Abstract
It is known that angiogenesis plays an important role in bone regeneration and that release of angiogenic and osteogenic growth factors can enhance bone formation. Multiple growth factors play key roles in processes that lead to tissue formation/regeneration during natural tissue development and repair. Therefore, treatments aiming to mimic tissue regeneration can benefit from multiple growth factor release, and there remains a need for simple clinically relevant approaches for dual growth factor release. We hypothesized that mineral coatings could be used as a platform for controlled incorporation and release of multiple growth factors. Specifically, mineral-coated scaffolds were “dip coated” in multiple growth factor solutions, and growth factor binding and release were dictated by the growth factor-mineral binding affinity. Beta tricalcium phosphate (β-TCP) scaffolds were fabricated using indirect solid-free form fabrication techniques and coated with a thin conformal mineral layer. Mineral-coated β-TCP scaffolds were sequentially dipped in recombinant human vascular endothelial growth factor (rhVEGF) and a modular bone morphogenetic peptide, a mineral-binding version of bone morphogenetic protein 2 (BMP2), solutions to allow for the incorporation of each growth factor. The dual release profile showed sustained release of both growth factors for over more than 60 days. Scaffolds releasing either rhVEGF alone or the combination of growth factors showed an increase in blood vessel ingrowth in a dose-dependent manner in a sheep intramuscular implantation model. This approach demonstrates a “modular design” approach, in which a controllable biologics carrier is integrated into a structural scaffold as a thin surface coating.
Introduction
Therapeutic strategies in tissue engineering often rely on the delivery of growth factors in an effort to mimic the natural microenvironments of tissue formation and repair. Growth factors can stimulate cellular activities, such as migration, proliferation, and differentiation to repair or regenerate damaged tissue.1–4 An extensive number of in vitro and in vivo studies in multiple tissue types have shown that the delivery of single growth factors can promote tissue regeneration.5–8 However, during natural tissue development and regeneration, multiple growth factors play key roles in processes that lead to tissue formation. Therefore, treatments aiming to mimic tissue regeneration could benefit from the release of multiple therapeutic agents. For example, in bone tissue engineering research, recent studies have shown that the combined delivery of osteogenic and angiogenic growth factors enhances bone tissue regeneration compared with single growth factor delivery.9–11 Similarly, dual release of angiogenic and arteriogenic growth factors can enhance stable blood vessel formation in cardiac tissue engineering approaches.12 These studies suggest that local release of multiple growth factors is a promising approach for regenerative medicine.
One current strategy for dual growth factor release involves loading a growth factor into microparticles by diffusion followed by incorporation of the loaded microparticles into a hydrogel containing a second growth factor.9 Another approach involves incorporating a growth factor into polymer microspheres, which are later incorporated into a hollow scaffold coated with gelatin containing a second growth factor.10 These techniques have been successful in demonstrating the advantage of dual growth factor release. Although these techniques have demonstrated proof-of-concept for multiple growth factor delivery, clinical translation of this concept may be facilitated by simpler techniques that can be applied in the operating room. Here, we report one such simpler approach that exploits differential affinity between growth factors and a coating material, where higher affinity leads to slower release kinetics and lower affinity leads to faster release kinetics. Specifically, we report controlled sequential delivery of an angiogenic and an osteogenic growth factor by simply “double dipping” a scaffold material in growth factor solutions.
The application of bone morphogenetic proteins (BMPs) has been the gold standard in bone tissue engineering research because of the ability of BMPs to enhance bone formation and facilitate healing. rhBMP-2 and BMP-2 mimics are particularly potent osteogenic growth factors capable of regenerating bone tissue in orthotopic13–15 and ectopic16–18 sites. Thus, there is strong interest in delivering BMPs in a sustained manner from tissue engineering scaffolds. However, engineering of many tissue types, including bone, also relies on creating a functional vascular network. Inadequate bone vascularity is associated with decreased bone formation and bone mass.19–21 Angiogenesis, the formation of new blood vessels from pre-existing ones, is critical to deliver nutrients, remove waste products, and provide cells and biological mediators during fracture healing. Several factors, including vascular endothelial growth factor (VEGF), have been identified as critical regulators of angiogenesis.22–25 Previous studies have demonstrated that the development of a robust blood vessel network depends on the timing, concentration gradients, and prolonged tissue exposure to proangiogenic growth factors.26,27 Thus, there is a need for controllable sustained VEGF release strategies to stimulate angiogenesis in tissue engineering scaffolds. The pro-osteogenic efficacy of BMPs and the pro-angiogenic efficacy of recombinant human VEGF (rhVEGF) suggest that controlled delivery of these factors may encourage vascularized bone regeneration.
In this study, we aimed to control delivery of a pro-angiogenic and a pro-osteogenic growth factor from custom-designed tissue engineering scaffolds using a simple “double dipping” process. We hypothesized that growth factor-scaffold binding affinity could be used to dictate the growth factor release rate, enabling controllable incorporation and release of multiple growth factors. Porous beta tricalcium phosphate (β-TCP) scaffolds were fabricated using indirect solid-free form fabrication (SFF) and then coated with a nanoporous mineral layer using an approach described in a variety of previous studies.3,28–34 Calcium phosphate ceramics such as β-TCP are widely used as bone substitutes as they are bioactive and osteoconductive.35 Coated scaffolds were then loaded with two biologically active growth factors: rhVEGF protein and a modular peptide version of BMP2 (mBMP), which contains a high affinity mineral-binding sequence and a BMP-2-derived biologically active sequence.36–38 We hypothesized that rhVEGF would bind with lower affinity to mineral-coated β-TCP scaffolds resulting in faster release kinetics, whereas mBMP would bind with strong affinity resulting in slower release kinetics.
Materials and Methods
In vivo experimental design
All experimental protocols were approved by the Institutional Animal Use and Care Committee. Ten Hampshire mature female sheep, ranging in age from 2 to 5 years and weighing between 60 and 100 kg (mean±SD, 66.4±8.6 kg), were used in the study.
The experimental group consisted of scaffolds delivering different dosages of rhVEGF (generous gift from the NIH) or mBMP from mineral-coated β-TCP scaffolds or the combination of growth factors. The control group consisted of mineral-coated scaffolds with no growth factor. In groups releasing rhVEGF, 0.5, 1.0, 5.0, or 10 μg were surface bound on the mineral-coated scaffolds. A dosage of 50 μg was bound to scaffolds releasing mBMP. The dual group had 0.5, 1.0, 5.0, or 10 μg of rhVEGF and 50 μg mBMP, respectively.
All sheep received a scaffold from each of the 10 conditions (experimental and control groups). Five scaffolds were implanted in each side of the spine, in the longissimus lumborum, giving us a total of 10 implant sites per sheep. Placement of the scaffold was randomized. Five sheep were euthanized at 2 weeks and five sheep at 4 weeks, and scaffolds were collected for histological analysis.
β-TCP scaffold fabrication and incubation in modified simulated body fluids
100% β-TCP ceramic suspensions were utilized in the fabrication of scaffolds used in this study, produced by indirect SFF methods. In short, image-based design and three-dimensional (3D) printing techniques were employed to achieve scaffolds with controlled architecture: square, orthogonal interconnected pores with a 40% volume void fraction (porosity). In their final fabricated state, scaffolds measured 6×4 mm, with a pore size of 753±57 μm.
A 40% by volume ceramic slip was prepared by mixing as-received β-TCP powder (Plasma Biotal) with appropriate deflocculants and acrylate binders. The slurry was then cast into the designed molds generated via the ModelMaker II (Solidscape), which were removed after ceramic curing was established. After binder burnout scaffolds were sintered in air at 1100°C for 5 h and furnace cooled.
The scaffolds were incubated at 37°C in modified simulated body fluids (mSBF) for periods of 7 days under continuous rotation. Each scaffold was incubated in 15 mL of mSBF. The mSBF solution had a similar composition to that of human plasma but with double the concentration of calcium and phosphate to enhance mineral growth and was prepared as previously reported.33 Specifically, the following reagents were added to ddH2O heated to 37°C in the order shown; 141 mM NaCl, 4.0 mM KCl, 0.5 mM MgSO4, 1.0 mM MgCl2, 4.2 mM NaHCO3, 20.0 mM HEPES, 5.0 mM CaCl2, and 2.0 mM KH2PO4. The solution was then adjusted to a final pH of 6.8. The mSBF solution was renewed daily in order to maintain a consistent ionic strength throughout the experiment. The surface morphology of the biomineral coating formed on the β-TCP scaffolds was investigated by scanning electron microscopy. Mineral-coated β-TCP scaffolds were mounted on aluminum stubs and sputter coated with a thin layer of gold. Samples were imaged under high vacuum using a JEOL JSM-6100 scanning electron microscope operating at 10 kV.
Dip coating, dual binding, and release of rhVEGF and mBMP
Dip coating demonstration
mBMP (sequence KIPKASSVPTELSAISTLYL-AAAA-γEPRRγEVAγEL) was synthesized by RS Synthesis. Mineral-coated scaffolds were dipped for ∼10 s in 10 mL of rhodamine-labeled mBMP solution at a concentration of 1 mg/mL. During the dip coating process, a movie was captured using a Nikon D90 digital camera with a Tamron SP 60 mm f/2 Macro Lens. In addition, binding over time was characterized. At 10, 20, 30, 40, and 60 min, still images of the scaffolds were captured using a Nikon D90 digital camera with a Tamron SP 60 mm f/2 Macro Lens.
Binding studies
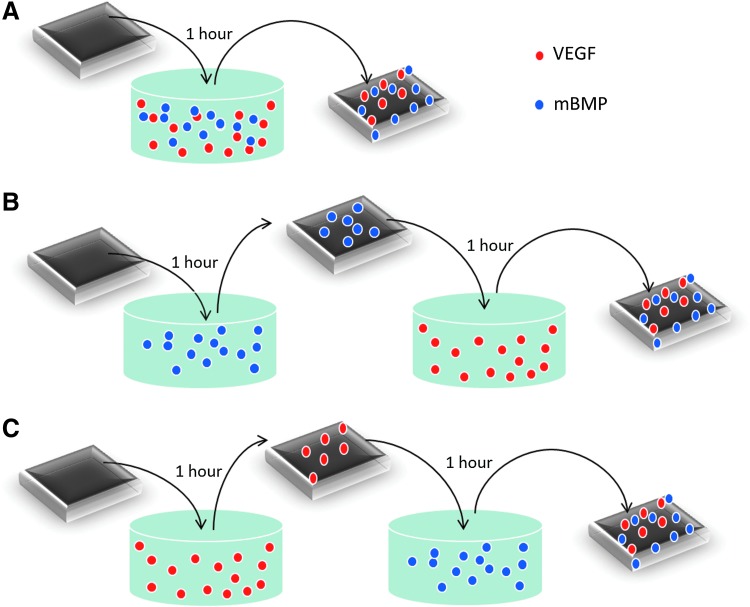
125I-labeled rhVEGF from Perkin Elmer and rhodamine-labeled mBMP were used to characterize binding of growth factors to the mineral-coated scaffolds (Fig. 1). Binding was characterized by incubating mineral-coated scaffolds in 400 μL of a cocktail solution of both growth factors (rhVEGF and mBMP) for 1 h or by a two-step incubation in 400 μL of solutions containing individual growth factors for 1 h each incubation step. The rhVEGF solution used for binding was 1 μg/mL, 0.35% 125I-labeled rhVEGF, and the mBMP solution, 200 μg/mL rhodamine-labeled mBMP. The radioactivity or the fluorescence intensity of the solution used for binding was measured to determine the percentage of growth factor bound using a Packard Cobra II Gamma Counter and a BioTek Synergy plate reader (excitation/emission: 494/519), respectively. In our release and in vivo studies, dual binding was performed by the two-step incubation starting with rhVEGF and subsequent mBMP incubation.
FIG. 1.
Experimental conditions used to evaluate dual binding of rhVEGF and mBMP. In (A), scaffolds were dipped in a cocktail solution of both growth factors for 1 h. In (B), scaffolds were dipped in an mBMP solution for 1 h, rinsed, and dipped in an rhVEGF solution for an additional hour. In (C), scaffolds were dipped in an rhVEGF solution for 1 h, rinsed, and dipped in an mBMP solution for an additional hour. mBMP, modular bone morphogenetic peptide; rhVEGF, recombinant human vascular endothelial growth factor. Color images available online at www.liebertpub.com/tea
Release studies
After binding of rhVEGF and mBMP, scaffolds were rinsed in diH2O, transferred, and incubated in either 500 or 1000 μL of Dulbecco's modified Eagle's medium and simulated body fluids (SBF, pH 7.4) at 37°C to characterize the release of mBMP and rhVEGF, respectively. At the indicated time points, the radioactivity or fluorescence intensity of the release medium was measured using a Packard Cobra II Gamma Counter and a BioTek Synergy plate reader (excitation/emission: 494/519), respectively.
Preparation of coated scaffolds for implantation
Scaffolds were mineral coated as described above and sterilized using ethylene oxide. Growth factor solutions were prepared by mixing the appropriate amount of rhVEGF or mBMP in phosphate-buffered saline. Growth factor solutions were sterilized by 0.2-μL syringe filtering. Before surgery, β-TCP scaffolds releasing single growth factor (rhVEGF or mBMP) were incubated in 500 μL of different rhVEGF solutions (5, 10, 50, and 100 μg/mL) or 500 μL of mBMP (100 μg/mL) for 1 h. Scaffolds from the DUAL group were incubated for 1 h in an rhVEGF solution, 100 μg/mL; rinsed and subsequently incubated in 500 μL of mBMP (100 μg/mL) for 1 h as well. After binding, scaffolds were directly used in the surgery. The rhVEGF dosages explored in this study are based on previous studies, which indicate that 2–3 μg rhVEGF released from polymer scaffolds in a sustained manner over 2–4 weeks induce angiogenesis at 2 and 4 weeks postimplantation.39,40 The mBMP-2 dosage used in this study was more difficult to choose since there are numerous studies releasing rhBMP2 but fewer using the modular mineral binding version of BMP2, and the information available in large animal models was limited as well. However, we based our decision on previous subcutaneous implantation studies from multiple investigators, in which ∼5 μg BMP-2 has been released over 7–30 days from various materials, including β-TCP,16 and resulted in a significant induction of osteogenesis and increased the dosage by an order of magnitude.
Surgical procedure
Each sheep was sedated using xylazine (0.1 mg/kg, IM) and shaved around the spine area. Ketamine (2–5 mg/kg, IV) in combination with diazepam (0.1 mg/kg, IV) were given intravenously through a cephalic catheter to induce anesthesia. A dose of procaine penicillin G (20,000 IU/kg, IM) was then administered. The sheep were maintained using isoflurane (0–4%, intratracheally). Ten implants were placed in the longissimus lumborum (five either side of the spine) of the sheep. A total of 10 longitudinal incisions of ∼20 mm in length were performed in either sides of the spine. A “pocket” was formed in the longissimus lumborum. Each pocket spaces 50 mm on each side. Scaffolds were placed in each muscle pocket, and the muscle was sutured and closed with a nonabsorbable suture that also served as a reference point for later implant retrieval. The subcutaneous tissue and skin were closed in a routine manner. After the surgery, a second dose of procaine penicillin G (20,000 IU/kg, IM) was given. Phenylbutazone (4.4 mg/kg, PO q24hr for 3 days) was administered for pain beginning the same day at the surgery and continued for 3 days.
Implant retrieval and scaffold preparation for histology
Animals were euthanized using sodium pentobarbital (Beuthanasia) 200 mg/kg, IV (n=5 per time point), and the longissimus lumborum removed at 2 and 4 weeks. The scaffolds collected at the 2 weeks time point were fixed in Zamboni's fixative (Newcomer Supply) for 48 h, decalcified in EDTA/sucrose for 7 days, paraffin embedded, and sectioned. Two-week tissue sections were stained for von Willebrand factor (vWF) using a Lab Vision autostainer (serial# LVMA-LV1.3-0237) and imaged with a Nikon Optiphot microscope and an AxioCam (Zeiss) digital camera. Blood vessels, indicated by vWF staining, were counted manually at 10×magnification in the pores of the scaffold area and normalized to tissue area with the use of AxioVision 4.7 software.
The scaffolds collected at the 4 weeks time point were fixed in 10% neutral buffered formalin, dehydrated in an ethanol series, and infiltrated with methylmethacrylate monomer. Following polymerization, samples were sectioned using a Leica SP1600-Saw Microtome. Resulting sections were stained with Goldner's Trichrome and Sanderson's Rapid Bone Stain (Surgipath) and counterstained with acid fuchsin (Sigma). Sections from 2 and 4 weeks were also stained with hematoxilyn and eosin and were imaged using a Nikon Optiphot microscope.
Results
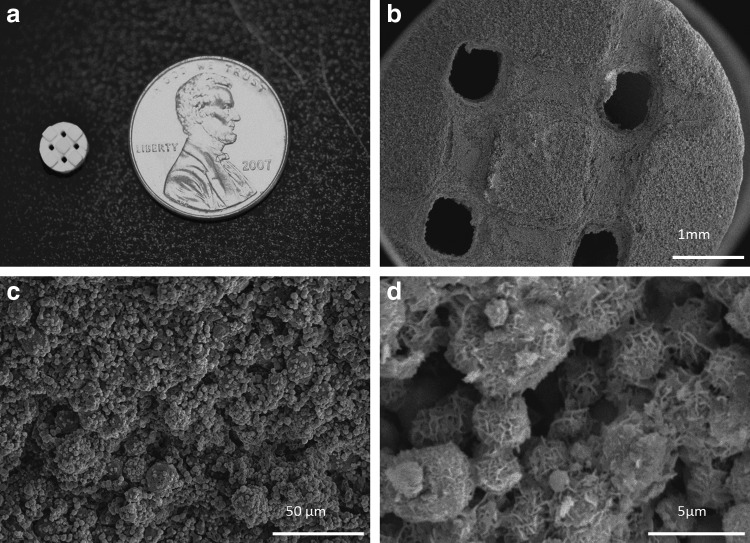
Porous β-TCP scaffolds were fabricated via 3D printing, an indirect SFF technique, with controlled periodic porous architecture (Fig. 2a). Incubation of β-TCP scaffolds in mSBF resulted in the formation of a continuous mineral layer on the material (Fig. 2b, c). The mineral displayed a plate-like nanostructure (Fig. 2d) consistent with a hydroxyapatite coating as previously characterized.3,34,41
FIG. 2.
(a) β-TCP scaffold fabricated via SFF. (b–c) Incubation of β-TCP scaffolds in mSBF for 7 days resulted in the formation of a continuous coating. (d) The morphology of the mineral displayed a plate-like nanostructure characteristic of hydroxyapatite. β-TCP, beta tricalcium phosphate; mSBF, modified simulated body fluids; SFF, solid-free form fabrication.
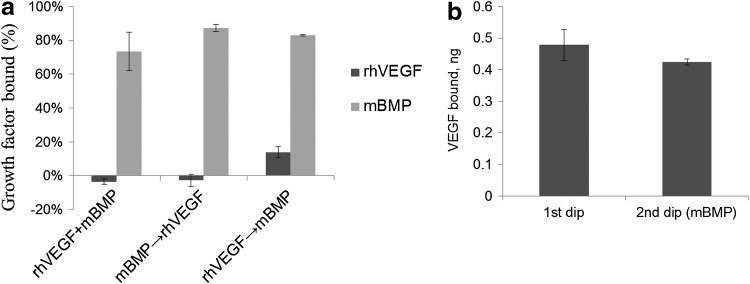
Dip coating scaffolds in a solution of rhodamine-labeled mBMP (1 mg/mL) results in binding of the growth factor within seconds (Fig. 3a and Supplementary Movie S1). Further evaluation of binding over time suggested that the amount of growth factor bound may increase with longer incubation times (Fig. 3b). Both rhVEGF and mBMP could be bound to mineral-coated scaffolds after a simple 2-step dipping process (Fig. 1). When scaffolds were dipped in a solution containing a cocktail of both rhVEGF (1 μg/mL) and mBMP (200 μg/mL), 73%±11% of mBMP bound to the mineral coating, whereas rhVEGF did not bind (Fig. 4a). This result indicates that mBMP's higher binding affinity can inhibit rhVEGF binding. Similarly, when scaffolds were first dipped in an mBMP containing solution, followed by an rhVEGF containing solution, 87%±2% of mBMP bound but we still observed no binding of rhVEGF (Fig. 4a). However, when scaffolds were first dipped in an rhVEGF solution, followed by an mBMP containing solution, 14%±3% of rhVEGF bound and 83%±1% of mBMP bound to the mineral coating (Fig. 4a). Thus, rhVEGF and mBMP can be loaded into the same mineral coating, but only by first incubating in rhVEGF followed by mBMP. We next characterized this “double dipping” process by exploring whether rhVEGF was competitively removed during the second dipping step (i.e., during mBMP binding). Our results demonstrated that the amount of bound rhVEGF was not significantly decreased after the mBMP binding step, indicating no competitive removal (Fig. 4b).
FIG. 3.
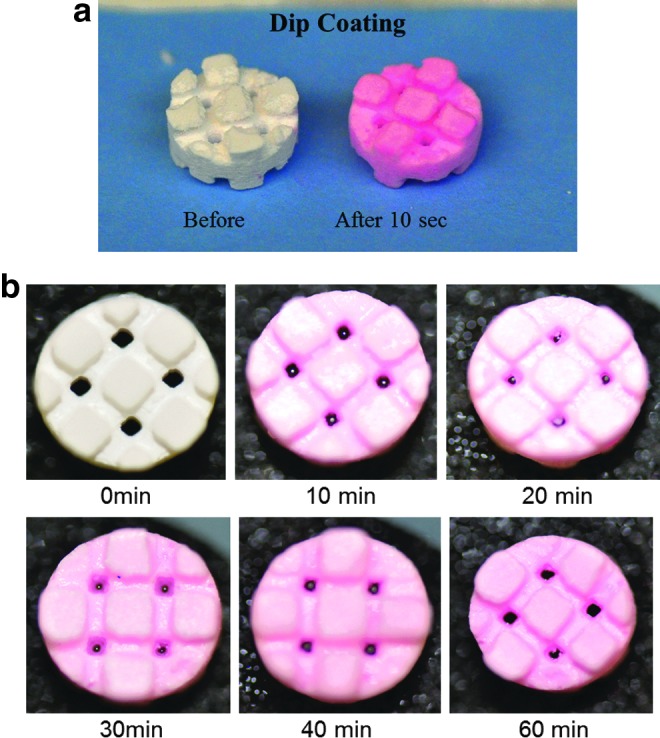
Visual demonstration of dip coating process. (a) Scaffolds dipped in a 1 mg/mL mBMP solution for 10 s changed color demonstrating binding of rhodamine-labeled mBMP to the mineral-coated scaffold (see Supplementary Movie S1). (b) Characterization of binding over time at the mBMP concentration used for the in vivo studies demonstrated that the amount of growth factor bound depends on the time scaffolds are incubated in the growth factor solution. Color images available online at www.liebertpub.com/tea
FIG. 4.
Characterization of dual binding of rhVEGF and mBMP to mineral-coated scaffolds. (a) Scaffolds were dipped in a cocktail of rhVEGF and mBMP, in rhVEGF followed by mBMP, and in rhVEGF followed by mBMP. The percentage of growth factor bound was characterized in (a) for all these conditions. Dual growth factor binding was obtained after the sequential dipping of scaffolds in rhVEGF initially, followed by mBMP. (b) The amount of rhVEGF bound to the scaffolds after the first dipping was not significantly changed during the second dipping step.
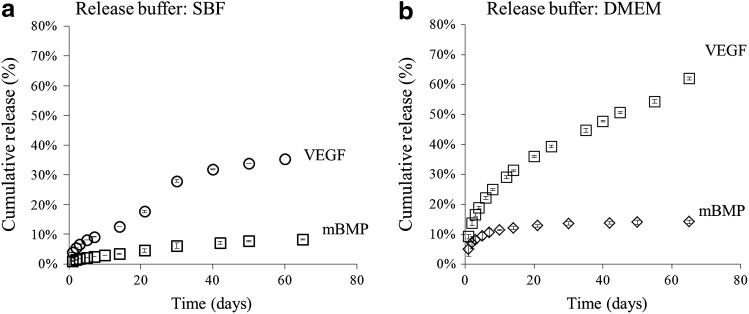
Release of the bound rhVEGF and mBMP was sustained for over 60 days in either the cell culture medium or SBF. The release profile over a 65 day period was strongly dependent on the mineral-binding affinity, as the mBMP molecule (designed for high affinity mineral binding) released less than 15%, whereas the rhVEGF (with no specific mineral-binding component) released over 60% (Fig. 5). The actual amount of growth factor released at different time points is shown in Supplementary Tables S1 and S2 for each buffer. Although the percentage of mBMP is lower from that of rhVEGF, the total amount of mBMp released is higher as a higher dosage (biologically relevant) of mBMP was chosen. The amount of released growth factor also depended on the buffer used for the release study. In SBF, scaffolds released less total growth factor (35%±0.3% of rhVEGF and 9%±0.1% of mBMP over 60 days) than in the cell culture medium (62%±0.5% of rhVEGF and 14%±0.5% of mBMP over 60 days) (Fig. 5).
FIG. 5.
Dual release of rhVEGF and mBMP in (a) SBF and (b) DMEM. Release of both growth factors was sustained for over 2 months. The release of both growth factors was slower in SBF, which contains the same ionic constituents of blood plasma. DMEM, Dulbecco's modified Eagle's medium.
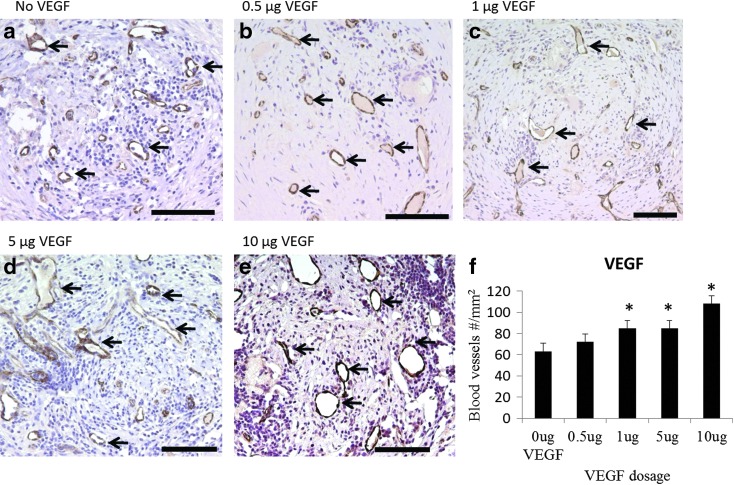
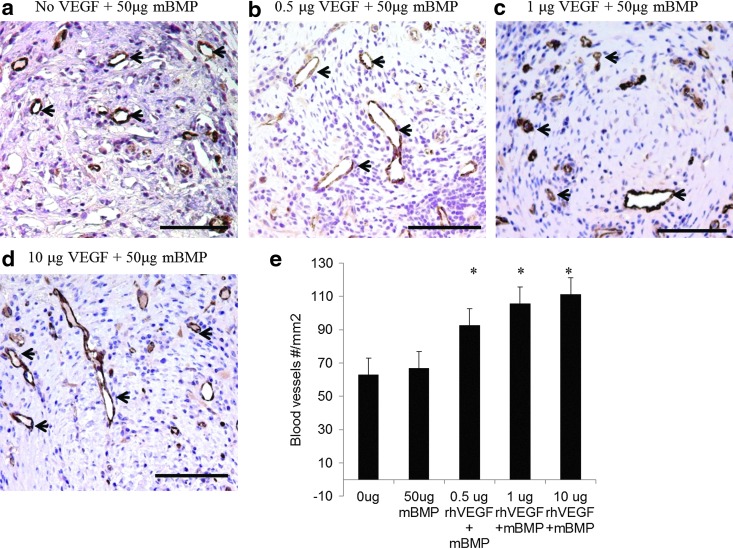
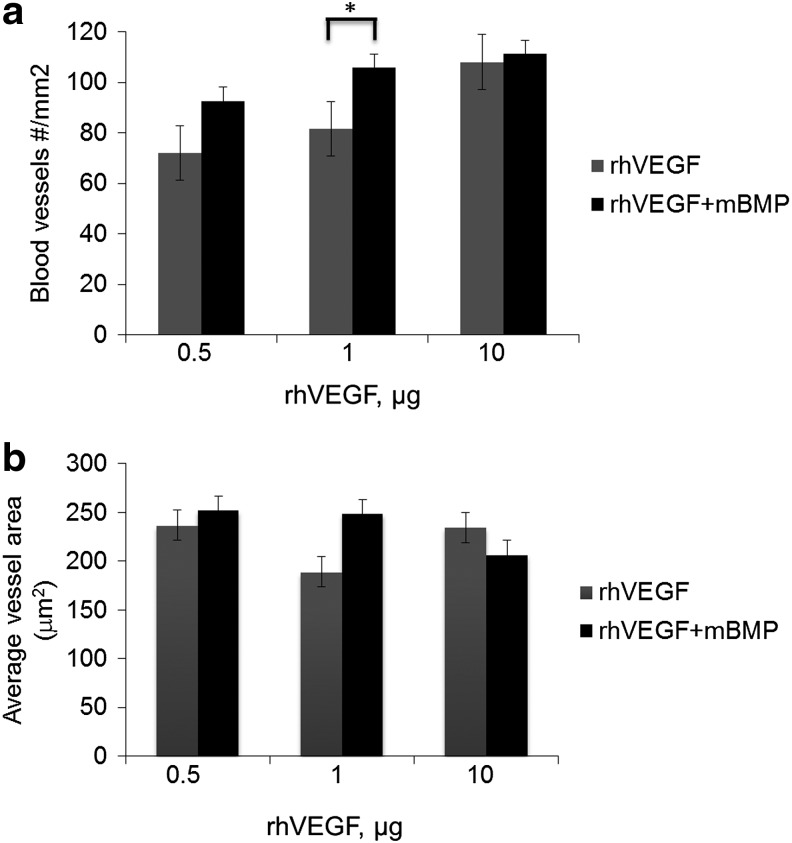
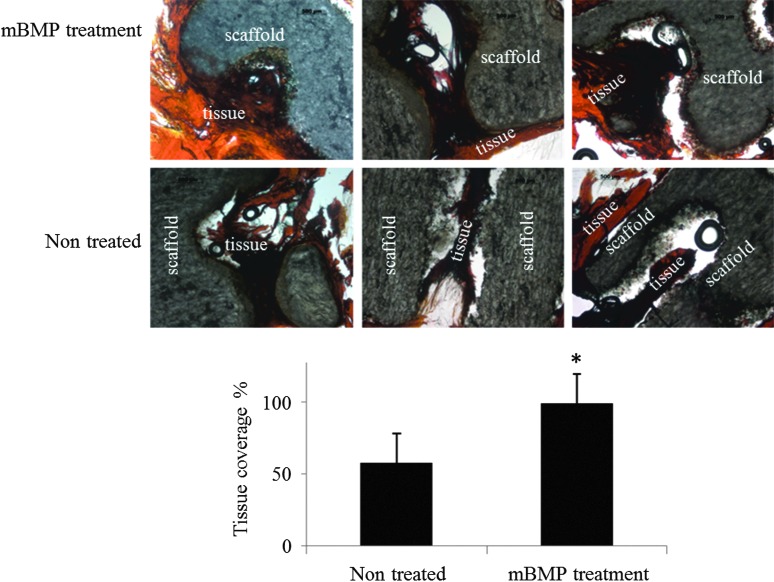
Scaffolds releasing rhVEGF stimulated an increase in blood vessel density within the scaffolds, and the stimulatory effect of released rhVEGF was dose dependent. The density of blood vessels within scaffolds releasing rhVEGF at dosages of 1, 5, and 10 μg significantly increased compared to blank scaffolds (Fig. 6). Similarly, the scaffolds releasing both rhVEGF and mBMP demonstrated an rhVEGF-dependent increase in blood vessel density (Fig. 7), and the rhVEGF-stimulated blood vessel infiltration was not significantly influenced by the release of mBMP (Fig. 8). The scaffolds were well tolerated and engendered no apparent immune reaction, and the presence of rhVEGF and mBMP did not influence the implant response. At 2 weeks, a thin fibrous capsule was observed around the outside of each of the scaffolds, as well as around the individual pores (Fig. 9). The release of mBMP resulted in significantly increased tissue infiltration into the scaffold at 4 weeks (Fig. 10) but did not stimulate apparent ectopic bone formation.
FIG. 6.
In vivo effect of released rhVEGF from single growth factor releasing scaffolds. rhVEGF release enhanced blood vessel ingrowth (black arrows) 2 weeks after implantation. (a–e) vWF immunostaining of blood vessels within sections of implanted β-TCP scaffolds releasing (a) No rhVEGF; (b) 0.5 μg rhVEGF; (c) 1.0 μg rhVEGF; (d) 5 μg rhVEGF; and (e) 10 μg rhVEGF. Positive vWF staining is brown, and circular vWF staining represents a blood vessel. (f) Quantification of the number of blood vessels within the pores of the scaffold. *p<0.003 relative to the no growth factor condition. Scale bars=100 μm. vWF, von Willebrand factor. Color images available online at www.liebertpub.com/tea
FIG. 7.
In vivo effect of released rhVEGF from dual growth factor releasing scaffolds. rhVEGF release enhanced blood vessel ingrowth (black arrows) 2 weeks after implantation. (a–d) vWF immunostaining of blood vessels within sections of implanted β-TCP scaffolds releasing (a) No rhVEGF; (b) 0.5 μg rhVEGF; (c) 1.0 μg rhVEGF; (d) 10 μg rhVEGF. Positive vWF staining is brown, and circular vWF staining represents a blood vessel. (e) Quantification of the number of blood vessels within the pores of the scaffold. *p<0.009 relative to the no growth factor condition. Scale bars=100 μm. Color images available online at www.liebertpub.com/tea
FIG. 8.
Comparison of blood vessel number and area between scaffolds releasing single versus dual growth factor. (a) Blood vessel infiltration was not significantly different between single and dual releasing scaffolds in the 0.5 and 10 μg rhVEGF. There was statistical significance at the 1 μg dose (p=0.03). (b) Blood vessel area was not significantly different (p>0.18), suggesting that the presence of mBMP did not influence the effect of rhVEGF in blood vessel infiltration and the area of the blood vessels formed.
FIG. 9.
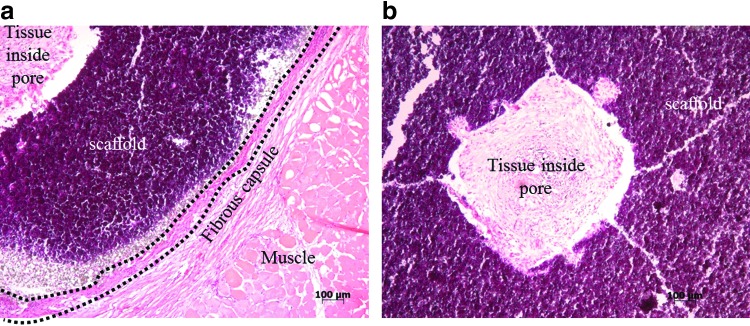
H&E staining of sections of scaffolds at 2 weeks. (a) Formation of a thin fibrous capsule was observed in all conditions. This image is from an mBMP releasing scaffold, but all treatment groups showed the formation of a thin fibrous capsule. (b) Fibrous tissue infiltration was also observed in all conditions. The image presented is from the no growth factor group. H&E, hematoxylin and eosin. Color images available online at www.liebertpub.com/tea
FIG. 10.
Tissue sections of scaffolds at 4 weeks stained for Masson's trichrome. mBMP released in vivo resulted in significant differences in the percentage of tissue coverage within the scaffold pores. mBMP improved tissue-material interactions. Asterisk denotes significance from the nontreated group (p<0.05). Color images available online at www.liebertpub.com/tea
Discussion
The objective of this study was to deliver angiogenic and osteogenic growth factors using a simple adaptable double dipping process. We hypothesized that two different growth factors would bind to mineral-coated scaffolds and would exhibit sustained release kinetics dependent on the growth factor-scaffold binding affinity. To address this hypothesis, we chose two growth factors: one with relatively low mineral binding affinity (rhVEGF) and one with uniquely high mineral binding affinity (mBMP). The surface of the mineral coating was highly porous (Fig. 2d) and contained charged calcium and phosphate components. Therefore, we hypothesized that the coating would be capable of efficiently binding growth factors via electrostatic interactions and would release growth factors for extended periods of time.
We achieved binding of rhVEGF and mBMP by sequentially dipping scaffolds in rhVEGF and mBMP solutions, respectively. The mBMP was engineered to contain a mineral binding domain that strongly interacts with the mineral surface. The affinity of this engineered peptide was characterized previously by Lee et al. and is due to the presence of an osteocalcin-inspired peptide sequence containing three γ-carboxylated glutamic acid resides that coordinate with calcium ions in the mineral crystal lattice.42 Previous studies have demonstrated an ability to systematically vary the peptide-mineral binding affinity and release.42,43 Here, we chose to use the highest affinity mineral-binding sequence to extend mBMP release over a much longer time frame than rhVEGF release, as a clear demonstration of our dual release concept. Analysis of the release profile of the growth factors from mineral-coated β-TCP biomaterials showed that both rhVEGF and mBMP can be released for over 60 days in different mediums (Fig. 5). Growth factor release from mineral coatings is influenced by the surrounding solution characteristics. Since SBF is supersaturated with calcium and phosphate ions, mineral dissolution and reprecipitation can both occur. As the coating dissolves in SBF, the released protein may re-bind to existing or newly formed mineral or may become encapsulated within a growing mineral coating. Each of these scenarios would result in slower protein release kinetics. These mechanisms for decreased protein release would likely be less prevalent in the cell culture medium as it has a lower concentration of calcium and phosphate ions when compared to SBF. As expected, mBMP released with slower release kinetics (14%±1.6% over 60 days) when compared to rhVEGF release kinetics (62%±1.5% over 60 days).
Dual growth factor release has been explored successfully by others in previous studies.9,10,44 Patel et al. released rhVEGF and BMP2 from gelatin microparticles into a critical-sized rat calvarial defect.9 This approach resulted in significantly higher bone formation in the dual release group, but loading of the growth factor into gelatin by diffusion required a 20 h incubation step.9 Similarly, Kempen et al. used dual release of rhVEGF and BMP2 to enhance ectopic bone formation in rats.10 The Kempen et al. study created PLG microspheres loaded with BMP2 and embedded them in a scaffold that was coated with a VEGF-loaded gelatin hydrogel.10 These studies clearly demonstrate the impact of dual growth factor delivery. Here, we endeavored to create a relatively simple and adaptable approach for sustained release of multiple bioactive growth factors based on scaffold binding affinity. Mineral-coated scaffolds were dipped in growth factor solutions intraoperatively—immediately before scaffold implantation. The amount of rhVEGF bound to the mineral coating was not significantly decreased during the subsequent dipping in the mBMP solution (Fig. 4b). Interestingly, when scaffolds were incubated in mixed solutions with both rhVEGF and mBMP, or mBMP followed by rhVEGF, there was negligible rhVEGF binding. We can attribute this to more rapid and efficient mBMP binding relative to rhVEGF. Therefore, this intraoperative “double dipping” process could be used for dual growth factor incorporation, but only in the case where the lower affinity binder (rhVEGF) was added before the higher affinity binder (mBMP).
Previous studies by our group have successfully used mineral coatings on various devices for the release of bioactive single growth factors in vitro and in vivo, suggesting growth factors are not denatured.3,43,45 In a study by Suarez-Gonzalez et al., rhVEGF released from mineral-coated β-TCP scaffolds similar to those explored in our current study was capable of binding to VEGF antibody and was capable of promoting human umbilical vein endothelial cell (HUVEC) proliferation in vitro. VEGF-induced HUVEC proliferation required a lower concentration of released rhVEGF when compared to bolus rhVEGF.3 Lee et al. delivered basic fibroblast growth factor (bFGF) from mineral-coated sutures in a chronic rotator cuff repair model in sheep and the local release of bFGF improved the load at failure of the repaired tendon.46 Lu et al. delivered mBMP from mineral-coated bioresorbable interference screws in a sheep tendon-bone healing model and demonstrated that the group receiving mBMP had improved histological scores of early tendon-bone healing.45 Taken together, these studies and others suggest that the mechanism of growth factor-mineral binding and subsequent release maintains the growth factor biological activity and serves as a simple and perhaps broadly applicable technique.3,34,41,45,47,48
Importantly, mineral coatings can be grown on β-TCP scaffolds fabricated via indirect SFF. SFF could prove highly useful for the construction of scaffolds possessing patient-specific anatomies and interior porous architectures derived from computational design optimizations. This fabrication technique allows precise control over properties, such as pore size, porosity, permeability, and stiffness.49 Control over these characteristics may enhance cell infiltration and mass transport of nutrients and metabolic waste throughout the scaffold. We observed tissue infiltration within the pores of β-TCP scaffolds implanted after 2 and 4 weeks (Figs. 9 and 10). This is consistent with previous work with other porous implants in which fibrous and/or bone tissue inflitration has been observed.35,50 At both 2 and 4 weeks, we observed a thin fibrous capsule around the outer surface of the scaffold as well as around the pores (Fig. 9). Formation of a thin capsule has been observed in other studies as well and suggests a mild foreign body reaction. The potential to mineral coat structurally optimized β-TCP scaffolds allows for “modular design” of scaffolds, in which the biologics carrier (i.e., the coating) is integrated into the structural device without negatively impacting scaffold physical properties. This type of modular design approach may be particularly useful for design of bone tissue engineering scaffolds, in which there is a clear need for optimized physical and biological properties.51
Release of rhVEGF from β-TCP scaffolds resulted in an increase in blood vessel tissue ingrowth (Figs. 6 and 7). The number of blood vessels increased as the dosage of rhVEGF increased from 0.5 to 10 μg (Fig. 6). The same effect of released rhVEGF in blood vessel tissue ingrowth was observed in the dual releasing scaffolds (Fig. 7), indicating that the presence of mBMP does not affect the bioactivity of the released rhVEGF in vivo. mBMP enhanced tissue infiltration to the scaffold suggesting improved tissue-material interactions, but mBMP did not induce any apparent ectopic bone formation in this study (Fig. 10). Although our previous studies have shown robust orthotopic bone formation in response to mBMP,47 ectopic bone formation was not observed in any of the groups releasing mBMP in this study. There are numerous possible reasons for this observation. Previous studies that have used the BMP2 mimicking peptide portion of mBMP to stimulate ectopic bone formation used dosages on the order of milligrams (3 mg) in rats.37 From the in vitro release data, we observed slow release of mBMP, and the initial amount of mBMP loaded into scaffolds was 50 μg. Thus, it is possible that the mBMP amount released in vivo was not sufficient to elicit a response in a sheep intramuscular implantation model. It is also possible that high densities of mBMP are required for biological activity. mBMP is a monomer and may require high epitope presentation to promote receptor multimerization and activation. Higher dosages of mBMP, high-density mBMP presentation on a biomaterial, prolonged time points for implant retrieval, and/or the use of mBMP in an orthotopic defect may be promising approaches for future studies. However, the lack of ectopic bone formation induced in the current study may be desirable when combined with our previous observations of orthotopic bone formation in response to mBMP in sheep models.45,47 In particular, the ability of mBMP to stimulate orthopic bone formation without stimulating ectopic bone formation could be advantageous, as heterotopic bone growth has led to significant side effects during clinical use of bone growth factors, such as rhBMP2.52,53 Further studies will be needed to more completely define the effects of mBMP in ectopic versus orthotopic sites.
Conclusions
This study presented a simple and adaptable approach for dual growth factor release from β-TCP scaffolds formed via solid free-form fabrication. Scaffolds were mineral-coated via incubation in mSBF, and the coating was used as a template for growth factor binding and release. In the present study, the simple approach used for dual binding consisted of sequentially dip coating mineral scaffolds in growth factor solutions. This approach allowed for control over the dosage by changing the growth factor concentration in the solution used for the dip coating process. Both growth factors tested were released in a sustained manner, with release kinetics commensurate with scaffold-growth factor affinity. When implanted in vivo, intramuscularly in sheep, rhVEGF stimulated an increase in blood vessel density. The effect was enhanced as the dosage of rhVEGF increased, and the effect was not influenced by simultaneous release of mBMP. mBMP stimulated a significant increase in tissue infiltration in the scaffold but did not stimulate ectopic bone formation.
Supplementary Material
Acknowledgments
We acknowledge the comparative orthopedic research laboratory for the preparation of the histological samples. We also thank our sources of funding, AO Research Foundation (Focus Grant # F-07-65M), National Institutes of Health (R01HL093282, R01AR059916, and T-32 DC009401), and the National Science Foundation (DMR 0906817).
Disclosure Statement
No competing financial interests exist.
References
- 1.Forte G., Minieri M., Cossa P., Antenucci D., Sala M., Gnocchi V., et al. . Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells 24,23, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Sieg D.J., Hauck C.R., Ilic D., Klingbeil C.K., Schaefer E., Damsky C.H., et al. . FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2,249, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Suarez-Gonzalez D., Lee J.S., Lan Levengood S.K., Vanderby R., Jr., and Murphy W.L.Mineral coatings modulate beta-TCP stability and enable growth factor binding and release. Acta Biomater 8,1117, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanada K., Dennis J.E., and Caplan A.I.Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J Bone Miner Res 12,1606, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Wang E.A., Rosen V., D'Alessandro J.S., Bauduy M., Cordes P., Harada T., et al. . Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A 87,2220, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Beuningen H.M., van der Kraan P.M., Arntz O.J., and van den Berg W.B.Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest 71,279, 1994 [PubMed] [Google Scholar]
- 7.Rosenblatt-Velin N., Lepore M.G., Cartoni C., Beermann F., and Pedrazzini T.FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest 115,1724, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano S., Tateya I., Kishimoto Y., Kanemaru S., and Ito J.Clinical trial of regeneration of aged vocal folds with growth factor therapy. Laryngoscope 122,327, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Patel Z.S., Young S., Tabata Y., Jansen J.A., Wong M.E., and Mikos A.G.Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43,931, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempen D.H., Lu L., Heijink A., Hefferan T.E., Creemers L.B., Maran A., et al. . Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 30,2816, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Huang Y.C., Kaigler D., Rice K.G., Krebsbach P.H., and Mooney D.J.Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res 20,848, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Kim J.H., Jung Y., Kim S.H., Sun K., Choi J., Kim H.C., et al. . The enhancement of mature vessel formation and cardiac function in infarcted hearts using dual growth factor delivery with self-assembling peptides. Biomaterials 32,6080, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Liao S.S., Guan K., Cui F.Z., Shi S.S., and Sun T.S.Lumbar spinal fusion with a mineralized collagen matrix and rhBMP-2 in a rabbit model. Spine 28,1954, 2003 [DOI] [PubMed] [Google Scholar]
- 14.McKay W.F., Peckham S.M., and Badura J.M.A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int Orthop 31,729, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards R.B., 3rd, Seeherman H.J., Bogdanske J.J., Devitt J., Vanderby R., Jr., and Markel M.D.Percutaneous injection of recombinant human bone morphogenetic protein-2 in a calcium phosphate paste accelerates healing of a canine tibial osteotomy. J Bone Joint Surg Am 86-A,1425, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kim C.S., Kim J.I., Kim J., Choi S.H., Chai J.K., Kim C.K., et al. . Ectopic bone formation associated with recombinant human bone morphogenetic proteins-2 using absorbable collagen sponge and beta tricalcium phosphate as carriers. Biomaterials 26,2501, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Aspenberg P., and Turek T.BMP-2 for intramuscular bone induction: effect in squirrel monkeys is dependent on implantation site. Acta Orthop Scand 67,3, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Jingushi S., Urabe K., Okazaki K., Hirata G., Sakai A., Ikenoue T., et al. . Intramuscular bone induction by human recombinant bone morphogenetic protein-2 with beta-tricalcium phosphate as a carrier: in vivo bone banking for muscle-pedicle autograft. J Orthop Sci 7,490, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Burkhardt R., Kettner G., Bohm W., Schmidmeier M., Schlag R., Frisch B., et al. . Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone 8,157, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Carano R.A., and Filvaroff E.H.Angiogenesis and bone repair. Drug Discov Today 8,980, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Glowacki J.Angiogenesis in fracture repair. Clin Orthop Relat Res 355Suppl,S82, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Maharaj A.S., and D'Amore P.A.Roles for VEGF in the adult. Microvasc Res 74,100, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., et al. . VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161,1163, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara N., Gerber H.P., and LeCouter J.The biology of VEGF and its receptors. Nat Med 9,669, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Bao P., Kodra A., Tomic-Canic M., Golinko M.S., Ehrlich H.P., and Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res 153,347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozawa C.R., Banfi A., Glazer N.L., Thurston G., Springer M.L., Kraft P.E., et al. . Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest 113,516, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simons M., and Ware J.A.Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov 2,863, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Li P., Ohtsuki C., Kokubo T., Nakanishi K., Soga N., Nakamura T., and Yamamuro T.Apatite formation induced by silica gel in a simulated body fluid. J Am Ceram Soc 75,2094, 1992 [Google Scholar]
- 29.Habibovic P.Biomimetic hydroxyapatite coating on metal implants. J Am Ceram Soc 85,517, 2002 [Google Scholar]
- 30.Murphy W.L., and Mooney D.J.Bioinspired growth of crystalline carbonate apatite on biodegradable polymer substrata. J Am Chem Soc 124,1910, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Zhang R., and Ma P.X.Biomimetic polymer/apatite composite scaffolds for mineralized tissue engineering. Macromol Biosci 4,100, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., Ni M., Zhang M., and Ratner B.Calcium phosphate-chitosan composite scaffolds for bone tissue engineering. Tissue Eng 9,337, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Suarez-Gonzalez D., Barnhart K., Saito E., Vanderby R., Jr., Hollister S.J., and Murphy W.L.Controlled nucleation of hydroxyapatite on alginate scaffolds for stem cell-based bone tissue engineering. J Biomed Mater Res A 95,222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suarez-Gonzalez D., Barnhart K., Migneco F., Flanagan C., Hollister S.J., and Murphy W.L.Controllable mineral coatings on PCL scaffolds as carriers for growth factor release. Biomaterials 33,713, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sohier J., Daculsi G., Sourice S., de Groot K., and Layrolle P.Porous beta tricalcium phosphate scaffolds used as a BMP-2 delivery system for bone tissue engineering. J Biomed Mater Res Part A 92,1105, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Saito A., Suzuki Y., Kitamura M., Ogata S., Yoshihara Y., Masuda S., et al. . Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an alpha-tricalcium phosphate scaffold. J Biomed Mater Res A 77,700, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Saito A., Suzuki Y., Ogata S., Ohtsuki C., and Tanihara M.Prolonged ectopic calcification induced by BMP-2-derived synthetic peptide. J Biomed Mater Res A 70,115, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Lee J.S., Wagoner Johnson A.J., and Murphy W.L.A modular, hydroxyapatite-binding version of vascular endothelial growth factor. Adv Mater 22,5494, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Richardson T.P., Peters M.C., Ennett A.B., and Mooney D.J.Polymeric system for dual growth factor delivery. Nat Biotechnol 19,1029, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Murphy W.L., Simmons C.A., Kaigler D., and Mooney D.J.Bone regeneration via a mineral substrate and induced angiogenesis. J Dent Res 83,204, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Lee J.S., Suarez-Gonzalez D., and Murphy W.L.Mineral coatings for temporally controlled delivery of multiple proteins. Adv Mater 23,4279, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J.S., Lee J.S., and Murphy W.L.Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta Biomater 6,21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J.S., Lee J.S., Wagoner-Johnson A., and Murphy W.L.Modular peptide growth factors for substrate-mediated stem cell differentiation. Angew Chem Int Ed Engl 48,6266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young S., Patel Z.S., Kretlow J.D., Murphy M.B., Mountziaris P.M., Baggett L.S., et al. . Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A 15,2347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Y., Markel M.D., Nemke B., Lee J.S., Graf B.K., and Murphy W.L.Influence of hydroxyapatite-coated and growth factor-releasing interference screws on tendon-bone healing in an ovine model. Arthroscopy 25,1427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J.S., Lu Y., Baer G.S., Markel M.D., and Murphy W.L.Controllable protein delivery from coated surgical sutures. J Mater Chem 20,8894, 2010 [Google Scholar]
- 47.Lu Y., Lee J.S., Nemke B., Graf B.K., Royalty K., Illgen R. 3rd, et al. . Coating with a modular bone morphogenetic peptide promotes healing of a bone-implant gap in an ovine model. PLoS One 7,e50378, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jongpaiboonkit L., Franklin-Ford T., and Murphy W.L.Growth of hydroxyapatite coatings on biodegradable polymer microspheres. ACS Appl Mater Interfaces 1,1504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams J.M., Adewunmi A., Schek R.M., Flanagan C.L., Krebsbach P.H., Feinberg S.E., et al. . Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 26,4817, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Maus U., Andereya S., Gravius S., Ohnsorge J.A., Niedhart C., and Siebert C.H.BMP-2 incorporated in a tricalcium phosphate bone substitute enhances bone remodeling in sheep. J Biomater Appl 22,559, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Hollister S.J., and Murphy W.L.Scaffold translation: barriers between concept and clinic. Tissue Eng Part B Rev 17,459, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cahill K.S., Chi J.H., Day A., and Claus E.B.Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA 302,58, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Shields L.B., Raque G.H., Glassman S.D., Campbell M., Vitaz T., Harpring J., et al. . Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976) 31,542, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.