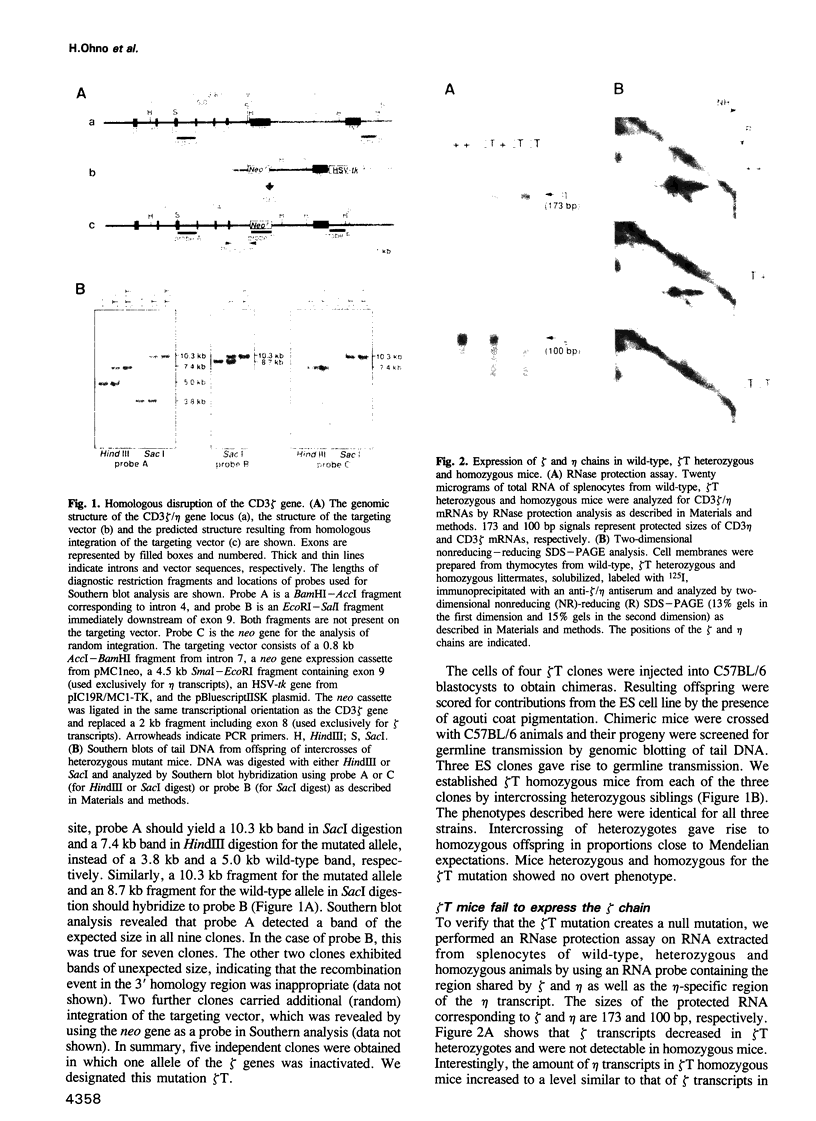
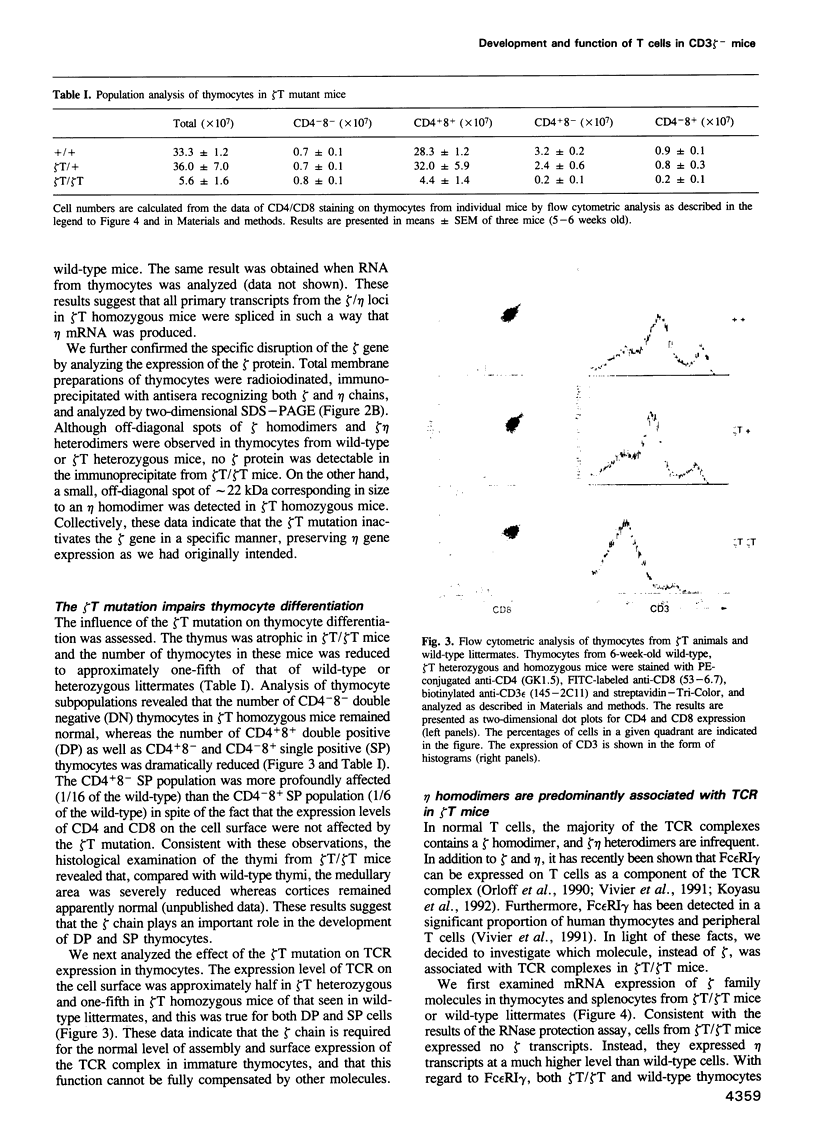
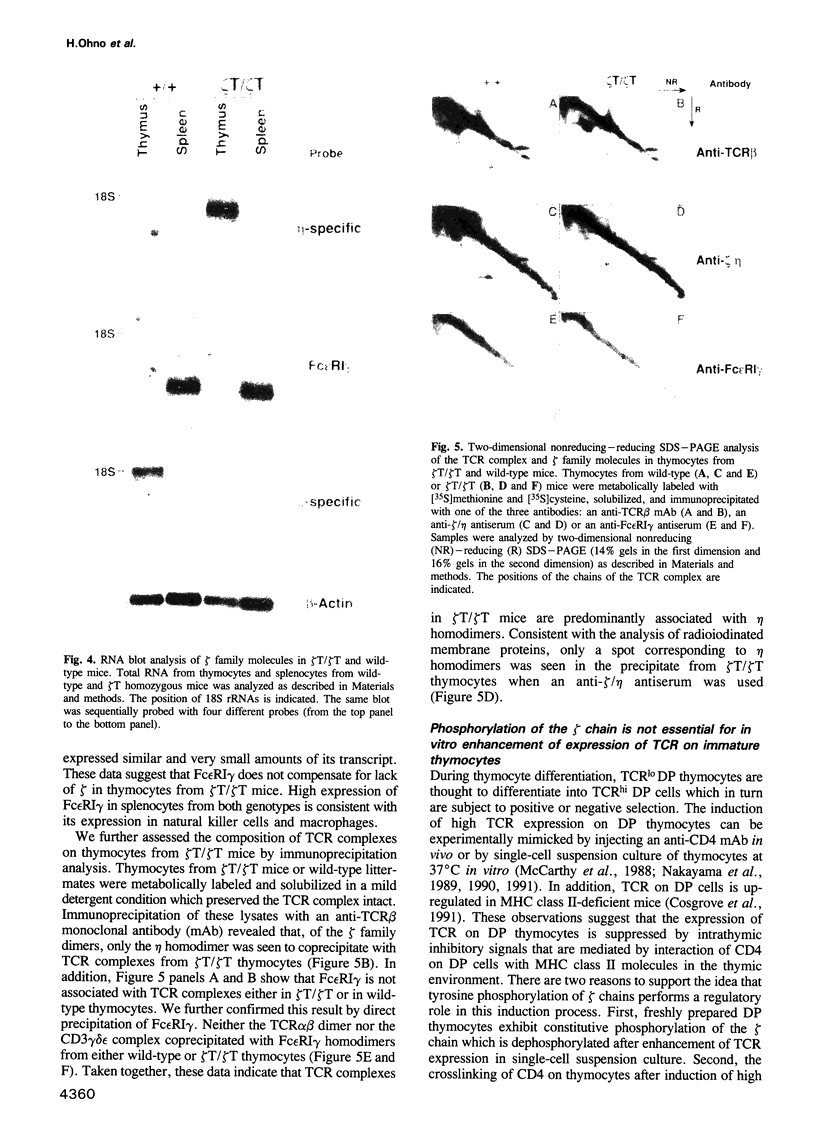
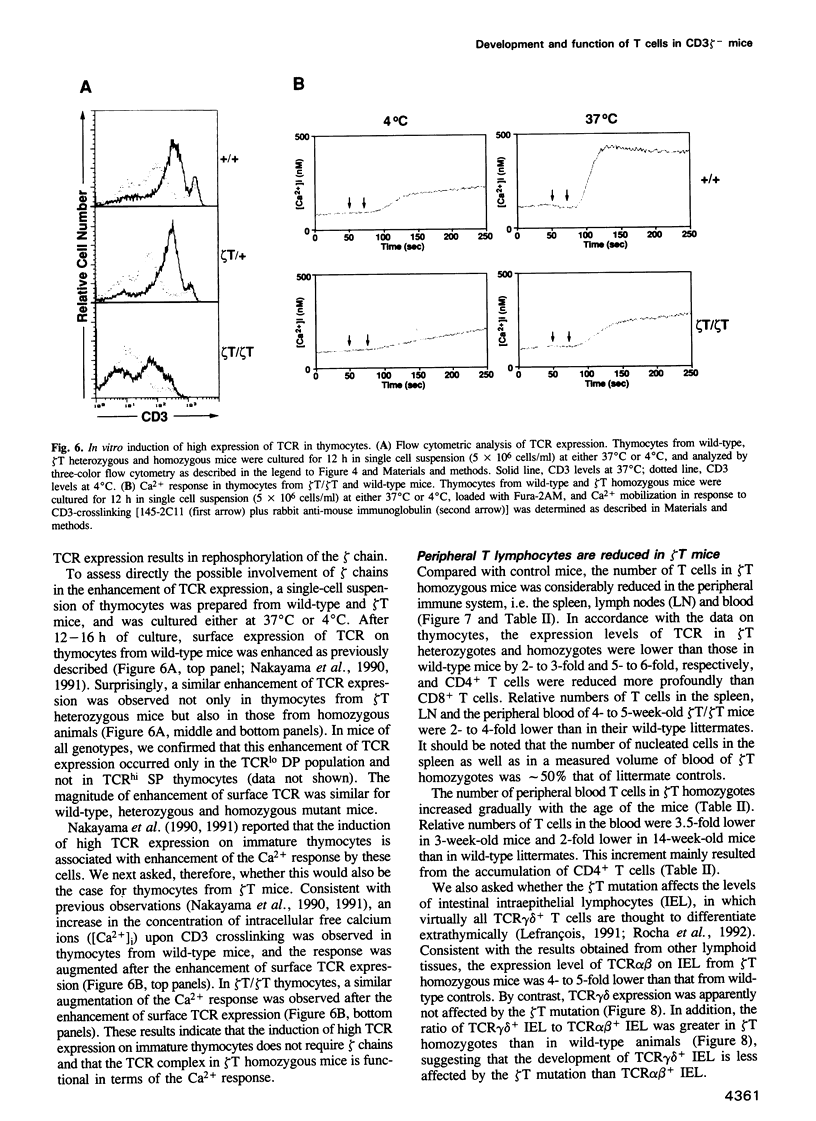
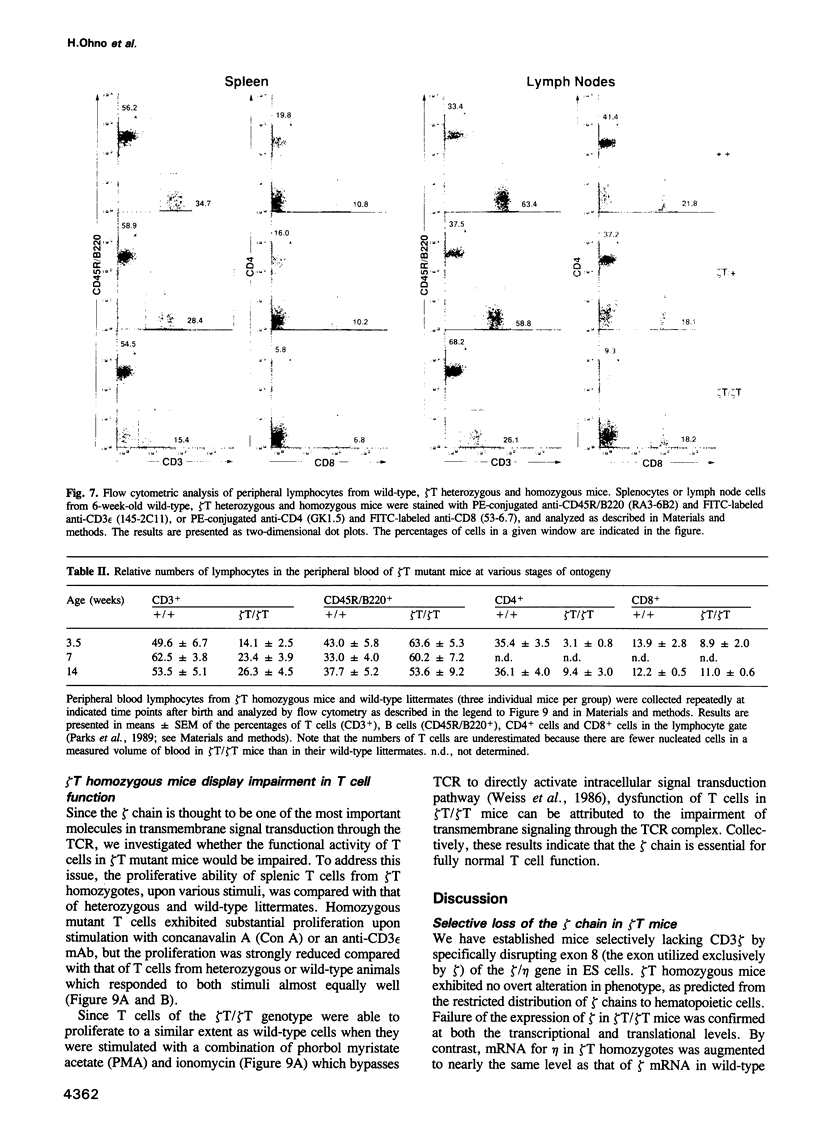
CD3 zeta is a component of the T cell antigen receptor (TCR) complex and is important for signal transduction. We have established mice selectively lacking CD3 zeta but able to express CD3 eta, a polypeptide produced from the same locus through alternative splicing, using the method of gene targeting in embryonic stem cells. In homozygous mutant mice, the numbers of thymocytes and peripheral T cells were greatly reduced and the expression levels of TCR on these cells were 5-fold lower than those on wild-type cells. By contrast, TCR gamma delta+ intestinal intraepithelial lymphocytes were not obviously affected by the mutation. T cells from homozygous mutants exhibited an impaired proliferative response. These results imply that CD3 zeta has a critical role in the development and signal transduction of T cells in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A., McConkey D. J., Howard F. D., Clayton L. K., Novick D., Koyasu S., Reinherz E. L. Differential signal transduction via T-cell receptor CD3 zeta 2, CD3 zeta-eta, and CD3 eta 2 isoforms. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3842–3846. doi: 10.1073/pnas.88.9.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M., Kappler J., Marrack P. The role of the T cell receptor in positive and negative selection of developing T cells. Science. 1990 Jun 15;248(4961):1335–1341. doi: 10.1126/science.1972592. [DOI] [PubMed] [Google Scholar]
- Blumberg R. S., Ley S., Sancho J., Lonberg N., Lacy E., McDermott F., Schad V., Greenstein J. L., Terhorst C. Structure of the T-cell antigen receptor: evidence for two CD3 epsilon subunits in the T-cell receptor-CD3 complex. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7220–7224. doi: 10.1073/pnas.87.18.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., McCarthy S. A., Maguire J. E., Nakayama T., Singer D. S., Klausner R. D., Singer A. Novel post-translational regulation of TCR expression in CD4+CD8+ thymocytes influenced by CD4. Nature. 1990 Mar 15;344(6263):247–251. doi: 10.1038/344247a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clayton L. K., D'Adamio L., Howard F. D., Sieh M., Hussey R. E., Koyasu S., Reinherz E. L. CD3 eta and CD3 zeta are alternatively spliced products of a common genetic locus and are transcriptionally and/or post-transcriptionally regulated during T-cell development. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5202–5206. doi: 10.1073/pnas.88.12.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton L. K., Diener A. C., Lerner A., Tse A. G., Koyasu S., Reinherz E. L. Differential regulation of T-cell receptor processing and surface expression affected by CD3 theta, an alternatively spliced product of the CD3 zeta/eta gene locus. J Biol Chem. 1992 Dec 25;267(36):26023–26030. [PubMed] [Google Scholar]
- Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., Mathis D. Mice lacking MHC class II molecules. Cell. 1991 Sep 6;66(5):1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991 Mar 8;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kearse K. P., Wiest D. L., Singer A. Subcellular localization of T-cell receptor complexes containing tyrosine-phosphorylated zeta proteins in immature CD4+CD8+ thymocytes. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2438–2442. doi: 10.1073/pnas.90.6.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinet J. P. The gamma-zeta dimers of Fc receptors as connectors to signal transduction. Curr Opin Immunol. 1992 Feb;4(1):43–48. doi: 10.1016/0952-7915(92)90122-u. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992 May 29;69(5):823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Koning F., Maloy W. L., Coligan J. E. The implications of subunit interactions for the structure of the T cell receptor-CD3 complex. Eur J Immunol. 1990 Feb;20(2):299–305. doi: 10.1002/eji.1830200211. [DOI] [PubMed] [Google Scholar]
- Kosugi A., Weissman A. M., Ogata M., Hamaoka T., Fujiwara H. Instability of assembled T-cell receptor complex that is associated with rapid degradation of zeta chains in immature CD4+CD8+ thymocytes. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9494–9498. doi: 10.1073/pnas.89.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S., D'Adamio L., Arulanandam A. R., Abraham S., Clayton L. K., Reinherz E. L. T cell receptor complexes containing Fc epsilon RI gamma homodimers in lieu of CD3 zeta and CD3 eta components: a novel isoform expressed on large granular lymphocytes. J Exp Med. 1992 Jan 1;175(1):203–209. doi: 10.1084/jem.175.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S., D'Adamio L., Clayton L. K., Reinherz E. L. T-cell receptor isoforms and signal transduction. Curr Opin Immunol. 1991 Feb;3(1):32–39. doi: 10.1016/0952-7915(91)90073-a. [DOI] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Lefrançois L. Extrathymic differentiation of intraepithelial lymphocytes: generation of a separate and unequal T-cell repertoire? Immunol Today. 1991 Dec;12(12):436–438. doi: 10.1016/0167-5699(91)90015-L. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- McCarthy S. A., Kruisbeek A. M., Uppenkamp I. K., Sharrow S. O., Singer A. Engagement of the CD4 molecule influences cell surface expression of the T-cell receptor on thymocytes. Nature. 1988 Nov 3;336(6194):76–79. doi: 10.1038/336076a0. [DOI] [PubMed] [Google Scholar]
- Merćep M., Bonifacino J. S., Garcia-Morales P., Samelson L. E., Klausner R. D., Ashwell J. D. T cell CD3-zeta eta heterodimer expression and coupling to phosphoinositide hydrolysis. Science. 1988 Oct 28;242(4878):571–574. doi: 10.1126/science.2845582. [DOI] [PubMed] [Google Scholar]
- Merćep M., Weissman A. M., Frank S. J., Klausner R. D., Ashwell J. D. Activation-driven programmed cell death and T cell receptor zeta eta expression. Science. 1989 Dec 1;246(4934):1162–1165. doi: 10.1126/science.2531464. [DOI] [PubMed] [Google Scholar]
- Minami Y., Weissman A. M., Samelson L. E., Klausner R. D. Building a multichain receptor: synthesis, degradation, and assembly of the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1987 May;84(9):2688–2692. doi: 10.1073/pnas.84.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A. R., Rudnicki M. A., Iacomini J., Itohara S., Lafaille J. J., Wang L., Ichikawa Y., Jaenisch R., Hooper M. L. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992 Nov 19;360(6401):225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Mortensen R. M., Conner D. A., Chao S., Geisterfer-Lowrance A. A., Seidman J. G. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992 May;12(5):2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Kühn R., Rajewsky K. Major histocompatibility complex class II hyperexpression on B cells in interleukin 4-transgenic mice does not lead to B cell proliferation and hypergammaglobulinemia. Eur J Immunol. 1991 Apr;21(4):921–925. doi: 10.1002/eji.1830210410. [DOI] [PubMed] [Google Scholar]
- Nakayama T., June C. H., Munitz T. I., Sheard M., McCarthy S. A., Sharrow S. O., Samelson L. E., Singer A. Inhibition of T cell receptor expression and function in immature CD4+CD8+ cells by CD4. Science. 1990 Sep 28;249(4976):1558–1561. doi: 10.1126/science.2120773. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Samelson L. E., Nakayama Y., Munitz T. I., Sheard M., June C. H., Singer A. Ligand-stimulated signaling events in immature CD4+CD8+ thymocytes expressing competent T-cell receptor complexes. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9949–9953. doi: 10.1073/pnas.88.22.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Singer A., Hsi E. D., Samelson L. E. Intrathymic signalling in immature CD4+CD8+ thymocytes results in tyrosine phosphorylation of the T-cell receptor zeta chain. Nature. 1989 Oct 19;341(6243):651–654. doi: 10.1038/341651a0. [DOI] [PubMed] [Google Scholar]
- Ohno H., Saito T. CD3 zeta and eta chains are produced by alternative splicing from a common gene. Int Immunol. 1990;2(11):1117–1119. doi: 10.1093/intimm/2.11.1117. [DOI] [PubMed] [Google Scholar]
- Orloff D. G., Ra C. S., Frank S. J., Klausner R. D., Kinet J. P. Family of disulphide-linked dimers containing the zeta and eta chains of the T-cell receptor and the gamma chain of Fc receptors. Nature. 1990 Sep 13;347(6289):189–191. doi: 10.1038/347189a0. [DOI] [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989 Mar 30;338(6214):383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- Rocha B., Vassalli P., Guy-Grand D. The extrathymic T-cell development pathway. Immunol Today. 1992 Nov;13(11):449–454. doi: 10.1016/0167-5699(92)90074-H. [DOI] [PubMed] [Google Scholar]
- Rodewald H. R., Moingeon P., Lucich J. L., Dosiou C., Lopez P., Reinherz E. L. A population of early fetal thymocytes expressing Fc gamma RII/III contains precursors of T lymphocytes and natural killer cells. Cell. 1992 Apr 3;69(1):139–150. doi: 10.1016/0092-8674(92)90125-v. [DOI] [PubMed] [Google Scholar]
- Roes J., Rajewsky K. Cell autonomous expression of IgD is not essential for the maturation of conventional B cells. Int Immunol. 1991 Dec;3(12):1367–1371. doi: 10.1093/intimm/3.12.1367. [DOI] [PubMed] [Google Scholar]
- Romeo C., Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991 Mar 8;64(5):1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Weissman A. M., Robey F. A., Berkower I., Klausner R. D. Characterization of an anti-peptide antibody that recognizes the murine analogue of the human T cell antigen receptor-T3 delta-chain. J Immunol. 1986 Nov 15;137(10):3254–3258. [PubMed] [Google Scholar]
- Sussman J. J., Bonifacino J. S., Lippincott-Schwartz J., Weissman A. M., Saito T., Klausner R. D., Ashwell J. D. Failure to synthesize the T cell CD3-zeta chain: structure and function of a partial T cell receptor complex. Cell. 1988 Jan 15;52(1):85–95. doi: 10.1016/0092-8674(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Taguchi T., Aicher W. K., Fujihashi K., Yamamoto M., McGhee J. R., Bluestone J. A., Kiyono H. Novel function for intestinal intraepithelial lymphocytes. Murine CD3+, gamma/delta TCR+ T cells produce IFN-gamma and IL-5. J Immunol. 1991 Dec 1;147(11):3736–3744. [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Vivier E., Rochet N., Kochan J. P., Presky D. H., Schlossman S. F., Anderson P. Structural similarity between Fc receptors and T cell receptors. Expression of the gamma-subunit of Fc epsilon RI in human T cells, natural killer cells and thymocytes. J Immunol. 1991 Dec 15;147(12):4263–4270. [PubMed] [Google Scholar]
- Wegener A. M., Letourneur F., Hoeveler A., Brocker T., Luton F., Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992 Jan 10;68(1):83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Williams R. L., Hilton D. J., Pease S., Willson T. A., Stewart C. L., Gearing D. P., Wagner E. F., Metcalf D., Nicola N. A., Gough N. M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988 Dec 15;336(6200):684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- de la Hera A., Müller U., Olsson C., Isaaz S., Tunnacliffe A. Structure of the T cell antigen receptor (TCR): two CD3 epsilon subunits in a functional TCR/CD3 complex. J Exp Med. 1991 Jan 1;173(1):7–17. doi: 10.1084/jem.173.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]