Abstract
Objective: One of the factors preventing clinical application of regenerative medicine to degenerative cartilage diseases is a suitable source of cells. Chondrocytes, the only cell type of cartilage, grown in vitro under culture conditions to expand cell numbers lose their phenotype along with the ability to generate hyaline cartilaginous tissue. In this study we determine that a serum- and growth-factor-free three-dimensional (3D) culture system restores the ability of the passaged chondrocytes to form cartilage tissue in vitro, a process that involves sox9.
Methods: Bovine articular chondrocytes were passaged twice to allow for cell number expansion (P2) and cultured at high density on 3D collagen-type-II-coated membranes in high glucose content media supplemented with insulin and dexamethasone (SF3D). The cells were characterized after monolayer expansion and following 3D culture by flow cytometry, gene expression, and histology. The early changes in signaling transduction pathways during redifferentiation were characterized.
Results: The P2 cells showed a progenitor-like antigen profile of 99% CD44+ and 40% CD105+ and a gene expression profile suggestive of interzone cells. P2 in SF3D expressed chondrogenic genes and accumulated extracellular matrix. Downregulating insulin receptor (IR) with HNMPA-(AM3) or the PI-3/AKT kinase pathway (activated by insulin treatment) with Wortmannin inhibited collagen synthesis. HNMPA-(AM3) reduced expression of Col2, Col11, and IR genes as well as Sox6 and -9. Co-immunoprecipitation and chromatin immunoprecipitation analyses of HNMPA-(AM3)-treated cells showed binding of the coactivators Sox6 and Med12 with Sox9 but reduced Sox9–Col2a1 binding.
Conclusions: We describe a novel culture method that allows for increase in the number of chondrocytes and promotes hyaline-like cartilage tissue formation in part by insulin-mediated Sox9–Col2a1 binding. The suitability of the tissue generated via this approach for use in joint repair needs to be examined in vivo.
Introduction
Development of new biological treatments for cartilage degradation has been hampered by lack of sufficient numbers of cells that exhibit the appropriate phenotype of articular chondrocytes. The most commonly used method to increase the number of cells is to culture them in monolayer (ML) in vitro. However, under these conditions the chondrocyte phenotype changes; cells obtain a spindled morphology and lose their chondrocytic characteristics resulting in an inability to form cartilage tissue in vitro unless manipulated in some way such as coculture with differentiated chondrocytes.1,2 Use of passaged chondrocytes for cartilage repair is FDA approved and has been utilized clinically for autologous chondrocyte transplant for more than 10 years, although with limited success in part because of their inability to form articular cartilage in vivo.3 Therefore, developing a method that uses passaged chondrocytes to generate hyaline cartilage without any supplementation is particularly attractive as it could shorten the time to clinical translation.
It has been suggested that, with passage, as chondrocytes lose their phenotype they acquire some characteristics of mesenchymal stromal cells (MSCs) as defined by spindled morphology; higher collagen type I gene expression; cell surface profile of CD105+, CD90+, CD73+, CD44+, CD45−, and CD34−; and the ability to differentiate to tissues of mesenchymal lineage.4–7 A number of conditions have been used to redifferentiate these passaged cells to chondrocytes, such as coculture or three-dimensional (3D) culture.8,9 All of these approaches require the presence of fetal bovine serum (FBS), exogenous hormones, and/or growth factors of the transforming growth factor (TGF)β family—conditions that can trigger hypertrophic differentiation or fibrocartilage formation.10,11 Proteomic analysis shows that FBS contains fibronectin, collagen type 2, and collagen type 1 that may promote cell attachment. It also contains growth factors, such as FGF, TGFβ1, glial growth factor, and prepro-insulin-like growth factor 1 (PIlGF—signal peptide containing IGF-1, functionally similar to insulin), in lot dependent concentrations.12 Hence, with FBS supplementation reproducibility becomes dependent on the batch of serum. The xenogeneic nature of FBS is also a concern for tissue engineering both in terms of the potential for inducing an immune response and/or transmitting disease.13 The serum substitute ITS+ (insulin, transferrin, selenium, and dexamethasone; BD Bioscience) is often used to circumvent these limitations and is commonly used together with TGFβ to differentiate MSCs to chondrocytes. This may be due in part to the presence of insulin, a hormone highly conserved among vertebrates. It is a component of ITS+ and is known to have effects on cell survival, proliferation, differentiation, metabolism, and during development.14 Insulin binds to the insulin receptor (IR) that activates cytoplasmic substrates insulin receptor substrate 1 (IRS-1) and/or Shc that in turn activate ERK-MAPK, PI-3 kinase, and/or Akt signal transduction pathways.15 These pathways have been implicated in regulating chondrogenesis, although Akt activation has been shown to favor chondrocyte hypertrophy.16 Importantly chondrocytes express functional IRs and levels may be altered in osteoarthritic chondrocytes.17 Recently insulin has been shown to regulate Sox9 expression in mesenchymal cells.18 Sox9, the master transcription factor regulating chondrogenesis, is expressed at the earliest stage of cartilage anlagen formation by chondrocytes.19 Sox9 requires additional coactivators, such as Sox5, Sox6, Med12, and SP1, to form a functional transcriptional complex20,21 that regulates gene expression of many cartilage-related molecules, including Col2a1, Col9a2, Col11a2, aggrecan, and cartilage oligomeric matrix protein (COMP).20,22–24
This study demonstrates that in the presence of media supplemented with ITS+ instead of FBS, passaged bovine chondrocytes when grown on collagen-type-II-coated membrane inserts revert back to articular chondrocytes that form hyaline cartilage tissue. The insulin in the culture media contributes to the ability of the redifferentiating cells to form cartilage tissue by sox9-mediated collagen II gene and protein expression. Understanding the mechanism(s) regulating redifferentiation will allow identification of the conditions that will support this redifferentiation process in human chondrocytes.
Materials and Methods
Cell isolation and culture
Bovine articular chondrocytes (BACs) were harvested by enzymatic digestion from cartilage obtained from bovine metacarpo-phalangeal joints (6–9 months old) as described previously.19 BACs (2000 cells/cm2) were cultured in ML in 5% FBS and passaged twice (P2) to attain up to 200-fold increase in cell number. P2 (1.5×106 cells) were seeded onto type-II-collagen-coated Millicell® culture inserts (60 mm2; Millipore) and cultured for up to 4 weeks in serum-free 3D culture (SF3D) consisting of high glucose content medium (HG) Dulbecco's modified Eagle's medium (DMEM; 4.5 g/L), ITS+ (10 μg/mL insulin, 0.5 μg/mL transferrin, 0.67 ng/mL selenium, 5.35 μg/mL linoleic acid, and 1.25 mg/mL BSA; BD Bioscience, MA), proline (40 μg/mL), pyruvate (110 μg/mL), dexamethasone (0.1 μM), and ascorbate-2-PO4 (50 μg/mL). As controls, cells were grown in serum-containing 3D culture (SC3D) with 20% FBS.
Inhibition studies
Twenty-four hours after seeding, the P2 cells were serum or ITS+ starved for 18 h and treated with the IR inhibitor HNMPA-(AM3) (100 μM; Enzo Life Sciences)25 and PI-3 kinase (upstream of AKT) inhibitors Wortmannin (5 ng/mL; Sigma-Aldrich) or LY294002 (10 μM; Millipore). Insulin was added after 2 h and tissue was harvested after 24 h. Controls were cultures that received carrier (DMSO) only.
Flow cytometry
Cells were harvested using trypsin (1× for 5 min), allowed to recover for 30 min in 2% FBS containing phosphate-buffered saline (PBS), and stained with either CD105-PE or CD44-PE (12-1057 and 12-0441; eBioscience). Cells were analyzed by EPICS XL FACS and Kaluza analysis software (Beckman Coulter).
Histology and immunohistochemistry
Tissues were fixed in 10% formalin and embedded in paraffin, and 5-μm sections were cut. Representative sections were stained with toluidine blue. For immunohistochemistry deparaffinized sections were digested at RT for 10 min with 0.4% pepsin (w/v) (Sigma-Aldrich) in TBS-HCl (pH 2.0), blocked with 20% goat serum (v/v) (Sigma-Aldrich), and incubated overnight at 4°C with either type I collagen (1:100, T59103R; Meridian) or type II collagen (1:100, MAB8887; Millipore) antibody. Immunoreactivity was detected by Alexa-488 goat anti-rabbit or Alex-594 goat anti-mouse secondary antibody (1:300, 1 h; Invitrogen). Nuclei were visualized by DAPI (1:10,000; Invitrogen). IgG was used as the negative control. Images were collected using a 40× objective (Nikon Eclipse C1si).
Tissue analysis
The tissues were digested in papain (40 μg/mL in 20 mM ammonium acetate, 1 mM ethylenediaminetetraacetic acid [EDTA], and 2 mM DTT; Sigma-Aldrich) for 48 h at 65°C and assayed as described previously.2 Briefly, DNA content was quantified by Hoechst dye 33258 assay (Polysciences, Inc.) and fluorometry (excitation λ=365 nm and emission λ=458 nm). Proteoglycan (PG) content was estimated by the dimethylmethylene blue dye binding assay (λ=525 nm) (Polysciences, Inc.). Collagen content was quantified by chloramine-T/Ehrlich's reagent assay (λ=560 nm).
RNA isolation and relative quantitative PCR
Total RNA was extracted with RNeasy® kit (Qiagen) and reverse transcribed with 200 units of SuperscriptIII (Invitrogen). SYBR green dye I, 0.2 μM of primers (Table 1), and realplex2 Master cycler (Eppendorf) were used for relative quantitative PCR (qPCR).
Table 1.
List of Sequences of Primers Used in Quantitative PCR
| Gene | Forward (5′) | Reverse (5′) |
|---|---|---|
| Sox6 | tcctggcagcgcatgatgaaca | agcaaggtccatttgctgccgt |
| Sox9 | acacacagctcactcgaccttg | agggaattctggttggtcctct |
| Col1a1 | cggctcctgctcctctac | cacacgtctctctcggtcatggta |
| Col2a1 | gtgtcacggccaggatgtc | gcagaggacagtcccagtgt |
| Col9a1 | aacgacggaggccttacagacgg | aattcctggagaaccttgcgg |
| Col10al | tccaaaatacaggtctgagc | cctgttaattgtcagaacag |
| Col11al | acagttgtgagtgcgggggct | tcccagagccaccgtttcgt |
| TenacinC | aagtcatccggcacaagcagca | acgtgattgaacaccaccggct |
| Wnt9a | acaacctcgtgggtgtgaaggt | tcgtacttgtgcttcaggcgct |
| Gdf5 | tggtgtttggccgcaccaagaa | aagttgacatgcagcgccttcc |
| Gli3 | tcggccagatgtcagcgagaaa | tgtggctgcatagtgactgcgt |
| Aggrecan | tgggactgaagttcttggaga | gcgagttgtcatggtctgaa |
| COMP | tgcctgtgacaactgtcctcagaa | ttgtctaccaccttgtccgcatca |
| CD44 | gatccaccccaattccatctgt | aaccgcgagaatcaaagccaa |
| IR | tccgcacactcggtttctcctt | agggttcaaaccttgcagggca |
| IGF1R | ccgagttcccagagctgtgcagtta | gatggtccacactgggcaagacc |
PCR, polymerase chain reaction.
Radiolabeling
Synthesis of collagens and PGs was evaluated by assaying the incorporation of [3H]-proline and [35S]-SO4 (2 μCi; PerkinElmer), respectively, 3 days after cell seeding. Cells were labeled for 24 h, washed in PBS, and digested in papain as described previously. Collagen and PG were precipitated from the media with 70% ammonium sulfate or 100% cold ethanol, respectively, and centrifuged at 14,000 rpm for 30 min at 4°C. Pellets were washed with cold 70% ethanol, and centrifuged for 10 min at 14,000 rpm. The precipitated collagen was resuspended in 10% sodium dodecyl sulfate (SDS) and the PGs were solubilized in 4 M guanidine hydrochloride and quantified using a β-scintillation counter (Beckman Coulter).
To determine glucose uptake, cells were cultured in SF3D-HG, SF3D-low glucose content media (LG), or SC3D-HG for 24 h; serum or ITS and glucose starved for 2 h; and then incubated with [3H]-deoxyglucose (2D-G 3H; 10 μCi/mL) for 30 min at 37°C. The cultures were rinsed with PBS, and digested with papain (40 μg/mL) for 24 h. Glucose uptake was quantified by measuring 3H incorporation using a β-scintillation counter and normalized to DNA content.
Immunoblotting
Cells were harvested in RIPA buffer containing 1% NP40, 0.1% SDS, 2 mM EDTA, 0.5% sodium deoxycholate (NaDC), and 150 mM NaCl in 50 mM Tris (pH 7.8). Fifteen micrograms of protein was separated on 12% SDS–polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane by iblot (Invitrogen). Membranes were blocked in 1% milk for 1 h and incubated with antibodies reactive with Sox9 (1:1000, ab3697; Abcam), β-actin (1:2000, A5441; Sigma-Aldrich), Shc, pSHC, pAKT, AKT, pERK, or ERK (1:1000, respectively, 2432, 2434, 9611s, 9272, 9102, and 9101; Cell Signalling) overnight. Washed membranes were incubated with HRP-conjugated secondary antibodies for 1 h and immunoreactivity was detected by ECL+ (GE Healthcare).
Co-immunoprecipitation
Lysis buffer containing 1% Triton X-100, 10% glycerol, 137 mM NaCl, 1 mM Na-orthovanadate, and 1× protease inhibitors (Complete mini; Roche) was used to extract protein. Cell lysates (120 μg) were centrifuged at 15,000 g for 20 min and the supernatant was precleared with protein A/G beads (Millipore) for 2 h and then incubated for 4 h with antibody reactive with Sox9 (1:1000, ab3697; Abcam) at 4°C. The immune complex was harvested by incubation with Protein A/G beads overnight at 4°C. Beads were washed in lysis buffer and boiled for 10 min in mercaptoethanol containing sample buffer and immunoblotted (see immunoblotting section) with antibodies reactive with Sox6 and Med12 (1:1000, ab66316 and ab49053; Abcam).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP)–qPCR analysis was carried out using genomic DNA. Briefly, 2-day-old cultures were crosslinked in situ with 0.75% formaldehyde and harvested in 50 μL/106 cells of lysis buffer (50 mM HEPES-KOH [pH 7.5], 140 mM NaCl, 1 mM EDTA [pH 8.0], 1% Triton X-100, 1% SDS, and 0.1% NaDC). DNA was sheared by sonication at 25% amp (30 s ON and 60 s OFF 20×; Vibracell) to 250–900 bp fragments (confirmed by agarose gel); a fraction was used as input control. Twenty-five micrograms of DNA was diluted 1:10 in RIPA and immunoprecipitated with antibody reactive with Sox9 and Protein A/G beads overnight at 4°C. Samples were reverse crosslinked in the presence of 5 μL Proteinase K at 65°C overnight. Co-immunoprecipitated DNA was purified with equal volume of 1:1 phenol:chloroform and washed with 3× volume of ethanol containing 10 μL of glycogen (5 mg/mL). The air-dried pellet was resuspended in 100 μL of water; qPCR with Col2a1 primers (f-5′ TTCCAGATGGGGCTGAAACGCT, r-5′ TGGGGCTTTTCTCGAGCACACA) located in the Sox9 binding region was carried out (see Table 1).
Statistical analysis
Each experiment was done using cartilage tissues obtained from a single animal. The results are expressed as the mean of three to five independent experiments. Each condition was done in triplicate. Pearson's chi-square test was used to verify the Gaussian distribution and independence of the data. One-way analysis of variance followed by Tukey's post hoc test was used for all pair-wise comparisons between groups. p-Values≤0.05 were considered to be statistically significant. Data are presented as mean with 95% confidence interval (95% CI).
Results
Passaged chondrocytes lose their chondrocytic phenotype and do not form cartilage tissue in 3D culture in the presence of serum containing media
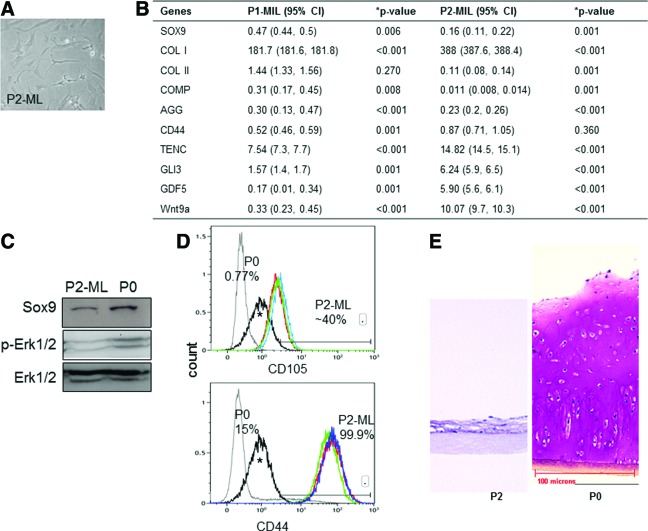
Cell passaging in ML culture resulted in an increase in cell number but cells lost their polygonal shape and acquired a spindled morphology (Fig. 1A). Gene expression analysis after each passage showed progressive loss of Col2a1, Sox9, aggrecan, and COMP, and gain of Col1a1 expression. In addition the cells showed a significant increase in the expression of interzone cell markers—Gli3, tenascinC, Gdf5, and Wnt9a (Fig. 1B). Sox9 and p-ERK-1/2 protein levels were decreased in passaged cells (P2) as determined by western blot analysis (Fig. 1C). Flow cytometry showed that >99% of P2 cells expressed the hyaluronan receptor CD44+ and nearly 40% of the cells were CD105+, a marker of marrow stromal cells (Fig. 1D). The P2 cells were placed in SC3D, and histological analysis after 4 weeks showed that they were unable to form cartilage tissue, in contrast to the PG-rich tissue formed by the primary (P0) chondrocytes under the same conditions (Fig. 1E).
FIG. 1.
Loss of chondrocytic phenotype in culture-expanded cells. (A) Spindle-shaped morphology of P2 cells in monolayer (ML). n=5 experiments. (B) Gene expression profile of cells after each passage compared with P0 levels. A significant increase in Col1a1 was observed after the first passage along with downregulation of cartilage-associated gene aggrecan and cartilage oligomeric matrix protein (COMP) whereas a decrease in Col2a1 was seen only after the second passage. Genes expressed by interzone cells—TenacinC, Gli3, GDF5, and Wnt9a—were significantly upregulated in P2 cells compared with P0 or P1 cells. Data are expressed as mean with the uncertainty estimated by 95% confidence interval (95% CI; lower and upper limits are within brackets). n=5 experiments. P0, primary chondrocytes; P1-ML or P2-ML, passage 1 or 2 cells, respectively, harvested from ML. (C) Immunoblot analysis showed a decrease in Sox9 and p-ERK-1/2 levels in P2-ML compared with P0. n=3 experiments. (D) FACS analysis of surface markers showed 40% CD105+ and 99% CD44+ cells after two passages. n=3 experiments. ^Nonspecific background staining in P2 cells. (E) Photomicrographs of toluidine-blue-stained tissues formed by P2 cells cultured in serum containing three-dimensional conditions (SC3D) for 4 weeks show loss of capacity to accumulate cartilaginous matrix by P2 cells unlike P0 that retained abundant proteoglycan (PG)–rich matrix. n=5 experiments. ¶Collagen-type-II-coated membrane insert. Color images available online at www.liebertpub.com/tea
Passaged chondrocytes undergo phenotype reversal and form cartilage tissue in 3D culture in serum-free media
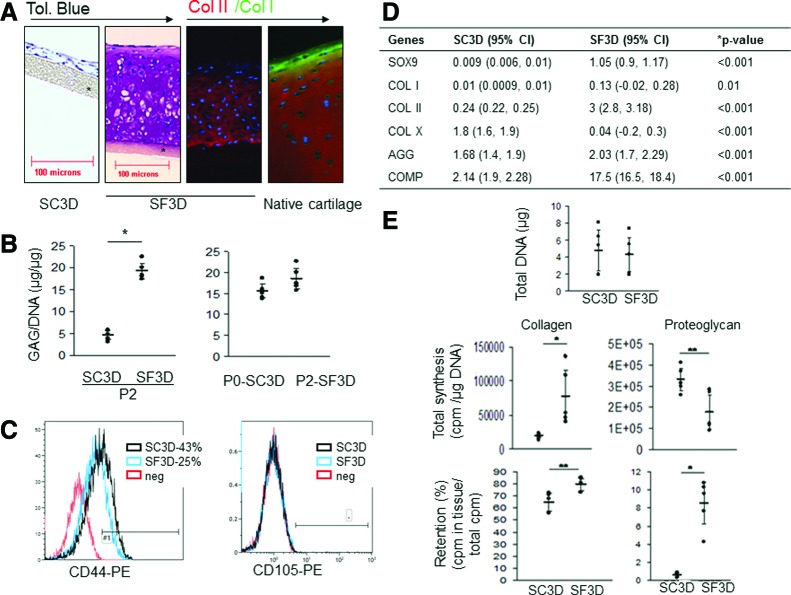
In SF3D, P2 reacquired the ability to form cartilage tissue as compared with cells grown in the presence of serum. After 4 weeks of culture P2 had accumulated abundant hyaline cartilaginous extracellular matrix that contained sulfated PGs (Fig. 2A) and type II collagen. Type I collagen was not detected by immunostaining (Fig. 2A). The amount of glycosaminoglycans was similar to that accumulated by P0 grown in SC3D culture (Fig. 2B). FACS analysis of the P2 at 48 h of SF3D culture revealed that only 25% of P2 in SF3D were CD44+ and 100% had lost CD105 antigen (Fig. 2C). Gene expression analysis at 3 weeks showed that Col2a1, Sox9, aggrecan, and COMP were higher in SF3D compared with P2 cells grown in SC3D. Col1a1 gene expression was lower in both conditions. Col10a1 was significantly lower in SF3D (Fig. 2D). Col10a1 levels were in the same range as that found in primary chondrocytes (data not shown). Cells grown under either culture conditions showed similar DNA content (Fig. 2E). Analysis of [3H]-proline and [35S]-SO4 incorporation at day 2 of culture showed that P2 in SF3D synthesized and retained significantly more collagens. PG synthesis was higher in the SC3D culture conditions; however, significantly more PGs were retained in SF3D (Fig. 2E). This suggested that collagen synthesis and retention was important for tissue formation and this parameter was used as the marker for short-term assessments in subsequent experiments.
FIG. 2.
Assessment of the tissue formed by the P2 cells in SF3D. (A) Photomicrographs of 4-week-old tissue formed by P2 cells cultured in SC3D (serum containing media) show no matrix accumulation (far left panel) while growth in SF3D (serum-free media) shows accumulation of matrix rich in PGs (toluidine blue; second panel). SF3D-generated tissue stained positively for collagen type II (red) and was predominantly negative for collagen type I (green) (third panel) similar to native cartilage tissue stained with the same antibodies (fourth panel). n=5 experiments. (B) More PGs (glycosaminoglycan) were accumulated in tissues formed by P2 in SF3D than in SC3D (left) and was comparable to that accumulated by P0 cells in SC3D (right) at 4 weeks of culture. Each dot represents an independent experiment; horizontal lines represent mean and 95% CI. n=5 experiments. (C) FACS analysis after 2 days of culture shows complete loss of CD105 in both the conditions while only 25% of the cells retained CD44 in SF3D in comparison to 43% in SC3D. n=3 experiments. (D) After 3 weeks the gene expression profile relative to freshly harvested P2-ML cells also shows that P2 in SF3D expressed significantly higher cartilage-related genes and lower Col10a1 expression compared with the cells in SC3D. Data are shown as mean with the uncertainty estimated by 95% CI (lower and upper limits are within brackets). n=3 experiments. (E) Total DNA content of tissues was similar between the conditions. At 48 h, P2 in SF3D synthesized and retained more collagen and, although PG retention was higher in SF3D, more was synthesized in SC3D. ¶ Collagen-type-II-coated membrane inserts; all images are of the same magnification. Each dot represents the mean of an independent experiment; horizontal lines represent mean and 95% CI. n=5 experiments. *p≤0.01, **p≤0.05 compared with SC3D. Color images available online at www.liebertpub.com/tea
Redifferentiation is insulin, dexamethasone, and HG dependent
To evaluate the contribution of the individual media components, P2 cells were cultured under various media conditions and histological analysis at 4 weeks of culture showed that insulin, dexamethasone (synthetic glucocorticoid), and high glucose content DMEM were all essential for cartilage growth (Fig. 3). In contrast to the thin fibrous layer of tissue formed by the P2 cells grown in the absence of insulin or in low glucose, P2 cells grown in insulin and HG resulted in thick hyaline-like cartilage tissue rich in PGs. The presence of dexamethasone also contributed to cartilage formation but was insufficient in the absence of HG or insulin to support generation of this tissue (Fig. 3). IGF-1 could not replace insulin as no tissue formed in the presence of this growth factor, even when HG and dexamethasone were present. The serum substitute ITS+ was replaceable by insulin. DNA content was measured after 48 h of culture and was similar in both HG and LG culture conditions, indicating that there were no differences in cell attachment and survival (data not shown).
FIG. 3.
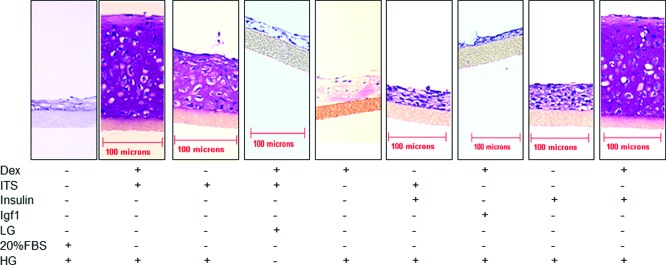
Insulin, HG, and Dex are essential components of SF3D. Photomicrographs of the histological appearance of tissue formed by P2 under different media conditions after 4 weeks of culture. Dex, dexamethasone; HG, high glucose content media; LG, low glucose content media. ¶ Collagen-type-II-coated membrane inserts. n=3 experiments. Color images available online at www.liebertpub.com/tea
Characterization of cells during early redifferentiation
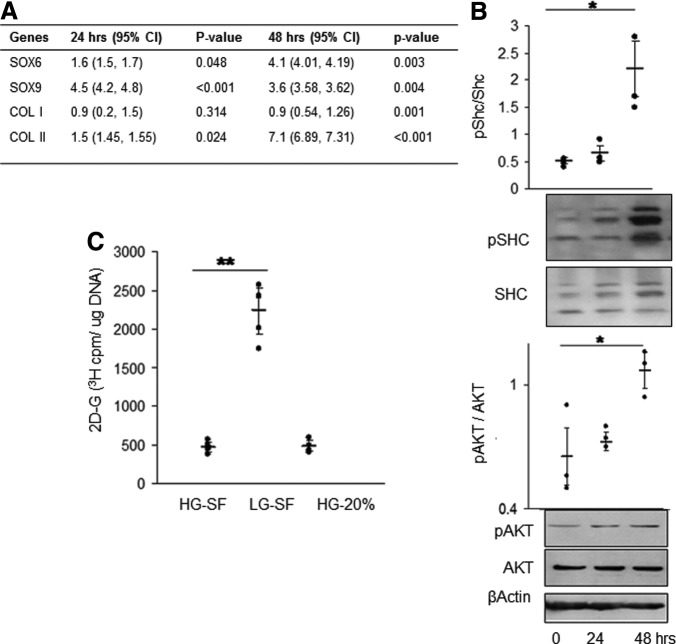
Redifferentiation was evident as early as 48 h as shown by upregulation of Sox9 and Col2a1 gene expression and downregulation of Col1a1 (Fig. 4A). Western blot analysis of protein extracts from 48-h-old SF3D cultures shows increased phosphorylation of the insulin/IGF signaling molecules Shc and AKT (Fig. 4B). Based on these results the remaining analyses were done within 48 h. Quantification of intracellular glucose levels showed that cells exhibited glucose uptake although those cultured in low-glucose media had significantly higher levels than in HG-SF or SC (20% FBS) condition (Fig. 4C).
FIG. 4.
Redifferentiation of P2 in SF3D. (A) Quantitative PCR analysis showed that cartilage-differentiation-associated genes in P2 cells were upregulated early. Col1a1 was downregulated by 48 h of culture when compared with freshly harvested P2-ML cells. Data are shown as mean with 95% CI (lower and upper limits are within brackets). n=3 experiments. (B) Representative immunoblots and densitometry of extracts from redifferentiating P2 cells in SF3D over the first 2 days of culture showed upregulation of insulin/IGF-pathway-related signaling molecules. (C) Glucose assay demonstrated higher uptake as indicated by increased intracellular glucose (2D-G 3H) by P2 cells cultured under LG serum-free conditions. HG-SF, serum-free high glucose content medium containing ITS; LG-SF, low glucose content media containing ITS; HG-20%, high glucose content medium with 20% fetal bovine serum. Each dot represents the mean of an independent experiment; horizontal lines represent mean and 95% CI. n=5 experiments. *p≤0.01, **p≤0.05.
Insulin signaling is involved in Sox9-mediated chondrogenic differentiation
The role of insulin in this process was evaluated using pharmacological agents known to inhibit insulin signaling. Synthesis of collagens was significantly reduced by HNMPA-(AM3) treatment, a drug known to inhibit the IR (Fig. 5A). PG synthesis was also inhibited by this treatment (data not shown). We confirmed by qPCR and immunoblotting that HNMPA-(AM3) treatment downregulated IR gene [control=35 (33.9, 36) vs. HNMPA-(AM3) treated=14 (13.4, 14.5; mean±95% CI)] and protein levels (Fig. 5B) and that the activity of Shc, a signaling pathway downstream of IR, was also reduced after IR inhibition (Fig. 5C). We then examined the levels of ERK/MAPK that lies downstream of Shc. Phosphorylation of 44-kDa ERK-1 subunit was unaffected after inhibition; however, the 42-kDa ERK-2 subunit was significantly more phosphorylated, potentially implicating ERK-2 as a mediator of the insulin effect (Fig. 5D).
FIG. 5.
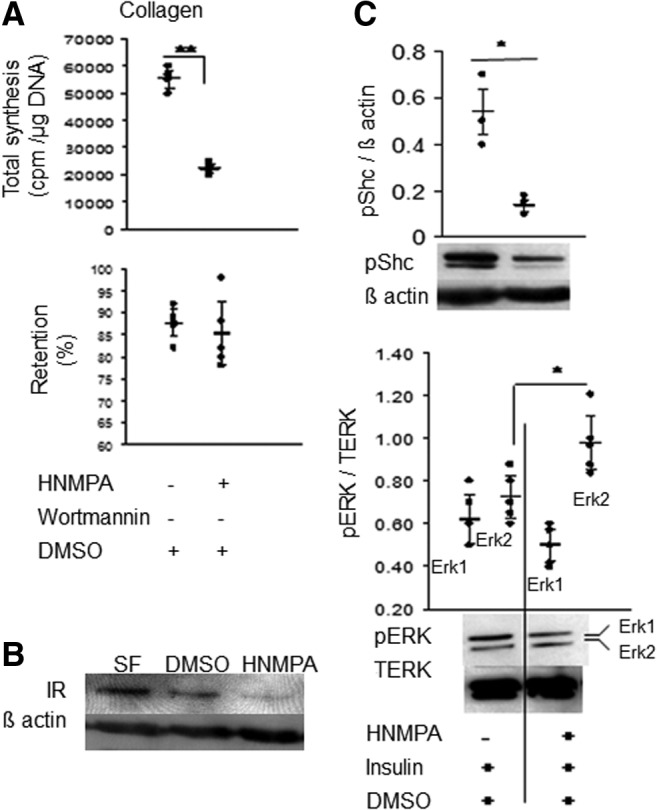
Effect of inhibition of insulin receptor (IR). (A) Radioisotope incorporation studies show inhibition of total collagen synthesis but no change in % retention with HNMPA-(AM3) treatment. (B) Immunoblotting shows that HNMPA-(AM3) decreased IR protein levels. n=3 experiments. (C, D) Immunoblots followed by densitometry shows downregulation of Shc phosphorylation (C) and upregulation of 42-kDa ERK (D) in HNMP-treated cells. Each dot represents the mean of an independent experiment; horizontal lines represent mean and 95% CI. n=3 or 5 experiments. *p≤0.01, **p≤0.05 compared with DMSO control.
Both the gene and protein levels of sox9 and -6 were also significantly decreased by HNMPA-(AM3) treatment (Fig. 6). Gene expression analysis of P2 showed downregulation of cartilage-related collagens, Col2a1 and col11a1 (Fig. 6B). The Sox9 transcription complex likely remained unaffected as Sox6 and Med12 co-immunoprecipitated with Sox9 after HNMPA-(AM3) treatment (Fig. 6C). Given the decreased levels of Sox9 protein, we determined whether Sox9 binding to its target gene Col2a1 was affected. ChIP analysis showed that Sox9 binds to the Col2a1 gene at similar levels as in primary cells cultured in chondrogenic condition but the binding was significantly decreased in the treated P2 cells as would be expected given the decreased Sox9 levels (Fig. 6D). This suggested that insulin-regulated Sox9 levels influenced collagen synthesis by P2 cells.
FIG. 6.
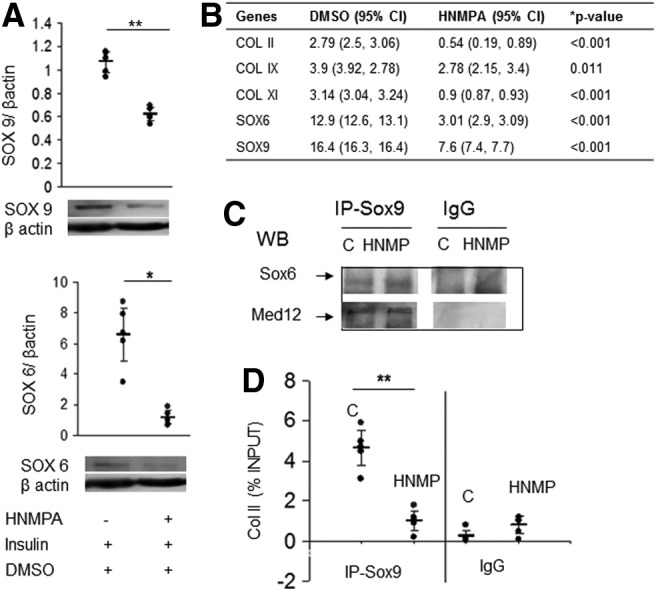
Insulin mediates SOX9 levels and col2a expression. (A) Immunoblot and densitometry show that treatment with HNMPA-(AM3) leads to lower protein levels of Sox6 and Sox9 in P2 grown in SF3D. Each dot represents the mean of an independent experiment; horizontal lines represent mean and 95% CI. n=5 experiments. (B) Gene expression also shows decreases in cartilage-associated genes after downregulating IR. Data are expressed relative to P2-ML and are shown as mean with 95% CI (lower and upper limits). *p≤0.01, **p≤0.05. n=3 experiments. (C) Physical interaction of transcription factor Sox6 and binding protein MED12 with Sox9 is unaffected by HNMPA-(AM3) treatment as seen in co-IP analysis. n=5 independent experiments. (D) Chromatin immunoprecipitation extraction of Sox9-bound DNA showed decreased binding to Col2a1 after HNMPA-(AM3) treatment (IR downregulation). Each dot represents the mean of an independent experiment; horizontal lines represent mean and 95% CI. n=5 experiments. *p≤0.01. C, control; co-IP, co-immunoprecipitation.
Discussion
The data shows that chondrocytes serially passaged in ML culture (P2-ML) lost their chondrocyte phenotype and acquired a “progenitor-like” phenotype with a gene expression profile suggestive of interzone cells. These cells could be redifferentiated to chondrocytes that form hyaline-like cartilage tissue using a defined serum-free culture system (SF3D) in the absence of exogenous factors, such as TGFβ, as demonstrated by the ability of the cells to form cartilaginous hyaline tissue that appears qualitatively and quantitatively similar to the tissue formed by primary chondrocytes.26 The redifferentiated cells showed elevated gene and protein levels of sox9 and mRNA levels of the cartilaginous matrix molecules Col2a1, COMP, and aggrecan and significantly lower Col1a1 expression compared with P2-ML. Expression of Col10 was low in SF3D and immunostaining of tissue sections also did not show collagen type 10 in tissue formed in SF3D at 3 weeks (data not shown). These findings suggest that the redifferentiated chondrocytes have an articular phenotype.27 This redifferentiation in HG appears to be regulated in part by a signal transduction cascade involving insulin that upregulated Sox9 expression and modulated col2a expression and collagen synthesis. These findings are consistent with other studies that show that chondrocyte phenotype can be changed by modulating the microenvironment and/or culture conditions.10,26,28
SF3D culture provides a sufficient chondro-conducive environment for the P2 cells that obviates the need for addition of exogenous TGFβ family molecules. Interestingly, SF3D does not invoke the same response (formation of cartilage tissue) in primary chondrocytes (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). To our knowledge, almost every study on redifferentiation of passaged chondrocytes has supplemented media with BMP, TGFβ, or some other type of growth factor.11,29 In this SF3D system addition of TGFβ3 or inhibition of TGFβ3 signaling (SB431542) showed no effect on collagen synthesis by P2 cells further confirming the independence of this redifferentiation process on exogenous TGFβ (Supplementary Fig. S2) and that the cells were likely not producing TGFβ endogenously. It is possible that P2 cells in SF3D do not require exogenous growth factors, such as TGFβ, due to their unique progenitor-like phenotype with interzone features. A number of previous studies have also shown that passaged cells acquire some features of either MSCs5,6,30 or interzone cells, such as GDF5 protein expression.31 This type of differentiation may impart a unique phenotype on bovine P2 cells, which may explain their independence of growth factors. Additional studies are needed to further characterize this phenotype.
While type-II-collagen-coated membranes are part of the SF3D system, the critical components of the DMEM media are insulin, dexamethasone, and high glucose. Since all the three media components are essential for the tissue formation, it is difficult to determine whether they support each other or act independently. Glucose levels are important as culturing in low glucose prevented cartilage tissue formation. The mechanism by which glucose regulates redifferentiation and matrix accumulation is not clear; especially as in this system glucose uptake by the redifferentiating cells was greater in the presence of low-glucose media. HG levels have been shown to promote chondrogenesis in MSCs by downregulating ERK and upregulating p38. Glucose can have other effects such as inducing O-glycosylation of EGF domains in PGs32,33 and macromolecular crosslinking that may alter matrix retention.34 Further studies will be required to delineate the role of glucose in this system. It is highly likely that dexamethasone acted by influencing matrix synthesis because the tissue formed by the cells cultured without dexamethasone contained much less matrix compared with the cells grown in the presence of dexamethasone (Fig. 3A). In support of this, blocking the effect of dexamethasone by treating cells with the glucocorticoid receptor antagonist led to decreased collagen and PG synthesis (data not shown). Interestingly, glucocorticoids have been shown to induce IR mRNA levels in other cell types.35
It was not entirely unexpected to find that insulin promotes chondrogenesis in passaged cells as this effect has been reported in other cell types, such as MSCs and the ATDC5 cells (mouse teratocarcinoma cell line that differentiates to chondrocytes in the presence of insulin).36,37 Proteomic studies suggest that insulin levels in FBS are much lower than that used in SF3D, which might explain why the presence of 20% FBS in SC3D was insufficient to promote matrix formation and accumulation by P2 cells.12 The involvement of insulin was supported by the observation that treatment with HNMPA-(AM3) downregulated IR gene and protein levels, decreased sox9 and -6 levels, and decreased synthesis of collagens. However, HNMPA-(AM3) is known to have other effects that could affect chondrogenesis. It can block prostaglandin synthesis by chondrocytes,38 which has been shown to affect chondrogenesis; hence, HNMPA-(AM3) could be working by mechanisms other than just modulating insulin effects.39 In our culture system insulin may act via Shc as the levels of pShc and downstream ERK-MAPK were modulated by HNMPA-(AM3). In keeping with this observation microarray analysis during redifferentiation showed upregulation of Shc binding protein and PRKCSH (data not shown), both involved in Shc-mediated cascade.40 Interestingly only the ERK-2 (42 kDa) and not the ERK-1 (44 kDa) subunit was affected by HMNPA-(AM3) treatment. Differential ERK-1/2 responses have been described by others as well.41,42 Several studies have shown that under physiological conditions ERK-2 can exist in predominantly a monomeric form (not dimerized to ERK-1) and is capable of regulating downstream signaling pathways.42 It was unexpected that ERK-2 levels would increase with IR inhibition by HNMPA-(AM3) treatment, but others have also observed that insulin can inhibit ERK, although in neuronal cells.43 Further, our results are consistent with those that have shown that high levels of ERK-2 inhibit differentiation in mouse embryonic stem cells.44
The data also suggests that the level of Sox9 may be important for redifferentiation, which would be consistent with the study on liver cells where Sox9 was shown to affect differentiation in a dose-dependent manner.45 In our system the redifferentiating cells have higher levels of Sox9 and subsequently higher association with Col2a1 on chromatin when compared with cells treated with HMNPA-(AM3), a condition that prevented the increase in collagen synthesis. In addition, this treatment—although affecting levels of sox9 and sox6—did not seem to affect the formation of the transcriptional complex, at least as investigated in this study, as the cotranscriptional molecules sox6 and Med12 were detected. This suggests that insulin-induced increases in Sox9 levels may be involved in the redifferentiation and/or accumulation of matrix by passaged cells.
In summary we describe a novel serum- and TGFβ-free culture system that requires insulin, dexamethasone, and HG to generate hyaline-like cartilage tissue from passaged bovine chondrocytes grown in 3D on collagen-type-II-coated membrane inserts. This is an important step toward the ultimate goal of developing autologous, patient-specific tissue-engineered hyaline-like cartilage tissue suitable to use for repair/replacement of damaged articular cartilage. Further studies are required to translate what has been learnt from this SF3D study for use with human chondrocytes.
Supplementary Material
Acknowledgments
The authors thank Mr. Harry Bojarski and Ryding-Regency Meat Packers for providing bovine tissues. The project was supported by funding from U.S. Department of Defense. N.A. received a fellowship from the Samuel Lunenfeld Research Institute Special Opportunities Fund. J.I. was supported by OGSST, ON. C.E.B. was funded by BRAVO!/MHIRT and by NIH grant No. MD 001427.
Disclosure Statement
No competing financial interests exist.
References
- 1.Holtzer H., Abbott J., Lash J., and Holtzer S.The loss of phenotypic traits by differentiated cells in vitro, I. Dedifferentiation of cartilage cells. Proc Natl Acad Sci U S A 46,1533, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gan L., and Kandel R.A.In vitro cartilage tissue formation by co-culture of primary and passaged chondrocytes. Tissue Eng 13,831, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Kon E., Filardo G., Di Martino A., and Marcacci M.ACI and MACI. J Knee Surg 25,17, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Mackay A.M., Beck S.C., Murphy J.M., Barry F.P., Chichester C.O., and Pittenger M.F.Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4,415, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Tallheden T., Dennis J.E., Lennon D.P., Sjogren-Jansson E., Caplan A.I., and Lindahl A.Phenotypic plasticity of human articular chondrocytes. J Bone Joint Surg Am 85-A(Suppl 2),93, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Fickert S., Fiedler J., and Brenner R.E.Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther 6,R422, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. . Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Bonaventure J., Kadhom N., Cohen-Solal L., Ng K.H., Bourguignon J., Lasselin C., et al. . Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res 212,97, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Chaipinyo K., Oakes B.W., and van Damme M.P.Effects of growth factors on cell proliferation and matrix synthesis of low-density, primary bovine chondrocytes cultured in collagen I gels. J Orthop Res 20,1070, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Quarto R., Campanile G., Cancedda R., and Dozin B.Thyroid hormone, insulin, and glucocorticoids are sufficient to support chondrocyte differentiation to hypertrophy: a serum-free analysis. J Cell Biol 119,989, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narcisi R., Quarto R., Ulivi V., Muraglia A., Molfetta L., and Giannoni P.TGF beta-1 administration during ex vivo expansion of human articular chondrocytes in a serum-free medium redirects the cell phenotype toward hypertrophy. J Cell Physiol 227,3282, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Zheng X., Baker H., Hancock W.S., Fawaz F., McCaman M., and Pungor E., Jr.Proteomic analysis for the assessment of different lots of fetal bovine serum as a raw material for cell culture. Part, I.V. Application of proteomics to the manufacture of biological drugs. Biotechnol Prog 22,1294, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Sensebe L., Krampera M., Schrezenmeier H., Bourin P., and Giordano R.Mesenchymal stem cells for clinical application. Vox Sang 98,93, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Siddle K.Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol 47,R1, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Uhles S., Moede T., Leibiger B., Berggren P.O., and Leibiger I.B.Selective gene activation by spatial segregation of insulin receptor B signaling. FASEB J 21,1609, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Ikegami D., Akiyama H., Suzuki A., Nakamura T., Nakano T., Yoshikawa H., et al. . Sox9 sustains chondrocyte survival and hypertrophy in part through Pik3ca-Akt pathways. Development 138,1507, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Rosa S.C., Rufino A.T., Judas F., Tenreiro C., Lopes M.C., and Mendes A.F.Expression and function of the insulin receptor in normal and osteoarthritic human chondrocytes: modulation of anabolic gene expression, glucose transport and GLUT-1 content by insulin. Osteoarthritis Cartilage 19,719, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Malafaya P.B., Oliveira J.T., and Reis R.L.The effect of insulin-loaded chitosan particle-aggregated scaffolds in chondrogenic differentiation. Tissue Eng Part A 16,735, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Bi W., Huang W., Whitworth D.J., Deng J.M., Zhang Z., Behringer R.R., et al. . Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A 98,6698, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lefebvre V., Huang W., Harley V.R., Goodfellow P.N., and de Crombrugghe B.SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol 17,2336, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiyama H., and Lefebvre V.Unraveling the transcriptional regulatory machinery in chondrogenesis. J Bone Miner Metab 29,390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Q., Eberspaecher H., Lefebvre V., and De Crombrugghe B.Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn 209,377, 1997 [DOI] [PubMed] [Google Scholar]
- 23.de Crombrugghe B., Lefebvre V., Behringer R.R., Bi W., Murakami S., and Huang W.Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol 19,389, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Oh C.D., Maity S.N., Lu J.F., Zhang J., Liang S., Coustry F., et al. . Identification of SOX9 interaction sites in the genome of chondrocytes. PLoS One 5,e10113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baltensperger K., Lewis R.E., Woon C.W., Vissavajjhala P., Ross A.H., and Czech M.P.Catalysis of serine and tyrosine autophosphorylation by the human insulin receptor. Proc Natl Acad Sci U S A 89,7885, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed N., Gan L., Nagy A., Zheng J., Wang C., and Kandel R.A.Cartilage tissue formation using redifferentiated passaged chondrocytes in vitro. Tissue Eng Part A 15,665, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Leung V.Y., Gao B., Leung K.K., Melhado I.G., Wynn S.L., Au T.Y., et al. . SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet 7,e1002356, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenzweig D.H., Chicatun F., Nazhat S.N., and Quinn T.M.Cartilaginous constructs using primary chondrocytes from continuous expansion culture seeded in dense collagen gels. Acta Biomater 9,9360, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Narcisi R., Signorile L., Verhaar J.A., Giannoni P., and van Osch G.J.TGFbeta inhibition during expansion phase increases the chondrogenic re-differentiation capacity of human articular chondrocytes. Osteoarthritis Cartilage 20,1152, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Polacek M., Bruun J.A., Elvenes J., Figenschau Y., and Martinez I.The secretory profiles of cultured human articular chondrocytes and mesenchymal stem cells: implications for autologous cell transplantation strategies. Cell Transplant 20,1381, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Schlegel W., Albrecht C., Eckl P., Freudenthaler H., Berger A., Vecsei V., et al. . Dedifferentiation of human articular chondrocytes is associated with alterations in expression patterns of GDF-5 and its receptors. J Cell Mol Med 13,3398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han Y.S., Bang O.S., Jin E.J., Park J.H., Sonn J.K., and Kang S.S.High dose of glucose promotes chondrogenesis via PKCalpha and MAPK signaling pathways in chick mesenchymal cells. Cell Tissue Res 318,571, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Whitworth G.E., Zandberg W.F., Clark T., and Vocadlo D.J.Mammalian notch is modified by D-Xyl-alpha1-3-D-Xyl-alpha1-3-D-Glc-beta1-O-Ser: implementation of a method to study O-glucosylation. Glycobiology 20,287, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Mentink C.J., Hendriks M., Levels A.A., and Wolffenbuttel B.H.Glucose-mediated cross-linking of collagen in rat tendon and skin. Clin Chim Acta 321,69, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Mamula P.W., McDonald A.R., Brunetti A., Okabayashi Y., Wong K.Y., Maddux B.A., et al. . Regulating insulin-receptor-gene expression by differentiation and hormones. Diabetes Care 13,288, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Laron Z.Insulin—a growth hormone. Arch Physiol Biochem 114,11, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Phornphutkul C., Wu K.Y., and Gruppuso P.A.The role of insulin in chondrogenesis. Mol Cell Endocrinol 249,107, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Jacques C., Holzenberger M., Mladenovic Z., Salvat C., Pecchi E., Berenbaum F., et al. . Pro-inflammatory actions of visfatin/Nampt involve regulation of insulin signaling pathway and Nampt enzymatic activity. J Biol Chem 287,15100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyamoto M., Ito H., Mukai S., Kobayashi T., Yamamoto H., Kobayashi M., et al. . Simultaneous stimulation of EP2 and EP4 is essential to the effect of prostaglandin E2 in chondrocyte differentiation. Osteoarthritis Cartilage 11,644, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Ravichandran K.S.Signaling via Shc family adapter proteins. Oncogene 20,6322, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Davis R.J.Transcriptional regulation by MAP kinases. Mol Reprod Dev 42,459, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Kaoud T.S., Devkota A.K., Harris R., Rana M.S., Abramczyk O., Warthaka M., et al. . Activated ERK2 is a monomer in vitro with or without divalent cations and when complexed to the cytoplasmic scaffold PEA-15. Biochemistry 50,4568, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Heide L.P., Hoekman M.F., Biessels G.J., and Gispen W.H.Insulin inhibits extracellular regulated kinase 1/2 phosphorylation in a phosphatidylinositol 3-kinase (PI3) kinase-dependent manner in Neuro2a cells. J Neurochem 86,86, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Kim M.O., Kim S.H., Cho Y.Y., Nadas J., Jeong C.H., Yao K., et al. . ERK1 and ERK2 regulate embryonic stem cell self-renewal through phosphorylation of Klf4. Nat Struct Mol Biol 19,283, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Seymour P.A., Freude K.K., Dubois C.L., Shih H.P., Patel N.A., and Sander M.A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Dev Biol 323,19, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.